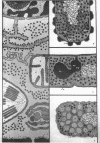
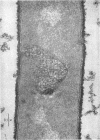
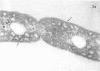
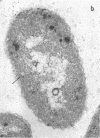
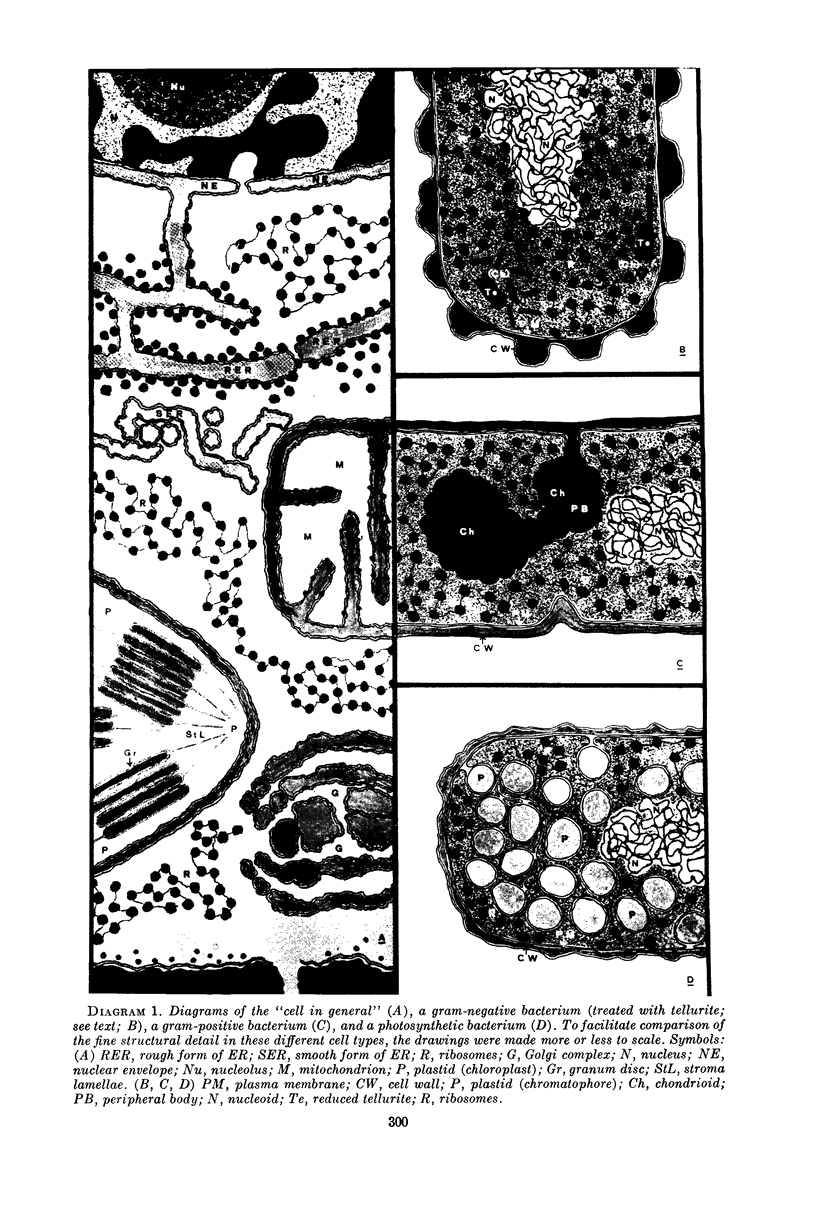
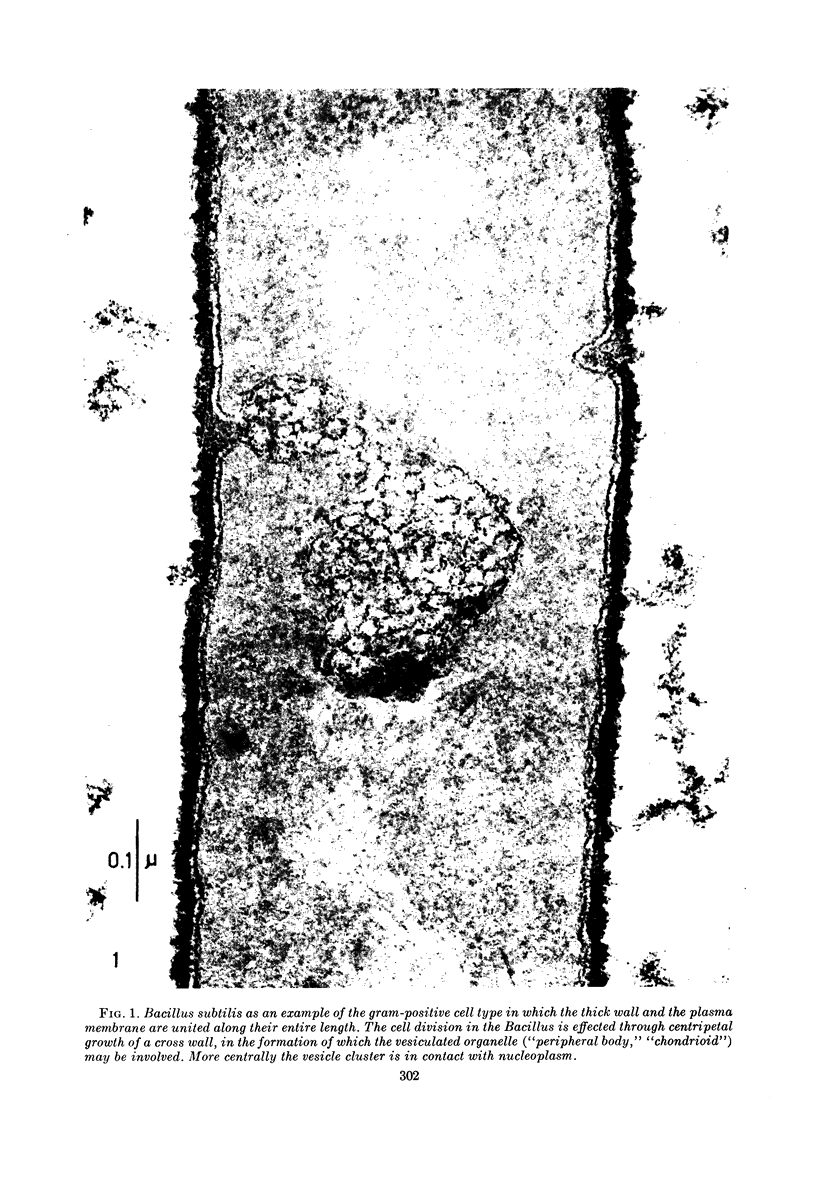
Full text
PDF

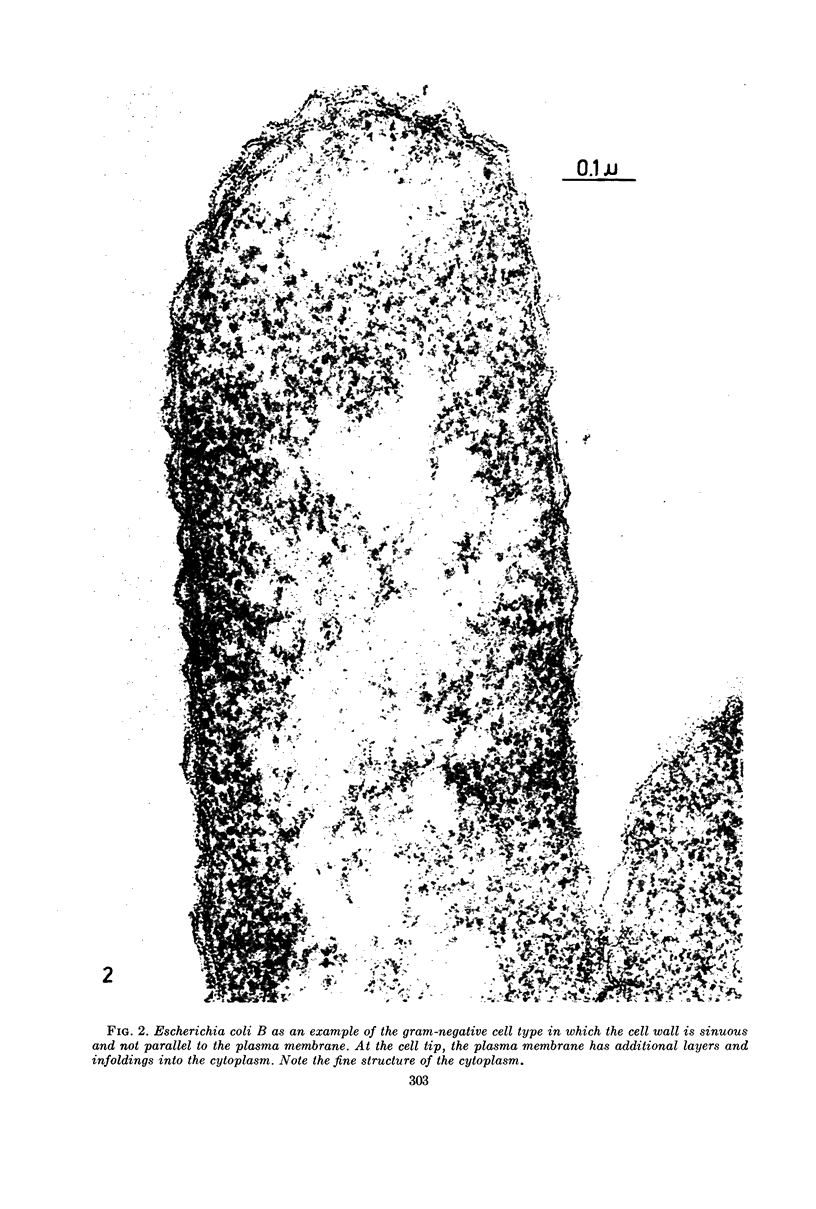
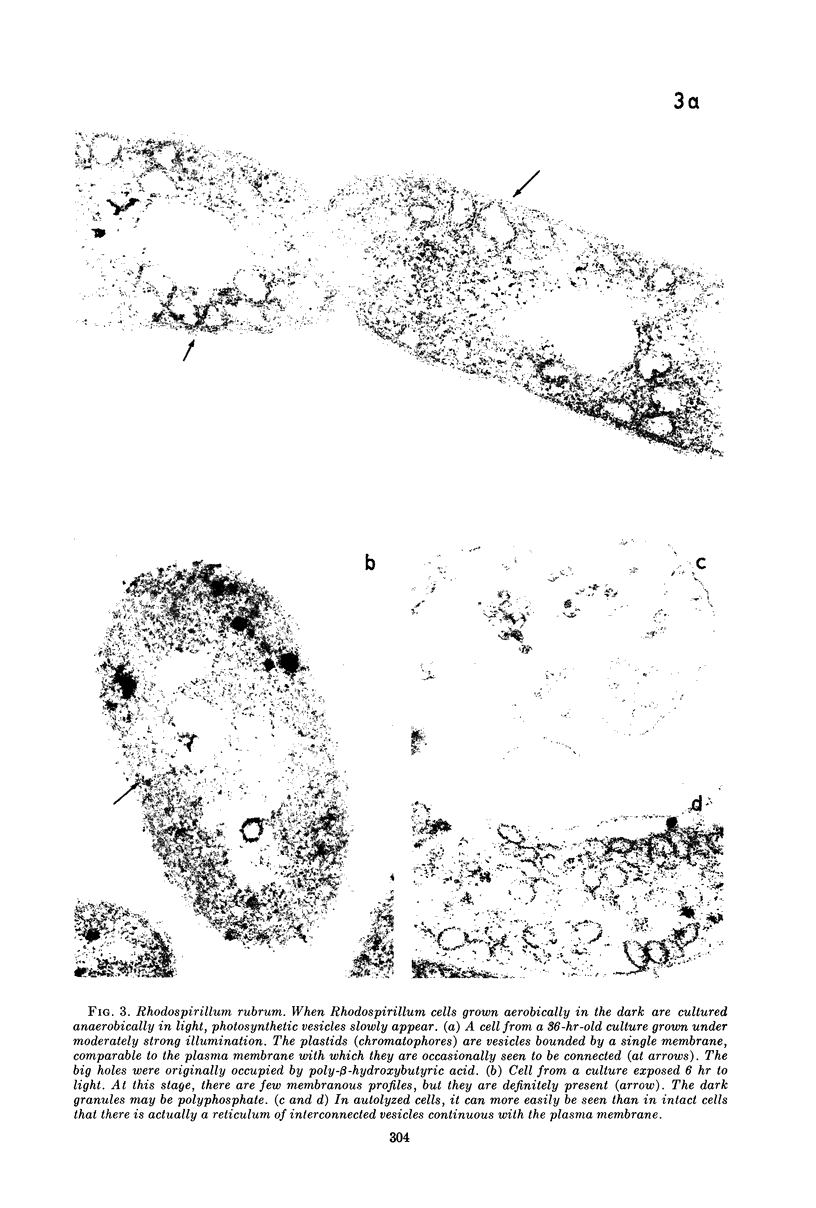
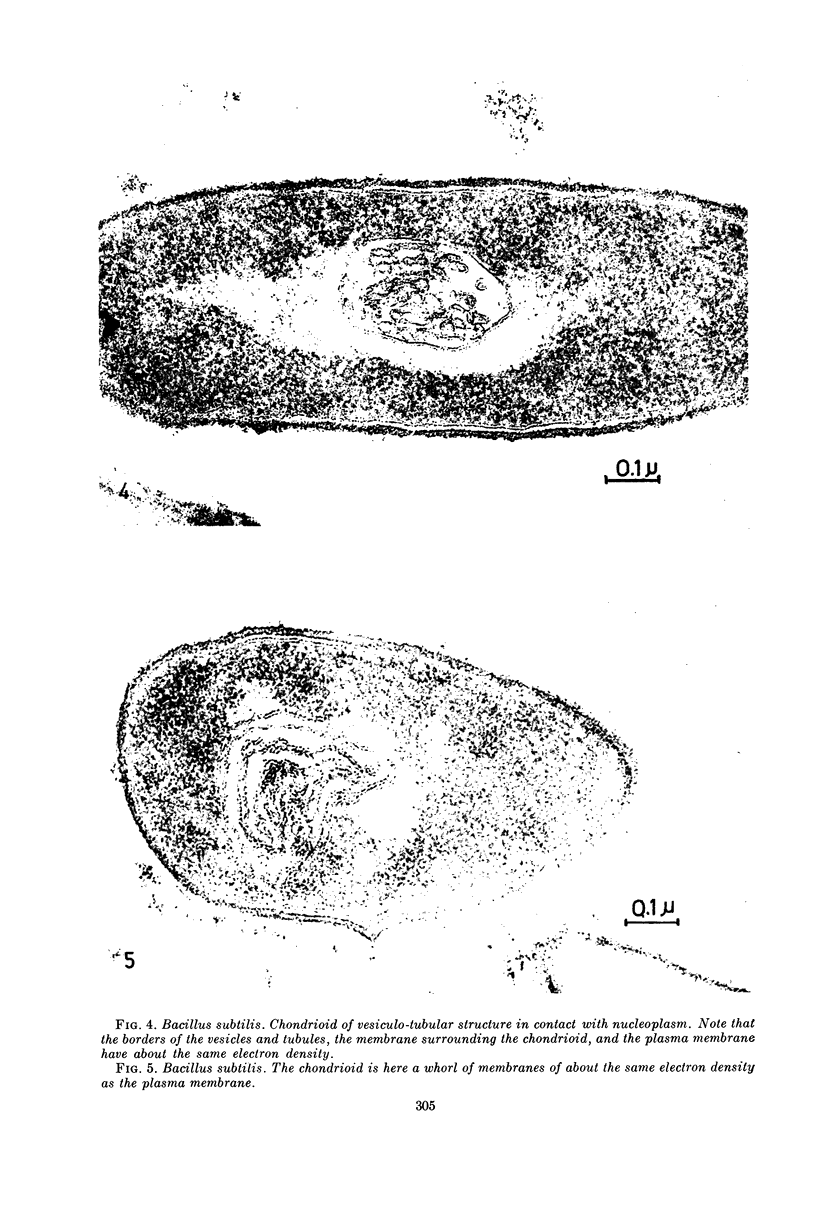

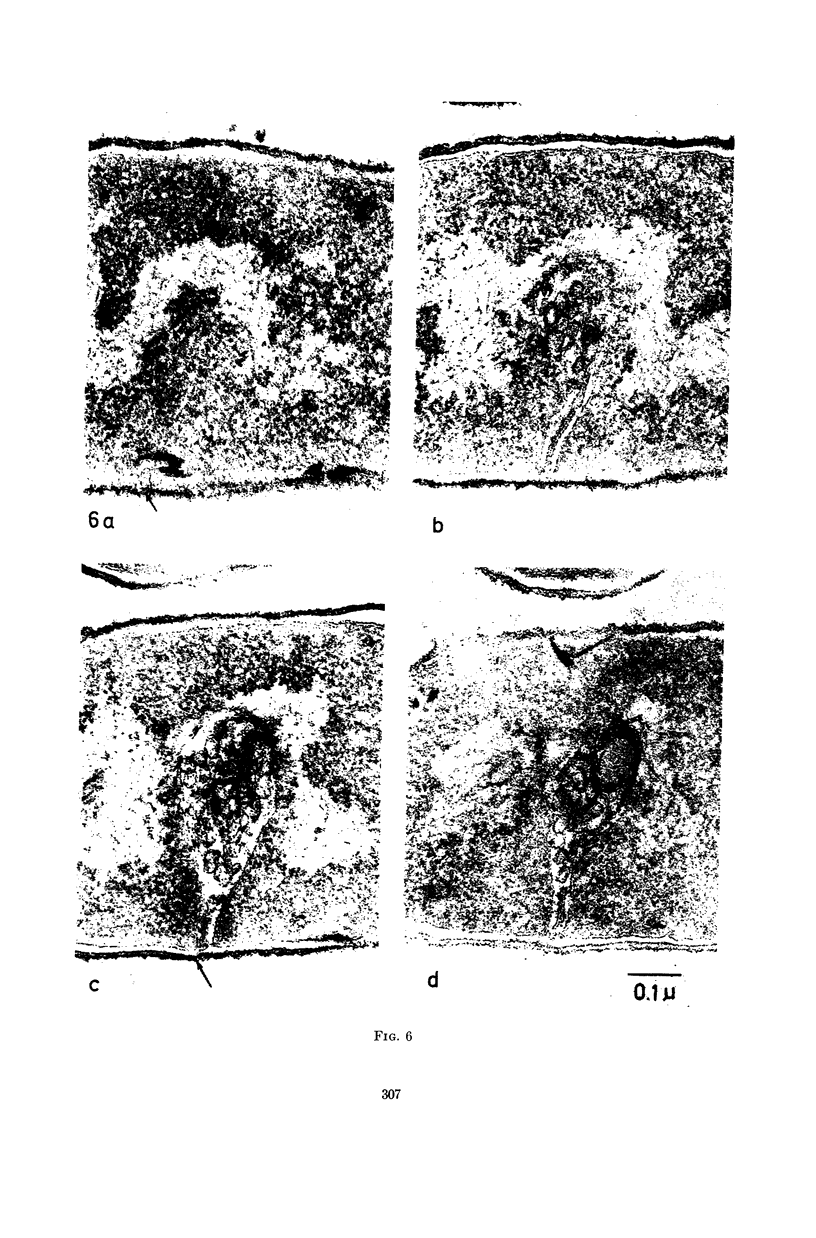

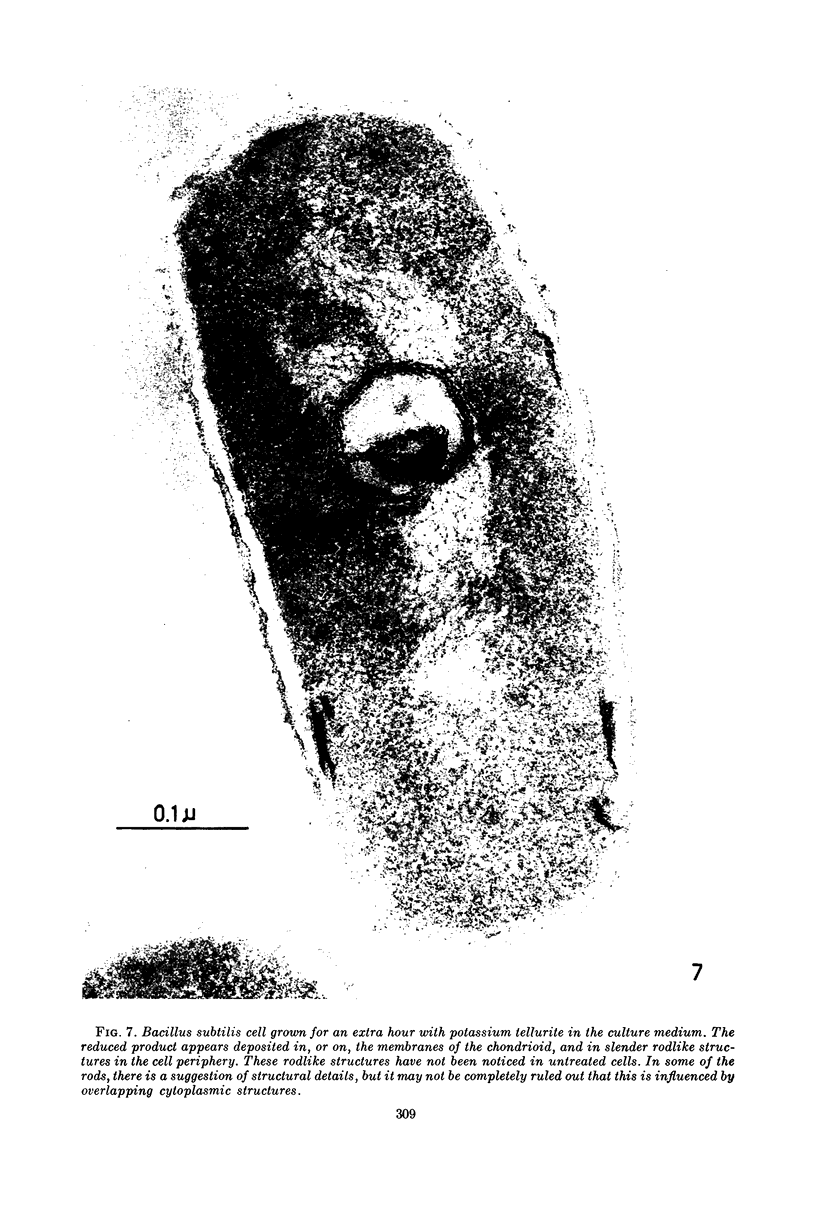


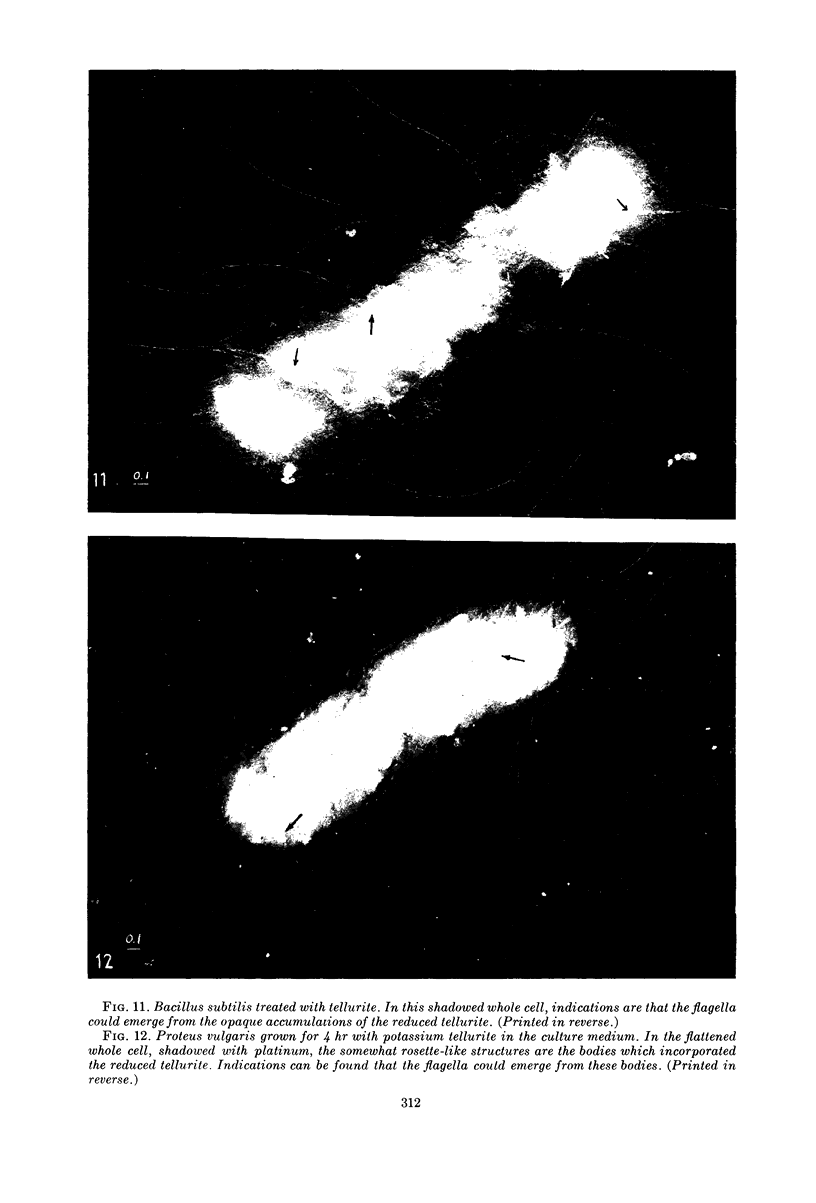


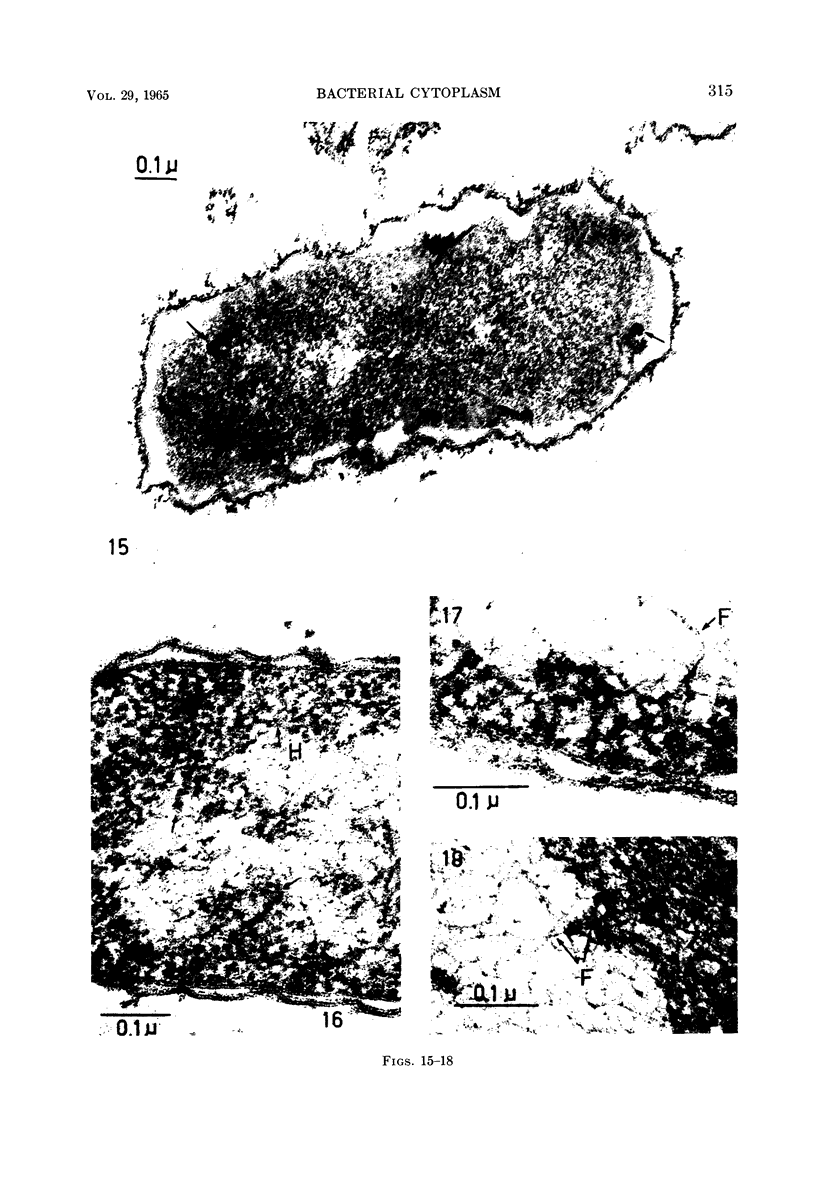

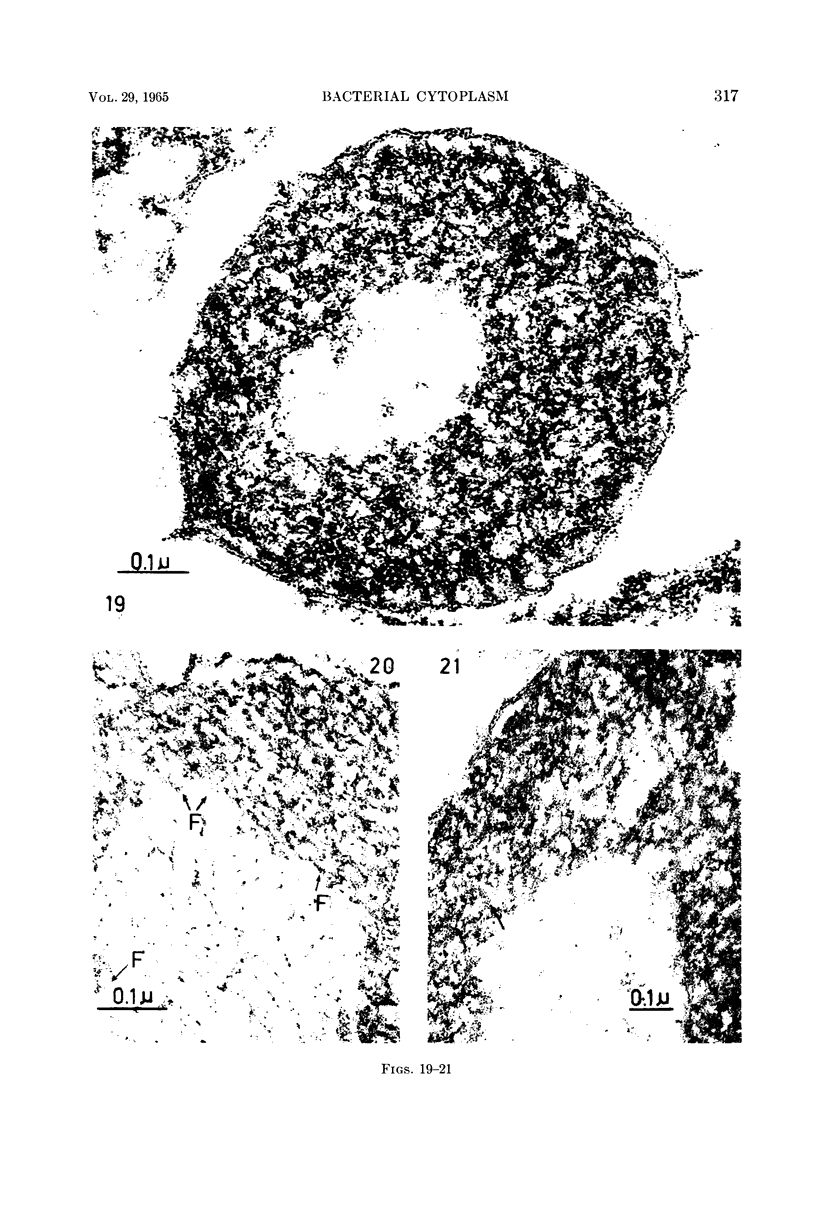

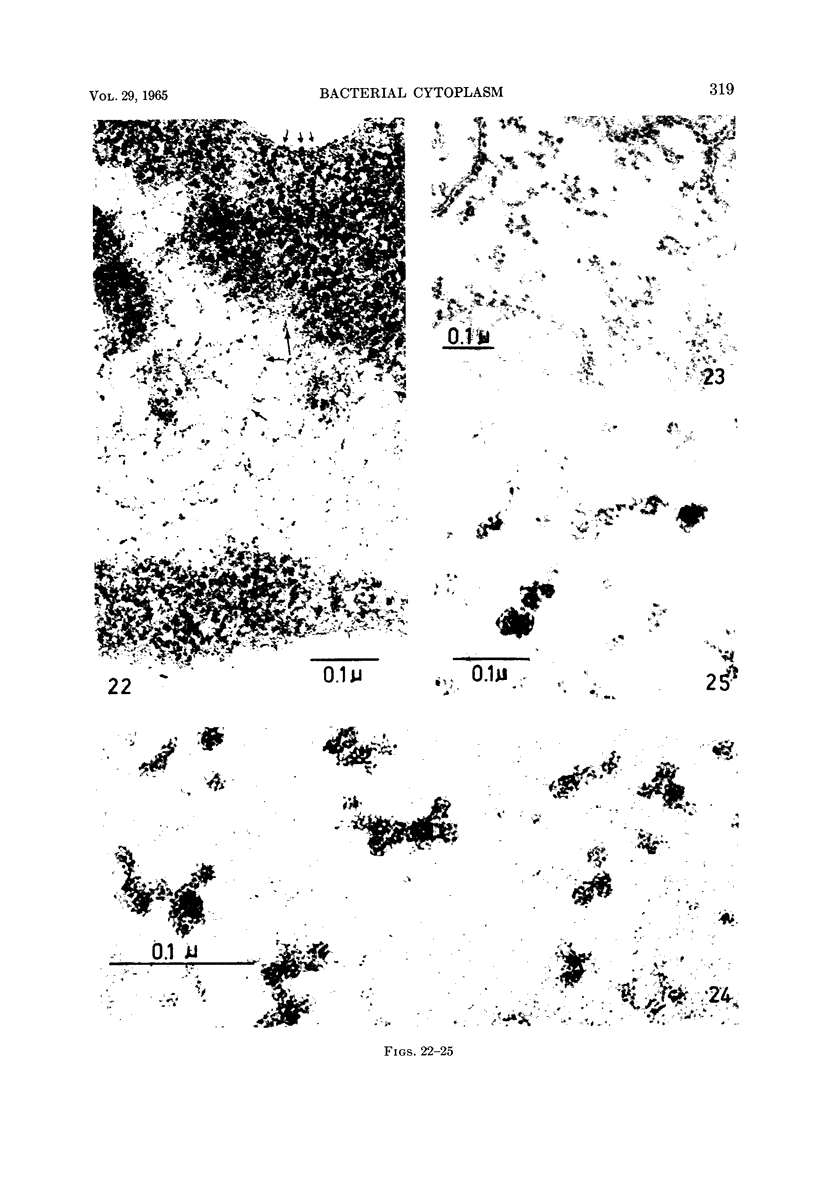
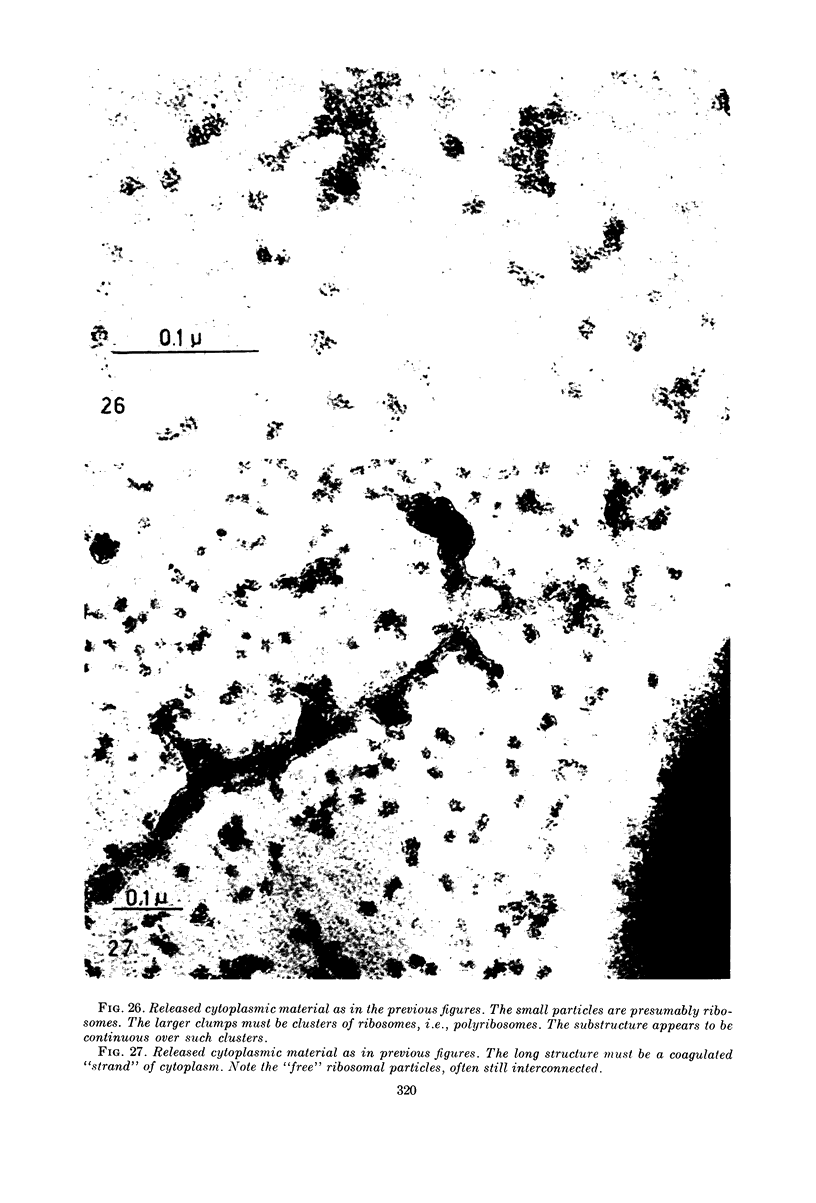
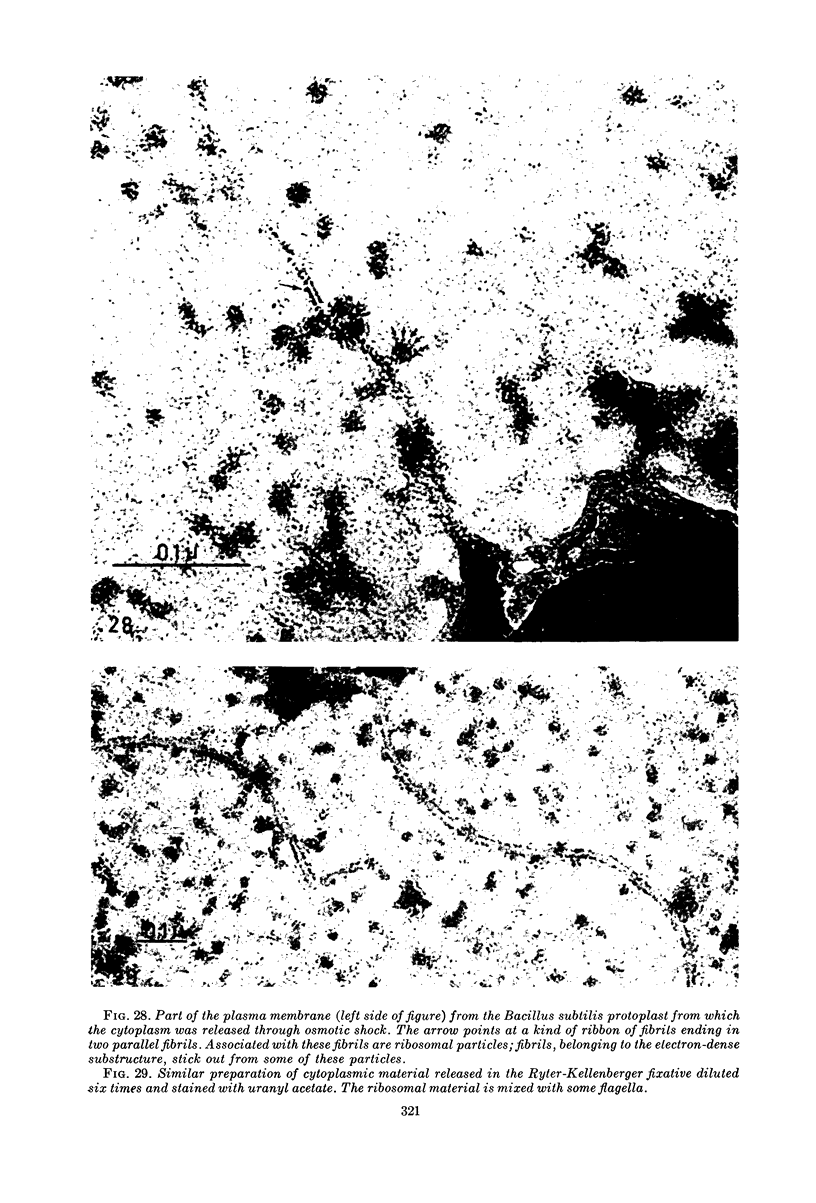




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A., NIELSEN L., THAEMERT J. RAPIDLY SYNTHESIZED RIBONUCLEIC ACID IN MEMBRANE GHOSTS FROM STREPTOCOCCUS FECALIS PROTOPLASTS. Biochim Biophys Acta. 1964 Feb 17;80:325–337. doi: 10.1016/0926-6550(64)90104-5. [DOI] [PubMed] [Google Scholar]
- BARRNETT R. J., PALADE G. E. Histochemical demonstration of the sites of activity of dehydrogenase systems with the electron microscope. J Biophys Biochem Cytol. 1957 Jul 25;3(4):577–588. doi: 10.1083/jcb.3.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYLEY S. T. PHYSICAL STUDIES ON RIBOSOMES FROM PEA SEEDLINGS. J Mol Biol. 1964 Feb;8:231–238. doi: 10.1016/s0022-2836(64)80132-7. [DOI] [PubMed] [Google Scholar]
