Abstract
BACKGROUND AND OBJECTIVES:
Despite previous studies demonstrating no difference in mortality or morbidity, the various surgical approaches for necrotizing enterocolitis (NEC) in infants have not been evaluated economically. Our goal was to compare total in-hospital cost and mortality by using propensity score–matched infants treated with peritoneal drainage alone, peritoneal drainage followed by laparotomy, or laparotomy alone for surgical NEC.
METHODS:
Utilizing the California OSHPD Linked Birth File Dataset, 1375 infants with surgical NEC between 1999 and 2007 were retrospectively propensity score matched according to intervention type. Total in-hospital costs were converted from longitudinal patient charges. A multivariate mixed effects model compared adjusted costs and mortality between groups.
RESULTS:
Successful propensity score matching was performed with 699 infants (peritoneal drainage, n = 101; peritoneal drainage followed by laparotomy, n = 172; and laparotomy, n = 426). Average adjusted cost for peritoneal drainage followed by laparotomy was $398 173 (95% confidence interval [CI]: 287 784–550 907), which was more than for peritoneal drainage ($276 076 [95% CI: 196 238–388 394]; P = .004) and similar to laparotomy ($341 911 [95% CI: 251 304–465 186]; P = .08). Adjusted mortality was highest after peritoneal drainage (56% [95% CI: 34–75]) versus peritoneal drainage followed by laparotomy (35% [95% CI: 19–56]; P = .01) and laparotomy (29% [95% CI: 19–56]; P < .001). Mortality for peritoneal drainage was similar to laparotomy.
CONCLUSIONS:
Propensity score–matched analysis of surgical NEC treatment found that peritoneal drainage followed by laparotomy was associated with decreased mortality compared with peritoneal drainage alone but at significantly increased costs.
Keywords: cost analysis, mortality, necrotizing enterocolitis, prematurity, surgery
What’s Known on This Subject:
Mortality rates and health care expenditures are high among infants requiring surgery for necrotizing enterocolitis. The impact of different surgical managements on mortality remains equivocal. Adjusted economic differences for various surgical treatments may exist but have not been elucidated.
What This Study Adds:
After performing a relatively large-scale, adjusted analysis of cost and mortality for surgical managements currently used for treating necrotizing enterocolitis, a cost-benefit for a particular surgical approach was demonstrated while accounting for comorbidities and group assignment bias.
Necrotizing enterocolitis (NEC) is a highly morbid, life-threatening condition affecting infants, particularly preterm neonates.1,2 Despite modern advances in neonatal care, mortality remains frequent, with rates reported between 18% and 63%.2–8 Clinical management of critically ill neonates,9 and with NEC in particular, carries considerable financial costs.10,11
If medical management fails, operative management is pursued, including either laparotomy or placement of a percutaneous peritoneal drain. Ein et al12 originally described peritoneal drainage as a technique to physiologically stabilize infants and bridge them to definitive laparotomy. Recently, peritoneal drains have been used more broadly. Drains are now commonly used in 3 ways: as intended definitive management; as an initial management attempt with an interval laparotomy only if the infant fails to improve; or finally as a planned bridge to definitive laparotomy.13–15
Prospective studies have found no difference in mortality between laparotomy and peritoneal drainage for surgical NEC.16,17 In these studies, however, a large and variable percentage of patients in the peritoneal drainage group eventually received a laparotomy (35%–74%), potentially limiting the validity of the intention-to-treat analyses.18 Given the crossover observed in these studies, as well as the restrictive inclusion criteria of clinical trials, a new approach may be valuable. Ultimately, the 3 options for surgical management of NEC must be compared: initial peritoneal drainage, peritoneal drainage followed by laparotomy, and laparotomy alone. The highest value treatment of these 3 surgical management options may best be identified by using an as-treated comparison.
In addition to the important issue of mortality, NEC and its management are extremely costly. Two studies have estimated that the average charge in the United States associated with a case of surgical NEC is $400 000 to $500 000.5,11 One prospective study, which considered hospital cost, found that the costs of surgical NEC were between $300 000 and $660 000.15 The choice of surgical treatment for NEC may significantly affect hospital costs. Peritoneal drain placement is typically a short, bedside procedure and may be less costly than a formal laparotomy, which requires operating room time and specialized staff. Peritoneal drainage followed by laparotomy may be even more costly than either procedure alone.15,19 Although NEC represents a significant burden on infant health and health care dollars, the highest value intervention has never been identified.
Cost and mortality are informative benchmarks for guiding surgical decision-making. The primary goal of the present study was to identify whether hospital costs for infants with NEC varied significantly according to the type of surgical approach used. Given the considerable mortality associated with surgical NEC, the secondary goal was to compare mortality rates among propensity score–matched infants with surgical NEC managed by peritoneal drainage alone, peritoneal drainage followed by laparotomy, or laparotomy alone.
