Abstract
Rationale
Decision-making is a complex cognitive process that is mediated, in part, by subregions of the medial prefrontal cortex (PFC). Decision-making is impaired in a number of psychiatric conditions including schizophrenia. Notably, people with schizophrenia exhibit reductions in GABA function in the same PFC areas that are implicated in decision-making. For example, expression of the GABA-synthesizing enzyme GAD67 is reduced in the dorsolateral PFC of people with schizophrenia.
Objectives
The goal of this experiment was to determine whether disrupting cortical GABA transmission impairs decision-making using a rodent gambling task (rGT).
Methods
Rats were trained on the rGT until they reached stable performance and then were implanted with guide cannulae aimed at the medial PFC. Following recovery the effects of intra-PFC infusions of the GABAA receptor antagonist bicuculline methiodide (BMI) or the GABA synthesis inhibitor L-allylglycine (LAG) on performance on the rGT were assessed.
Results
Intra-cortical infusions of BMI (25 ng/μl/side), but not LAG (10 μg/μl/side), altered decision-making. Following BMI infusions, rats made fewer advantageous choices. Follow-up experiments suggested that the change in decision-making was due to a change in the sensitivity to the punishments, rather than a change in the sensitivity to reward magnitudes, associated with each outcome. LAG infusions increased premature responding, a measure of response inhibition, but did not affect decision-making.
Conclusions
Blocking GABAA receptors, but not inhibiting cortical GABA synthesis, within the medial PFC affects decision-making in the rGT. These data provide proof-of-concept evidence that disruptions in GABA transmission can contribute to the decision-making deficits in schizophrenia.
Keywords: GABAA receptor, GAD67, decision-making, response inhibition, medial prefrontal cortex, schizophrenia, rat, bicuculline, L-allylglycine
Introduction
Decision-making is suboptimal in a number of psychiatric conditions, including attention-deficit disorder (Sonuga-Barke and Fairchild, 2012), substance abuse (Brevers et al., 2014; Kohno et al., 2014; Rogers and Robbins, 2001), bipolar disorder (Adida et al., 2011; Mason et al., 2014) and schizophrenia (Hutton et al., 2002; Beninger et al., 2003; Ritter et al., 2004; Shurman et al., 2005; Yip et al., 2009; Struglia et al., 2011). It is likely that impairments in decision-making contribute to poor functional outcomes that can be associated with each of these disorders. Thus, understanding the biological basis of decision-making is of the utmost importance.
Risk-based decision-making is a complex cognitive process that involves assessing various options and selecting from those options a response that will likely result in the most optimal outcome (Ernst and Paulus, 2005). In the laboratory decision-making is frequently tested using “gambling” tasks that are designed to simulate real-life decisions in terms of uncertainty, reward, and punishment (Floresco et al., 2008). In humans the Iowa Gambling Task (IGT; Bechara et al., 1999) is amongst the most popular tasks of risk-reward decision-making. Winstanely and colleagues (Zeeb et al., 2009) developed a rodent version of this task. Like the human task, there are 4 response options, each of which is associated with a different reward/punishment contingency. In the rodent task, two of the response options are associated with smaller rewards (food pellets) and shorter, infrequent punishments (omission of reward and delay until the next trial). The other two response options are associated with larger rewards and longer, more frequent punishments. These response contingencies result in two apertures being more “advantageous” than the other two “disadvantageous” apertures. Similar to humans, intact rats develop a preference for the small reward/small punishment advantageous choices over the large reward/large punishment disadvantageous choices (Zeeb et al., 2009; Paine et al., 2013).
Subregions of the prefrontal cortex (PFC) regulate risk-based decision-making in both humans and rodents. For example, human lesion and functional magnetic resonance imaging studies implicate the ventromedial (Bechara et al., 2000; Bechara, 2004), orbitofrontal (Bechara, 2004; Lawrence et al., 2009) and dorsolateral PFC (Bechara et al., 1999; Bembich et al., 2014; Fellows et al., 2005; Manes et al., 2002) in decision-making. Indeed people with damage to these areas fail to develop optimal decision-making strategies in the IGT, meaning that they either fail to develop a preference for the advantageous options or they prefer the disadvantageous options (e.g., Bechara et al., 1999; Fellows et al., 2005). Consistent with these findings, rodents with either medial PFC or orbitofrontal cortex lesions are delayed in developing an advantageous decision-making strategy (Rivalan et al., 2011; Zeeb and Winstanely, 2011). Similarly, excitotoxic lesions and temporary inactivation of the medial PFC alter established decision-making strategies in rodents (Paine et al., 2013; de Visser et al., 2011b). In contrast, lesions to the orbitofrontal cortex do not affect established decision-making strategies (Zeeb and Winstanely, 2011). Finally, rodents characterized as “poor” decision-makers exhibit increased activation of both dorsal and ventral regions of the medial PFC (de Visser et al., 2011a). Combined these data support a role for the medial PFC in maintaining optimal decision-making strategies in rodents.
As noted earlier, humans with schizophrenia exhibit deficits in decision-making (Struglia et al., 2011; Yip et al., 2009; Ritter et al., 2004; Beninger et al., 2003). Notably people with schizophrenia also exhibit abnormalities in GABA function in the dorsolateral PFC (Lewis, 2014; Nakazawa et al., 2012). For example, reductions in both the mRNA (Curley et al., 2011; Volk et al., 2000) and protein content (Hashimoto et al., 2003) of the 67kDa isoform of glutamic acid decarboxylase (GAD67) have been observed in the dorsolateral PFC during post-mortem analyses. Furthermore, decreased expression of the GABA reuptake transporter GAT1 (Volk et al., 2001; Pierri et al., 1999; Woo et al., 1998), and increased expression GABAA receptor α2 subunits have been observed (Beneyto et al., 2011; Volk et al., 2002). Combined these results have been interpreted to suggest that GABA synthesis and/or release are decreased in the dorsolateral PFC of people with schizophrenia, an effect that is incompletely compensated for by a decrease in reuptake and an increase in receptor subunit expression (Lewis, 2014).
Given the abnormalities in GABA function observed in the dorsolateral PFC in schizophrenia and the role of this brain area in decision-making, we speculated that cortical GABA dysfunction might contribute to the decision-making deficits in this, and other, psychiatric conditions. In order to test this hypothesis we conducted a proof-of-concept experiment in which we infused the GABAA receptor antagonist bicuculline methiodide (BMI) or the GABA synthesis inhibitor L-allylglycine (LAG) into the medial PFC of rodents as a means of modeling the GABA abnormalities observed in schizophrenia. We then assessed the effects on decision-making using the rGT. We have previously shown that both BMI and LAG infusions cause widespread increases in the protein expression of the immediate early gene c-fos within the medial PFC. These data have been interpreted to suggest that both manipulations decrease GABA tone and thereby increase neuronal activity within the PFC (Paine et al., 2011; Asinof and Paine, 2013).