- BOATMAN E. S. OBSERVATIONS ON THE FINE STRUCTURE OF SPHEROPLASTS OF RHODOSPIRILLUM RUBRUM. J Cell Biol. 1964 Feb;20:297–311. doi: 10.1083/jcb.20.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barondes S. H., Nirenberg M. W. Fate of a Synthetic Polynucleotide Directing Cell-Free Protein Synthesis II. Association with Ribosomes. Science. 1962 Nov 16;138(3542):813–817. doi: 10.1126/science.138.3542.813. [DOI] [PubMed] [Google Scholar]
- CHAPMAN G. B., HANKS J. H., WALLACE J. H. An electron microscope study of the disposition and fine structure of Mycobacterium lepraemurium in mouse spleen. J Bacteriol. 1959 Feb;77(2):205–211. doi: 10.1128/jb.77.2.205-211.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B., HILLIER J. Electron microscopy of ultra-thin sections of bacteria I. Cellular division in Bacillus cereus. J Bacteriol. 1953 Sep;66(3):362–373. doi: 10.1128/jb.66.3.362-373.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., KUNISAWA R. The fine structure of Rhodospirillum rubrum. J Cell Biol. 1963 Feb;16:401–419. doi: 10.1083/jcb.16.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREWS G. [Electron microscopic studies on Mycobacterium phlei. (Structure and formation of metachromatic granula)]. Arch Mikrobiol. 1960;35:53–62. [PubMed] [Google Scholar]