Methods
Data Source and Measures
The present study examined the Linked Birth File Dataset obtained from the California Office of Statewide Health Planning and Development (OSHPD), which collects information on all infant and associated maternal hospital discharges in California combined with infant vital statistics records. Each record includes patient demographic characteristics (age, gender, county of residence, and race/ethnicity), diagnoses (up to 24 per admission), procedures (up to 20 per admission) and procedure dates, disposition including death or discharge from the hospital, total charges, and expected source of payment. Diagnoses and procedures are coded by using the International Classification of Diseases, Ninth Revision (ICD-9) codes; pertinent ICD-9 codes used for this study are listed in Supplemental Table 5. Near 100% deterministic and probabilistic linkage of longitudinal patient admissions was included by OSHPD for up to 1 year of age in the data set. The Birth Linked Cohort Dataset was merged with the cost-to-charge ratio files from the Healthcare Cost and Utilization Project (HCUP), as well as the American Academy of Pediatrics designation of NICU level by hospital. The study was approved by the University of California, Los Angeles institutional review board and the Committee for the Protection of Human Subjects of the California Health and Human Services Agency.
Any infant treated in high-level NICUs for surgical NEC recorded in the data set between 1999 and 2007 was eligible for inclusion. Exclusion criteria were: (1) treatment at low-level NICUs, defined as level I, level II, and level IIIA, which may have inconsistent access to pediatric surgical capabilities; and (2) fatal congenital abnormalities such as trisomy 13, trisomy 18, and significant cardiac defects.
The 2 outcomes of interest were mortality and cost. In-hospital mortality was extracted directly from the data set. Because the United States has a multipayer system, 3 dollar amounts are generally ascribed to any health service: (1) the cost to the hospital system (hospital and providers) for production of care; (2) the charge that the hospital system requests from the payer (patient and insurance); and (3) the actual payment to the hospital system as an expense by the payer. The present analysis focused only on the first 2 metrics: costs and charges. Every fiscal year, hospitals report their annual sum of costs and charges. This information is used to construct publicly available cost-to-charge ratios for each hospital for each fiscal year. Costs were calculated from the total patient charges in the OSHPD data set (summed across all transfers and inflated to 2013 dollars by using the Medicare market basket index) multiplied by the hospital-specific, fiscal year cost-to-charge ratio.
The primary predictor of interest was the type of surgical management; it was identified by using ICD-9 procedure codes (Supplemental Table 5). Surgical management included: (1) peritoneal drainage alone; (2) peritoneal drainage followed by laparotomy; and (3) laparotomy alone. Secondary predictors were age, birth weight, gestational age, gender, race, and congenital comorbid diagnoses. The comorbidities were selected based on their potential for affecting mortality and were used as dichotomous variables. Gestational age and birth weight were converted into categorical variables based on ICD-9 coding because the relationship with the outcomes was assumed to be nonlinear; this action enabled imputation of missing data points derived from diagnosis data. After this imputation, 5.2% of infants’ gestational age and 0.4% of infants’ birth weight were excluded from further analysis due to missing data.
Propensity Score Matching
Considerable selection bias is present when the surgical treatment is chosen.20 To overcome this bias, propensity score matching was used.21 The following initial analyses were performed to identify the variables necessary to include in the propensity score matching. First, a χ2 test was used to identify any significant differences among the 3 surgical treatment groups for the categorical variables. A Kruskal-Wallis test was used to compare continuous variables among treatment groups. Propensity score matching was used to limit the effect of selection bias in the final comparison of outcomes among treatment groups. The outcome for the propensity score model was the probability of initially undergoing peritoneal drainage. To obtain 3 propensity score–matched cohorts, another propensity score model was run for the probability of undergoing peritoneal drainage followed by laparotomy. Variables used in the probability formulas for matching were age at surgery, birth weight, gestational age, gender, race, and congenital comorbid diagnoses. One to 2 matching was performed to conserve sample size.
Statistical Analyses
All subsequent analyses were performed with the propensity score–matched cohorts. A negative binomial mixed effects model was used to analyze cost because its distribution was highly skewed. A logistic mixed effects model was used to analyze mortality. The fixed effects in each model included the surgical treatment group, the primary predictor, as well as age at initial surgery, birth weight, gestational age, gender, race, and congenital comorbid diagnoses. To account for hospital-specific variation in costs and mortality, a hospital random intercept was included in both the negative binomial and the logistic models. The least squares mean cost and the least squares mean mortality of each surgical treatment group were calculated and compared among treatment groups adjusting for multiple comparisons after controlling for age, birth weight, gestational age, gender, race, and congenital comorbid diagnoses.
