Abstract
Cell-cell adhesion is fundamental to multicellular life and is mediated by a diverse array of cell surface proteins. However, the adhesive interactions for many of these proteins are poorly understood. Here we present a simple, rapid method for characterizing the adhesive properties of putative homophilic cell adhesion molecules. Cultured HEK293 cells are transfected with DNA plasmid encoding a secreted, epitope-tagged ectodomain of a cell surface protein. Using functionalized beads specific for the epitope tag, the soluble, secreted fusion protein is captured from the culture medium. The coated beads can then be used directly in bead aggregation assays or in fluorescent bead sorting assays to test for homophilic adhesion. If desired, mutagenesis can then be used to elucidate the specific amino acids or domains required for adhesion. This assay requires only small amounts of expressed protein, does not require the production of stable cell lines, and can be accomplished in 4 days.
Keywords: Bioengineering, Issue 92, adhesion, aggregation, Fc-fusion, cadherin, protocadherin
Introduction
Cell-cell adhesion is essential for the development and integrity of multicellular organisms and is mediated by a diverse array of cell surface molecules. Many of these adhesion molecules have been identified and characterized, though many remain to be discovered. Several methods are used to investigate the properties of cell adhesion molecules (CAMs), including cell sorting assays, cell aggregation assays1-8 and biophysical methods, such as atomic force microscopy and surface force spectroscopy9-12.
The complexity of even simplified in vitro systems using cell lines makes it difficult to determine the adhesive properties of a putative CAM. Typically, a molecule is considered a CAM if it induces cell aggregation when transfected into a non-adhesive cell line. However, it is clear that this is not direct evidence of adhesive activity. For instance, facilitating the cell surface delivery or stability of a CAM would also result in increased cell aggregation1,13. Moreover, a true CAM may fail to mediate cell aggregation if the cell line lacks other co-factors required for cell surface delivery or stabilization.
To avoid these complicating factors, more direct assays can be employed that are based on the idea that adhesive interactions should be an intrinsic biochemical property of the extracellular domain. While beads were initially used to characterize Ng-CAM2, these assays have been extended in order to investigate cadherin-mediated adhesion12,14,15. Using fusion of the C-cadherin ectodomain-Fc fusions, the Gumbiner lab showed that multiple cadherin repeats contribute to homophilic interactions14. Using comparable bead aggregation assays, E-cadherin and N-cadherin adhesion have also been characterized12,15, as have a number of protocadherins1,15-18 and Dscam isoforms from Drosophila19. Here we describe a relatively simple and rapid assay for characterizing the adhesive activity of secreted, epitope-tagged ectodomains of putative homophilic CAMs (Figure 1). We have used this assay primarily to characterize members of the cadherin superfamily.
Protocol
1. Cell Preparation (Day -2 to 0)
Split HEK293 cells 1:5 using 0.05% Trypsin-EDTA solution and incubate in Growth Media at 37 °C with 5% CO2 until 60 - 80% confluent (2 - 3 days). For each condition, culture cells in 2 x 100 mm dishes.
2. Cell Transfection (Day 1)
Transfect HEK293 cells with plasmid encoding Fc-fusion using a transfection reagent such as Lipofectamine. Alternative methods that result in comparable transfection efficiencies may also be used.
Return transfected cells to incubator for 24 hr.
3. Cell Propagation (Day 2)
Preheat Growth Media without Fetal Bovine Serum (-FBS) to 37 °C.
Rinse dishes 2x with 10 ml Growth Media -FBS, and return the dishes to the 37 °C incubator for 1 hr.
Rinse the cultures dishes one more time with 10 ml Growth Media -FBS, for a total of 3 washes.
Incubate the transfected HEK293 cells at 37 °C for another 48 hr without FBS before collecting media.
4. Bead Aggregation (Day 4)
To collect media from culture dishes, transfer media from each pair of dishes to 50 ml conical tubes and spin at 500 x g for 5 min to pellet cellular debris.