Methods
Animals
Thirty-eight male Sprague-Dawley rats born at Oberlin College and derived from rats obtained from Hilltop Laboratories (Scottdale, PA) were used. Rats were maintained on a 14-h/10-h light-dark cycle (lights on at 0700h) and were group housed until post-natal day (PND) 55; during this time they had unlimited access to food (Purina Rat Chow) and water. On PND 55 rats were pair housed and remained pair housed until the time of surgery (see below). Following surgery rats were housed singly. Upon pair housing and throughout the experiment rats were food restricted to ~85% of their free feeding weight; rats were fed after daily training sessions. Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011) and Oberlin College policies.
Drugs
Bicuculline methiodide (BMI) and L-Allylglycine (LAG) were purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in physiological saline (0.9%). BMI doses were based on salt weight and LAG doses were based on the weight of the base.
Surgery
Rats were treated with the non-steroidal inflammatory analgesic drug ketoprofen (5 mg/kg, SC, dissolved in 50% DMSO, Sigma-Aldrich, St. Louis, MO) approximately 15 min prior to surgery. Rats were anesthetized sodium pentobarbital (65 mg/kg, IP) and implanted with bilateral guide cannulae (26-gauge; Plastics One, Roanoke, VA) aimed at the border of infralimbic (IL) and prelimbic cortex (PrL) cortices (relative to bregma: AP = + 2.8, ML = ± 0.75, DV = −1.8 mm from dura (Paxinos and Watson, 2009). Skull screws and dental acrylic secured the guide cannulae in place. Obturators and injector needles (33-gauge) extended 1.5 mm below the guide cannulae.
Standard Rodent Gambling Task (rGT)
The rGT was based on that developed by Winstanely and colleagues (Zeeb et al., 2009) and which we have found to be sensitive to medial PFC lesions (Paine et al., 2013). Rats were trained and tested in 5-hole operant conditioning chambers enclosed in sound attenuating chambers (Med Associates, Vermont); the center hole was occluded throughout all experiments.
Rats were first trained to retrieve food pellets (45-mg, Purified, Dustless Precision Pellets F0021, Bio-Serv, Frenchtown NJ) from the food magazine over two consecutive days; during these sessions all holes were occluded. Next, rats were trained to nose poke in the four holes under a continuous reinforcement schedule. In order to encourage equal nose poking in all four holes the two outer holes were occluded on the first session and the two inner holes occluded on the second session. On the third and subsequent sessions all 4 holes were open. Occasionally a rat would exhibit a strong preference for one hole (>70% responses); in that case the preferred hole was occluded for one or more sessions in order to encourage nose-poking in the remaining holes. The continuous reinforcement task began with the delivery of 1 food pellet and the illumination of the houselight and magazine light. Retrieval of the food pellet caused the magazine light to extinguish and initiated a 5-sec inter-trial interval (ITI). At the end of the ITI, lights at the rear of the holes (aperture lights) were illuminated and rats had 10 sec (limited hold) to make a response in any hole. Responses in an illuminated aperture resulted in the delivery of a single food pellet reward. Failing to respond within the limited hold was scored as an omission and responses that occurred during the ITI were scored as premature responses. Omissions and premature responses were punished with a 5-sec time out (TO); the houselight, magazine light and all aperture lights were extinguished during the TO. Rats were trained on the continuous reinforcement task until they received 100 rewards with less than 20 omissions in ~30 min.
Once rats reached criterion performance on the continuous reinforcement task they underwent 4 days of forced choice training on the full rGT. In each forced choice session only a single hole was available; this measure ensured that rats had experience with the contingencies associated with each hole prior to allowing rats to choose freely (see below). For the full rGT, sessions began with the delivery of a single food pellet and illumination of the houselight and the magazine light. Pellet retrieval extinguished the magazine light and initiated a 5-sec ITI. At the end of the ITI the 4 aperture lights were illuminated and rats had 10 sec (limited hold) to make a response in any hole. A response resulted in the assigned outcome (reward/punishment) for that hole (see Table 1 for example). On rewarded trials the aperture lights were all extinguished, the magazine light was illuminated and the appropriate number of pellets was delivered to the magazine. On punished trials no pellets were delivered and the houselight and all aperture lights, except for the one in the chosen hole, were extinguished for the duration of the punishment period (i.e., the chosen hole remained illuminated throughout the punishment period). The next trial commenced at the end of the punishment period or once the rat retrieved the food pellet reward. As above, failing to respond within the limited hold was scored as an omission and responses occurring during the ITI were scored as premature responses; both of which were punished with a 5-sec TO. Each session lasted for a total of 30 min.
Table 1.
Example of response contingencies across nose poke holes
| Hole (H) 1 | H2 | H3 | H4 | |
|---|---|---|---|---|
| Standard Task | ||||
| # of Rewards | 1 | 2 | 3 | 4 |
| Punishment Duration (sec) |
5 | 10 | 30 | 40 |
| Probability of Punishment |
0.1 | 0.2 | 0.5 | 0.6 |
|
| ||||
| Punishment Task | ||||
| # of Rewards | 2 | 2 | 2 | 2 |
| Punishment Duration (sec) |
5 | 10 | 30 | 40 |
| Probability of Punishment |
0.1 | 0.2 | 0.5 | 0.6 |
|
| ||||
| Reward Task | ||||
| # of Rewards | 1 | 2 | 3 | 4 |
| Punishment Duration (sec) |
10 | 10 | 10 | 10 |
| Probability of Punishment |
0.2 | 0.2 | 0.2 | 0.2 |
Note: For each task there were 4 different spatial arrangements of outcomes; one such arrangement is depicted here. An individual rat was assigned to a single arrangement for the duration of the experiment. On rewarded trials, the number of pellets indicated in the table were delivered; on punished trials no pellets were delivered.
Four different spatial arrangements of the outcomes across holes were used. An individual rat however, was only trained and tested on a single spatial arrangement of outcomes. Two of the holes had relatively “advantageous” outcomes (increased total number of pellets over the entire 30 min session) due to the low probability (0.1-0.2) and short duration (5-10 sec) of punishment (see Table 1). The advantageous choices were associated with smaller rewards (1-2 pellets); with the most advantageous option being the hole that resulted in the delivery of 2 pellets. Two holes were associated with relatively “disadvantageous” outcomes due to the high probability (0.5-0.6) and long duration (30-40 sec) of punishment. The disadvantageous choices were associated with larger rewards (3-4 pellets). The % responses at each hole and % advantageous responses ((# advantageous responses/total responses) * 100) were used as indicators of decision-making. Once % advantageous responding stabilized (< 5% variability across 5 days), rats underwent surgery (described above).