To identify whether medical futility was disproportionately present in the peritoneal drainage group, a subanalysis of cost according to mortality outcome was performed. Three mortality outcomes were defined: mortality before 29 days of age (ie, early mortality), mortality 29 days to 1 year of age (ie, late mortality), and survival. The mortality rate and total cost at each time point were compared among surgical treatment groups by using χ2 and Kruskal-Wallis tests, respectively. Finally, the cost per day was analyzed to determine whether the intensity of treatment (ie, quantity of health care resources per day) differed among surgical groups; a negative binomial mixed effects model was used with an offset for length of stay. All analyses and data management were performed by using SAS/STAT version 9.3 (SAS Institute, Inc, Cary, NC; 2002–2010).
Results
The initial cohort before propensity score matching consisted of 1375 infants; 186 underwent peritoneal drainage alone, 202 underwent peritoneal drainage followed by laparotomy, and 987 underwent laparotomy alone. Of note, 54% of infants who underwent peritoneal drainage also later had a laparotomy. Birth weight, gestational age, respiratory distress syndrome, cardiovascular abnormality, and pulmonary hemorrhage differed significantly among the 3 unmatched groups (Table 1). Preliminary analysis found that the smaller, sicker infants were significantly more likely to undergo initial peritoneal drainage compared with healthier infants. A total of 699 infants were successfully propensity score matched; 101 underwent peritoneal drainage alone, 172 underwent peritoneal drainage followed by laparotomy, and 426 underwent laparotomy alone. After matching, no differences were found between treatment groups with regard to age, birth weight, gestational age, gender, race, or rates of congenital comorbidities (Table 2). All subsequent reported analyses are of these propensity score–matched cohorts.
TABLE 1.
Demographic Characteristics, Comorbidities, and Unadjusted Outcomes of 1375 Surgical NEC Infants Without Propensity Score Matching
| Variable | Peritoneal Drainage Alone (n = 186) | Peritoneal Drainage and Laparotomy (n = 202) | Laparotomy Alone (n = 987) | P |
|---|---|---|---|---|
| Gestational age, wk | 26 (25–30) | 28 (25–33) | 29 (26–35) | <.001 |
| Birth weight, g | 765 (627–1065) | 840 (682–1515) | 1077 (765–1942) | <.001 |
| Age at initial procedure, d | 21 (9–46) | 15 (8–30) | 18 (8–41) | .6 |
| Gender, % male | 55 | 63 | 61 | .3 |
| White | 62 | 58 | 60 | .9 |
| African American | 12 | 13 | 12 | .9 |
| Hispanic | 46 | 50 | 50 | .7 |
| Level IIIC NICU | 28 | 18 | 22 | .1 |
| Sepsis | 76 | 73 | 69 | .2 |
| Thrombocytopenia | 59 | 61 | 53 | .06 |
| Pulmonary hemorrhage | <10 | <10 | <10 | .03 |
| Respiratory distress syndrome | 88 | 84 | 78 | .01 |
| Cardiovascular abnormalitya | 62 | 50 | 46 | .002 |
| Intraventricular hemorrhage grades 3–4 | <10 | <10 | <10 | .06 |
| Mortality | 57 | 35 | 30 | <.001 |
| Charges, $ | 469 991 (205 553–1 069 932) | 829 804 (358 800–1 354 421) | 588 551 (246 213–1 025 573) | .16 |
| Length of stay, d | 55 (20–76) | 103 (46–117) | 79 (31–97) | <.001 |
Data are presented as median (25%–75% interquartile range) or percentages, small value disclosure is limited due to data use agreement.
Includes patent ductus arteriosus, ventricular septal defect, and/or atrial septal defect.
TABLE 2.
Demographic Characteristics and Comorbidities of Infants According to Propensity Score–Matched Treatment Group
| Variable | Peritoneal Drainage Alone (n = 101) | Peritoneal Drainage and Laparotomy (n = 172) | Laparotomy Alone (n = 426) | P |
|---|---|---|---|---|
| Gestational age, wk | 27 (25–30) | 28 (25–33) | 27 (25–32) | .33 |
| Birth weight, g | 770 (627–1075) | 845 (687–1531) | 867 (694–1486) | .06 |
| Age at initial procedure, d | 23 (10–48) | 15 (8–30) | 21 (8–44) | .37 |
| Gender, % male | 54 | 62 | 61 | .34 |
| White | 63 | 57 | 60 | .58 |
| African American | 10 | 13 | 12 | .77 |
| Hispanic | 45 | 49 | 50 | .68 |
| Level IIIC NICU | 27 | 19 | 23 | .25 |
| Sepsis | 52 | 59 | 51 | .20 |
| Disseminated intravascular coagulopathy | 12 | <10 | <10 | .14 |
| Thrombocytopenia | 33 | 36 | 35 | .82 |
| Pulmonary hemorrhage | <10 | <10 | <10 | .21 |
| Respiratory distress syndrome | 76 | 64 | 66 | .06 |
| Cardiovascular abnormalitya | 62 | 50 | 52 | .08 |
| Intraventricular hemorrhage grades 3–4 | <10 | <10 | <10 | .06 |
Data are presented as median (25%–75% interquartile range) or percentages, small value disclosure is limited due to data use agreement.
