Abstract
Throughout life, hematopoietic stem cells (HSCs) sustain the blood cell supply through their capacities for self-renewal and multilineage differentiation. These processes are regulated within a specialized microenvironment termed the ‘niche’. Here, we show a novel mechanism for regulating HSC function that is mediated by nephroblastoma overexpressed (Nov/CCN3), a matricellular protein member of the CCN family. We found that Nov contributes to the maintenance of long-term repopulating (LTR) activity through association with integrin αvβ3 on HSCs. The resultant β3 integrin outside-in signaling is dependent on thrombopoietin (TPO), a crucial cytokine involved in HSC maintenance. TPO was required for Nov binding to integrin αvβ3, and stimulated Nov expression in HSCs. However, in the presence of IFNγ, a cytokine known to impair HSC function, not only was TPO-induced expression of Nov suppressed, but the LTR activity was conversely impaired by TPO-mediated ligation of integrin αvβ3 with exogenous ligands, including Nov, as well. Thus, Nov/integrin αvβ3-mediated maintenance of HSCs appears to be modulated by simultaneous stimulation by other cytokines. Our finding suggests that this system contributes to the regulation of HSCs within the bone marrow niche.
Keywords: Hematopoietic stem cell, Nephroblastoma overexpressed, Integrin αvβ3, Thrombopoietin
Introduction
Hematopoietic stem cells (HSCs) are responsible for the life-long maintenance of the hematopoietic system both by sustaining HSCs through self-renewal and by giving rise to the various blood cell lineages via stepwise differentiation. To maintain this capacity, HSCs reside in a specialized microenvironment within the bone marrow (BM) termed a ‘niche,’ where their functions are appropriately regulated via complex combinations of mechanisms, including cytokine signaling, signaling via cell-cell contact, and cell-extracellular matrix (ECM) adhesion [1].
We recently reported a novel mechanism of integrin αvβ3 outside-in signaling that is indispensable for the regulation of the long-term repopulating (LTR) activity of HSCs [2]. This integrin function was dependent on the presence of thrombopoietin (TPO), an essential cytokine that contributes to HSC maintenance [3–5] and promotes αvβ3 integrin activation (a conformational change increasing its ligand affinity) through induction of integrin inside-out signaling (a pathway that activates integrins in response to extracellular stimulation) [2]. These findings established a mechanistic link between integrin αvβ3 signaling and TPO in maintaining the LTR activity of HSCs.
Nephroblastoma overexpressed (Nov/CCN3) is a soluble factor that belongs to the CCN family and is reportedly involved in modulating several biological processes including cell proliferation, cell adhesion, wound healing, and angiogenesis, through association with integrins, including integrin αvβ3 [6–8]. Recently, human HSCs were reported to express higher Nov levels than other progenitor cells, suggesting that Nov expression may be a hallmark of HSCs [9, 10]. In addition, endogenous Nov expression in HSCs seems to be required for the maintenance of HSC function in vivo because the knockdown of Nov expression impaired the repopulating activity of human HSCs [9]. Furthermore, the addition of recombinant Nov was shown to enhance the repopulating activity of human HSCs cultured ex vivo [9]. These data suggest that Nov acts as a positive regulator of HSC function, however, the underlying mechanism by which Nov regulates HSC function remains unclear.
Interferon-γ (IFNγ) is a cytokine that modulates immune systems and inflammation. While IFNγ reportedly promotes HSC proliferation in vivo by prompting dormant HSCs to enter the cell cycle, this response is accompanied by an impaired maintenance of LTR activity [11]. The negative regulation of LTR activity by IFNγ is mediated through STAT1 activation. Another recent report showed that IFNγ directly impairs the proliferative capacity of HSCs in vitro, thereby suppressing LTR activity [12]. It thus appears that IFNγ contributes to the maintenance of hematopoietic homeostasis through negative regulation of HSC function.
In this study, we identify a novel mechanism through which Nov regulates murine HSC function. Nov functions as a ligand of integrin αvβ3 on HSCs, thereby contributing to the maintenance of LTR activity, which is dependent on TPO. In the presence of IFNγ however, the ligation of integrin αvβ3 with Nov or ECM exerts negative influences on HSC function. These findings demonstrate for the first time that Nov regulates HSC function via integrin αvβ3 in the presence of simultaneous stimulation by other cytokines.
Materials and methods
Animals
The animals used in this study are described in “Supplemental Methods”.
Antibodies
The antibodies used in this study are described in “Supplemental Methods”.
Cell sorting and flow cytometric analyses
A MoFlo XDP or Gallios flow cytometer (Beckman Coulter Inc., Brea, CA) was used for cell sorting and flow cytometric analyses as described previously [2].
Estimation of Nov binding on HSCs
CD34−KSL HSCs were cultured in S-Clone SF-03 medium (Eidia Co., Ltd., Tokyo, Japan) supplemented with 0.5 % bovine serum albumin (Sigma-Aldrich Corporation, St. Louis, MO), 1 mM MnCl2 (Wako Junyaku, Osaka, Japan), 50 ng/ml TPO (R&D Systems, Minneapolis, MN), and/or 5 ng/ml IFNγ Shenandoah Biotechnology Inc., Warwick, PA) for either 1 or 18 h prior to treatment with 2 µg/ml recombinant mouse (rm)Nov tagged with oligohistidine (His-10) (R&D Systems) for 1 h. To identify bound rmNov, cultured cells were stained with an Alexa Fluor 647-conjugated mouse antibody recognizing the His-tag (AbD Serotec, Kidlington, UK). Subsequently, the stained cells were subjected to flow cytometric analyses after they were washed twice with PBS. For the inhibitory experiments, 200 µM of an Arg-Gly-Asp-Ser (RGDS) synthetic peptide (Life Technologies, Inc., Carlsbad, CA) or 50 µg/ml of antibodies against the integrins β3 or αv were added to the culture media, 1 h prior to the addition of rmNov. The Arg-Gly-Glu-Ser (RGES) peptide (Life Technologies, Inc.), Hamster IgG, or Rat IgG served as controls. All antibodies were purchased from BioLegend (San Diego, CA) unless otherwise indicated.
HSC cultures
Sorted CD150+CD34−KSL HSCs were cultured for 5 days in S-Clone SF-03 serum-free medium supplemented with 50 ng/ml rmNov, 50 ng/ml mouse TPO, 50 ng/ml mouse SCF (R&D Systems), and/or 5 ng/ml mouse IFNγ. Vitronectin (VN)-coated plates were prepared as described previously [2]. Following ex vivo culture, the total cell numbers were quantified by phase contrast microscopy, and subjected to the following experiments.
Long-term competitive repopulation assays
Long-term competitive repopulation assays were performed as described previously [13]. Briefly, cultured HSCs together with 2 × 105 whole BM competitor cells were transplanted into lethally irradiated (10 Gy) C57BL/6-Ly5.2 or C57BL/6-Ly5.1 congenic mice. Twenty weeks after transplantation, donor cell chimerism in the recipient mice was analyzed by flow cytometry. Recipient mice with donor cell chimerism (>1.0 % for myeloid and B- and T-lymphoid lineages) were considered to be multilineage-reconstituted mice.
Estimation of the number of retained HSCs after culture (limiting dilution assay)
Samples containing 20, 40, 80, or 150 CD150+CD34−KSL HSCs were sorted into individual wells of a 96-well plate and cultured for 5 days under the indicated conditions. Subsequently, whole cultured cells from each well were used for the transplantation assays as described above. Twenty weeks after transplantation, the percentage of unreconstructed mice (negative mice) was determined and the number of primary CD150+CD34−KSL cells required to retain one cell with LTR activity after culture was estimated based on the Poisson distribution [14].
Quantitative real-time RT-PCR
Using 5,000 sorted cells from each sample, mRNA expression was analyzed by quantitative real-time RT-PCR as previously described [2, 13]. This procedure is described in detail in the “Supplemental Methods”.
Analyses of STAT activation
After cytokine stimulation, the STAT5 or STAT1 activation levels were determined by flow cytometric analyses. This procedure is described in detail in the “Supplemental Methods”.
Statistical analysis
The results are expressed as mean ± standard deviation (SD). Statistical significance between two groups was assessed by the unpaired Student’s t test. P values <0.05 were considered significant.