Filter media from 50 ml conical tube into a centrifugal filter using a 30 ml syringe and 0.45 µm syringe filter.
Spin centrifugal filters at 4,000 x g and 4 °C until volume of concentrated culture media is approximately 500 µl (approximately 15 min). Repeat until all culture media has been added and concentrated.
Add 1.5 µl of Protein G magnetic beads to 1 ml of ice cold Binding Buffer in a 1.5 ml microcentrifuge tube for each sample, place on a magnet and remove buffer. Immediately add the concentrated culture media to the Protein G magnetic beads.
Rotate tubes at 4 °C for 2 hr.
Place tubes on a magnet and remove media. Quickly wash beads twice with ice cold 1 ml Binding Buffer, and then resuspend beads in 300 µl Binding Buffer.
Split resuspended beads into 2 tubes, 150 µl into each tube, and then add 1.5 µl of 200 mM CaCl2 or 200 mM EDTA for the “calcium” and “no calcium” conditions, respectively.
Transfer 100 µl from each condition to a depression well slide and collect micrographs using a transmitted light microscope (Figure 2). Collect images from 5 fields of view for each experiment at each desired time point.
5. Data Analysis
Using ImageJ, or comparable image analysis software, open one of the five image datasets using the Import/Image Sequence… from the File pulldown menu. These will be opened as an image stack.
In the Image/Properties… dialog box, change the image units to pixels and set Pixel Width and Pixel Height to 1.0.
Convert the images to binary using the Adjust/Threshold… command in the Image pulldown menu. Set the threshold to include pixels that contribute to beads or bead aggregates, but that exclude background and small particles. Apply to all images in the stack.
In the Set Measurements dialog box, check the Area and Stack Position boxes.
Run Analyze Particles… in the Analyze pulldown menu. This will generate a list of identified particles, including their size (area) and the image in which they were identified.
Repeat this process for which experiment and experimental condition.
Representative Results
An example experiment is presented in Figure 2, which shows calcium-dependent bead aggregation by the ectodomain of N-cadherin fused to Fc (NcadEC-Fc). In the absence of calcium, beads exhibit little or no tendency to aggregate and there is no increase in aggregate size with time (Figure 1A,C). In the presence of calcium, beads coated with NcadEC-Fc show robust aggregation, with aggregate size increasing over time (Figure 1B,C). This experiment was repeated three times, with each instance consisting of images from five non-overlapping fields. The particle size in pixel area was determined for each aggregate in the five fields. These data were averaged for each time-point in each experiment. The data from the three experiments were averaged to determine means and standard errors of measurement at each time point (Figure 2C).
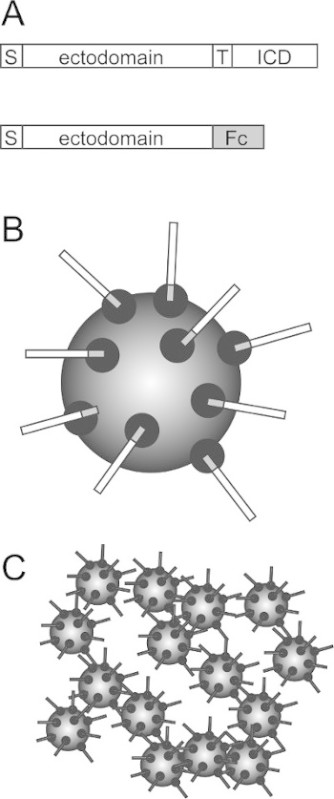 Figure 1. Use of secreted, epitope-tagged ectodomains in bead aggregation assays. (A) Schematic showing the organization of a typical, single-pass transmembrane cell adhesion molecule (top), which has a signal sequence (S), an ectodomain, a transmembrane domain (T) and an intracellular domain (ICD). To generate a secreted form of the ectodomain, a segment that lacks the transmembrane and intracellular domains is fused to the Fc region of human IgG (bottom). (B) When transfected into cultured cells, the ectomain-Fc fusion is expressed and secreted into the culture medium, where it can be captured and purified on Protein A or Protein G magnetic beads. Protein A or Protein G are shown as black circles on the beads. (C) After washing, the ectodomain-Fc coated magnetic beads are allowed to aggregate, as a test of homophilic adhesive interactions. Please click here to view a larger version of this figure.