- EDWARDS M. R., STEVENS R. W. FINE STRUCTURE OF LISTERIA MONOCYTOGENES. J Bacteriol. 1963 Sep;86:414–428. doi: 10.1128/jb.86.3.414-428.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C. MORPHOLOGY OF SPORE DEVELOPMENT IN CLOSTRIDIUM PECTINOVORUM. J Bacteriol. 1962 Jul;84(1):104–114. doi: 10.1128/jb.84.1.104-114.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIERER A. Function of aggregated reticulocyte ribosomes in protein synthesis. J Mol Biol. 1963 Feb;6:148–157. doi: 10.1016/s0022-2836(63)80131-x. [DOI] [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. I. Ribosomes and the active complex. J Mol Biol. 1963 May;6:374–388. doi: 10.1016/s0022-2836(63)80050-9. [DOI] [PubMed] [Google Scholar]
- GILBERT W. Polypeptide synthesis in Escherichia coli. II. The polypeptide chain and S-RNA. J Mol Biol. 1963 May;6:389–403. doi: 10.1016/s0022-2836(63)80051-0. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., BRIEGER E. M., ALLEN J. M. The fine structure of vegetative cells of Bacillus subtilis. Exp Cell Res. 1961 Jan;22:73–85. doi: 10.1016/0014-4827(61)90087-8. [DOI] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. A membranous component of the cytoplasm in Streptomyces coelicolor. J Biophys Biochem Cytol. 1959 Dec;6:515–516. doi: 10.1083/jcb.6.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. The fine structure of Streptomyces coelicolor. I. The cytoplasmic membrane system. J Biophys Biochem Cytol. 1960 Jun;7:479–488. doi: 10.1083/jcb.7.