Abstract
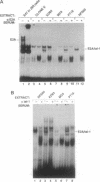
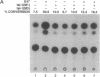
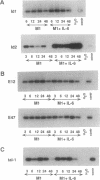
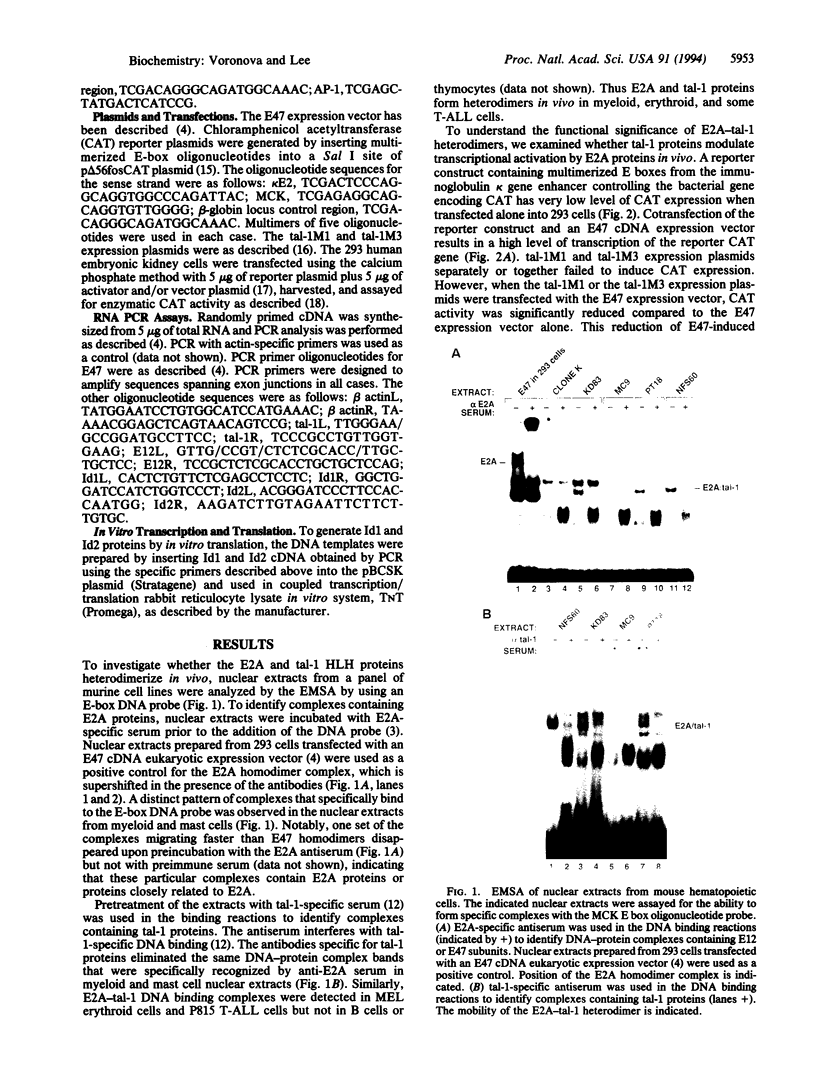
The immunoglobulin enhancer-binding proteins, E12 and E47, encoded by the E2A gene belong to the basic helix-loop-helix (bHLH) family of regulatory proteins and act as transcriptional activators. In addition to their critical role in B-lymphocyte development, the E12 and E47 proteins have been implicated in the induction of myogenesis as heterodimeric partners of myogenic bHLH proteins, MyoD and myogenin. Here we demonstrate that the E2A proteins form heterodimers with the bHLH oncoprotein tal-1 in myeloid and erythroid cells and that these heterodimers specifically bind to the CANNTG DNA motif. Heterodimerization with tal-1 represses transactivation by E47 and could function to prevent the expression of immunoglobulin genes in cells other than B lymphocytes. DNA binding by E2A-tal-1 heterodimers in the M1 mouse myeloid cell line is abrogated upon terminal macrophage differentiation induced by the cytokine interleukin 6. The loss of E2A-tal-1 DNA binding is correlated with elevated expression of mRNA encoding the dominant negative HLH proteins, Id1 and particularly Id2. Moreover, recombinant Id proteins inhibit the E2A-tal-1-specific DNA binding activity from undifferentiated M1 cells. These results suggest that E2A-tal-1 heterodimers may play a role in preventing terminal differentiation in the myeloid lineage and provide a possible explanation for oncogenic transformation induced by ectopic tal-1 expression in acute T-cell lymphoblastic leukemias.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayer D. E., Kretzner L., Eisenman R. N. Mad: a heterodimeric partner for Max that antagonizes Myc transcriptional activity. Cell. 1993 Jan 29;72(2):211–222. doi: 10.1016/0092-8674(93)90661-9. [DOI] [PubMed] [Google Scholar]
- Begley C. G., Aplan P. D., Denning S. M., Haynes B. F., Waldmann T. A., Kirsch I. R. The gene SCL is expressed during early hematopoiesis and encodes a differentiation-related DNA-binding motif. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10128–10132. doi: 10.1073/pnas.86.24.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Biggs J., Murphy E. V., Israel M. A. A human Id-like helix-loop-helix protein expressed during early development. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1512–1516. doi: 10.1073/pnas.89.4.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Yang C. Y., Tsan J. T., Xia Y., Ragab A. H., Peiper S. C., Carroll A., Baer R. Coding sequences of the tal-1 gene are disrupted by chromosome translocation in human T cell leukemia. J Exp Med. 1990 Nov 1;172(5):1403–1408. doi: 10.1084/jem.172.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. T., Cobb M. H., Baer R. Phosphorylation of the TAL1 oncoprotein by the extracellular-signal-regulated protein kinase ERK1. Mol Cell Biol. 1993 Feb;13(2):801–808. doi: 10.1128/mcb.13.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C. P., Lee F. IL-6 is a differentiation factor for M1 and WEHI-3B myeloid leukemic cells. J Immunol. 1989 Mar 15;142(6):1909–1915. [PubMed] [Google Scholar]