Results
Nov contributes to the maintenance of the TPO-dependent LTR activity of HSCs through integrin αvβ3
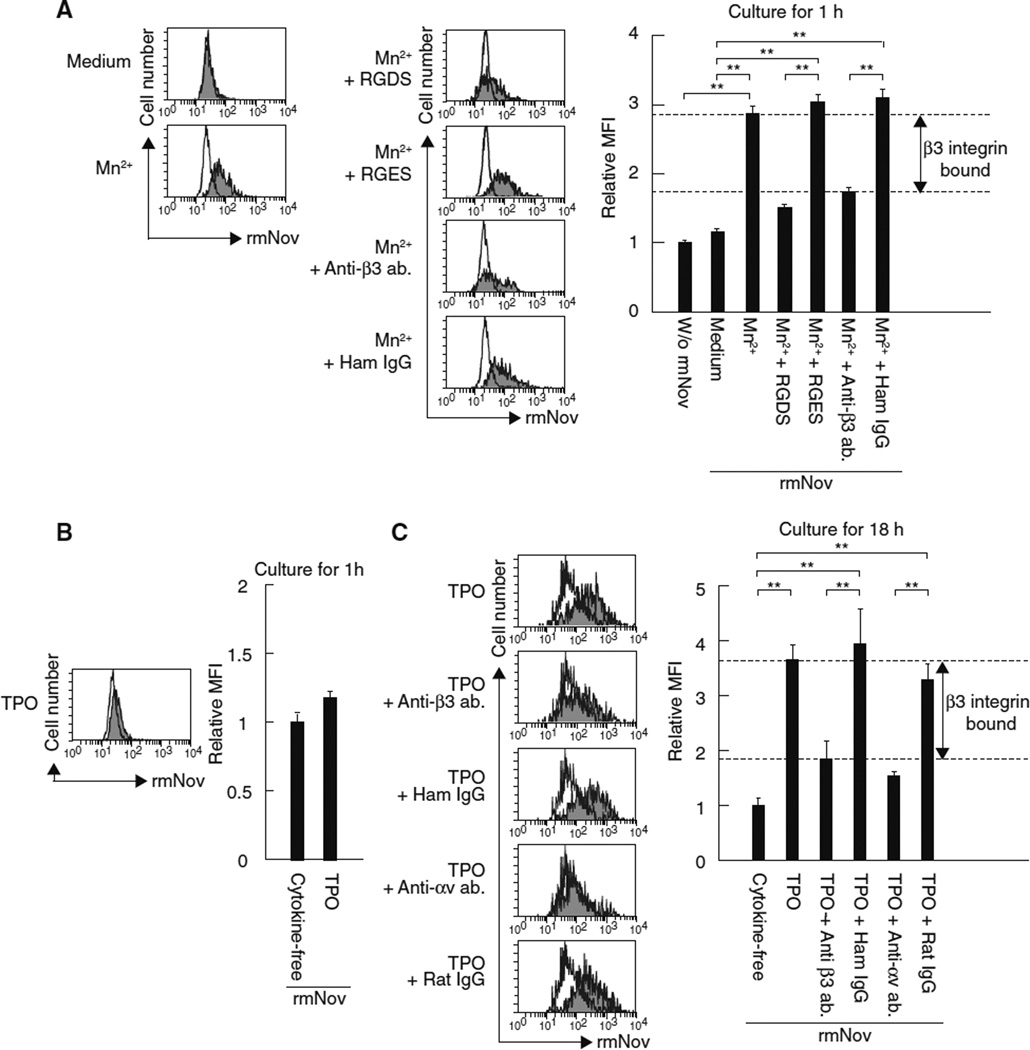
We previously demonstrated that outside-in signaling via integrin αvβ3 plays a key role in the maintenance of HSC LTR activity [2]. Because Nov is a known integrin αvβ3 ligand [6], we hypothesized that Nov acts via integrin αvβ3 to regulate LTR activity. To address this possibility, we first confirmed that Nov associates with integrin αvβ3 using flow cytometric analyses of CD34−KSL HSCs treated with His-tagged rmNov. By staining with an antibody recognizing the His-tag, enhanced binding of rmNov could be detected when HSCs were simultaneously treated with rmNov and Mn2+, a known integrin activator [15], whereas little binding of rmNov to HSCs was observed in the absence of Mn2+ (Fig. 1a). In addition, the effect of Mn2+ was inhibited by the Arg-Gly-Asp-Ser (RGDS) peptide, an integrin αvβ3 recognition sequence, but not by the Arg-Gly-Glu-Ser (RGES) control peptide. Furthermore, an anti-integrin β3 blocking antibody [16, 17] recapitulated the inhibitory effect of the RGDS peptide on Mn2+-dependent binding of rmNov to HSCs. These results indicate that Nov is capable of associating with integrin αvβ3 on HSCs, which is dependent on the activation of this integrin. We previously demonstrated that TPO contributes to the activation of integrin αvβ3 on HSCs through inside-out signaling [2], suggesting that TPO may mediate the association between Nov and integrin αvβ3 on HSCs. To confirm this hypothesis, we examined whether rmNov binds to cultured HSCs in the absence or presence of TPO. Although treatment with TPO for 1 h had little influence on rmNov binding to HSCs (Fig. 1b), extending the treatment with this cytokine to 18 h significantly enhanced rmNov ligation (Fig. 1c). In addition, the effect of TPO was inhibited in the presence of blocking antibodies against either the β3 or αv integrin subunits (Fig. 1c) [16, 18]. These results indicate that TPO enables the association between Nov and integrin αvβ3 on HSCs and suggest that TPO and/or outside-in signaling via the integrin αvβ3 are involved in the Nov-mediated regulation of LTR activity.
Fig. 1.
TPO is required for Nov binding to the integrin αvβ3. a CD34−KSL cells were treated for 1 h with or without His-tagged rmNov in the absence or presence of Mn2+ and subsequently stained with an antibody recognizing the His-tag prior to flow cytometric analyses. For the inhibitory experiments, an RGDS synthetic peptide or an anti-β3 integrin antibody was employed. An RGES peptide or Hamster (Ham) IgG served as controls, respectively. The histograms depict the fluorescence intensities of the bound rmNov: white, cells cultured in the absence of rmNov; and gray, cells treated with rmNov. The graph depicts the relative mean fluorescence intensity (MFI); the fluorescence intensity in the absence of rmNov served as a control. The data represent the mean ± SD (**P < 0.01, n = 3). In addition, CD34 KSL cells cultured for 1 h (b) or 18 h (c) were treated with His-tagged rmNov and subsequently treated with an antibody recognizing the His-tag before flow cytometric analyses as described above. Some cells were treated with the indicated antibodies during culture. Ham or Rat IgG was utilized as controls for the anti-β3 or αv integrin antibodies, respectively. The histograms depict the fluorescence intensities of bound rmNov: white, cells cultured in the absence of cytokine; and gray, cells cultured in the presence of TPO. The graph depicts the relative MFI; the fluorescence intensity in the absence of cytokine served as a control. The data represent the mean ± SD (**P < 0.01, n = 3)
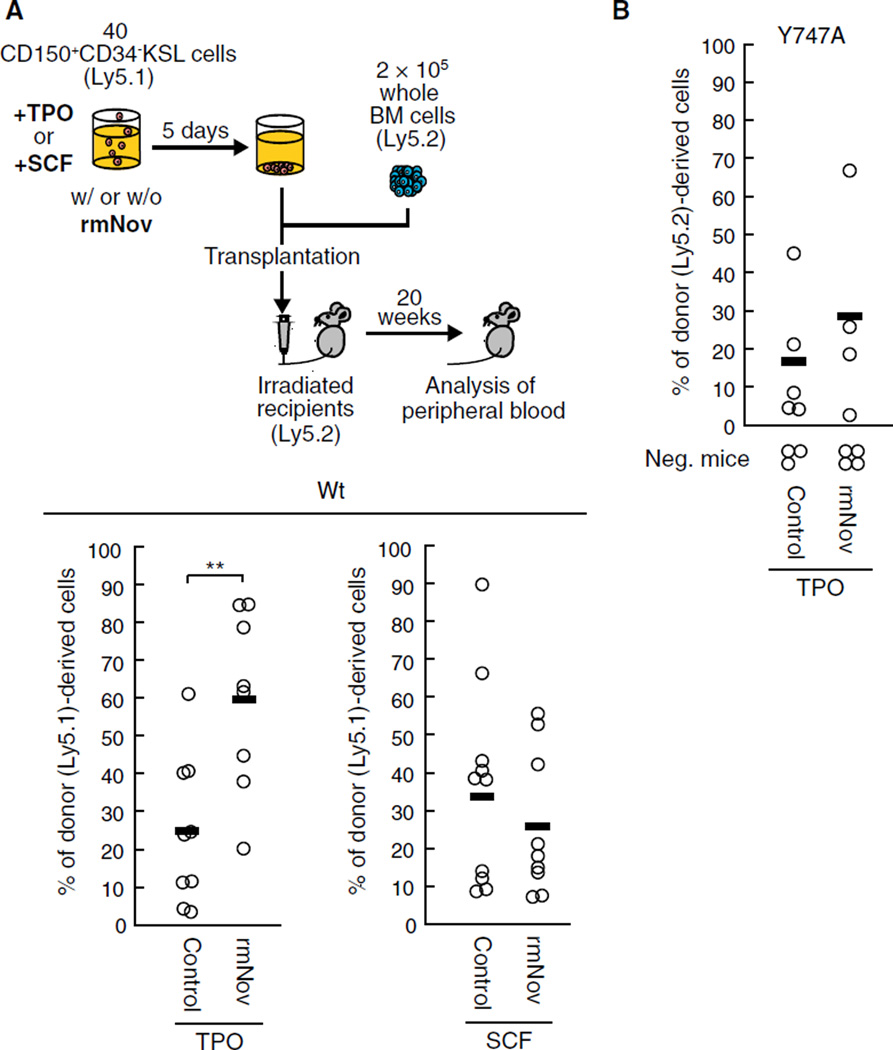