Figure 1. Use of secreted, epitope-tagged ectodomains in bead aggregation assays. (A) Schematic showing the organization of a typical, single-pass transmembrane cell adhesion molecule (top), which has a signal sequence (S), an ectodomain, a transmembrane domain (T) and an intracellular domain (ICD). To generate a secreted form of the ectodomain, a segment that lacks the transmembrane and intracellular domains is fused to the Fc region of human IgG (bottom). (B) When transfected into cultured cells, the ectomain-Fc fusion is expressed and secreted into the culture medium, where it can be captured and purified on Protein A or Protein G magnetic beads. Protein A or Protein G are shown as black circles on the beads. (C) After washing, the ectodomain-Fc coated magnetic beads are allowed to aggregate, as a test of homophilic adhesive interactions. Please click here to view a larger version of this figure.
 Figure 2. Bead aggregation assay. (A) The classical cadherins, such as N-cadherin and E-cadherin, mediate calcium-dependent, homophilic adhesion. In the absence of calcium (no added calcium and 2 mM EDTA), cadherins fail to mediate adhesive interactions. Shown here is an image of Protein G magnetic beads coated with the ectodomain of zebrafish N-cadherin fused to Fc (NcadEC-Fc). (B) NcadEC-Fc coated beads were allowed to aggregate for 1 hr in the presence of 2 mM CaCl2. Homophilic adhesion by the N-cadherin ectodomains is apparent from the formation of large bead aggregates. (C) As a semi-quantitative measure of adhesion, the size of aggregates can be measured. One method to accomplish this is to measure the area occupied by distinct aggregates in transmitted light images. The mean size of aggregate area can be plotted as a function of time, as shown here, or the ratio of mean aggregate area in the presence or absence of calcium can be calculated. The aggregation of NcadEC-Fc coated beads in the presence of calcium (closed circles) and in the absence of calcium (open circles) was determined at 15 min intervals over the course of 1 hr. Please click here to view a larger version of this figure.
Figure 2. Bead aggregation assay. (A) The classical cadherins, such as N-cadherin and E-cadherin, mediate calcium-dependent, homophilic adhesion. In the absence of calcium (no added calcium and 2 mM EDTA), cadherins fail to mediate adhesive interactions. Shown here is an image of Protein G magnetic beads coated with the ectodomain of zebrafish N-cadherin fused to Fc (NcadEC-Fc). (B) NcadEC-Fc coated beads were allowed to aggregate for 1 hr in the presence of 2 mM CaCl2. Homophilic adhesion by the N-cadherin ectodomains is apparent from the formation of large bead aggregates. (C) As a semi-quantitative measure of adhesion, the size of aggregates can be measured. One method to accomplish this is to measure the area occupied by distinct aggregates in transmitted light images. The mean size of aggregate area can be plotted as a function of time, as shown here, or the ratio of mean aggregate area in the presence or absence of calcium can be calculated. The aggregation of NcadEC-Fc coated beads in the presence of calcium (closed circles) and in the absence of calcium (open circles) was determined at 15 min intervals over the course of 1 hr. Please click here to view a larger version of this figure.