Includes patent ductus arteriosus, ventricular septal defect, and/or atrial septal defect.
Despite balance on clinical variables, comparison of the propensity score–matched treatment groups demonstrated a significant difference in unadjusted outcomes (Table 3). Unadjusted mortality was highest in the peritoneal drainage group compared with the peritoneal drainage followed by laparotomy and laparotomy alone groups. Unadjusted in-hospital charges did not significantly differ among groups, likely as a result of the large variance. Unadjusted in-hospital cost and length of stay were significantly greater in infants who underwent peritoneal drainage followed by laparotomy compared with peritoneal drainage alone and laparotomy alone. In subanalysis comparing time to mortality, the majority of infant mortalities in the peritoneal drainage group had early mortality that occurred before 29 days of age. In contrast, the majority of infant mortalities with peritoneal drainage followed by laparotomy and laparotomy alone occurred at ≥29 days (late mortality).
TABLE 3.
Unadjusted Mortality and Resource Use of Infants According to Propensity Score–Matched Treatment Group
| Unadjusted Outcome | Peritoneal Drainage Alone (n = 101) | Peritoneal Drainage and Laparotomy (n = 172) | Laparotomy Alone (n = 426) | P |
|---|---|---|---|---|
| Mortality | 55 | 35 | 32 | <.001 |
| Early mortality (<29 d) | 29 | <10 | 12 | |
| Late mortality (≥29 d) | 26 | 28 | 19 | |
| Total charges, $a | 543 278 (368 251–1 368 820) | 880 443 (604 185–1 714 674) | 796 276 (440 616–1 540 211) | .78 |
| Total costs, $a | 225 650 (95 965–480 343) | 318 259 (160 148–623 663) | 282 854 (131 376–464 985) | <.001 |
| Length of stay, d | 51 (23–115) | 104 (49–146) | 91 (41–129) | <.001 |
Data are presented as percentages or median (25%–75% interquartile range), small value disclosure is limited due to data use agreement.
Charges, costs, and length of stay are for in-hospital to initial discharge from the hospital or in-hospital death.
Mortality and cost were then adjusted for clinical variables to derive the best estimates of treatment effect after propensity matching. Infants who underwent peritoneal drainage alone had significantly higher adjusted mortality compared with laparotomy alone and peritoneal drainage followed by laparotomy (Table 4); adjusted mortality after laparotomy alone and peritoneal drainage followed by laparotomy was not significantly different. Average adjusted in-hospital costs of the laparotomy alone group and the peritoneal drainage alone group were comparable. However, the group of infants with peritoneal drainage followed by laparotomy had significantly higher costs than those who underwent peritoneal drainage alone. In summary, peritoneal drainage followed by laparotomy was the most costly management strategy after adjustment for comorbidities and potential treatment group bias.
TABLE 4.
Adjusted Mortality and Total In-hospital Cost of Infants According to Propensity Matched–Treatment Group
| Adjusteda Outcome | Peritoneal Drainage Alone (n = 101) | Peritoneal Drainage and Laparotomy (n = 172) | Laparotomy Alone (n = 426) |
|---|---|---|---|
| Mortality, % | 56 (34–75)REF | 35 (19–56)b | 29 (16–48)c |
| Total costs, $ | 276 076 (196 238–388 394)REF | 398 173 (287 784–550 907)d | 341 911 (251 304–465 186)e |
Data are least squares mean (95% confidence interval). Costs are for in-hospital to initial discharge or in-hospital death. REF, referent group for comparison.
Adjusted for age, birth weight, gestational age, gender, race, respiratory distress syndrome, other respiratory disorders, intraventricular hemorrhage, cardiovascular abnormality, pulmonary hemorrhage, sepsis, disseminated intravascular coagulation, and thrombocytopenia.
P = .01 compared with referent group.
P < .001 compared with referent group.
P = .004 compared with referent group.
P = .14 compared with referent group.
