Abstract
Ovarian cycling continues to similar ages in women and chimpanzees yet our nearest living cousins become decrepit during their fertile years and rarely outlive them. Given the importance of estrogen in maintaining physiological systems aside from fertility, similar ovarian aging in humans and chimpanzees combined with somatic aging differences indicates an important role for nonovarian estrogen. Consistent with this framework, researchers have nominated the adrenal androgen dehydroepiandrosterone (DHEA) and its sulfate (DHEAS), which can be peripherally converted to estrogen, as a biomarker of aging in humans and other primates. Faster decline in production of this steroid with age in chimpanzees could help explain somatic aging differences. Here, we report circulating levels of DHEAS in captive female chimpanzees and compare them with published levels in women. Instead of faster, the decline is slower in chimpanzees, but from a much lower peak. Levels reported for other great apes are lower still. These results point away from slowed decline but toward increased DHEAS production as one of the mechanisms underlying the evolution of human longevity.
Keywords: dehydroepiandrosterone sulfate, senescence, aging rates, human longevity
Humans have remarkably long lives compared to other members of the great ape clade (Raisz, 1999; Robson et al., 2006). Survival well beyond menopause is a distinctive feature of human life history (Bogin and Smith, 1996) and contrasts with patterns observed in most other mammals including all nonhuman primates (Levitis and Lackey, 2011). Because human life expectancies have nearly doubled in some populations since the nineteenth century (Oeppen and Vaupel, 2002), the human pattern is widely assumed to be a novelty of recent history. But those recent changes are largely due to reductions in infant and juvenile mortality (Oeppen and Vaupel, 2002). Where nutritional and technological advances responsible for the reduced old age mortality of some contemporary populations (Kirkwood, 2008; Hawkes, 2010) are absent, women have continued to be economically productive well beyond the fertile ages (Hamilton, 1966; Hawkes et al., 1989, 1997; Kaplan et al., 2000; Blurton Jones et al., 2002; Kaplan et al., 2010) and in hunter-gatherer socioecologies they show little decline in strength into their sixties (Blurton Jones and Marlowe, 2002; Walker and Hill, 2003).
Hypotheses about why natural selection favored slower aging in many human somatic systems (Hawkes et al., 1998; Kaplan et al., 2000; Hawkes, 2003; Kaplan et al., 2010) are silent on the physiological mechanisms that make it possible. This mechanism question is especially pressing because, aside from fertility, the steroid hormones collectively referred to as estrogen affect diverse tissues and cells (osteal: Raisz, 1999; cardiovascular: Kim and Levin, 2006; Turgeon et al., 2006; immunological: Wise et al., 2009; neurological: Lacreuse, 2006; Wise et al., 2005). While men produce testosterone, which is locally converted to estrogen in peripheral tissues throughout life, women produce ovarian estrogen only as ovarian follicles grow from a nonrenewing stock that begins declining before birth (Peters et al., 1978; McGee and Hseuth, 2000).
When follicle stocks fall below a threshold needed to support ovulation, cycling stops (Faddy and Gosden, 1996; McGee and Hsueh, 2000) and, estrogen secretion plummets to levels so low that it remains controversial whether postmenopausal ovaries produce any (Labrie et al., 2011). Many aspects of somatic aging in Western women have been linked with this drop (e.g., Riggs et al., 1998; Pfeilschifter et al., 1978; Turgeon et al., 2006; Stevenson and Thornton, 2007; Wise et al., 2009; Gibbs, 2010; Henn, 2010). Yet postmenopausal declines in physiological competence are not large enough to cause an inflection in mortality (Hamilton, 1966; Gavrilov and Gavrilova, 1991) or stop postmenopausal women from continuing high levels of economic productivity (e.g., Hawkes et al., 1989, 1997). If estrogen is important for physiological maintenance, postmenopausal women must produce it from nonovarian sources. Other steroids that can be converted to estrogen in peripheral tissues are obvious candidates.
After cholesterol, the adrenal androgen dehydroepiandrosterone (DHEA) and its sulfate ester dehydroepiandrosterone sulfate (DHEAS) are the most abundant steroids circulating in young human adults. They are the main products of the human adrenal gland (Longcope, 1986), circulating at nanomolar and micromolar concentrations respectively (Longcope, 1995; Baulieu, 1996; Longcope, 1996). Peripherally, DHEA and DHEAS are interconverted by sulfotransferase enzymes present in a wide variety of tissues (Fujikawa et al., 1997; Dalla Valle et al., 2006). As DHEAS has a longer circulating half-life and concentration several orders of magnitude higher, it is generally considered to be a reservoir for DHEA (Longcope, 1986; Rosenfeld et al., 1975; but see Hammer et al., 2005; Siiteri, 2005 for debate). Circulating levels are “1,000 to 10,000 times higher than those of estradiol” in women (Labrie et al., 1998:322), so that intracrine conversion of DHEA in peripheral target tissues may be responsible for “75% of estrogen before menopause and close to 100% after menopause” (Labrie, 1991:C116).