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., HOPWOOD D. A. The fine structure of Streptomyces violaceoruber (S. coelicolor). III. The walls of the mycelium and spores. J Biophys Biochem Cytol. 1961 Aug;10:505–516. doi: 10.1083/jcb.10.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M. The fine structure of bacteria. Br Med Bull. 1962 Sep;18:245–250. doi: 10.1093/oxfordjournals.bmb.a069988. [DOI] [PubMed] [Google Scholar]
- GODSON G. N., HUNTER G. D., BUTLER J. A. Cellular components of Bacillus megaterium and their role in protein biosynthesis. Biochem J. 1961 Oct;81:59–68. doi: 10.1042/bj0810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HART R. G. Electron microscopy of the 50-S ribosomes of Escherichia coli. Biochim Biophys Acta. 1962 Jul 16;60:629–637. doi: 10.1016/0006-3002(62)90881-8. [DOI] [PubMed] [Google Scholar]
- HENDLER R. W. A model for protein synthesis. Nature. 1962 Mar 3;193:821–823. doi: 10.1038/193821a0. [DOI] [PubMed] [Google Scholar]
- HENDLER R. W., BANFIELD W. G., TANI J., KUFFEL ON THE CYTOLOGICAL UNIT FOR PROTEIN SYNTHESIS IN VIVO IN E. COLI. III. ELECTRON MICROSCOPIC AND ULTRACENTRIFUGAL EXAMINATION OF INTACT CELLS AND FRACTIONS. Biochim Biophys Acta. 1964 Feb 17;80:307–314. [PubMed] [Google Scholar]
- HENDLER R. W., TANI J. ON THE CYTOLOGICAL UNIT FOR PROTEIN SYNTHESIS IN VIVO IN E. COLI. II. STUDIES WITH INTACT CELLS OF TYPE B. Biochim Biophys Acta. 1964 Feb 17;80:294–306. doi: 10.1016/0926-6550(64)90101-x. [DOI] [PubMed] [Google Scholar]