Initially, to confirm whether TPO is involved in regulation of HSC functions by Nov, we performed transplantation assays using HSCs incubated with or without rmNov in the presence of TPO or stem cell factor (SCF), an essential cytokine for HSC maintenance that acts independently of integrin αvβ3 [2] (Fig. 2a). Twenty weeks after transplantation, HSCs cultured with rmNov in the presence of TPO showed greater chimerism than cells cultured in the presence of TPO alone (Fig. 2a). In contrast, rmNov had no effect on the LTR activity of HSCs cultured in the presence of SCF (Fig. 2a). These results indicate that the positive effect of Nov on LTR activity is dependent on the presence of TPO. In addition, after the culture with TPO, rmNov had no effect on the total cell number, or on the frequency of KSL and CD48−KSL cells, which are the HSC/hematopoietic progenitor cell (HPC) fraction, or the HSC-enriched populations, respectively (Supplemental Fig. 1) [19]. Thus, Nov is not involved in HSC expansion, at least during ex vivo culture. Collectively, these results suggest that Nov positively regulates the LTR activity of individual HSCs, but it has no effect on HSC expansion. This behavior is reminiscent of the behavior of other integrin αvβ3 ligands [2] and strongly supports the hypothesis that Nov acts via integrin αvβ3 to regulate HSC function.
Fig. 2.
Exogenous Nov enhances LTR activity via the β3 integrin in the presence of TPO. a Forty CD150+CD34−KSL cells isolated from wild type (Wt) mice (Ly5.1) were cultured for 5 days with or without rmNov in the presence of TPO or SCF. The cultured cells were then transplanted into lethally irradiated mice (Ly5.2) along with 2 × 105 whole bone marrow (BM) competitor cells (Ly5.2). Twenty weeks after transplantation, the percentage of donor cells (Ly5.1) in the peripheral blood was determined, b Forty CD150+CD34−KSL cells derived from β3 integrin Y747A knock-in mutant mice (Ly5.2) were cultured with or without rmNov in the presence of TPO and subsequently transplanted along with 2 × 105 BM competitor cells (Ly5.1) into lethally irradiated recipient mice (Ly5.1) as described above. The plots represent the percentage of donor (Ly5.1 or Ly5.2)-derived cells in the peripheral blood of individual mice 20 weeks after transplantation. The bars depict the mean values (**P < 0.01). Recipient mice with donor cell chimerism <1.0 % for any lineage were not considered to be reconstituted (negative mice)
We have previously shown that outside-in signaling is indispensable for the sustained LTR activity mediated by β3 integrin [2]. Therefore, to directly confirm that outside-in signaling via integrin αvβ3 is crucial for the positive effect of Nov on LTR activity, we carried out transplantation assays using HSCs derived from β3 integrin Y747A knock-in mutant mice. In these mice, an alanine was substituted for tyrosine 747 in the β3 integrin subunit, thereby eliminating outside-in signaling [20–22]. We found that the positive effect of rmNov on LTR activity was almost completely lost in HSCs expressing the Y747A-mutated β3 integrin, even in the presence of TPO (Fig. 2b). These data suggest that, such as TPO, outside-in signaling via integrin αvβ3 is indispensable for the Nov-mediated regulation of LTR activity.
TPO contributes to the maintenance of expression of Nov in HSCs
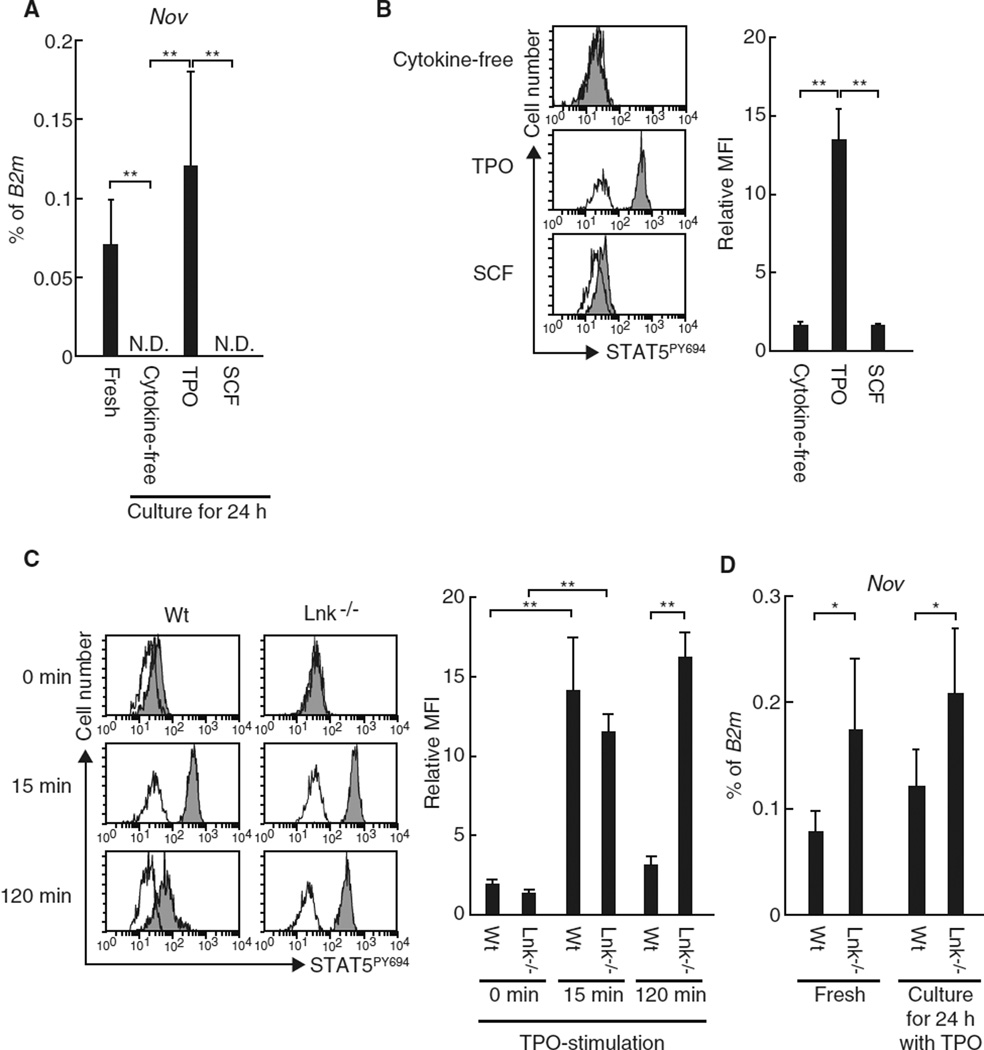
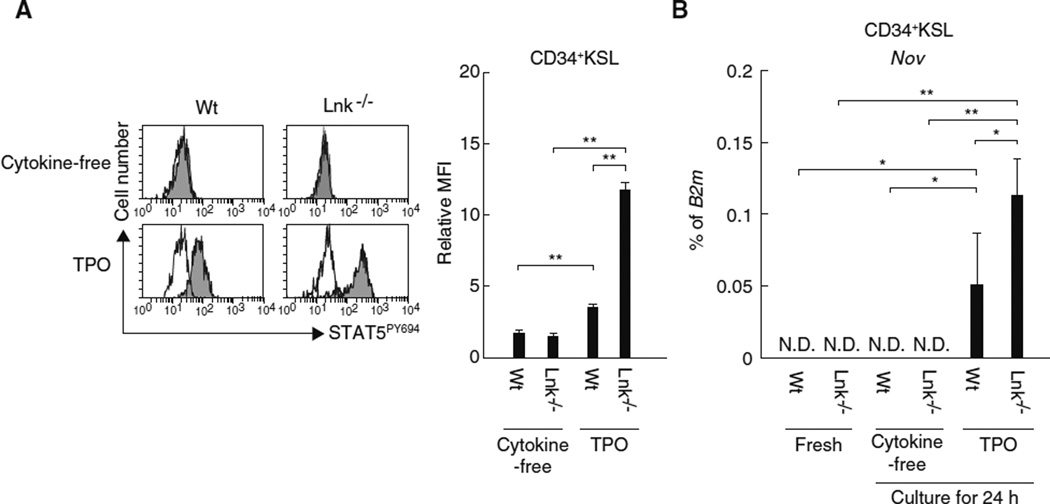
We have thus demonstrated the linkage between Nov, integrin αvβ3, and TPO in HSCs. The expression of Nov is regarded as a hallmark of HSCs [9, 10] and is prompted by STAT5 activation [23]. Because TPO is capable of inducing STAT5 activation in HSCs [24], we suggest a further linkage between TPO and Nov in regard to the regulation of Nov expression in HSCs. To test this hypothesis, we first used quantitative real-time RT-PCR to determine whether TPO stimulates the expression of Nov in cultured HSCs. We found that HSCs cultured for 24 h in the presence of TPO exhibited sustained levels of Nov expression compared to freshly isolated HSCs (Fig. 3a). TPO treatment was also associated with STAT5 phosphorylation (Fig. 3b). Furthermore, Lnk−/− HSCs, which display prolonged activation of STAT5 in response to TPO treatment (Fig. 3c) [25], exhibited enhanced Nov expression compared to Wt HSCs not only after treatment with TPO but also in vivo (Fig. 3d). In contrast, HSCs cultured in the presence of SCF or under cytokine-free conditions do not express Nov and exhibit minimal STAT5 activation (Fig. 3a, b). These data suggest that TPO enables the induction of Nov expression in HSCs likely through STAT5 activation, as predicted from the previous reports [23, 24]. Interestingly, although neither freshly isolated Wt nor Lnk−/− CD34+KSL HPCs expressed Nov, TPO treatment of HPCs in vitro induced Nov expression and STAT5 activation. These effects were more pronounced in Lnk-deficient HPCs (Fig. 4). TPO thus appears to have the capacity to induce Nov expression in both HSCs and HPCs, suggesting that expression of Nov is dependent on external stimulation rather than the cell-autonomous phenotypes.