Following surgery, rats (n=18) were allowed to recover for 5-7 days and then were re-stabilized for a minimum of 5 days prior to drug testing. To habituate rats to the infusion procedure they were first infused with vehicle (VEH, 0.9% physiological saline); data from this session were used to assign rats to BMI and LAG groups. On test days rats were infused with BMI (n=9; 0, 25, or 50 ng/μl) 5 min prior to testing or LAG (n=9; 0, 10, or 20 μg/μl) 25 min prior to testing. The doses of BMI and LAG were based on prior research from our lab (Paine et al., 2011; Asinof and Paine, 2013). Half of the rats received the drug doses in an ascending order and half of the rats received the drug dose in a descending order. There was a minimum of two drug-free days between drug doses.
Punishment rGT
Rats (n = 8) completed training on the punishment version of the rGT. The punishment version of the rGT was similar to the standard rGT except that all holes gave the same number of rewards and only differed in their punishment contingency (see Table 1). Once rats reached stable levels of performance (< 5% variability in % advantageous responses across 5 days), they were implanted with guide cannulae and allowed to recover. Following recovery and restabilization of % advantageous responses rats were habituated to the infusion procedure (as above) and then infused with BMI (0 or 50 ng/μl) 5 min prior to testing. The order of 0 ng and 50 ng BMI infusions was counter-balanced across rats.
Reward rGT
Rats (n = 12) completed training on the reward version of the rGT. The reward version of the rGT was similar to the standard rGT except that each hole was associated with the same punishment and only differed in the number of rewards associated with each response (see Table 1). Once rats reached stable levels of performance (< 5% variability in % advantageous responses across 5 days), they were implanted with guide cannulae and allowed to recover. Following recovery and restabilization of % advantageous responses rats were habituated to the infusion procedure (as above) and then infused with BMI (0 or 50 ng/μl) 5 min prior to testing. The order of 0 ng and 50 ng BMI infusions was counter balanced across rats.
Histology
Following testing rats were anesthetized with sodium pentobarbital (100 mg/kg, IP) and transcardially perfused with 0.9% NaCl followed by 4% paraformaldehyde. Following perfusions, brains were removed, post-fixed for 24 h and then cryoprotected in 20% sucrose prior to slicing on a microtome. Sections (40 m) were mounted on slides, stained with cresyl violet and the cannula placements assessed.
Statistical Analyses
Performance measures of interest included: % advantageous responses ((# most advantageous response/total response)*100), % choice per hole ((# responses per hole/total responses)*100), % omissions ((# omissions/total responses)*100) and % premature responses ((# premature responses/total responses)*100) and magazine entries.
Performance on the standard version of the rGT was analyzed using repeated measures ANOVAs with Dose (and % choice per hole) as the within-subjects factor(s). The sphericity of all within-subjects variables was determined using Mauchly’s sphericity test. When the sphericity assumption was violated the degrees of freedom were adjusted using the Huynh-Feldt correction and the adjusted degrees of freedom were used to determine the significance of the F-value. For simplicity, non-corrected degrees of freedom are reported. Significant within-subjects effects were further analyzed using pairwise comparisons with a Bonferroni correction.
Because only two doses of BMI were tested in the punishment and reward versions of the task, these data were analyzed using paired samples t-tests (% advantageous, % omissions, % premature, magazine entries) or a two-way within-subjects ANOVA with Dose and % choice per hole as within-subjects factors. Significant within-subjects effects were further analyzed using pairwise comparisons with a Bonferroni correction. Data were analyzed using IBM SPSS Statistics™.
Results
Histology
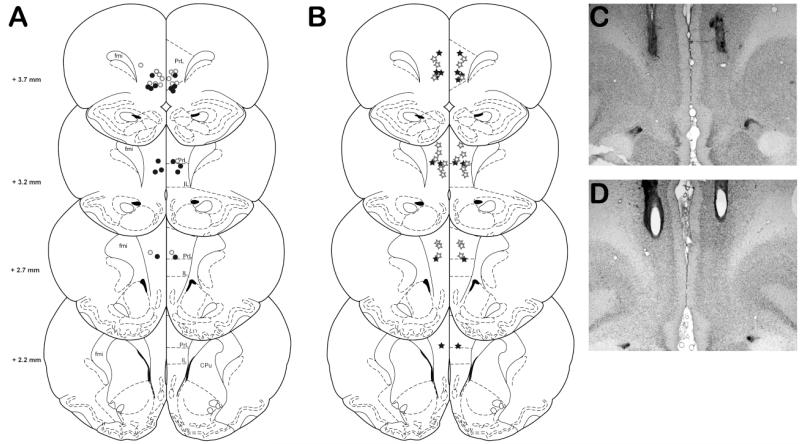
Of the thirty-eight rats that successfully completed training on the standard, punishment or reward versions of the rGT, two rats tested on the standard task (n=1, BMI; n=1, LAG) were removed from the statistical analysis due to inappropriate cannulae placements. Cannulae placements for the remaining rats are shown in Figure 1.
Fig 1.
Location of injector tips for rats tested in all versions of the rGT. A) Location of injector tips for rats tested with either BMI (filled circles, n=8) or LAG (open circles) in the standard rGT task. B) Location of injector tips for rats tested in the punishment (filled stars, n=8) and reward (open stars, n=12) versions of the rGT. C and D) Representative photomicrographs of cannulua placements within the prefrontal cortex. Rats with cannula placements outside of the PrL and IL were excluded from analysis (not shown). fmi (forceps minor corpus callosum); PrL (prelimbic cortex), IL (infralimbic cortex), CPu (caudate putamen). Adapted from Paxinos and Watson (2009).
Standard Task
Bicuculline
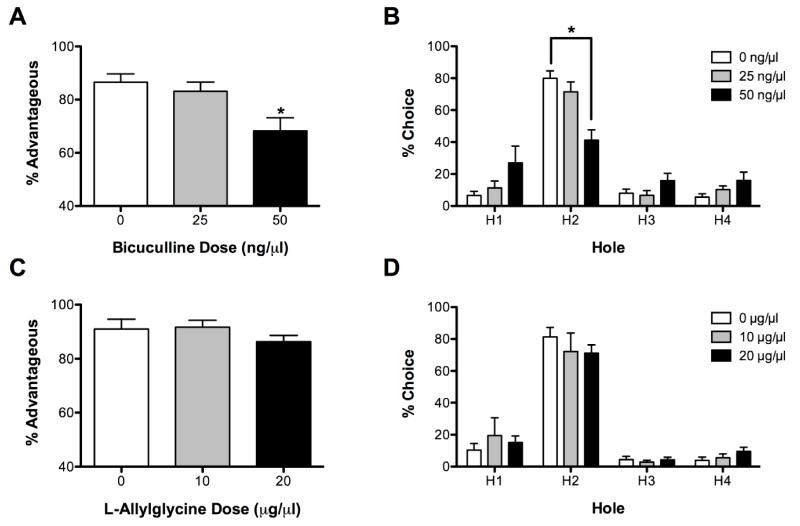
The effects of BMI on performance on the standard version of the rGT are shown in Figure 2. BMI administration significantly affected % choice of advantageous options (F(2, 14) = 11.67, P < 0.05, see Fig 2A). Post-hoc analysis determined that the choice of the most advantageous options was significantly lower following administration of the 50 ng dose compared to the 0 ng dose (P < 0.05), suggesting that BMI administration impaired decision-making. There was also a significant effect of BMI on the % choice of each hole (dose X hole interaction F(6,42) = 5.90, P < 0.01, see Fig 2B). For all three doses of BMI, rats chose aperture 2 more than any other hole (P < 0.05). Following BMI (50 ng) administration however, rats chose aperture 2 significantly less than they did following vehicle administration (P < 0.05).