Faster decline in adrenal androgen production across adulthood might help explain why chimpanzees become decrepit while their ovaries are still secreting estrogen. Follicle stocks decline with age at the same rate in chimpanzees and humans (Jones et al., 2007); and, like us, they can have last pregnancies into their forties (Roof et al., 2005; Emery Thompson et al., 2007). But chimpanzees display geriatric symptoms in their thirties (Goodall, 1986; Huffman, 1990; Nishida et al., 2003; Matsuzawa, 2007). Even in captivity where mortality is reduced (Dyke et al., 1995; Hill et al., 2001), chimpanzees rarely live beyond their cycling years (Lacreuse et al., 2008; Herndon et al., 2012).
Declines in circulating levels of DHEAS have been measured in several primate species; and those declines have been proposed as biomarkers of aging in humans and nonhuman primates (Lane et al., 1997; Kemnitz et al., 2000; Roth et al., 2002). Ingram et al. (2001:1030–1) say that
“The rate of age-related change in a candidate biomarker should be proportional to differences in lifespan among related species. For example, the rate of change in a candidate biomarker of aging in chimpanzees should be twice that of humans (60 vs. 120 years maximum lifespan); in rhesus monkeys about three times that of humans (40 vs. 120 years maximum lifespan).”
This expectation is consistent with general scaling assumptions and supported empirically for circulating DHEAS data on captive rhesus and humans (Lane et al., 1997). Building on these observations we hypothesized that circulating levels of DHEAS would decline twice as fast with age in female chimpanzees when compared to published levels in women.
MATERIALS AND METHODS
To test this hypothesis, we requested blood samples from female chimpanzees at Yerkes National Primate Research Center. Samples were drawn only when subjects were sedated for reasons unrelated to this project with a protocol approved by IACUCs at the University of Utah and Yerkes. During 2007, 2009, 2010, and 2011, samples were taken from 70 females then immediately processed for serum and kept frozen until analyzed for DHEAS by the Biomarkers Core Lab at Yerkes using RIA for samples from 2007 and LC-MS thereafter (Supporting Information Table S1). Here, we compare the results from 65 chimpanzee females over the age of 15 with a combined sample of published DHEAS levels for 71 Czech women between the ages of 20 and 80 reported by Sulcova et al. (1997), 68 Italian women between the ages of 19 and 78 reported by Ravaglia et al. (1996), and 530 Australian women aged 20–76 reported by Davison et al. (2005) (see Supporting Information Table S2). We used these sources because their published figures allowed recovery of individual DHEAS levels, not just age class means and because they provided DHEAS levels across adulthood.
Some of our chimpanzee measurements came from subjects on hormonal contraception (Supporting Information Table S1). As exogenous hormone supplements reduce DHEAS levels 26–32% in women (White et al., 2005), we looked for a similar effect in chimpanzees by splitting our sample into the 53 measurements taken when a subject was not on hormone contraception and the 12 when on. Comparison of models fitted to these subsamples (Supporting Information Fig. S1) showed no substantial differences.
Methods differed slightly among the human studies, so we used R statistical package (R Development Core R Development Core Team, 2011) to fit models of DHEAS concentrations against age for each dataset separately (Supporting Information Fig. S2) and found that the 95% confidence intervals overlap. To evaluate representativeness of our sample for women, we extracted 5-year age class means of DHEAS levels from figures reported by Orentreich et al. (1984), fit a model using the procedure described above, and found very close agreement between models of human DHEAS decline in our combined human sample and that of Orentreich and colleagues (Table 1).
TABLE 1.
Parameter estimates, sample size, and estimated maximum DHEAS at the start of adulthood for models of DHEAS loss in human and chimpanzee females
| Sample | N | Functional form | A (95% CI) | B (95% CI) | Max. DHEAS (μg/dL) |
|---|---|---|---|---|---|
| Chimpanzee Table S1 (Supporting Information) | 65 | y = A + B *ln(x) | 180.73 (114.71, 250.44) | −33.86 (−55.20, −13.64) | 89.04 |
| Human Table S2 (Supporting Information) | 698 | y = eˆ(A + B × x) | 6.24 (6.10, 6.35) | −0.03 (−0.03, −0.03) | 281.46 |
| Human standard | 11 | y = eˆ(A + B × x) | 6.14 (6.02, 6.25) | −0.03 (−0.04, −0.03) | 254.68 |
In all three models, x corresponds to years of age. The second row is a model fitted to data from Ravaglia et al. (1996), Sulcova et al. (1997), and Davison et al. (2005) detailed in Table S2 (Supporting Information). The third row describes a model fitted to 11 age-class means reported by Orentreich and colleagues (1984), which serves as check on the generality of our individual-based human model. To estimate maximum DHEAS (i.e., expected DHEAS concentration at the start of adulthood) reported in the last column, we evaluated our chimpanzee model at 15 years and the human models at 20 years.