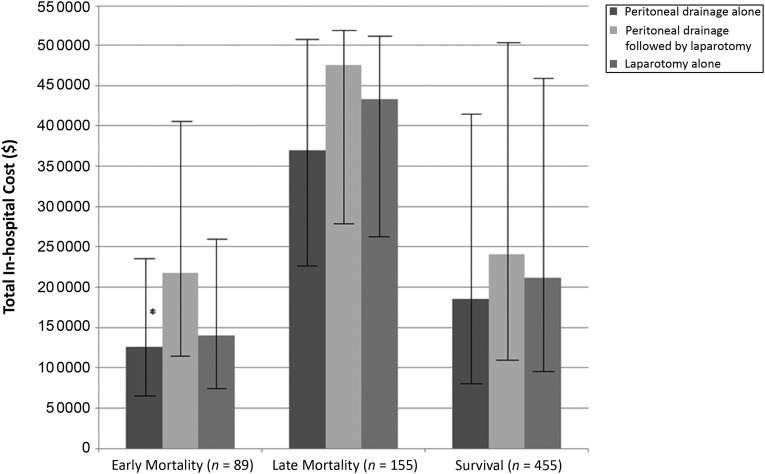
Not only did more infants undergoing peritoneal drainage alone experience early mortality, but this early mortality was less costly compared with early mortality in infants undergoing peritoneal drainage followed by laparotomy ($125 755 vs $218 015; P = .019) (Fig 1). No differences in cost were seen among infants who developed late mortality and among infants who survived. This finding demonstrates that the lower costs associated with early mortality in infants undergoing peritoneal drainage alone may be what drives the lower costs in patients undergoing peritoneal drainage procedures overall.
FIGURE 1.
Total in-hospital cost according to propensity score–matched treatment group for infants with early mortality, late mortality, or survival. Comparison of least squares mean cost among these groups was performed by using Tukey’s method for multiple comparisons of propensity score–matched cohorts of infants with surgical NEC according to time to outcome. Error bars represent 95% confidence intervals. *P = .02 for peritoneal drainage alone versus peritoneal drainage followed by laparotomy.
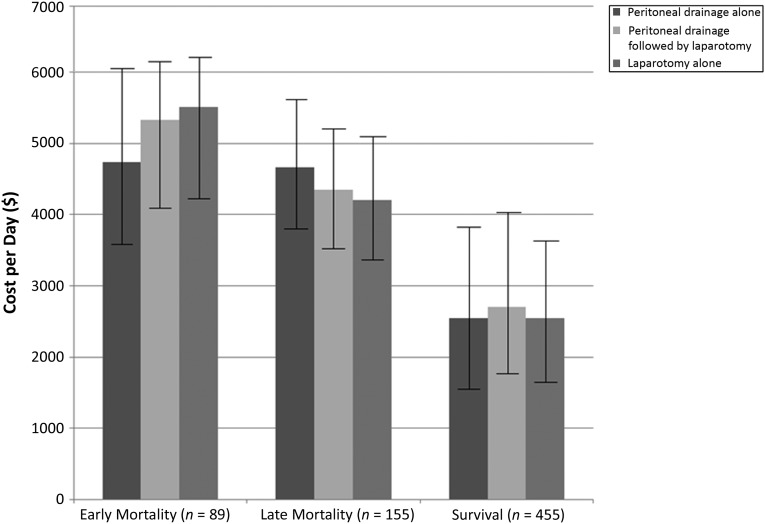
To assess if this difference in cost of early mortality in infants in the peritoneal drainage alone group was the result of differences in the provision of health care, cost per day was analyzed as a proxy for intensity of care. However, the cost per day was not significantly different among surgical treatment groups for infants who died early (Fig 2). Thus, these findings illustrate that among matched surgical treatment groups, infants who died early received comparable intensity of care.
FIGURE 2.
Cost per day according to propensity score–matched treatment group for infants with early mortality, late mortality, or survival. Comparison of least squares mean cost per day were performed among the treatment groups by using Tukey’s method for multiple comparisons. Error bars represent 95% confidence intervals.
Discussion
According to the World Health Organization, US health care costs are ∼17% of 2012 gross domestic product.22 Fundamental markers of health (eg, infant mortality rate) in the US remain worse than most of Western Europe. To make more explicit value-based decisions, outcomes and their associated costs must be considered. In the present propensity score–matched analysis, infants who underwent laparotomy alone and infants who underwent peritoneal drainage followed by laparotomy had comparably lower mortality rates than infants who underwent peritoneal drainage alone. The total in-hospital costs of the laparotomy alone and peritoneal drainage alone management strategies were not significantly different. However, peritoneal drainage followed by laparotomy demonstrated significantly higher total in-hospital costs than peritoneal drainage alone. Due to the broad range of costs exhibited in a cohort of this size, other significant differences in total in-hospital costs may have been more elusive to capture. Future analyses examining other patient-centered, disease-specific outcome measures may additionally guide surgical management strategies.23 An economic analysis of documented long-term neurodevelopmental24 outcomes and associated resource use may provide some of this valuable information.