Fig. 3.
TPO stimulates Nov expression in HSCs via STAT5 activation. a The expression of Nov mRNA was examined using quantitative real-time RT-PCR in uncultured CD150+CD34−KSL cells (fresh) or in cells cultured for 24 h under the indicated conditions. The graph depicts the levels of Nov mRNA expression normalized to the expression of B2m. The data represent the mean ± SD (**P < 0.01, n = 5). ND represents samples in which Nov expression could not be detected. b CD34−KSL cells were stimulated for 15 min with the indicated cytokines prior to flow cytometric analyses to examine STAT5 activation. The histograms depict the levels of STAT5 phosphorylation at Y694: white, isotype control; and gray, anti-STAT5PY694 antibody. The graph depicts the relative mean fluorescence intensity (MFI). Samples stained with control antibodies served as controls for each condition. The data represent the mean ± SD (**P < 0.01, n = 3). c CD34 KSL cells derived from wild type (Wt) or Lnk−/− mice were stimulated with TPO for the indicated times and STAT5 activation was analyzed by flow cytometry. The histograms represent the levels of STAT5 phosphorylation at Y694: white, isotype control; and gray, anti-STAT5PY694 antibody. The graph depicts the relative MFI. Samples stained with isotype control antibody served as the controls for each condition. The data represent the mean ± SD (**P < 0.01, n = 3). d CD150+CD34−KSL cells derived from Wt or Lnk−/− mice were subjected to quantitative real-time RT-PCR before (fresh) or after culturing for 24 h in the presence of TPO. The graph depicts the levels of Nov mRNA expression normalized to the levels of B2m expression. The data represent the mean ± SD (*P < 0.05, n = 5)
Fig. 4.
TPO stimulates Nov expression in CD34+KSL HPCs. a After CD34+KSL cells derived from wild type (Wt) or Lnk−/− mice were stimulated with TPO for 15 min, the activation of STAT5 was analyzed using a flow cytometer. The histograms represent the levels of STAT5 phosphorylation at Y694: white, isotype control; and gray, anti-STAT5PY694 antibody. The graph depicts the relative mean fluorescence intensity (MFI). Samples stained with isotype control antibody served as the controls for each condition. The data represent the mean ± SD (**P < 0.01, n = 3). b The expression of Nov was analyzed by quantitative real-time RT-PCR in uncultured Wt or Lnir’- CD34+KSL cells (fresh) or in cells cultured under the indicated conditions for 24 h. The graph depicts the levels of Nov mRNA expression normalized to the levels of B2m expression. The data represent the mean ± SD (**P < 0.01, *P < 0.05, n = 5). ND represents samples in which Nov expression could not be detected
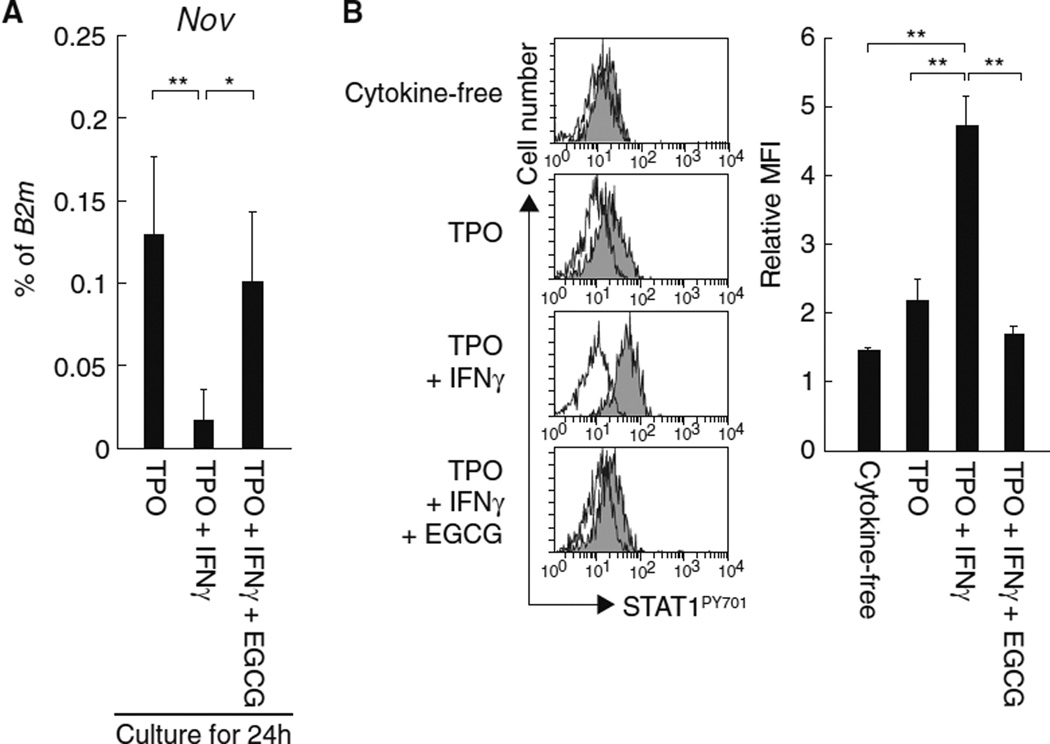
IFNγ impairs the TPO-induced expression of Nov in HSCs through the activation of STAT1
In response to cytokine stimulation, activated STAT5 is reportedly recruited into IFNγ-activated sequence (GAS) sites within the promoter region of Nov, and that this event correlates with Nov expression [23]. Because GAS sites were originally identified as STAT5 or STAT1 binding motifs that modulate IFNγ-activated transcription [26], we suspected that STAT1 was also involved in the regulation of STAT5-dependent Nov expression in HSCs. To test this possibility, we examined Nov expression in HSCs treated simultaneously with TPO and IFNγ, a known inducer of STAT1 activation. We found that IFNγ treatment impaired TPO-dependent Nov expression in both HSCs (Fig. 5a) and HPCs (Data not shown). Moreover, the suppressive effect of IFNγ was abrogated by treatment with epigallocatechin gallate (EGCG), an inhibitor of STAT1 activation (Fig. 5) [27]. Thus, the negative effect of IFNγ on Nov expression in HSCs appears to be dependent on STAT1 activation, supporting the possibility that Nov expression is dependent on exogenous stimulation in both HSCs and HPCs.