We then compared our combined human sample to our chimpanzee sample by fitting a number of functional forms, both linear and nonlinear, with maximum likelihood estimation in order to approximate the relationship between DHEAS concentrations and age. Using Akaike Information Criterion (Akaike, 1974) we determined the best fitting models for each species.
RESULTS
A logarithmic function where DHEAS (μg/dL) = 189.73−33.86 × ln(age) best fit the chimpanzee data, while an exponential model where DHEAS (μg/dL) = eˆ(6.24−0.03 × age) fit the human data best (Table 1). Using these models, we estimated peak concentrations of DHEAS at the start of adulthood (using 15-years-old for chimpanzees and 20 years for humans because these are close to the beginning of adulthood in each species and allow comparison of 5-year age class means; Table 1) and average rates of DHEAS decline across 5-year age classes (Table 2). We calculated the ratio of chimpanzee to human slope for each 5-year age class after peak in Table 2 to simplify comparison. Figure 1 shows the distribution of individual DHEAS concentrations by age and species, with the best-fit models for each.
TABLE 2.
Slope of declining human and chimpanzee DHEAS concentrations averaged over 5-year age intervals
| Chimpanzees
|
Humans
|
|||||
|---|---|---|---|---|---|---|
| Age range | N | Mean slope | Age range | N | Mean slope | Chimpanzee slope/human slope |
| 15–19 | 24 | −1.60 | 20–24 | 31 | −6.37 | 0.25 |
| 20–24 | 8 | −1.23 | 25–29 | 48 | −5.48 | 0.23 |
| 25–29 | 7 | −1.01 | 30–34 | 75 | −4.72 | 0.21 |
| 30–34 | 8 | −0.85 | 35–39 | 73 | −4.06 | 0.21 |
| 35–39 | 5 | −0.73 | 40–44 | 92 | −3.49 | 0.21 |
| 40–44 | 5 | −0.65 | 45–49 | 85 | −3.01 | 0.21 |
| 45–49 | 2 | −0.58 | 50–54 | 62 | −2.59 | 0.22 |
| 50–54 | 5 | −0.52 | 55–59 | 60 | −2.23 | 0.23 |
| – | – | 60–64 | 44 | −1.92 | ||
| – | – | 65–69 | 44 | −1.65 | ||
| – | – | 70–74 | 64 | −1.42 | ||
To calculate an age interval’s mean slope, we evaluated the relevant model (see Table 1 for parameter estimates) at the first and last years of the interval. We then subtracted the DHEAS estimate for the older age from that of the younger age and divided the resultant sum by five. The far right column shows the ratio of chimpanzee slope to human slope in each five-year interval following peak concentration.
Fig. 1.
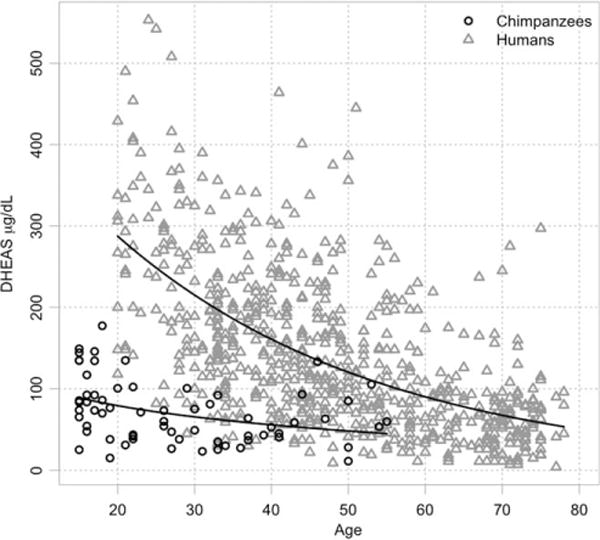
Concentrations of DHEAS (μg/dL) as a function of age in both human (n = 698) and chimpanzee (n = 65) females. Lines represent best-fit models of DHEAS decline with age in humans and chimpanzees. See Table 1 for parameter estimates.
Contrary to the hypothesis that circulating levels of DHEAS would decline twice as fast in chimpanzees as they do in humans, declines are more gradual in chimpanzees. Average declines in chimpanzee DHEAS range between 21 and 25% of human rates (Table 2). Compared to chimpanzees, women begin adulthood with more than three times the circulating levels of DHEAS (281.46 vs. 89.04 μg/dL for chimpanzee females; Table 1). Human concentrations do not fall to the highest chimpanzee levels until the tenth 5-year interval—starting at 65 and ending at 69.