The mortality rates in the present study were within the published ranges of 18% to 63%.2–8 Some retrospective studies have similarly demonstrated that infants undergoing peritoneal drainage alone may have higher morbidity and mortality.1,19,25–27 Prospective trials have not shown a difference, perhaps as a result of an intention-to-treat analysis with high rates of crossover (35%–74%) in which the peritoneal drainage group included infants who underwent peritoneal drainage followed by laparotomy.16,17
The total cost of care for a patient population is determined primarily according to 2 factors: the intrinsic costs of the surgical condition itself (inclusive of the comparatively small procedural costs), and the total quantity of care provided due to comorbidities. The total cost of care for infants with surgical NEC in the present study was comparable to that found in a previous single-center study (ie, $300 000–$600 000).15 The intrinsic costs of surgical NEC, or marginal costs, have been estimated to be between $22 328 and $198 040.10,28 The intrinsic costs of surgical NEC thus account for a fraction of the total costs of care once comorbidities are accounted for. The equivalence in cost between patients treated with peritoneal drainage alone and laparotomy alone in this study is likely a result of using matched cohorts with equivalent comorbidity rates. Nonetheless, the addition of a second surgical procedure (peritoneal drainage followed by laparotomy) significantly increased the total cost of care. This second surgical procedure was performed for more than one-half of the patients in this data set, which is consistent with the range reported in the literature (22%–74%).15–17,19,24,29,30 The known drivers of surgical costs in infants who underwent peritoneal drainage followed by laparotomy include increased length of stay19 and postdrainage clinical decompensation.15,31
The present study was limited by the administrative nature of the data set. It is difficult to capture severity of disease by using ICD-9 codes,32 which may be poorly reported and thus result in unmeasured variable bias. Such bias was minimized through the use of propensity score matching and postestimation comparisons.33 Second, because this study used administrative data, the treatment group definition depended on appropriate billing of the procedure codes. If the procedure codes were overcoded or undercoded, it is possible that patients were inaccurately categorized. This effect was likely minimized, however, because the study analyzed a specific neonatal diagnosis associated with a specific procedure, thereby increasing the likelihood of accurate capture. Third, the institutional cost-to-charge ratios available from the HCUP data set are an average of many individual cost centers’ cost-to-charge ratios. As a result, cost estimates may be less precise estimates of actual costs. Relatedly, certain costs may be less accurately reflected by billing charges (particularly the extent of postoperative care that is bundled into procedural charges, whether it is management of peritoneal drains or stomal complications). These costs may not be incorporated into the cost estimates derived from these administrative data and may be underestimated as a result. However, HCUP institutional cost-to-charge ratios are commonly used and are widely regarded as acceptable adjustments for estimating the cost of production of care.34 Fourth, the actual intent to treat of these infants was unknown, which limits the analysis that can be performed to a comparison of the ultimate management strategy or an as-treated comparison. Finally, external generalizability may be limited based on the data source. Although the state of California is large and ethnically diverse, the structure of the US health care system has decentralized care with a surplus of facilities seeking to provide neonatal intensive care, due largely to favorable reimbursement compared with actual hospital cost.35–37 As such, organizations with varying degrees of resources, support, and expertise provide vastly different care with different degrees of efficiency.38 In other settings, in which pediatric surgical care is more centralized to specific referral centers, care processes, outcomes, and costs may be more standardized, thus reducing the noise and possibly yielding a clearer signal-to-noise ratio of the potential differences in outcomes and costs of the surgical management of NEC.
The implications of the present research are twofold. First, it suggests that peritoneal drainage followed by laparotomy provides equally good outcomes as laparotomy alone for infants with surgical NEC. Second, it implies that the cost burden of peritoneal drainage followed by laparotomy is large enough that the need for performing both procedures should be considered before initial peritoneal drainage. As a result, an intent-to-treat type of analysis that includes costs is needed to gain further insight into which initial treatment is the highest value intervention.
Conclusions
Peritoneal drainage followed by laparotomy was associated with decreased mortality compared with peritoneal drainage alone but at significantly increased costs. These findings imply that economic analyses of prospectively gathered cost data are needed to determine not only which intervention has the best outcomes but the highest value.
Supplementary Material
Footnotes
Dr Stey participated in data acquisition, data analysis and interpretation, and drafting of the manuscript; Dr Barnert participated in conception and design and revised the manuscript critically for important intellectual content; Dr Leng participated in data analysis and interpretation and revised the manuscript critically for important intellectual content; Drs Tseng, Needleman, Keeler, and Kelley-Quon participated in providing conception and design and revising the manuscript critically for important intellectual content; and Dr Shew participated in providing conception and design, drafting the manuscript, and revising it critically for important intellectual content. All authors gave final approval of the version to be published, and all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
FINANCIAL DISCLOSURE: The Robert Wood Johnson Clinical Scholars Program provided support to Drs Stey and Barnert, and the National Institutes of Health and the Fubon Foundation provided support to Dr Shew; the other authors have indicated they have no financial relationships relevant to this article to disclose. The Robert Wood Johnson Foundation, the Fubon Foundation, and National Institutes of Health had no role in the preparation, review, or approval of the manuscript.