Fig. 5.
IFNγ-dependent activation of STAT1 impairs TPO-stimulated Nov expression in HSCs. a CD150+CD34−KSL cells were cultured for 24 h with or without IFNγ in the presence of TPO, and the expression of Nov mRNA was assessed using quantitative real-time RT-PCR. EGCG is an inhibitor of STAT1 activation. The graph depicts the levels of Nov mRNA expression normalized to the levels of B2m expression. The data represent the mean ± SD (**P < 0.01, *P < 0.05, n = 5). b CD34 KSL cells were stimulated for 15 min with the indicated cytokines in the presence or absence of EGCG and STAT1 phosphorylation was analyzed by flow cytometry. The histograms depict the levels of STAT1 phosphorylation at Y701: white, isotype control; and gray, anti-STAT1PY701 antibody. The graph depicts the relative mean fluorescence intensity (MFI). Samples stained with isotype control antibody served as controls for each condition. The data represent the mean ± SD (**P < 0.01, n = 3)
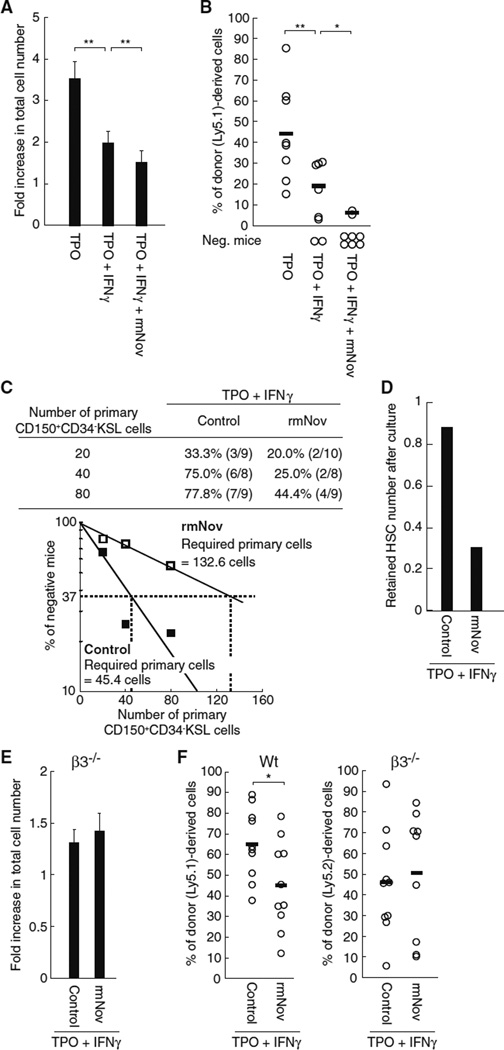
Nov inhibits LTR activity via integrin αvβ3 in the presence of IFNγ
IFNγ-mediated STAT1 activation reportedly leads to the suppression of HSC LTR activity as well as to an impaired HSC proliferation capacity in vitro [12]. Likewise, we confirmed that IFNγ negatively affects both the proliferation capacity (Fig. 6a) and the LTR activity of HSCs cultured in the presence of TPO (Fig. 6b). Our findings indicate that IFNγ dominantly impairs TPO-dependent expression of Nov (Fig. 5a), a positive regulator of LTR activity that may also be involved in the IFNγ-mediated suppression of the repopulating activity of HSCs (Fig. 6b) [9]. We therefore reasoned that the addition of rmNov to HSCs cultured in the presence of TPO likely prevented the IFNγ-dependent impairment of LTR activity by both offsetting the IFNγ-induced reduction in Nov expression and by acting as a positive regulator of LTR activity. To test this possibility, we performed transplantation assays using HSCs cultured with or without rmNov in the presence of both TPO and IFNγ Surprisingly, the addition of rmNov appeared to exert negative effects on both the proliferation capacity and the maintenance of HSCs, as evidenced by a decreased total cell number, suppressed chimerism and an increase in the percentage of unreconstructed mice (Fig. 6a, b). To quantify the negative effects on HSC maintenance, we next performed a limiting dilution assay based on the transplantation assays. Consequently, this assay showed that approximately threefold the number of primary CD150+CD34−KSL cells is required to retain one cell with LTR activity when cells are cultured in the presence of IFNγ and treated with rmNov (Fig. 6c). The number of HSCs decreased to approximately one-third compared with the control when cultured in the presence of IFNγ and treated with rmNov (Fig. 6d). Therefore, the suppressive actions of rmNov on cell proliferation (Fig. 6a) are less likely to contribute to its negative effects on HSC maintenance because they are insufficient to account for the decrease in the estimated HSC number after culture. Thus, these results indicate that Nov negatively affects the maintenance of LTR activity in the presence of IFNγ, even when TPO is present. In addition, we confirmed that IFNγ had little effect on the TPO-dependent association of rmNov with β3 integrin (Supplemental Fig. 2), suggesting that integrin αvβ3 is also involved in the negative actions of Nov observed in the presence of IFNγ. To confirm this hypothesis, we examined the IFNγ-dependent effects of rmNov in HSCs derived from integrin β3 mice. The addition of rmNov to integrin β3 HSCs cultured in the presence of both TPO and IFNγ had little effect on HSC proliferation (Fig. 6e). Similarly, integrin β3 deficiency abolished the IFNγ-dependent negative effects of rmNov on LTR activity that were observed in Wt HSCs (Fig. 6f). Thus, such as its positive effects on HSC function in the presence of TPO, the negative effects of Nov in the presence of IFNγ also appear to be mediated through integrin αvβ3.
Fig. 6.