DISCUSSION
Circulating levels of DHEAS in our chimpanzee sample do not support the hypothesis of a faster decline with age. Instead, when compared to women, female chimpanzees begin adulthood with DHEAS concentrations less than one-third as high that decrease at less than one fourth the rate. Assuming that DHEAS levels are an index of investment in somatic maintenance, we should have anticipated that maximum circulating levels would be substantially higher in humans. Evolutionary theories of aging link slower senescence and longer average adult life spans to increased somatic investment (Williams, 1957; Hamilton, 1966; Williams, 1966; Kirkwood and Rose, 1991). More investment in maintenance reduces vulnerability to mortality (e.g., Ricklefs, 1998), and selection favors more maintenance when that tradeoff increases lifetime fitness (Hamilton, 1966; Williams, 1966; Kirkwood and Rose, 1991; Hawkes, 2003).
The chimpanzee-human DHEAS comparison is consistent with the inference that adrenal steroids play an important role in somatic maintenance. In humans—and we assume in chimpanzees as well—estrogenic bioactivity is important for both fertility and somatic maintenance. This link between higher DHEA/S levels and increased somatic maintenance in humans is consistent with a broader hypothesis that higher circulating levels of DHEAS in primates versus nonprimate mammals (Labrie et al., 2001; Nguyen and Conley, 2008) contribute to greater longevity in our order (e.g., Austad and Fischer, 1992; Charnov and Berrigan, 1993). But this generalization warrants further scrutiny in light of complex variation in adrenal glands across the order (Conley et al., 2004; Nguyen and Conley, 2008). An implicit corollary of the hypothesis that DHEAS plays a role in primate longevity is that similarities in somatic maintenance between chimpanzees and other great apes derive partly from similarities in DHEAS levels. Bernstein and collaborators (Bernstein et al., 2012) recently presented data inconsistent with this corollary.
They reported serum levels of DHEA and DHEAS across the life span in captive great apes and showed, as do our data here, that circulating levels of DHEAS are much lower in chimpanzees than humans. Although they did not analyze changes across adulthood, their findings are generally concordant with those of our sample. Surprisingly they found marked differences between genus Pan and the other great apes. As ages at last birth and maximum lifespans are similar among the nonhuman great apes, we had assumed mechanisms of ovarian and somatic aging would be similar as well. But Bernstein and colleagues found otherwise. The level they calculated for gorillas (Gorilla gorilla)—again averaging both sexes—was only 34% that of Pan. Even more striking, orangutans (Pongo abelii and P. pygmaeus of both sexes) had average levels only 16% of Pan, the lowest average reported among catarrhines.
The differences in DHEAS levels across the nonhuman great apes suggest that androgens we have not investigated may be important in somatic maintenance. Lasley et al. (2012) further highlighted this possibility by suggesting the importance of another adrenal androgen, Androstenediol (Adiol), in perimenopausal women. They found (McConnell et al., 2012) that the transient increase in circulating DHEAS observed in perimenopausal women (Crawford et al., 2009) is accompanied by similar changes in the circulating levels of other adrenal androgens. Adiol is of particular interest because, in contrast to DHEA/S, it activates the estrogen receptor without intracrine conversion. Circulating at levels substantially higher than estrogen in postmenopausal women, Adiol may be vital to estrogenic bioactivity (Lasley et al., 2012).
Before concluding, we consider some limitations of our sample. Although often collapsed into age class averages, the individual variation in DHEAS levels in European, Australian, and American women is marked (see review in Enea et al., 2008). Yet Western women and captive chimpanzees do not represent the likely range of variation. For chimpanzees, captive conditions are known to affect ovarian hormone levels compared to those measured in the wild (Emery and Whitten, 2003; Emery Thompson, 2005) and adrenal steroid levels may vary between captivity and the wild as well. In the same way, human variation may be even wider if non-Western subjects were included. Ovarian hormone levels are known to differ between industrial and traditional populations, with covariates including diet, work, and disease load (e.g., Ellison et al., 1993; Ellison, 1994; Jasienska and Jasienski, 2008; Vitzthum, 2008). Adrenal steroid levels may also differ.
If research into variation in male steroid levels is a guide to the magnitude of differences in women, differences in adrenal androgen levels may be smaller than differences in gonadal levels. Campbell et al. (2006, 2007) reported levels of both for Turkana men in nomadic and settled communities. Levels were significantly different for testosterone, but—except in the oldest subjects—DHEAS levels were not. On the other hand, Crawford et al. (2009) found dramatic differences in both DHEAS level and rate of change with age for American women of different ethnic groups between the ages of 42 and 52. Through those ages some of the women in their dataset had DHEAS levels that overlap our chimpanzee sample. However, little difference between African American and Caucasian women has been found in studies of DHEAS levels in younger adults (e.g., Kitabchi et al., 1999; An et al., 2001), and levels in these younger women are substantially higher than those of chimpanzees.
With those caveats we conclude that declines in circulating levels of DHEAS are not steeper in female chimpanzees than in women; but levels are substantially lower in chimpanzees; and the human difference from the other great apes is even larger than the difference from genus Pan. Contrasts among the other hominids raise additional questions, but also further distinguish the high DHEAS production in humans, a distinction consistent with the likelihood that this mechanism contributes to the extraordinary longevity of our own lineage.