- HENSHAW E. C., BOJARSKI T. B., HIATT H. H. PROTEIN SYNTHESIS BY FREE AND BOUND RAT LIVER RIBOSOMES IN VIVO AND IN VITRO. J Mol Biol. 1963 Aug;7:122–129. doi: 10.1016/s0022-2836(63)80041-8. [DOI] [PubMed] [Google Scholar]
- Imaeda T., Convit J. ELECTRON MICROSCOPE STUDY OF MYCOBACTERIUM LEPRAE AND ITS ENVIRONMENT IN A VESICULAR LEPROUS LESION. J Bacteriol. 1962 Jan;83(1):43–52. doi: 10.1128/jb.83.1.43-52.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAVANAU J. L. Structure and functions of biological membranes. Nature. 1963 May 11;198:525–530. doi: 10.1038/198525a0. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., HUBER L. Contribution à l'étude des équivalents des mitochondries dans les bactéries. Experientia. 1953 Aug;9(8):289–291. doi: 10.1007/BF02156014. [DOI] [PubMed] [Google Scholar]
- KOIKE M., TAKEYA K. Fine structures of intracytoplasmic organelles of mycobacteria. J Biophys Biochem Cytol. 1961 Mar;9:597–608. doi: 10.1083/jcb.9.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGRIDGE R., HOLMES K. C. X-ray diffraction studies of concentrated gels of ribosomes from E. coli. J Mol Biol. 1962 Dec;5:611–617. doi: 10.1016/s0022-2836(62)80089-8. [DOI] [PubMed] [Google Scholar]