Exogenous Nov acts via β3 integrin to impair the maintenance β3 of LTR activity in the presence of IFNγ. Forty CD150+CD34−KSL cells were cultured in the presence of rmNov, TPO and/or IFNγ for 5 days. a The total number of cells was quantified. The graph shows the fold-increase in total cell number. The data represent the mean ± SD (**P < 0.01, n = 8). b The cultured cells were transplanted into lethally irradiated recipient mice along with 2 × 10 whole BM competitor cells. The plots depict the percentage of donor (Ly5.1)-derived cells in the peripheral blood of individual mice 20 weeks after transplantation. The bars represent the mean values (**P < 0.01, *P < 0.05). Recipient mice with donor cell chimerism <1.0 % for any lineage were not considered to be reconstituted (negative mice). c Groups of 20, 40 or 80 CD150+CD34−KSL cells were cultured for 5 days with or without rmNov in the presence of TPO and IFNγ. Afterward, the groups were individually transplanted as described above. The table shows the percentages of multi-lineage reconstituted mice. The numbers in parentheses denote multilineage reconstituted mice/tested mice. In addition, the percentages of unreconstructed mice (percentages of negative mice on the γ axis) were plotted versus the numbers of primary CD150+CD34−KSL cultured cells (white, rmNov treatment; and black, without rmNov treatment). The estimations of the theoretically required number of primary CD150+CD34−KSL cells required for retaining one cell with LTR activity after culture under indicated conditions are based on a Poisson distribution, d The graph represents the estimated HSC number after culture under the same conditions indicated in Fig. 6b. Two hundred CD150+CD34−KSL cells isolated from wild type (Wt) (Ly5.1) or integrin β3−/− mice (Ly5.2) were cultured for 5 days with or without rmNov in the presence of TPO and IFNγ. e The total number of cells was quantified. The graph shows the fold-increase in the total cell number. The data represent the mean ± SD (n = 8). f The transplantation assays were performed as described above. The plots depict the percentage of donor (Ly5.1 or Ly5.2)-derived cells in the peripheral blood of individual mice 20 weeks after transplantation. The bars represent the mean values (*P < 0.05)
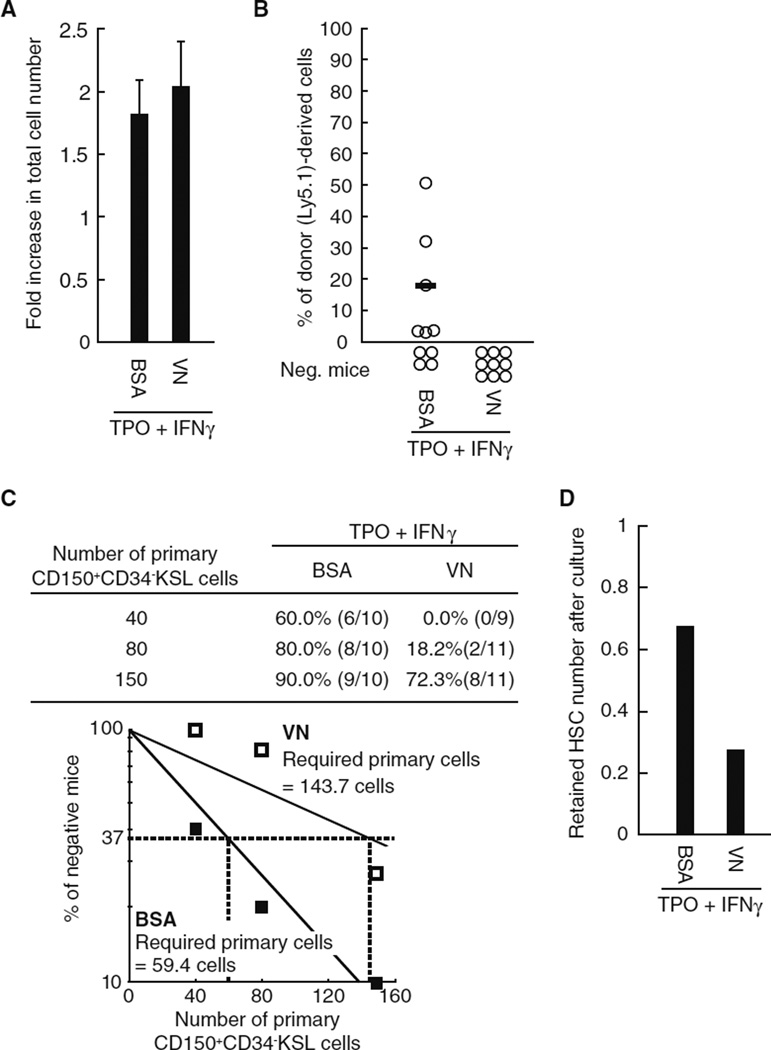
Finally, we confirmed whether another integrin αvβ3 ligand had similar negative effects on HSC function in the presence of IFNγ using HSCs cultured on plates coated with vitronectin (VN), a well-known integrin αvβ3 ligand. Although VN coating had little influence on the proliferative capacity of HSCs in the presence of IFNγ Fig. 7a), transplantation assays demonstrated that this ligand recapitulates the negative effects of rmNov on the maintenance of LTR activity (Fig. 7b – d). These results strongly suggest a novel function of integrin αvβ3 that negatively affects HSC maintenance in the presence of IFNγ.
Fig. 7.
Vitronectin (VN) impairs the maintenance of LTR activity in the presence of IFNγ. Forty CD150+CD34−KSL cells were cultured for 5 days in the presence of TPO and IFNγ on BSA- or VN-coated plates. α The total number of cells was quantified. The graph shows the fold-increase in total cell number. The data represent the mean ± SD (n = 8). b In addition, the cultured cells were transplanted into lethally irradiated recipient mice along with 2 × 105 whole BM competitor cells. The plots depict the percentage of donor (Ly5.1)-derived cells in the peripheral blood of individual mice 20 weeks after transplantation. The bars represent mean values. Recipient mice with donor cell chimerism <1.0 for any lineage were not considered to be reconstituted (negative mice). c Groups of 40, 80 or 150 sorted CD150+CD34−KSL cells were cultured for 5 days in the presence of TPO and IFNγ on BSA- or VN-coated plates and then used in transplantation assays as described above. The table shows the percentages of multi-lineage reconstituted mice and the numbers in parentheses denote the multilineage reconstituted mice/tested mice. In addition, the percentage of unreconstructed mice (percentage of negative mice on the γ axis) was plotted versus the numbers of primary CD150+CD34−KSL cultured cells (white, VN-coat; and black, BSA-coat). The estimates of the theoretically required number of primary CD150+CD34−KSL cells needed to retain one cell with LTR activity after culture are based on a Poisson distribution. d The graph represents the estimated number of HSCs cultured under the conditions indicated in b.
Discussion
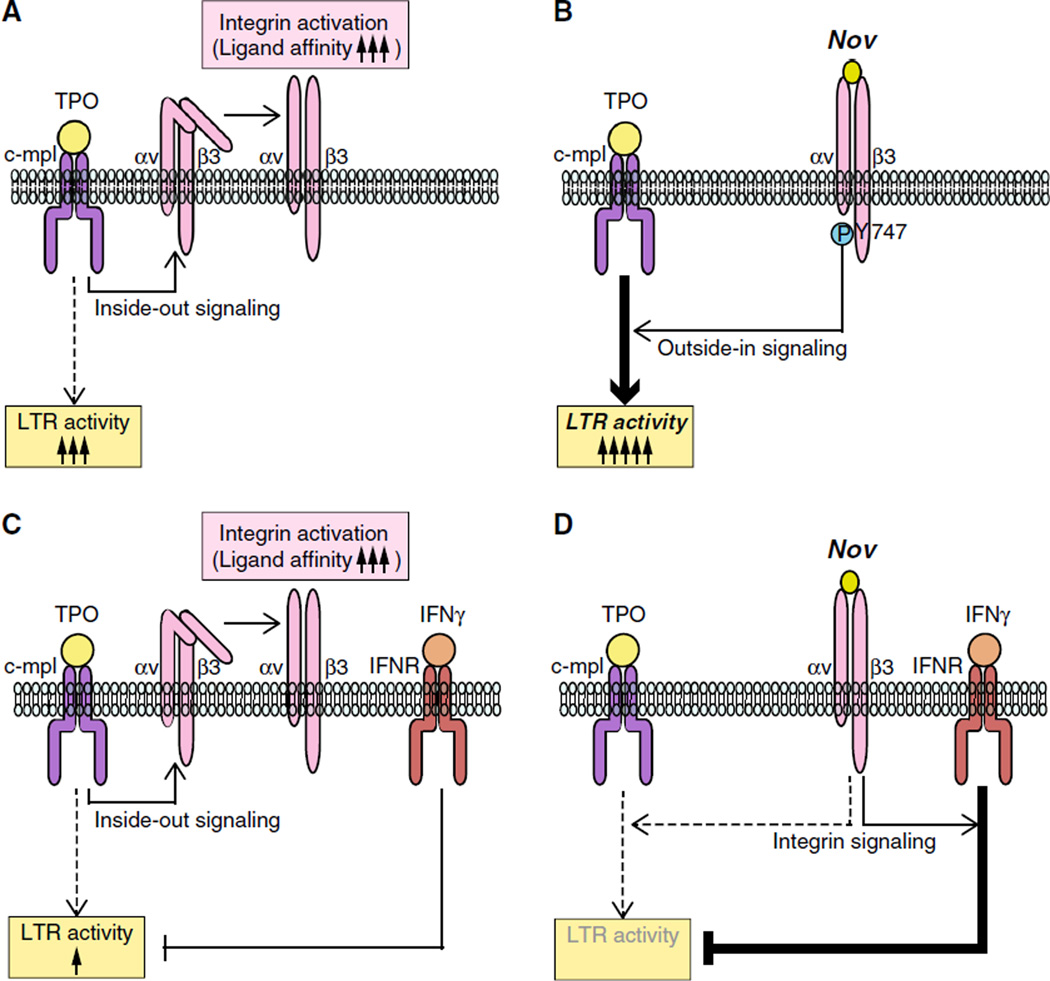
In this study, we demonstrate that Nov acts via integrin αvβ3 on HSCs to bidirectionally regulate LTR activity in vitro, which is dependent on TPO-dependent activation of this integrin as well as simultaneous stimulation by other cytokines (Fig. 8). The observed positive effects of Nov on the maintenance of HSC LTR activity in the presence of TPO (Fig. 2a) are consistent with earlier findings [2, 9], while our observation that Nov negatively affects LTR activity in the presence of IFNγ (Fig. 6) is novel. The negative behavior of Nov in the presence of IFNγ likely reflects a novel mechanism by which integrin αvβ3 regulates HSC function. Consistent with this possibility, VN, a well-known integrin αvβ3 ligand, also recapitulates the behavior of Nov in the presence of IFNγ (Fig. 7). Our findings thus provide a new understanding of the relationship between integrin and cytokine signaling in the regulation of HSC function.