Supplementary Material
Acknowledgments
The authors thank the keepers, veterinarians, and biological material procurement staff at Yerkes, the staff of the Biomarkers Core Lab, and CT Cloutier for bibliographic assistance.
Grant sponsor: NSF; Grant number: BCS—0717886; Grant sponsor: NIH; Grant numbers: P01AG026423 and P51RR000165.
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- Akaike H. A new look at the statistical-model identification. IEEE Trans Automatic Control. 1974;19:716–723. [Google Scholar]
- An P, Rice T, Gagnon J, Hong YL, Leon AS, Skinner JS, Wil-more JH, Bouchard C, Rao DC. Race differences in the pattern of familial aggregation for dehydroepiandrosterone sulfate and its responsiveness to training in the HERITAGE Family Study. Metabolism. 2001;50:916–920. doi: 10.1053/meta.2001.24926. [DOI] [PubMed] [Google Scholar]
- Austad SN, Fischer KE. Primate longevity—its place in the mammalian scheme. Am J Primatol. 1992;28:251–261. doi: 10.1002/ajp.1350280403. [DOI] [PubMed] [Google Scholar]
- Baulieu EE. Dehydroepiandrosterone (DHEA): a fountain of youth? J Clin Endocr Metab. 1996;81:3147–3151. doi: 10.1210/jcem.81.9.8784058. [DOI] [PubMed] [Google Scholar]
- Bernstein RM, Sterner KN, Wildman DE. Adrenal androgen production in catarrhine primates and the evolution of adrenarche. Am J Phys Anthropol. 2012;147:389–400. doi: 10.1002/ajpa.22001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton Jones NG, Hawkes K, O’Connell JF. Antiquity of post-reproductive life: are there modern impacts on hunter-gatherer postreproductive life spans? Am J Hum Biol. 2002;14:184–205. doi: 10.1002/ajhb.10038. [DOI] [PubMed] [Google Scholar]
- Blurton Jones NG, Marlowe FW. Selection for delayed maturity—Does it take 20 years to learn to hunt and gather? Hum Nature-Int Bios. 2002;13:199–238. doi: 10.1007/s12110-002-1008-3. [DOI] [PubMed] [Google Scholar]
- Bogin B, Smith BH. Evolution of the human life cycle. Am J Hum Biol. 1996;8:703–716. doi: 10.1002/(SICI)1520-6300(1996)8:6<703::AID-AJHB2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Campbell B, Leslie P, Campbell K. Age-related changes in testosterone and SHBG among Turkana males. Am J Hum Biol. 2006;18:71–82. doi: 10.1002/ajhb.20468. [DOI] [PubMed] [Google Scholar]
- Campbell BC, Leslie P, Campbell K. Age-related patterns of DHEAS among Turkana males of northern Kenya. Aging Male. 2007;10:203–209. doi: 10.1080/13685530701533151. [DOI] [PubMed] [Google Scholar]
- Charnov EL, Berrigan D. Why do female primates have such long lifespans and so few babies? or Life in the slow lane. Evol Anthropol. 1993;1:191–194. [Google Scholar]
- Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004;22:311–326. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocr Metab. 2009;94:2945–2951. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Valle L, Toffolo V, Nardi A, Fiore C, Bernante P, Di Liddo R, Parnigotto PP, Colombo L. Tissue-specific transcriptional initiation and activity of steroid sulfatase complementing dehydroepiandrosterone sulfate uptake and intracrine steroid activations in human adipose tissue. J Endocr. 2006;190:129–139. doi: 10.1677/joe.1.06811. [DOI] [PubMed] [Google Scholar]
- Davison SL, Bell R, Donath S, Montalto JG, Davis SR. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocr Metab. 2005;90:3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- Dyke B, Gage TB, Alford PL, Swenson B, Williams-Blangero S. Model life table for captive chimpanzees. Am J Primatol. 1995;37:25–37. doi: 10.1002/ajp.1350370104. [DOI] [PubMed] [Google Scholar]
- Ellison PT. Advances in human reproductive ecology. Annu Rev Anthropol. 1994;23:255–275. doi: 10.1146/annurev.an.23.100194.001351. [DOI] [PubMed] [Google Scholar]
- Ellison PT, Panterbrick C, Lipson SF, O’Rourke MT. The ecological context of human ovarian function. Hum Reprod. 1993;8:2248–2258. doi: 10.1093/oxfordjournals.humrep.a138015. [DOI] [PubMed] [Google Scholar]
- Emery MA, Whitten PL. Size of sexual swellings reflects ovarian function in chimpanzees (Pan troglodytes) Behav Ecol Sociobiol. 2003;54:340–351. [Google Scholar]
- Emery Thompson M. Reproductive endocrinology of wild female chimpanzees (Pan troglodytes schweinfurthii): methodological considerations and the role of hormones in sex and conception. Am J Primatol. 2005;67:137–158. doi: 10.1002/ajp.20174. [DOI] [PubMed] [Google Scholar]