- LANGRIDGE R. Ribosomes: a common structural feature. Science. 1963 May 31;140(3570):1000–1000. doi: 10.1126/science.140.3570.1000. [DOI] [PubMed] [Google Scholar]
- MUDD S. Cytology of bacteria. I. The bacterial cell. Annu Rev Microbiol. 1954;8:1–22. doi: 10.1146/annurev.mi.08.100154.000245. [DOI] [PubMed] [Google Scholar]
- McALEAR J. H., EDWARDS G. A. [Continuity of plasma membrane and nuclear membrane]. Exp Cell Res. 1959 Mar;16(3):689–692. doi: 10.1016/0014-4827(59)90139-9. [DOI] [PubMed] [Google Scholar]
- Mudd S. CELLULAR ORGANIZATION IN RELATION TO FUNCTION. Bacteriol Rev. 1956 Dec;20(4):268–271. doi: 10.1128/br.20.4.268-271.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASS M. M., NASS S. INTRAMITOCHONDRIAL FIBERS WITH DNA CHARACTERISTICS. I. FIXATION AND ELECTRON STAINING REACTIONS. J Cell Biol. 1963 Dec;19:593–611. doi: 10.1083/jcb.19.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASS S., NASS M. M. INTRAMITOCHONDRIAL FIBERS WITH DNA CHARACTERISTICS. II. ENZYMATIC AND OTHER HYDROLYTIC TREATMENTS. J Cell Biol. 1963 Dec;19:613–629. doi: 10.1083/jcb.19.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D. F. Mitochondrial Structure: Two Types of Subunits on Negatively Stained Mitochondrial Membranes. Science. 1963 May 31;140(3570):985–987. doi: 10.1126/science.140.3570.985. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON J. D. The ultrastructure of cell membranes and their derivatives. Biochem Soc Symp. 1959;16:3–43. [PubMed] [Google Scholar]