Fig 2.
Effects of BMI and LAG on performance on the standard rGT. BMI (50 ng) decreased choice of % advantageous responses (A) and choice of the most advantageous hole (B). LAG did not affect decision-making (C-D). *P < 0.05 from 0 ng (Vehicle).
BMI administration also affected other task parameters. BMI administration significantly increased omissions (F(2,14) = 12.23, P < 0.05, see Table 2); the highest dose of BMI significantly increased omissions relative to vehicle (P < 0.01). BMI administration also decreased magazine entries (F (2,14) = 3.98, P < 0.05, see Table 2), but no dose was significantly different from vehicle (all P > 0.05). BMI administration did not affect % premature responses (F(2,14) = 1.78, P > 0.05, see Table 2).
Table 2.
Effects of BMI and LAG on performance on the standard rGT
| % Omissions | % Premature Responses |
Magazine Entries | |
|---|---|---|---|
| BMI | |||
| Vehicle | 3.93 ± 1.66 | 42.86 ± 7.08 | 525.9 ± 91.1 |
| 25 ng | 8.59 ± 4.15 | 41.17 ± 7.85 | 406.5 ± 64.3 |
| 50 ng | 19.83 ± 3.54* | 26.24 ± 3.48 | 357.2 ± 59.4 |
|
| |||
| LAG | |||
| Vehicle | 2.35 ± 2.22 | 30.18 ± 2.52 | 480.1 ± 59.9 |
| 10 μg | 0.71 ± 0.45 | 46.20 ± 7.46 | 487.5 ± 58.8 |
| 20 μg | 1.87 ± 0.92 | 76.37 ± 12.78* | 367.8 ± 40.6 |
Note: P < 0.05 from respective Vehicle.
L-Allylglycine
The effects of LAG on performance on the standard version of the rGT are shown in Figure 2. LAG administration did not affect decision-making; there was no effect of LAG on either % advantageous responses (F(2,14) = 2.07, P > 0.05, see Fig 2C) or % choice of any hole (i.e., no dose X hole interaction (F(6,42) = 0.63, P > 0.05, Fig 2C). There was a significant effect of hole (F(3, 21) = 54.38, P < 0.05, see Fig 2C); regardless of LAG dose all rats chose hole 2 more than any other hole (all P < 0.05).
LAG did not significantly affect omissions (F(2,14) = 0.49, P > 0.05, see Table 2), but did increase % premature responses (F(2,14) = 9.57, P < 0.01, see Table 2). Administration of the highest dose of LAG (20 μg) resulted in a significant increase in the % premature responses compared to vehicle (P < 0.05). There was a trend for LAG to affect magazine entries (F(2,14) = 3.67, P = 0.05, see Table 2), but no dose was significantly different from vehicle (all P > 0.05).
Punishment rGT
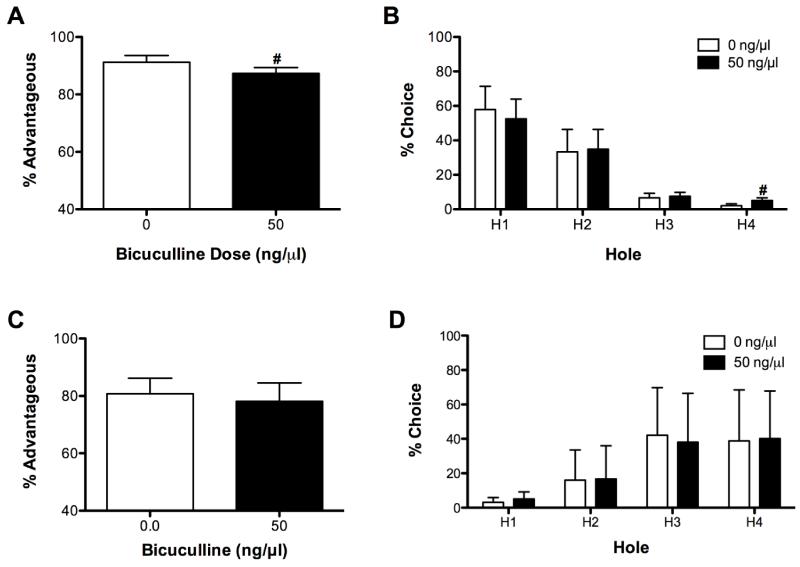
Because decision-making can be affected by either a change in the sensitivity to punishment or a change in the sensitivity to reward, we only assessed the effects of BMI, not LAG, on the punishment and reward versions of the rGT. On the punishment version of the task there was a trend for BMI (50 ng) to decrease % advantageous responses (t(7) = 2.26, P = 0.06; see Fig 3A). BMI did not affect other measures on the punishment version of the rGT [% omissions (t(7) = 1.02, P > 0.05), % premature responses (t(7) = 1.65, P > 0.05), magazine entries (t(7) = 0.00, P > 0.05); see Table 3].
Fig 3.
Effects of BMI on performance on the punishment and reward versions of the rGT. BMI administration had a tendency to decrease % advantageous responses (A) and to increase choice of hole 4 (B) on the punishment version of the task. BMI did not affect performance on the reward version of the task (C-D). #P = 0.06 from 0 ng (Vehicle).
Table 3.
Effects of BMI on the punishment and reward versions of the rGT
| % Omissions | % Premature Responses |
Magazine Entries | |
|---|---|---|---|
| Punishment | |||
| Vehicle | 2.54 ± 2.01 | 118.04 ± 43.86 | 301.9 ± 66.8 |
| 50 ng | 5.46 ± 2.58 | 49.60 ± 4.98 | 301.9 ± 83.2 |
|
| |||
| Reward | |||
| Vehicle | 16.18 ± 6.86 | 37.93 ± 12.91 | 160.6 ± 36.7 |
| 50 ng | 26.73 ± 5.88* | 24.07 ± 6.05 | 137.8 ± 21.5 |
Note: P < 0.05 from respective Vehicle.