- Emery Thompson M, Jones JH, Pusey AE, Brewer-Marsden S, Goodall J, Marsden D, Matsuzawa T, Nishida T, Reynolds V, Sugiyama Y, Wrangham RW. Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol. 2007;17:2150–2156. doi: 10.1016/j.cub.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enea C, Boisseau N, Diaz V, Dugue B. Biological factors and the determination of androgens in female subjects. Steroids. 2008;73:1203–1216. doi: 10.1016/j.steroids.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG. A model conforming the decline in follicle numbers to the age of menopause in women. Hum Reprod. 1996;11:1484–1486. doi: 10.1093/oxfordjournals.humrep.a019422. [DOI] [PubMed] [Google Scholar]
- Fujikawa H, Okura F, Kuwano Y, Sekizawa A, Chiba H, Shimodaira K, Saito H, Yanaihara T. Steroid sulfatase activity in osteoblast cells. Biochem Biophys Res Commun. 1997;231:42–47. doi: 10.1006/bbrc.1996.6038. [DOI] [PubMed] [Google Scholar]
- Gavrilov LA, Gavrilova NS. The biology of life span: a quantitative approach. New York: Harwood Academic Publishers; 1991. [Google Scholar]
- Gibbs RB. Estrogen therapy and cognition: a review of the cholinergic hypothesis. Endocr Rev. 2010;31:224–253. doi: 10.1210/er.2009-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J. The chimpanzees of Gombe: patterns of behavior. Cambridge, MA: Belknap Press; 1986. [Google Scholar]
- Hamilton WD. The moulding of senescence by natural selection. J Theor Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Hammer F, Subtil S, Lux P, Maser-Gluth C, Stewart PM, Allolio B, Arlt W. No evidence for hepatic conversion of dehydroepiandrosterone (DHEA) sulfate to DHEA: in vivo and in vitro studies. J Clin Endocr Metab. 2005;90:3600–3605. doi: 10.1210/jc.2004-2386. [DOI] [PubMed] [Google Scholar]
- Hawkes K. Grandmothers and the evolution of human longevity. Am J Hum Biol. 2003;15:380–400. doi: 10.1002/ajhb.10156. [DOI] [PubMed] [Google Scholar]
- Hawkes K. Colloquium paper: how grandmother effects plus individual variation in frailty shape fertility and mortality: guidance from human-chimpanzee comparisons. Proc Natl Acad Sci USA. 2010;107(Suppl 2):8977–8984. doi: 10.1073/pnas.0914627107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes K, O’Connell JF, Blurton Jones NG. Hardworking Hadza grandmothers. In: Standen V, Foley RA, editors. Comparative socioecology: the behavioural ecology of humans and other mammals. London: Basil Blackwell; 1989. pp. 341–366. [Google Scholar]
- Hawkes K, O’Connell JF, Blurton Jones NG. Hadza women’s time allocation, offspring provisioning, and the evolution of long postmenopausal life spans. Curr Anthropol. 1997;38:551–577. [Google Scholar]
- Hawkes K, O’Connell JF, Blurton Jones NG, Alvarez H, Charnov EL. Grandmothering, menopause, and the evolution of human life histories. Proc Natl Acad Sci USA. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn EW. Menopause and its effect on the female lower urinary tract. S Afr Fam Practice. 2010;52:405–408. [Google Scholar]
- Herndon JG, Paredes J, Wilson ME, Bloomsmith MA, Chennareddi L, Walker ML. Menopause occurs late in life in the captive chimpanzee (Pan troglodytes) Age (Dordr) 2012;34:1145–1156. doi: 10.1007/s11357-011-9351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K, Boesch C, Goodall J, Pusey A, Williams J, Wrangham R. Mortality rates among wild chimpanzees. J Hum Evol. 2001;40:437–450. doi: 10.1006/jhev.2001.0469. [DOI] [PubMed] [Google Scholar]
- Huffman MA. Some socio-behavioral manifestations of old-age in chimpanzees. In: Nishida T, editor. The chimpanzees of the Mahale Mountains: sexual and life history strategies. Tokyo: University of Tokyo; 1990. pp. 237–255. [Google Scholar]
- Ingram DK, Nakamura E, Smucny D, Roth GS, Lane MA. Strategy for identifying biomarkers of aging in long-lived species. Exp Gerontol. 2001;36:1025–1034. doi: 10.1016/s0531-5565(01)00110-3. [DOI] [PubMed] [Google Scholar]
- Jasienska G, Jasienski M. Interpopulation, interindivid-ual, intercycle, and intracycle natural variation in progesterone levels: a quantitative assessment and implications for population studies. Am J Hum Biol. 2008;20:35–42. doi: 10.1002/ajhb.20686. [DOI] [PubMed] [Google Scholar]
- Jones KP, Walker LC, Anderson D, Lacreuse A, Robson SL, Hawkes K. Depletion of ovarian follicles with age in chimpanzees: similarities to humans. Biol Reprod. 2007;77:247–251. doi: 10.1095/biolreprod.106.059634. [DOI] [PubMed] [Google Scholar]