Discussion
Cell adhesion is an essential feature of multicellular life and is mediated by a broad array of cell surface proteins. Of these, the detailed adhesive properties are understood for only a relatively small proportion. Here, we have described a simple, rapid protocol for investigating the homophilic adhesive capacity of secreted ectodomains fused to a convenient epitope tag. This approach has a number of important advantages. First, stable cell lines are not required14,20, as sufficient quantities of protein can be generated by transient transfection of 100 mm dishes. This allows the screening of larger numbers of constructs – e.g., point or deletion mutants – without the effort and expense of generating and maintaining large numbers of cell lines. Second, these bead assays are direct tests of the biochemical properties of putative adhesion molecules in a reduced system. Therefore, adhesion can be directly attributed to the protein under investigation, rather than potential indirect effects. Third, to investigate the effects of possible co-factors, cells can be co-transfected with secreted forms of the putative adhesion molecule and its putative co-factor, each with a distinct epitope tag. In addition, this approach is flexible, as it can be adapted for use with a variety of appropriate epitope tags. For example, uniformly-sized magnetic beads are available that are conjugated to Protein A or G (Fc tag), Streptavidin (Strep II tag), glutathione (GST tag), or TALON or Ni:NTA (6x His tag). Finally, the availability of fluorescent beads allows the same protocol to be adapted for simple sorting assays to investigate homophilic specificity. In this scenario, red and green fluorescent beads, each coated with a distinct CAM, are mixed and the degree of intermixing or segregation is assessed.
Despite these advantages, there are also caveats. First, protein fragments on beads in solution do not recapitulate the complexity of the in vivo situation. Individual cells express specific complements of cell surface proteins and their intracellular effectors. Each of these may have either direct or indirect effects on adhesion, and many of these effects will not be reflected in bead assays. For example, a molecule may mediate strong, heterophilic adhesion (it is a CAM), but would not induce bead aggregation in these assays. Second, bead assays are only semi-quantitative and do not provide reliable, quantitative information on the relative strength of adhesive interactions. Finally, all of the molecules that we have investigated are type I, single-pass membrane proteins in which the adhesive domain is present on a single, contiguous polypeptide. It is likely that many CAMs could be multi-pass transmembrane proteins that have an adhesive interface built from multiple ectodomain regions. This assay would not be easily adaptable to such molecules.
For bead assays to be reliable, the conditions need to be reproducible. There are two potential sources of variability in these assays. The first is variability in protein levels. If there is variability in expression levels, due to variation in transfection efficiency or a poorly-expressed protein, the beads may not be saturated and the results may be unreliable. Prior bead aggregation implementations use a defined amount of purified ectodomain-fusion protein. Typically, this fusion protein is purified in large batches from stable cell lines and can be used in multiple experiments. As the quantity of beads used in each experiment and the amount of protein required is very small, a comparable reproducibility can be achieved with transient transfection. The magnetic beads that we use have a binding capacity of ~250 ng/L of resuspended beads. A typical transfection of 2 x 100 mm dishes results in a vast excess of protein, which can be verified with a secondary binding step, using the supernatant after the initial binding of the fusion protein to the beads. The second potential source of variability is the quantity of beads used. The magnetic beads that we use are supplied as a 30 mg/ml slurry which settles quickly. For reproducibility, the beads must be resuspended completely and the relevant volume extracted quickly, before the beads begin to settle. One alternative is to pre-dilute a defined amount of beads in a larger volume. These pre-diluted beads can be added in a larger volume (15 L rather than 1.5 L) to avoid pipetting errors. If proper care isn't taken, there will be experiment-to-experiment variability in the quantity of beads used. This could affect the rate of aggregation, leading to differences in aggregate size at a specified time.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported by an NSF/ARRA award (IOS 0920357) and an award from the NIH (5R21MH098463).