In the punishment version of the rGT, rats preferred the options that resulted in the least amount of punishment (main effect of hole, F(3, 21) = 5.75, P < 0.05; see Fig 3B). The 2 holes with the least amount of punishment were chosen more frequently than the two holes with the most punishment (all P < 0.05). Although the hole X dose interaction was not statistically significant (F(3, 21) = 1.32, P > 0.05), we used post-hoc analyses to further explore this interaction because we expected BMI to have differential effects on choice across holes. Indeed, there was a trend for BMI (50 ng) administration to increase choice of hole 4 relative to Vehicle (0 ng, P = 0.06).
Reward rGT
BMI (50 ng) administration increased omissions relative to vehicle (t(10) = 2.51, P < 0.05), but did not affect other measures on the reward version of the rGT [% advantageous responses (t(10) = 0.96, P < 0.05), % premature responses (t(10) = 1.40, P > 0.05), magazine entries (t(10) = 0.67); see Fig 3C and Table 3].
In the reward version of the rGT, there was a significant effect of hole (F(3, 30) = 5.24, P < 0.05; see Fig 3D); rats preferred holes resulting in 3 or 4 pellets relative to holes resulting in 1 pellet (P < 0.05). Neither dose nor the dose X hole interaction were significant (both F < 0.71, P > 0.05).
Discussion
Disruption of GABA function within the medial PFC affected performance on the rodent gambling task. Blockade of GABA synthesis with LAG caused a deficit in response inhibition (i.e., it increased premature responding), while blockade of GABAA receptors altered decision-making on the standard version of the rGT. Following BMI administration rats chose the advantageous options less frequently. This resulted from a significant decrease in the choice of the most advantageous hole, and non-significant increases in the choice of the other response options. In order to investigate whether the change in decision-making was the result of a change in the sensitivity to reward and/or a change in the sensitivity to punishment, separate subsets of rats were tested on two modified versions of the task. We have previously observed that following intra-PFC BMI infusions rats are more willing to work for smaller amounts of reward in an intracranial self-stimulation task (Paine et al., 2011) suggesting that rats may be more reward-motivated. Thus, we speculated that, following BMI administration, the rats may have been drawn to the high risk/reward options in the standard rGT. In order to address this possibility rats were tested on a modified version of the rGT task in which the punishments remained constant across holes, but the number of rewards differed. In the reward task, BMI administration did not affect the choice of any individual hole or the % advantageous responses. These data suggest a change in reward sensitivity cannot account for the BMI-induced change in decision-making. Alternatively, a change in the decision-making strategy caused by intra-cortical BMI administration may be due to a change in the rats’ sensitivity to the punishments associated with each hole. Indeed, damage to the medial PFC can increase “risky” decision-making (St. Onge and Floresco, 2010; St. Onge et al., 2012). In order to determine if BMI administration affected sensitivity to punishment, rats were tested on a modified version of the task in which the number of rewards associated with each hole remained the same but the magnitude of the punishment varied. On the punishment version of the rGT, BMI administration tended to decrease the choice of the most advantageous apertures, in part due to a small increase in % choice of the most punished aperture. Thus, a decreased sensitivity to punishment (or increased “risky” decision-making) likely contributed to the change in decision-making caused by BMI administration. In sum, our data suggest that blockade of cortical GABAA receptors, but not blockade of GABA synthesis, impairs decision-making on the rGT; an effect that may be mediated by a decreased sensitivity to the negative outcomes associated with each option.
One limitation of the variants of the rGT used in the current experiment is that they resulted in different baseline (i.e., drug-free) levels of performance. For example, choice of the most optimal hole was ~80% in the standard task, but only 40-60% in the two task variants. Similarly, the number of omissions and premature responses also varied across tasks. Although some of the variability was likely the result of individual differences in the rats that performed the tasks (baseline levels of performance across groups of rats can vary), some of the variability likely resulted from differences in the task requirements. Indeed, to our surprise rats in the reward task had difficulty distinguishing between the two options that delivered the most rewards, despite extensive training. Furthermore, there was tremendous variability in the % choice of each of the high reward options because rats did not readily distinguish between the two high reward options. Despite our efforts to maintain the basic task structure across tasks, the difference in the baseline levels of performance between tasks may have minimized our ability to observe significant effects of BMI administration. Thus, some caution should be used in interpreting the data obtained using these tasks.
In addition to affecting measures of decision-making, BMI administration also affected other measures of task performance, namely omissions and magazine entries. Combined, an increase in omissions and a decrease in magazine entries (food seeking responses) might suggest that BMI affected the rats’ motivation to earn rewards and consequently their choice of the most advantageous options. However, BMI administration did not change % choice of any hole during the simplified task when only the number of rewards varied across holes. In fact, in this task all rats exhibited a preference for the holes that resulted in the most rewards. If rats exhibited decreased motivation for reward, it would be predicted that rats would not exhibit a preference for the most rewarded holes. Furthermore we have previously shown that following BMI administration rats need less, not more, reward to maintain lever pressing in a self-stimulation task (Paine et al., 2011). Thus, we do not believe that a change in motivation for the reward caused the increase in omissions, nor do we believe that a change in motivation can explain the change in decision-making observed following BMI administration. Rather, it is likely that the increase in omissions and decrease in magazine entries reflects a broad suppression of responding or an attention deficit.
We have previously observed that intra-cortical infusions of BMI impair attention (Paine et al., 2011). It is likely that the increase in omissions observed in the reward and punishment versions of the gambling task reflect this attentional deficit. Importantly, the observed changes in % advantageous responses and % choice per hole cannot be directly explained by the increase in omissions as these measures were calculated based only on trials that rats completed. That said, risk-reward decision-making is a complex cognitive process that involves planning, assessing risks, evaluating outcomes and choosing amongst options that which will provide the maximal benefit to the individual in the long run (Ernst and Paulus, 2005; Floresco et al., 2008). It is possible that an attentional deficit may impact the ability to make advantageous choices; to the best of our knowledge this has yet to be studied directly. It has been shown that other cognitive factors do affect decision-making in a gambling task. For example, rats that exhibit poor decision-making also displayed traits including increased risk-taking, reward-seeking and behavioral inflexibility (Rivalan et al., 2013). Thus, it is possible that attentional deficits could negatively impact decision-making. Future research is needed to determine the role of attention in decision-making in the rGT.