- Kaplan H, Gurven M, Winking J, Hooper PL, Stieglitz J. Learning, menopause, and the human adaptive complex. Ann NY Acad Sci. 2010;1204:30–42. doi: 10.1111/j.1749-6632.2010.05528.x. [DOI] [PubMed] [Google Scholar]
- Kaplan H, Hill K, Lancaster J, Hurtado AM. A theory of human life history evolution: diet, intelligence, and longevity. Evol Anthropol. 2000;9:156–185. [Google Scholar]
- Kemnitz JW, Roecker EB, Haffa ALM, Pinheiro J, Kurzman I, Ramsey JJ, MacEwen EG. Serum dehydroepiandrosterone sulfate concentrations across the life span of laboratory-housed rhesus monkeys. J Med Primatol. 2000;29:330–337. doi: 10.1034/j.1600-0684.2000.290504.x. [DOI] [PubMed] [Google Scholar]
- Kim JK, Levin ER. Estrogen signaling in the cardiovascular system. Nuclear receptor signaling. 2006;4:e013. doi: 10.1621/nrs.04013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TBL. A systematic look at an old problem. Nature. 2008;451:644–647. doi: 10.1038/451644a. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL, Rose MR. Evolution of senescence—late survival sacrificed for reproduction. Philos Trans R Soc B. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Kitabchi AE, Imseis RE, Bush AJ, Williams-Cleaves B, Pourmotabbed G. Racial differences in the correlation between gonadal androgens and serum insulin levels. Diabetes Care. 1999;22:1524–1529. doi: 10.2337/diacare.22.9.1524. [DOI] [PubMed] [Google Scholar]
- Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–C118. doi: 10.1016/0303-7207(91)90116-a. [DOI] [PubMed] [Google Scholar]
- Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez J-L, Candas B. DHEA and the intracrine formation of androgens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–328. doi: 10.1016/s0039-128x(98)00007-5. [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Labrie C, Simard J. DHEA and its transformation into androgens and estrogens in peripheral target tissues: intracrinology. Front Neuroendocrinol. 2001;22:185–212. doi: 10.1006/frne.2001.0216. [DOI] [PubMed] [Google Scholar]
- Labrie F, Martel C, Balser J. Wide distribution of the serum dehydroepiandrosterone and sex steroid levels in postmenopausal women: role of the ovary? Menopause. 2011;18:30–43. doi: 10.1097/gme.0b013e3181e195a6. [DOI] [PubMed] [Google Scholar]
- Lacreuse A. Effect of ovarian hormones on cognitive function in nonhuman primates. Neuroscience. 2006;138:859–867. doi: 10.1016/j.neuroscience.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Lacreuse A, Chennareddi L, Gould KG, Hawkes K, Wijatyawardana SR, Chen J, Easley KA, Herndon JG. Menstrual cycles continue into advanced old age in the common chimpanzee (Pan troglodytes) Biol Reprod. 2008;79:407–412. doi: 10.1095/biolreprod.108.068494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Ball SS, Roth GS. Dehydroepiandrosterone sulfate: a biomarker of primate aging slowed by calorie restriction. J Clin Endocr Metab. 1997;82:2093–2096. doi: 10.1210/jcem.82.7.4038. [DOI] [PubMed] [Google Scholar]
- Lasley BL, Chen JG, Stanczyk FZ, El Khoudary SR, Gee NA, Crawford S, McConnell DS. Androstenediol complements estrogenic bioactivity during the menopausal transition. Menopause: J N Am Menopause Soc. 2012;19:650–657. doi: 10.1097/gme.0b013e31823df577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitis DA, Lackey LB. A measure for describing and comparing postreproductive life span as a population trait. Methods Ecol Evol. 2011;2:446–453. doi: 10.1111/j.2041-210X.2011.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcope C. Adrenal and gonadal androgen secretion in normal females. Clinics Endocr Metab. 1986;15:213–228. doi: 10.1016/s0300-595x(86)80021-4. [DOI] [PubMed] [Google Scholar]
- Longcope C. Metabolism of dehydroepiandrosterone. Ann NY Acad Sci. 1995;774:143–148. doi: 10.1111/j.1749-6632.1995.tb17378.x. [DOI] [PubMed] [Google Scholar]
- Longcope C. Dehydroepiandrosterone metabolism. J Endocr. 1996;150(Suppl):S125–S127. [PubMed] [Google Scholar]
- Matsuzawa T. Comparative cognitive development. Dev Sci. 2007;10:97–103. doi: 10.1111/j.1467-7687.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- McConnell DS, Stanczyk FZ, Sowers MR, Randolph JF, Lasley BL. Menopausal transition stage-specific changes in circulating adrenal androgens. Menopause: J N Am Menopause Soc. 2012;19:658–663. doi: 10.1097/gme.0b013e31823fe274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJW. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Nguyen AD, Conley AJ. Adrenal androgens in humans and nonhuman primates: production, zonation and regulation. Endocr Dev. 2008;13:33–54. doi: 10.1159/000134765. [DOI] [PubMed] [Google Scholar]