Fig. 8.
A model depicting the regulation of Nov/integrin αvβ3-mediated maintenance of LTR activity of HSCs that requires simultaneous stimulation by other cytokines. a TPO is not only involved in HSC maintenance, but it also induces αvβ3 integrin activation (a conformational change that increases its ligand affinity). b Nov associates with the integrin αvβ3, thereby enhancing the TPO- dependent maintenance of LTR activity that is mediated by β3 integrin outside-in signaling, c IFNγ is capable of impairing the LTR activity of HSCs independently of the TPO-dependent activation of the αvβ3 integrin. d The presence of exogenous Nov induces β3 integrin-mediated signaling by acting in concert with IFNγ rather than via TPO, resulting in further suppression of LTR activity
Previously, Nov was thought to be exclusively expressed by HSCs in vivo and was thus considered a hallmark of those cells (Figs. 3a, 4) [9, 10]. However, our findings indicate that Nov expression does not seem to be an attribution of HSCs because Nov expression was evident in TPO-treated HPCs in vitro (Fig. 4) and SCF-treated cells lacking Nov expression were capable of engraftment (Figs. 2a, 3a). In addition, our results indicate that Nov expression in HSCs and HPCs is strongly influenced by cytokine stimulation (Figs. 3, 4, 5). These results suggest that the distribution of Nov expression in vivo is attributable to the environment surrounding each cell. Accordingly, we propose that TPO mediates Nov expression by HSCs in vivo because HSCs reportedly make contact with TPO-expressing cells within the osteoblastic niche [28]. Our data demonstrate that TPO supports both STAT5 activation and Nov expression in HSCs (Fig. 3). Moreover, Nov expression in vivo was increased by Lnk deficiency, which causes prolonged TPO-dependent STAT5 activation (Fig. 3c, d). These findings strongly suggest that TPO contributes to Nov expression in vivo. This possibility is also supported by a previous study showing that the positive effects of Lnk deficiency on the activation of JAK2, a signaling molecule that acts upstream of STAT5, are dependent on TPO stimulation but are unaffected by IL-3 or G-CSF [29].
Although other cells that likely compose the HSC niche, such as osteoblasts and vascular endothelial cells, also express Nov [30, 31], endogenous Nov expression in HSCs seems to have a stronger influence on the maintenance of HSC function within the BM niche because the knockdown of Nov expression in human HSCs leads to a decreased repopulating activity in vivo [9]. Indeed, this finding indicates that Nov secreted by the HSCs contributes to the maintenance of stem cell activity in vivo. Our findings demonstrate that TPO induces Nov expression in vitro (Fig. 3), suggesting that TPO may regulate Nov expression in vivo. In addition, the positive effects of Nov on LTR activity in the presence of TPO seem to be mainly attributable to β3 integrin outside-in signaling (Fig. 2b). Taken together, our findings suggest that endogenous Nov, along with external ligands, likely acts via αvβ3 integrin on HSCs to contribute to the maintenance of LTR activity within a TPO-enriched niche.
In the presence of IFNγ, a cytokine that impairs HSC function, rmNov and VN exerted suppressive effects on HSC function in vitro, even in the presence of TPO (Figs. 6, 7). These results imply that integrin αvβ3 ligands preferentially contribute to IFNγ-dependent suppression of HSC function over TPO-dependent maintenance of LTR activity. Therefore, TPO-induced expression of Nov was impaired in HSCs treated with IFNγ (Fig. 5a), which likely mediated the IFNγ-dependent suppressive effects of αvβ3 integrin. Under similar conditions, exogenous ligands may give rise to integrin αvβ3-mediated suppression of LTR activity. However because IFNγ seems to influence HSC regulation differently in vivo and in vitro [11, 12], it remains unclear whether the IFNγ-dependent effects of integrin ligands observed in vitro will be recapitulated in vivo.
We also found that Nov, but not VN, suppressed HSC proliferation in the presence of IFNγ (Figs. 6a, 7a). This suppressive effect of Nov was dependent on the presence of IFNγ, as rmNov had little effect on proliferation in the presence of TPO alone (Supplemental Fig. 1a). Thus, the differential integrin αvβ3 -mediated effects of Nov and VN on HSC function in the presence of IFNγ may be attributable to their ligand specificity. Unlike VN, Nov is able to associate with surface antigens other than integrin receptors (e.g., Notch 1 and Connexin 43) [32, 33], an ability that may contribute to its unique effects in the presence of IFNγ. Our results also indicate that Nov associates with additional unidentified receptors on HSCs because the binding of rmNov to HSCs was not completely compromised by treatment with the anti-integrin antibodies (Fig. 1, Supplemental Fig. 2). These additional Nov ligations are not likely to exert a substantial effect on HSC functions because the effects of rmNov on HSCs were blocked by either β3 integrin deficiency (Fig. 6e, f) or the impairment of β3 integrin outside-in signaling (Fig. 2b).
In conclusion, we have demonstrated a novel mechanism of Nov action on the regulation of HSC function. Our results show that TPO, IFNγ integrin αvβ3, and Nov act in concert to regulate HSC function and shed light on the complex regulatory mechanisms governing HSC function within its BM niche. Our findings also expand on the results of our previous study, furthering our understanding of the relationship between integrin and cytokine signaling in HSCs and supporting our previously proposed model of the integrin αvβ3-mediated expansion of HSCs in vitro that does not negatively affect LTR activity [2]. Thus, this study provides a basis for further characterization of the HSC niche and for the development of in vitro methods of HSCs manipulation for transplantation purposes.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. M. H. Ginsberg (University of California, San Diego, La Jolla, CA) and Bristol-Myers Squibb (New York, NY) for providing the β3 integrin Y747A knock-in mutant mice, all of the members of our research group for discussions, and the Institute of Laboratory Animals, Tokyo Women’s Medical University for their assistance with breeding mice. This work was supported by the Formation of Innovation Center for Fusion of Advanced Technologies in the Special Coordination Funds for Promoting Science and Technology Cell Sheet Tissue Engineering Center (M.Y. and T.O.) and Grant-in-Aid for JSPS Fellows (J.I.).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/sl2185-014-1534-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Jun Ishihara, Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University, 8-1 Kawadacho, Shinjuku-ku, Tokyo 162-8666, Japan; Division of Cellular Therapy, The Institute of Medical Science, The University of Tokyo, Tokyo 108-8639, Japan.
Terumasa Umemoto, Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University, 8-1 Kawadacho, Shinjuku-ku, Tokyo 162-8666, Japan.
Masayuki Yamato, Email: yamato.masayuki@twmu.ac.jp, Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University, 8-1 Kawadacho, Shinjuku-ku, Tokyo 162-8666, Japan.
Yoshiko Shiratsuchi, Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University, 8-1 Kawadacho, Shinjuku-ku, Tokyo 162-8666, Japan.
Satoshi Takaki, Department of Immune Regulation, Research Institute National Center for Global Health and Medicine, Chiba 272-8516, Japan.
Brian G. Petrich, Department of Medicine, University of California, San Diego, La Jolla, CA 92093-0726, USA
Hiromitsu Nakauchi, Division of Stem Cell Therapy, Center for Stem Cell Biology, The Institute of Medical Science, The University of Tokyo, Tokyo 108-8639, Japan.
Koji Eto, Department of Clinical Application, Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto 606-8507, Japan.