The current data are consistent with a role of the medial PFC in the regulation of decision-making. Here we show that a manipulation that increases c-fos immunoreactivity, an indirect measure of neuronal activity (Schilling et al., 1991), within both infralimbic and prelimbic regions of the medial PFC causes a reduction in advantageous decision-making. Consistent with this observation, poor performance on a similar rodent gambling task was associated with increased c-fos immunoreactivity in both the infralimbic and prelimbic regions of the medial PFC (de Visser et al., 2011a). Moreover, temporary inactivation (de Visser et al., 2011b) and excitotoxic lesions (Paine et al., 2013) of the medial PFC have previously been shown to impair performance on these tasks. These data are broadly consistent with experiments investigating decision-making in humans: damage to the dorsolateral PFC in humans (analogous to the rodent medial PFC (Uylings et al., 2003)) impairs performance on the IGT (Fellows et al., 2005; Manes et al., 2002) and the dorsolateral PFC is activated during IGT task performance (Bembich et al., 2014). Furthermore, our data are consistent with the hypothesis that lesions of the medial PFC increase “risky” decision-making (St. Onge and Floresco al., 2010; St. Onge et al., 2012). It should be noted that the infralimbic and prelimbic regions of the medial PFC have different connectivities (Vertes, 2004) and have been found to have disparate roles in other cognitive tasks (Cassaday et al., 2014); thus it is now imperative to determine if the infralimbic and prelimbic cortex provide unique contributions to decision-making. Indeed, specific patterns of deficits have been observed following damage to other subregions of the rodent PFC (Rivalan et al., 2011).
Although blockade of cortical GABA synthesis in the medial PFC did not affect decision-making per se, it did increase premature responding, a measure of response inhibition. We have previously observed impaired response inhibition following administration of LAG in the 5-choice serial reaction time task (5CSRTT; Asinof and Paine, 2013). Furthermore, our findings are consistent with other findings of impaired response inhibition in the 5CSRTT following disruption of the medial PFC (Paine et al., 2011; Pezze et al., 2009; Chudasama et al., 2009; Murphy et al., 2005). Moreover, disruption in PFC functioning has been observed to decrease response inhibition in the stop-signal reaction time task (Bari et al., 2005) and in a waiting task (Narayanan et al., 2006).
It is somewhat surprising that we observed disparate effects of BMI and LAG on measures of decision-making and response inhibition. We, and others, however have observed similar dissociations in cognitive function following parallel manipulations in similar brain regions. For example, attention was impaired by antagonism of cortical GABAA receptors (Paine et al., 2011; Pehrson et al., 2013), but not by inhibition of cortical GABA synthesis (Asinof and Paine, 2013; Pehrson et al., 2013). It is unlikely that the degree of disruption in cortical GABA transmission is sufficient to explain the different effects of BMI and LAG on behavior. First, in our previous research we assessed a wider range of doses of each drug and still did not find parallel effects on behavior between any of the doses tested (Asinof and Paine, 2013; Paine et al., 2011). Furthermore, we have previously found that the highest dose of each drug used in the current experiment causes similar increases in the expression of the immediate early gene c-fos (Asinof et al., 2013; Paine et al., 2011). Because c-fos expression is used as an indirect marker of neuronal activity (Schilling et al., 1991), a similar increase in c-fos expression could suggest that both drugs are disrupting GABA function to a similar degree. Although it is difficult to determine whether the doses of BMI and LAG used here in fact cause a similar disruption in GABA transmission, we do not believe that differences in the degree of disruption in GABA transmission can explain the disparate effects that BMI and LAG have on behavior.
Alternatively, it is possible that the decision-making deficit caused by BMI did not result from its effects on GABAA receptors, but because of the non-GABAA receptor-mediated effects of BMI. In addition to blocking GABAA receptors, BMI can inhibit afterhyperpolarizations mediated by Ca2+ and K+ channels in vivo (Seutin and Johnson, 1999). That said, it is unlikely these non-GABAA receptor-mediated actions contribute to the effects of BMI on decision-making because these effects only appear at drug concentrations that exceed those that are used here (Seutin and Johnson, 1999).
Finally, the discrepancy between blockade of GABAA receptors and inhibition of GABA synthesis on behavior may have to do with the differential effects of these manipulations on GABAB receptor activation. Blocking GABAA receptors with BMI would not be expected to directly affect the activation of GABAB receptors. In contrast, because LAG decreases GABA synthesis and release (Horton et al., 1978), it is possible that LAG administration decreases activation of both GABAA and GABAB receptors. Indeed, the effects of LAG on dopamine release in the nucleus accumbens have been found to be mediated primarily by GABAB, rather than GABAA, receptors (Saigusa et al., 2012). Interestingly, administration of GABAB receptor antagonists can improve cognitive performance in both humans and rodent models (Helm et al., 2005; Kleshchevnikov et al., 2012; Mondadori et al., 1996; Stäubli et al., 1999). Thus, it is possible that by decreasing GABA synthesis, LAG decreases activation of GABAB receptors and this effect may mitigate any decrease in decision-making caused by a reduction in GABAA receptor activation. Future research is necessary to investigate the effects of GABAB receptor activation on decision-making.
The current experiment supports the working hypothesis that reductions in the activation of cortical GABAA receptors contribute to the cognitive deficits in schizophrenia. There are a variety of different GABAA receptors (Hines et al., 2012); these receptors are comprised of different subunits, have different affinities for GABA, and are found in different subcellular locations (Hines et al., 2012; Rudolph et al., 2014). BMI is a broad spectrum GABAA receptor antagonist that, to the best of our knowledge, does not have a higher affinity for one particular receptor over another. That said, future research aimed at refining the role of cortical GABAA receptors in these cognitive deficits is warranted. The prefrontal cortex has a moderate to high level of expression of α1-, α2-, and α3-containing GABAA receptors (Rudolph et al., 2014). In schizophrenia an upregulation in α2 receptor subunits has been observed in the axon initial segment of pyramidal neurons, and a decrease in α1 receptor subunits has been observed in layer 3 pyramidal neurons (Glausier et al., 2011; reviewed in Lewis, 2014). While it is not yet clear which, if any, of these changes in subunit expression contribute to the abnormalities in cognition in schizophrenia, some data suggest that disrupting α2 receptor subunits impairs sensorimotor gating (Hines et al., 2013). Thus, future research, using more targeted genetic and/or pharmacological tools, is needed to delineate the relative contribution of GABAA receptors containing different receptor subunits to cognitive functions, including decision-making.
Acknowledgements
This work was supported by NIH grant R15MH098246 awarded to TAP.