- ROSA C. G., TSOU K. C. The use of tetranitro-blue tetrazolium for the cytochemical localization of succinic dehydrogenase. Cytochemical and cytological studies of sarcoma 37 ascites tumor cells. J Cell Biol. 1963 Mar;16:445–454. doi: 10.1083/jcb.16.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUDZINSKA M. A., SEDAR A. W. Mitochondria of protozoa. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):331–336. doi: 10.1083/jcb.2.4.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., JACOB F. ETUDE AU MICROSCOPE 'ELECTRONIQUE DES RELATIONS ENTRE M'ESOSOMES ET NOYAUX CHEZ BACILLUS SUBTILIS. C R Hebd Seances Acad Sci. 1963 Nov 13;257:3060–3063. [PubMed] [Google Scholar]
- SALTON M. R., CHAPMAN J. A. Isolation of the membranemesosome structures from Micrococcus lysodeikticus. J Ultrastruct Res. 1962 Jun;6:489–498. doi: 10.1016/s0022-5320(62)80004-5. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M. BACTERIAL POLYRIBOSOMES AND THEIR PARTICIPATION IN PROTEIN SYNTHESIS IN VIVO. J Mol Biol. 1963 Nov;7:561–568. doi: 10.1016/s0022-2836(63)80102-3. [DOI] [PubMed] [Google Scholar]
- SCHLESSINGER D. PROTEIN SYNTHESIS BY POLYRIBOSOMES ON PROTOPLAST MEMBRANES OF B. MEGATERIUM. J Mol Biol. 1963 Nov;7:569–582. doi: 10.1016/s0022-2836(63)80103-5. [DOI] [PubMed] [Google Scholar]