FUNDING: The Fubon Foundation and the National Institutes of Health (grant HD052885) provided support to the project. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Kelley-Quon LI, Tseng CH, Scott A, Jen HC, Calkins KL, Shew SB. Does hospital transfer predict mortality in very low birth weight infants requiring surgery for necrotizing enterocolitis? Surgery. 2012;152(3):337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eicher C, Seitz G, Bevot A, et al. Surgical management of extremely low birth weight infants with neonatal bowel perforation: a single-center experience and a review of the literature. Neonatology. 2012;101(4):285–292 [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Moore SW, Sidler D, Kirsten GF. Long-term outcome of surgically managed necrotizing enterocolitis in a developing country. Pediatr Surg Int. 2010;26(4):355–360 [DOI] [PubMed] [Google Scholar]
- 4.Fitzgibbons SC, Ching Y, Yu D, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. 2009;44(6):1072–1075, discussion 1075–1076 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Ortega G, Camp M, Osen H, Chang DC, Abdullah F. Necrotizing enterocolitis requiring surgery: outcomes by intestinal location of disease in 4371 infants. J Pediatr Surg. 2011;46(8):1475–1481 [DOI] [PubMed] [Google Scholar]
- 6.Pang KK, Chao NS, Wong BP, Leung MW, Liu KK. The clip and drop back technique in the management of multifocal necrotizing enterocolitis: a single centre experience. Eur J Pediatr Surg. 2012;22(1):85–90 [DOI] [PubMed] [Google Scholar]
- 7.Thyoka M, Eaton S, Kiely EM, et al. Outcomes of diverting jejunostomy for severe necrotizing enterocolitis. J Pediatr Surg. 2011;46(6):1041–1044 [DOI] [PubMed] [Google Scholar]
- 8.Sakellaris G, Partalis N, Dede O, et al. Gastrointestinal perforations in neonatal period: experience over 10 years. Pediatr Emerg Care. 2012;28(9):886–888 [DOI] [PubMed] [Google Scholar]
- 9.St John EB, Nelson KG, Cliver SP, Bishnoi RR, Goldenberg RL. Cost of neonatal care according to gestational age at birth and survival status. Am J Obstet Gynecol. 2000;182(1 pt 1):170–175 [DOI] [PubMed] [Google Scholar]
- 10.Ganapathy V, Hay JW, Kim JH. Costs of necrotizing enterocolitis and cost-effectiveness of exclusively human milk-based products in feeding extremely premature infants. Breastfeed Med. 2012;7(1):29–37 [DOI] [PubMed] [Google Scholar]
- 11.Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics. 2002;109(3):423–428 [DOI] [PubMed] [Google Scholar]
- 12.Ein SH, Marshall DG, Girvan D. Peritoneal drainage under local anesthesia for perforations from necrotizing enterocolitis. J Pediatr Surg. 1977;12(6):963–967 [DOI] [PubMed] [Google Scholar]
- 13.Ein SH, Shandling B, Wesson D, Filler RM. A 13-year experience with peritoneal drainage under local anesthesia for necrotizing enterocolitis perforation. J Pediatr Surg. 1990;25(10):1034–1036, discussion 1036–1037 [DOI] [PubMed] [Google Scholar]
- 14.Morgan LJ, Shochat SJ, Hartman GE. Peritoneal drainage as primary management of perforated NEC in the very low birth weight infant. J Pediatr Surg. 1994;29(2):310–314, discussion 314–315 [DOI] [PubMed] [Google Scholar]
- 15.Dimmitt RA, Meier AH, Skarsgard ED, Halamek LP, Smith BM, Moss RL. Salvage laparotomy for failure of peritoneal drainage in necrotizing enterocolitis in infants with extremely low birth weight. J Pediatr Surg. 2000;35(6):856–859 [DOI] [PubMed] [Google Scholar]
- 16.Rees CM, Eaton S, Kiely EM, Wade AM, McHugh K, Pierro A. Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann Surg. 2008;248(1):44–51 [DOI] [PubMed] [Google Scholar]
- 17.Moss RL, Dimmitt RA, Barnhart DC, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med. 2006;354(21):2225–2234 [DOI] [PubMed] [Google Scholar]
- 18.Raval MV, Hall NJ, Pierro A, Moss RL. Evidence-based prevention and surgical treatment of necrotizing enterocolitis—a review of randomized controlled trials. Semin Pediatr Surg. 2013;22(2):117–121 [DOI] [PubMed] [Google Scholar]