References
- Adida M, Jollant F, Clark L, Besnier N, Guillaume S, Kaladjian A, Mazzola-Pomietto P, Jeanningros R, Goodwin GM, Azorin JM, Courtet P. Trait-related decision-making impairment in the three phases of bipolar disorder. Biol Psychiatry. 2011;70:357–365. doi: 10.1016/j.biopsych.2011.01.018. doi: 10.1016/j.biopsych.2011.01.018. [DOI] [PubMed] [Google Scholar]
- Asinof SK, Paine TA. Inhibition of GABA synthesis in the prefrontal cortex increases locomotor activity but does not affect attention in the 5-choice serial reaction time task. Neuropharmacology. 2013;65:39–47. doi: 10.1016/j.neuropharm.2012.09.009. doi: 10.1016/j.neuropharm.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Mar AC, Theobald DE, Elands SA, Oganya KC, Eagle DM, Robbins TW. Prefrontal and monoaminergic contributions to stop-signal task performance in rats. J Neurosci. 2011;31:9254–9263. doi: 10.1523/JNEUROSCI.1543-11.2011. doi: 10.1523/JNEUROSCI.1543-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. The role of emotion in decision-making: evidence from neurological patients with orbitofrontal damage. Brain Cogn. 2004;55:30–40. doi: 10.1016/j.bandc.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. Erratum in: Brain (2009) 132:1993. [DOI] [PubMed] [Google Scholar]
- Bembich S, Clarici A, Vecchiet C, Baldassi G, Cont G, Demarini S. Differences in time course activation of dorsolateral prefrontal cortex associated with low or high risk choices in a gambling task. Front Hum Neurosci. 2014;8:464. doi: 10.3389/fnhum.2014.00464. doi: 10.3389/fnhum.2014.00464. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABAA receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21:999–1011. doi: 10.1093/cercor/bhq169. doi: 10.1093/cercor/bhq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beninger RJ, Wasserman J, Zanibbi K, Charbonneau D, Mangels J, Beninger BV. Typical and atypical antipsychotic medications differentially affect two nondeclarative memory tasks in schizophrenic patients: a double dissociation. Schizophr Res. 2003;61:281–292. doi: 10.1016/s0920-9964(02)00315-8. [DOI] [PubMed] [Google Scholar]
- Brevers D, Bechara A, Cleeremans A, Kornreich C, Verbanck P, Noël X. Impaired decision-making under risk in individuals with alcohol dependence. Alcohol Clin Exp Res. 2014;38:1924–1931. doi: 10.1111/acer.12447. doi: 10.1111/acer.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassaday HJ, Nelson AJ, Pezze MA. From attention to memory along the dorsal-ventral axis of the medial prefrontal cortex: some methodological considerations. Front Syst Neurosci. 2014;8:160. doi: 10.3389/fnsys.2014.00160. doi: 10.3389/fnsys.2014.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, Lewis DA. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psyc. 2011;168:921–929. doi: 10.1176/appi.ajp.2011.11010052. doi: 10.1176/appi.ajp.2011.11010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser L, Baars AM, Lavrijsen M, van der Weerd CM, van den Bos R. Decision-making performance is related to levels of anxiety and differential recruitment of frontostriatal areas in male rats. Neuroscience. 2011a;184:97–106. doi: 10.1016/j.neuroscience.2011.02.025. doi: 10.1016/j.neuroscience.2011.02.025. [DOI] [PubMed] [Google Scholar]
- de Visser L, Baars AM, van’t Klooster J, van den Bos R. Transient inactivation of the medial prefrontal cortex affects both anxiety and decision-making in male Wistar rats. Front Neurosci. 2011b;5:102. doi: 10.3389/fnins.2011.00102. doi: 10.3389/fnins.2011.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Different underlying impairments in decision-making following ventromedial and dorsolateral frontal lobe damage in humans. Cerebr Cortex. 2005;15:58–63. doi: 10.1093/cercor/bhh108. [DOI] [PubMed] [Google Scholar]
- Floresco SB, St Onge JR, Ghods-Sharifi S, Winstanley CA. Cortico-limbic-striatal circuits subserving different forms of cost-benefit decision making. Cogn Affect Behav Neurosci. 2008;8:375–389. doi: 10.3758/CABN.8.4.375. doi: 10.3758/CABN.8.4.375. [DOI] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Selective pyramidal cell reduction of GABA(A) receptor α1 subunit messenger RNA expression in schizophrenia. Neuropsychopharmacology. 2011;36:2103–2110. doi: 10.1038/npp.2011.102. doi: 10.1038/npp.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, Sampson AR, Lewis DA. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm KA, Haberman RP, Dean SL, Hoyt EC, Melcher T, Lund PK, Gallagher M. GABAB receptor antagonist SGS742 improves spatial memory and reduces protein binding to the cAMP response element (CRE) in the hippocampus. Neuropharmacology. 2005;48:956–964. doi: 10.1016/j.neuropharm.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Hines RM, Hines DJ, Houston CM, Mukherjee J, Haydon PG, Tretter V, Smart TG, Moss SJ. Disrupting the clustering of GABAA receptor α2 subunits in the frontal cortex leads to reduced γ-power and cognitive deficits. Proc Natl Acad Sci U S A. 2013;110:16628–16633. doi: 10.1073/pnas.1308706110. doi: 10.1073/pnas.1308706110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines RM, Davies PA, Moss SJ, Maguire J. Functional regulation of GABAA receptors in nervous system pathologies. Curr Opin Neurobiol. 2012;22:552–558. doi: 10.1016/j.conb.2011.10.007. doi: 10.1016/j.conb.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RW, Chapman AG, Meldrum BS. Regional changes in cerebral GABA concentration and convulsions produced by D and by L-allylglycine. J Neurochem. 1978;30:1501–1504. doi: 10.1111/j.1471-4159.1978.tb10484.x. [DOI] [PubMed] [Google Scholar]
- Hutton SB, Murphy FC, Joyce EM, Rogers RD, Cuthbert I, Barnes TRE, McKenna PJ, Sahakian BJ, Robbins TW. Decision making deficits in patients with first-episode and chronic schizophrenia. Schizophrenia Research. 2002;55:249–257. doi: 10.1016/s0920-9964(01)00216-x. [DOI] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Faizi M, Jacobs LF, Htun K, Shamloo M, Mobley WC. Deficits in cognition and synaptic plasticity in a mouse model of Down syndrome ameliorated by GABAB receptor antagonists. J Neurosci. 2012;32:9217–9227. doi: 10.1523/JNEUROSCI.1673-12.2012. doi: 10.1523/JNEUROSCI.1673-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry. 2014;71:812–820. doi: 10.1001/jamapsychiatry.2014.399. doi: 10.1001/jamapsychiatry.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA. Inhibitory neurons in human cortical circuits: substrate for cognitive dysfunction in schizophrenia. Curr Opin Neurobiol. 2014;26:22–26. doi: 10.1016/j.conb.2013.11.003. doi: 10.1016/j.conb.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, Robbins T. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Mason L, O’Sullivan N, Montaldi D, Bentall RP, El-Deredy W. Decision-making and trait impulsivity in bipolar disorder are associated with reduced prefrontal regulation of striatal reward valuation. Brain. 2014;137:2346–2355. doi: 10.1093/brain/awu152. doi: 10.1093/brain/awu152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondadori C, Moebius HJ, Zingg M. CGP 36,742, an orally active GABAB receptor antagonist, facilitates memory in a social recognition test in rats. Behav Brain Res. 1996;77:227–229. doi: 10.1016/0166-4328(95)00226-x. [DOI] [PubMed] [Google Scholar]