- Cleary M. L., Mellentin J. D., Spies J., Smith S. D. Chromosomal translocation involving the beta T cell receptor gene in acute leukemia. J Exp Med. 1988 Feb 1;167(2):682–687. doi: 10.1084/jem.167.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hsu H. L., Cheng J. T., Chen Q., Baer R. Enhancer-binding activity of the tal-1 oncoprotein in association with the E47/E12 helix-loop-helix proteins. Mol Cell Biol. 1991 Jun;11(6):3037–3042. doi: 10.1128/mcb.11.6.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreider B. L., Benezra R., Rovera G., Kadesch T. Inhibition of myeloid differentiation by the helix-loop-helix protein Id. Science. 1992 Mar 27;255(5052):1700–1702. doi: 10.1126/science.1372755. [DOI] [PubMed] [Google Scholar]
- Kretzner L., Blackwood E. M., Eisenman R. N. Myc and Max proteins possess distinct transcriptional activities. Nature. 1992 Oct 1;359(6394):426–429. doi: 10.1038/359426a0. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Davis R. L., Wright W. E., Kadesch T., Murre C., Voronova A., Baltimore D., Weintraub H. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell. 1991 Jul 26;66(2):305–315. doi: 10.1016/0092-8674(91)90620-e. [DOI] [PubMed] [Google Scholar]
- Mouthon M. A., Bernard O., Mitjavila M. T., Romeo P. H., Vainchenker W., Mathieu-Mahul D. Expression of tal-1 and GATA-binding proteins during human hematopoiesis. Blood. 1993 Feb 1;81(3):647–655. [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Murre C., Voronova A., Baltimore D. B-cell- and myocyte-specific E2-box-binding factors contain E12/E47-like subunits. Mol Cell Biol. 1991 Feb;11(2):1156–1160. doi: 10.1128/mcb.11.2.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. W., Gifford A. M., Baltimore D. Silencing of the expression of the immunoglobulin kappa gene in non-B cells. Mol Cell Biol. 1991 Mar;11(3):1431–1437. doi: 10.1128/mcb.11.3.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G. C., Lawe D., Ziff E. B. Association of Myn, the murine homolog of max, with c-Myc stimulates methylation-sensitive DNA binding and ras cotransformation. Cell. 1991 May 3;65(3):395–407. doi: 10.1016/0092-8674(91)90457-a. [DOI] [PubMed] [Google Scholar]
- Reddy P. M., Shen C. K. Protein-DNA interactions in vivo of an erythroid-specific, human beta-globin locus enhancer. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8676–8680. doi: 10.1073/pnas.88.19.8676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel M., Voronova A., Baltimore D. Helix-loop-helix transcription factor E47 activates germ-line immunoglobulin heavy-chain gene transcription and rearrangement in a pre-T-cell line. Genes Dev. 1991 Aug;5(8):1367–1376. doi: 10.1101/gad.5.8.1367. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. H., Copeland N. G., Jenkins N. A., Baltimore D. Id proteins Id1 and Id2 selectively inhibit DNA binding by one class of helix-loop-helix proteins. Mol Cell Biol. 1991 Nov;11(11):5603–5611. doi: 10.1128/mcb.11.11.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Brown L., Yang C. Y., Tsan J. T., Siciliano M. J., Espinosa R., 3rd, Le Beau M. M., Baer R. J. TAL2, a helix-loop-helix gene activated by the (7;9)(q34;q32) translocation in human T-cell leukemia. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11416–11420. doi: 10.1073/pnas.88.24.11416. [DOI] [PMC free article] [PubMed] [Google Scholar]