- 19.Choo S, Papandria D, Zhang Y, et al. Outcomes analysis after percutaneous abdominal drainage and exploratory laparotomy for necrotizing enterocolitis in 4,657 infants. Pediatr Surg Int. 2011;27(7):747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moss RL, Dimmitt RA, Henry MC, Geraghty N, Efron B. A meta-analysis of peritoneal drainage versus laparotomy for perforated necrotizing enterocolitis. J Pediatr Surg. 2001;36(8):1210–1213 [DOI] [PubMed] [Google Scholar]
- 21.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 pt 2):757–763 [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. United States of America. Available at: www.who.int/countries/usa/en/. Accessed February 27, 2015 [Google Scholar]
- 23.Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–2481 [DOI] [PubMed] [Google Scholar]
- 24.Blakely ML, Tyson JE, Lally KP, et al. NICHD Neonatal Research Network . Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: outcomes through 18 months adjusted age. Pediatrics. 2006;117(4). Available at: www.pediatrics.org/cgi/content/full/117/4/e680 [DOI] [PubMed] [Google Scholar]
- 25.Rakshasbhuvankar A, Rao S, Minutillo C, Gollow I, Kolar S. Peritoneal drainage versus laparotomy for perforated necrotising enterocolitis or spontaneous intestinal perforation: a retrospective cohort study. J Paediatr Child Health. 2012;48(3):228–234 [DOI] [PubMed] [Google Scholar]
- 26.Sola JE, Tepas JJ, 3rd, Koniaris LG. Peritoneal drainage versus laparotomy for necrotizing enterocolitis and intestinal perforation: a meta-analysis. J Surg Res. 2010;161(1):95–100 [DOI] [PubMed] [Google Scholar]
- 27.Tepas JJ, 3rd, Sharma R, Hudak ML, Garrison RD, Pieper P. Coming full circle: an evidence-based definition of the timing and type of surgical management of very low-birth-weight (<1000 g) infants with signs of acute intestinal perforation. J Pediatr Surg. 2006;41(2):418–422 [DOI] [PubMed] [Google Scholar]
- 28.Johnson TJ, Patel AL, Jegier BJ, Engstrom JL, Meier PP. Cost of morbidities in very low birth weight infants. J Pediatr. 2013;162(2):243–249.e1 [DOI] [PMC free article] [PubMed]
- 29.Rees CM, Eaton S, Pierro A. National prospective surveillance study of necrotizing enterocolitis in neonatal intensive care units. J Pediatr Surg. 2010;45(7):1391–1397 [DOI] [PubMed] [Google Scholar]
- 30.Rao SC, Basani L, Simmer K, Samnakay N, Deshpande G. Peritoneal drainage versus laparotomy as initial surgical treatment for perforated necrotizing enterocolitis or spontaneous intestinal perforation in preterm low birth weight infants. Cochrane Database Syst Rev. 2011;(6):CD006182. [DOI] [PubMed] [Google Scholar]
- 31.Rees CM, Eaton S, Khoo AK, Kiely EM, Pierro A, Members of NET Trial Group . Peritoneal drainage does not stabilize extremely low birth weight infants with perforated bowel: data from the NET Trial. J Pediatr Surg. 2010;45(2):324–328, discussion 328–329 [DOI] [PubMed] [Google Scholar]
- 32.Hannan EL, Kilburn H, Jr, Lindsey ML, Lewis R. Clinical versus administrative data bases for CABG surgery. Does it matter? Med Care. 1992;30(10):892–907 [DOI] [PubMed] [Google Scholar]
- 33.D’Agostino RB, Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281 [DOI] [PubMed] [Google Scholar]
- 34.Sun BC, Hsia RY, Weiss RE, et al. Effect of emergency department crowding on outcomes of admitted patients. Ann Emerg Med. 2013;61(6):605–611.e6 [DOI] [PMC free article] [PubMed]
- 35.Berman L, Rosenthal MS, Moss RL. The paradoxical effect of medical insurance on delivery of surgical care for infants with congenital anomalies. J Pediatr Surg. 2010;45(1):38–43, discussion 44 [DOI] [PubMed] [Google Scholar]
- 36.Gagnon D, Allison-Cooke S, Schwartz RM. Perinatal care: the threat of deregionalization. Pediatr Ann. 1988;17(7):447–452 [DOI] [PubMed] [Google Scholar]
- 37.Schwartz RM. Supply and demand for neonatal intensive care: trends and implications. J Perinatol. 1996;16(6):483–489 [PubMed] [Google Scholar]
- 38.Hein HA. Regionalization of perinatal health care: a lesson learned but lost. J Perinatol. 1999;19(8 pt 1):584–588 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.