- Murphy ER, Dalley JW, Robbins TW. Local glutamate receptor antagonism in the rat prefrontal cortex disrupts response inhibition in a visuospatial attentional task. Psychopharmacology (Berl) 2005;179:99–107. doi: 10.1007/s00213-004-2068-3. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology. 2012;62:1574–1583. doi: 10.1016/j.neuropharm.2011.01.022. doi: 10.1016/j.neuropharm.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139:865–876. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Edition National Academy Press; Washington, DC: 2011. [Google Scholar]
- Paine TA, Asinof SK, Diehl GW, Frackman A, Leffler J. Medial prefrontal cortex lesions impair decision-making on a rodent gambling task: reversal by D1 receptor antagonist administration. Behav Brain Res. 2013;243:247–254. doi: 10.1016/j.bbr.2013.01.018. doi: 10.1016/j.bbr.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, Slipp LE, Carlezon WA., Jr Schizophrenia-like attentional deficits following blockade of prefrontal cortex GABAA receptors. Neuropsychopharmacology. 2011;36:1703–1713. doi: 10.1038/npp.2011.51. doi: 10.1038/npp.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th Ed Academic Press; Amsterdam, Netherlands: 2009. [Google Scholar]
- Pehrson AL, Bondi CO, Totah NK, Moghaddam B. The influence of NMDA and GABA(A) receptors and glutamic acid decarboxylase (GAD) activity on attention. Psychopharmacology (Berl) 2013;225:31–39. doi: 10.1007/s00213-012-2792-z. doi: 10.1007/s00213-012-2792-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze MA, Dalley JW, Robbins TW. Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D(2/3) receptor antagonist sulpiride. Psychopharmacology (Berl) 2009;202:307–313. doi: 10.1007/s00213-008-1384-4. doi: 10.1007/s00213-008-1384-4. [DOI] [PubMed] [Google Scholar]
- Pierri JN, Chaudry AS, Woo T-UW, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- Ritter LM, Meador-Woodruff JH, Dalack GW. Neurocognitive measures of prefrontal cortical dysfunction in schizophrenia. Schizophr Res. 2004;68:65–73. doi: 10.1016/S0920-9964(03)00086-0. [DOI] [PubMed] [Google Scholar]
- Rivalan M, Valton V, Seriès P, Marchand AR, Dellu-Hagedorn F. Elucidating poor decision-making in a rat gambling task. PLoS One. 2013;8:e82052. doi: 10.1371/journal.pone.0082052. doi: 10.1371/journal.pone.0082052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivalan M, Coutureau E, Fitoussi A, Dellu-Hagedorn F. Inter-individual decision-making differences in the effects of cingulate, orbitofrontal, and prelimbic cortex lesions in a rat gambling task. Front Behav Neurosci. 2011;5:22. doi: 10.3389/fnbeh.2011.00022. doi: 10.3389/fnbeh.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Möhler H. GABAA receptor subtypes: Therapeutic potential in Down syndrome, affective disorders, schizophrenia, and autism. Annu Rev Pharmacol Toxicol. 2014;54:483–507. doi: 10.1146/annurev-pharmtox-011613-135947. doi: 10.1146/annurev-pharmtox-011613-135947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigusa T, Aono Y, Sekino R, Uchida T, Takada K, Oi Y, Koshikawa N, Cools AR. In vivo neurochemical evidence that newly synthesised GABA activates GABA(B), but not GABA(A), receptors on dopaminergic nerve endings in the nucleus accumbens of freely moving rats. Neuropharmacology. 2012;62:907–913. doi: 10.1016/j.neuropharm.2011.09.021. doi: 10.1016/j.neuropharm.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Schilling K, Luk D, Morgan JI, Curran T. Regulationof a fos lacZ fusion gene: a paradigm for quantitative analysis of stimulus-transcription coupling. Proc Natl Acad Sci USA. 1991;88:5665–5669. doi: 10.1073/pnas.88.13.5665. doi: 10.1073/pnas.88.13.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seutin V, Johnson SW. Recent advances in the pharmacology of quaternary salts of bicuculline. Trends Pharmacol Sci. 1999;20:268–270. doi: 10.1016/s0165-6147(99)01334-6. [DOI] [PubMed] [Google Scholar]
- Shurman B, Horan WP, Nuechterlein Schizophrenia patients demonstrate a distinctive pattern of decision-making impairment in the Iowa Gambling Task. Schizophr Res. 2005;72:215–224. doi: 10.1016/j.schres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Fairchild G. Neuroeconomics of attention-deficit/hyperactivity disorder: differential influences of medial, dorsal, and ventral prefrontal brain networks on suboptimal decision making? Biol Psychiatry. 2012;72:126–133. doi: 10.1016/j.biopsych.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Stäubli U, Scafidi J, Chun D. GABAB receptor antagonism: facilitatory effects on memory parallel those on LTP induced by TBS but not HFS. J Neurosci. 1999;19:4609–4615. doi: 10.1523/JNEUROSCI.19-11-04609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Stopper CM, Zahm DS, Floresco SB. Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. J Neurosci. 2012;32:2886–2899. doi: 10.1523/JNEUROSCI.5625-11.2012. doi: 10.1523/JNEUROSCI.5625-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Floresco SB. Prefrontal cortical contribution to risk-based decision making. Cereb Cortex. 2010;20:1816–1828. doi: 10.1093/cercor/bhp250. doi: 10.1093/cercor/bhp250. [DOI] [PubMed] [Google Scholar]
- Struglia F, Stratta P, Gianfelice D, Pacifico R, Riccardi I, Rossi A. Decision-making impairment in schizophrenia: Relationships with positive symptomatology. Neurosci Lett. 2011;502:80–83. doi: 10.1016/j.neulet.2011.07.017. doi: 10.1016/j.neulet.2011.07.017. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Volk DW, Pierri JN, Fritschy J-M, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: Decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase 67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Woo T-U, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal γ-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Sacco KA, George TP, Potenza MN. Risk/reward decision-making in schizophrenia: a preliminary examination of the influence of tobacco smoking and relationship to Wisconsin Card Sorting Task performance. Schizophr Res. 2009;110:156–164. doi: 10.1016/j.schres.2009.01.012. doi: 10.1016/j.schres.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Robbins TW, Winstanley CA. Serotonergic and dopaminergic modulation of gambling behavior as assessed using a novel rat gambling task. Neuropsychopharmacology. 2009;34:2329–2343. doi: 10.1038/npp.2009.62. doi: 10.1038/npp.2009.62. [DOI] [PubMed] [Google Scholar]
- Zeeb FD, Winstanley CA. Functional disconnection of the orbitofrontal cortex and basolateral amygdala impairs acquisition of a rat gambling task and disrupts animals’ ability to alter decision-making behavior after reinforcer devaluation. J Neurosci. 2013;33:6434–6443. doi: 10.1523/JNEUROSCI.3971-12.2013. doi: 10.1523/JNEUROSCI.3971-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]