References
- Chen X, Gumbiner BM. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J. Cell Biol. 2006;174(2):301–313. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M, Friedlander DR, Edelman GM. Evidence for the binding of Ng-CAM to laminin. Cell Adhes. Commun. 1993;1(2):177–190. doi: 10.3109/15419069309095693. [DOI] [PubMed] [Google Scholar]
- Hatta K, Nose A, Nagafuchi A, Takeichi M. Cloning and expression of cDNA encoding a neural calcium-dependent cell adhesion molecule: its identity in the cadherin gene family. J. Cell Biol. 1988;106(3):873–881. doi: 10.1083/jcb.106.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Nose A, Hatta K, Kawakami A, Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J. Cell Biol. 1987;105(6 Pt 1):2501–2510. doi: 10.1083/jcb.105.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54(7):993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J. Cell Biol. 1986;103(6 Pt 2):2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner D, Weiner JA. Combinatorial homophilic interaction between gamma-protocadherin multimers greatly expands the molecular diversity of cell adhesion. Proc. Natl. Acad. Sci. U. S. A. 2010;107(33):14893–14898. doi: 10.1073/pnas.1004526107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J. Cell Biol. 1977;75:464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankar S, Brieher W, Lavrik N, Gumbiner B, Leckband D. Direct molecular force measurements of multiple adhesive interactions between cadherin ectodomains. Proc. Natl. Acad. Sci. U. S. A. 1999;96(21):11820–11824. doi: 10.1073/pnas.96.21.11820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankar S, Gumbiner B, Leckband D. Direct measurements of multiple adhesive alignments and unbinding trajectories between cadherin extracellular domains. Biophys. J. 2001;80:1758–1768. doi: 10.1016/S0006-3495(01)76146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasankar S, Zhang Y, Nelson WJ, Chu S. Characterizing the initial encounter complex in cadherin adhesion. Structure. 2009;17(8):1075–1081. doi: 10.1016/j.str.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Sivasankar S, Nelson WJ, Chu S. Resolving cadherin interactions and binding cooperativity at the single-molecule level. Proc. Natl. Acad. Sci. U. S. A. 2009;106(1):109–114. doi: 10.1073/pnas.0811350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda S, et al. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2beta and p38 MAP kinases. Neuron. 2007;56(3):456–471. doi: 10.1016/j.neuron.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappuis-Flament S, Wong E, Hicks LD, Kay CM, Gumbiner BM. Multiple cadherin extracellular repeats mediate homophilic binding and adhesion. J. Cell Biol. 2001;154(1):231–243. doi: 10.1083/jcb.200103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emond MR, Biswas S, Blevins CJ, Jontes JD. A complex of Protocadherin-19 and N-cadherin mediates a novel mechanism of cell adhesion. J. Cell Biol. 2011;195(7):1115–1121. doi: 10.1083/jcb.201108115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Emond MR, Jontes JD. The clustered protocadherins Pcdhalpha and Pcdhgamma form a heteromeric complex in zebrafish. Neuroscience. 2012;219:280–289. doi: 10.1016/j.neuroscience.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins CJ, Emond MR, Biswas S, Jontes JD. Differential expression, alternative splicing, and adhesive properties of the zebrafish delta1-protocadherins. Neuroscience. 2011;199:523–534. doi: 10.1016/j.neuroscience.2011.09.061. [DOI] [PubMed] [Google Scholar]
- Morishita H, et al. Structure of the cadherin-related neuronal receptor/protocadherin-alpha first extracellular cadherin domain reveals diversity across cadherin families. J. Biol. Chem. 1074;281:33650–33663. doi: 10.1074/jbc.M603298200. [DOI] [PubMed] [Google Scholar]
- Wojtowicz WM, Flanagan JJ, Millard SS, Zipursky SL, Clemens JC. Alternative splicing of Drosophila Dscam generates axon guidance receptors that exhibit isoform-specific homophilic binding. Cell. 2004;118(5):619–633. doi: 10.1016/j.cell.2004.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees F, Reilein A, Nelson WJ. Cell-adhesion assays: fabrication of an E-cadherin substratum and isolation of lateral and Basal membrane patches. Methods Mol. Biol. 2005;294:303–320. doi: 10.1385/1-59259-860-9:303. [DOI] [PMC free article] [PubMed] [Google Scholar]