- Nishida T, Corp N, Hamai M, Hasegawa T, Hiraiwa-Hasegawa M, Hosaka K, Hunt KD, Itoh N, Kawanaka K, Matsumoto-Oda A, Mitani JC, Nakamura M, Norikoshi K, Sakamaki T, Turner L, Uehara S, Zamma K. Demography, female life history, and reproductive profiles among the chimpanzees of Mahale. Am J Primatol. 2003;59:99–121. doi: 10.1002/ajp.10068. [DOI] [PubMed] [Google Scholar]
- Oeppen J, Vaupel JW. Broken limits to life expectancy. Science. 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocr Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Peters H, Byskov AG, Grinsted J. Follicular growth in fetal and prepubertal ovaries of humans and other primates. Clinics in Endocrinology and Metabolism. 1978;7:469–485. doi: 10.1016/s0300-595x(78)80005-x. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Raisz LG. Physiology and pathophysiology of bone remodeling. Clin Chem. 1999;45:1353–1358. [PubMed] [Google Scholar]
- Ravaglia G, Forti P, Maioli F, Boschi F, Bernardi M, Pratelli L, Pizzoferrato A, Gasbarrini G. Journal of Clinical Endocrinology and Metabolism. 1996;81:1173–1178. doi: 10.1210/jcem.81.3.8772596. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE. Evolutionary theories of aging: confirmation of a fundamental prediction, with implications for the genetic basis and evolution of life span. Am Nat. 1998;152:24–44. doi: 10.1086/286147. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Khosla S, Melton LJ. A unitary model for involutional osteoporosis: Estrogen deficiency causes both Type I and Type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13:763–773. doi: 10.1359/jbmr.1998.13.5.763. [DOI] [PubMed] [Google Scholar]
- Robson SL, van Schaik CP, Hawkes K. The derived features of human life history. In: Hawkes K, Paine RR, editors. The evolution of human life history. Sante Fe: SAR Press; 2006. pp. 17–44. [Google Scholar]
- Roof KA, Hopkins WD, Izard MK, Hook M, Schapiro AJ. Maternal age, parity, and reproductive outcome in captive chimpanzees (Pan troglodytes) Am J Primatol. 2005;67:199–207. doi: 10.1002/ajp.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld RS, Rosenberg BJ, Fukushima DK, Hellman L. 24-hour secretory pattern of dehydroisoandrosterone and dehydroisoandrosterone sulfate. J Clin Endocr Metab. 1975;40:850–855. doi: 10.1210/jcem-40-5-850. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811–811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Siiteri PK. Editorial: The continuing saga of dehydroepiandrosterone (DHEA) J Clin Endocr Metab. 2005;90:3795–3796. doi: 10.1210/jc.2005-0852. [DOI] [PubMed] [Google Scholar]
- Stevenson S, Thornton J. Effect of estrogens on skin aging and the potential role of SERMs. Clin Interv Aging. 2007;2:283–297. doi: 10.2147/cia.s798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulcova J, Hill M, Hampl R, Starka L. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. J Endocr. 1997;154:57–62. doi: 10.1677/joe.0.1540057. [DOI] [PubMed] [Google Scholar]
- Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: insights from basic science and clinical studies. Endocr Rev. 2006;27:575–605. doi: 10.1210/er.2005-0020. [DOI] [PubMed] [Google Scholar]
- Vitzthum VJ. Evolutionary models of women’s reproductive functioning. Annu Rev Anthropol. 2008;37:53–73. [Google Scholar]
- Walker R, Hill K. Modeling growth and senescence in physical performance among the Ache of eastern Paraguay. Am J Hum Biol. 2003;15:196–208. doi: 10.1002/ajhb.10135. [DOI] [PubMed] [Google Scholar]
- White T, Jain JK, Stanczyk FZ. Effect of oral versus transdermal steroidal contraceptives on androgenic markers. Am J Obstet Gynecol. 2005;192:2055–2059. doi: 10.1016/j.ajog.2005.02.067. [DOI] [PubMed] [Google Scholar]
- Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the Women’s health initiative. Endocrine reviews. 2005;26:308–312. doi: 10.1210/er.2004-0014. [DOI] [PubMed] [Google Scholar]
- Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- Williams GC. Natural selection, the cost of reproduction, and a refinement of Lack’s principle. Am Nat. 1966;199:687–690. [Google Scholar]
- Wise PM, Suzuki S, Brown CM. Estradiol: a hormone with diverse and contradictory neuroprotective actions. Dialogues Clin Neurosci. 2009;11:297–303. doi: 10.31887/DCNS.2009.11.3/pmwise. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