Toshio Kitamura, Division of Cellular Therapy, The Institute of Medical Science, The University of Tokyo, Tokyo 108-8639, Japan.
Teruo Okano, Institute of Advanced Biomedical Engineering and Science, Tokyo Women’s Medical University, 8-1 Kawadacho, Shinjuku-ku, Tokyo 162-8666, Japan.
References
- 1.Takubo K, Suda T. Roles of the hypoxia response system in hematopoietic and leukemic stem cells. Int J Hematol. 2012;95:478–483. doi: 10.1007/s12185-012-1071-4. [DOI] [PubMed] [Google Scholar]
- 2.Umemoto T, Yamato M, Ishihara J, Shiratsuchi Y, Utsumi M, Morita Y, et al. Integrin-αvβ3 regulates thrombopoietin-mediated maintenance of hematopoietic stem cells. Blood. 2012;119:83–94. doi: 10.1182/blood-2011-02-335430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimura S, Roberts AW, Metcalf D, Alexander WS. Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buza-Vidas N, Antonchuk J, Qian H, Månsson R, Luc S, Zandi S, et al. Cytokines regulate postnatal hematopoietic stem cell expansion: opposing roles of thrombopoietin and LNK. Genes Dev. 2006;20:2018–2023. doi: 10.1101/gad.385606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013;98:10–23. doi: 10.1007/s12185-013-1382-0. [DOI] [PubMed] [Google Scholar]
- 6.Lin CG, Leu S-J, Chen N, Tebeau CM, Lin S-X, Yeung C-Y, et al. CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem. 2003;278:24200–24208. doi: 10.1074/jbc.M302028200. [DOI] [PubMed] [Google Scholar]
- 7.Lin CG, Chen CC, Leu SJ, Grzeszkiewicz TM, Lau LF. Integrin-dependent functions of the angiogenic inducer NOV (CCN3): implication in wound healing. J Biol Chem. 2005;280:8229–8237. doi: 10.1074/jbc.M404903200. [DOI] [PubMed] [Google Scholar]
- 8.Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol. 2001;54:57–79. doi: 10.1136/mp.54.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta R, Hong D, Iborra F, Sarno S, Enver T. NOV (CCN3) functions as a regulator of human hematopoietic stem or progenitor cells. Science. 2007;316:590–593. doi: 10.1126/science.1136031. [DOI] [PubMed] [Google Scholar]
- 10.Bruno L, Hoffmann R, McBlane F, Brown J, Gupta R, Joshi C, et al. Molecular signatures of self-renewal, differentiation, and lineage choice in multipotential hemopoietic progenitor cells in vitro. Mol Cell Biol. 2004;24:741–756. doi: 10.1128/MCB.24.2.741-756.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-y in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Bruin AM, Demirel Ö, Hooibrink B, Brandts CH, Nolte MA. Interferon-γ impairs proliferation of hematopoietic stem cells in mice. Blood. 2013;121:3578–3585. doi: 10.1182/blood-2012-05-432906. [DOI] [PubMed] [Google Scholar]
- 13.Umemoto T, Yamato M, Shiratsuchi Y, Terasawa M, Yang J, Nishida K, et al. Expression of Integrin β3 is correlated to the properties of quiescent hemopoietic stem cells possessing the side population phenotype. J Immunol. 2006;177:7733–7739. doi: 10.4049/jimmunol.177.11.7733. [DOI] [PubMed] [Google Scholar]
- 14.Szilvassy SJ, Humphries RK, Lansdorp PM, Eaves AC, Eaves CJ. Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci USA. 1990;87:8736–8740. doi: 10.1073/pnas.87.22.8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell. 2002;110:599–611. doi: 10.1016/s0092-8674(02)00935-2. [DOI] [PubMed] [Google Scholar]
- 16.Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, et al. CD31/PECAM-1 is a ligand for alpha v beta 3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–861. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Nakamura T, Koyanagi M, Kato K, Hashimoto Y, Yagita H, et al. A murine very late activation antigen-like extracellular matrix receptor involved in CD2-and lymphocyte function-associated antigen-1-independent killer-target cell interaction. J Immunol. 1990;145:4371–4379. [PubMed] [Google Scholar]
- 19.Noda S, Horiguchi K, Ichikawa H, Miyoshi H. Repopulating activity of ex vivo-expanded murine hematopoietic stem cells resides in the CD48−c-Kit+Sca-l+lineage marker−cell population. Stem Cells. 2008;26:646–655. doi: 10.1634/stemcells.2007-0623. [DOI] [PubMed] [Google Scholar]
- 20.Takizawa H, Nishimura S, Takayama N, Oda A, Nishikii H, Morita Y, et al. Lnk regulates integrin αIIbβ3 outside-in signaling in mouse platelets, leading to stabilization of thrombus development in vivo. J Clin Invest. 2010;120:179–190. doi: 10.1172/JCI39503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tahiliani PD, Singh L, Auer KL, LaFlamme SE. The role of conserved amino acid motifs within the integrin β3 cytoplasmic domain in triggering focal adhesion kinase phosphorylation. J Biol Chem. 1997;272:7892–7898. doi: 10.1074/jbc.272.12.7892. [DOI] [PubMed] [Google Scholar]
- 22.Petrich BG, Fogelstrand P, Partridge AW, Yousefi N, Ablooglu AJ, Shattil SJ, et al. The antithrombotic potential of selective blockade of talin-dependent integrin alpha IIb beta 3 (platelet GPIIb-IIIa) activation. J Clin Invest. 2007;117:2250–2259. doi: 10.1172/JCI31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura A, Martin C, Robinson GW, Simone JM, Chen W, Wickre MC, et al. The gene encoding the hematopoietic stem cell regulator CCN3/NOV is under direct cytokine control through the transcription factors STAT5A/B. J Biol Chem. 2010;285:32704–32709. doi: 10.1074/jbc.M110.141804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seita J, Ema H, Ooehara J, Yamazaki S, Tadokoro Y, Yamasaki A, et al. Lnk negatively regulates self-renewal of hematopoietic stem cells by modifying thrombopoietin-mediated signal transduction. Proc Natl Acad Sci USA. 2007;104:2349–2354. doi: 10.1073/pnas.0606238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh ST, Simonds EF, Jones C, Hale MB, Goltsev Y, Gibbs KD, Jr, et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood. 2010;116:988–992. doi: 10.1182/blood-2010-02-270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saha B, Jyothi Prasanna S, Chandrasekar B, Nandi D. Gene modulation and immunoregulatory roles of interferon y. Cytokine. 2010;50:1–14. doi: 10.1016/j.cyto.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Menegazzi M, Tedeschi E, Dussin D, de Prati AC, Cavalieri E, Mariotto S, et al. Anti-interferon y action of epigallocatechin-3-gallate mediated by specific inhibition of STAT1 activation. FASEB J. 2001;15:1309–1311. doi: 10.1096/fj.00-0519fje. [DOI] [PubMed] [Google Scholar]
- 28.Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Bersenev A, Wu C, Balcerek J, Tong W. Lnk controls mouse hematopoietic stem cell self-renewal and quiescence through direct interactions with JAK2. J Clin Invest. 2008;118:2832–2844. doi: 10.1172/JCI35808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsushita Y, Sakamoto K, Tamamura Y, Shibata Y, Minamizato T, Kihara T, et al. CCN3 protein participates in bone regeneration as an inhibitory factor. J Biol Chem. 2013;288:19973–19985. doi: 10.1074/jbc.M113.454652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Z, Natesan V, Shi H, Hamik A, Kawanami D, Hao C, et al. A novel role of CCN3 in regulating endothelial inflammation. J Cell Commun Signal. 2010;4:141–153. doi: 10.1007/s12079-010-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakamoto K, Yamaguchi S, Ando R, Miyawaki A, Kabasawa Y, Takagi M, et al. The nephroblastoma overexpressed gene (NOV/ccn3) protein associates with Notchl extracellular domain and inhibits myoblast differentiation via Notch signaling pathway. J Biol Chem. 2002;277:29399–29405. doi: 10.1074/jbc.M203727200. [DOI] [PubMed] [Google Scholar]
- 33.Gellhaus A, Wotzlaw C, Otto T, Fandrey J, Winterhager E. More insights into the CCN3/Connexin43 interaction complex and its role for signaling. J Cell Biochem. 2010;110:129–140. doi: 10.1002/jcb.22519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.