- SIEKEVITZ P., PALADE G. E. A cytochemical study on the pancreas of the guinea pig. 5. In vivo incorporation of leucine-1-C14 into the chymotrypsinogen of various cell fractions. J Biophys Biochem Cytol. 1960 Jul;7:619–630. doi: 10.1083/jcb.7.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEKEVITZ P., PALADE G. E. A cytochemical study on the pancreas of the guinea pig. 6. Release of enzymes and ribonucleic acid from ribonucleoprotein particles. J Biophys Biochem Cytol. 1960 Jul;7:631–644. doi: 10.1083/jcb.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAYTER H. S., WARNER J. R., RICH A., HALL C. E. THE VISUALIZATION OF POLYRIBOSOMAL STRUCTURE. J Mol Biol. 1963 Dec;7:652–657. doi: 10.1016/s0022-2836(63)80112-6. [DOI] [PubMed] [Google Scholar]
- SPYRIDES G. J., LIPMANN F. Polypeptide synthesis with sucrose gradient fractions of E. coli ribosomes. Proc Natl Acad Sci U S A. 1962 Nov 15;48:1977–1983. doi: 10.1073/pnas.48.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOECKENIUS W. Some observations on negatively stained mitochondria. J Cell Biol. 1963 May;17:443–454. doi: 10.1083/jcb.17.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGANUMA A. SOME FEATURES OF MEMBRANOUS STRUCTURES IN STAPHYLOCOCCUS AUREUS. J Infect Dis. 1963 Nov-Dec;113:179–185. doi: 10.1093/infdis/113.3.179. [DOI] [PubMed] [Google Scholar]
- TANI J., HENDLER R. W. ON THE CYTOLOGICAL UNIT FOR PROTEIN SYNTHESIS IN VIVO IN E. COLI. I. STUDIES WITH SPHEROPLASTS OF TYPE K-12. Biochim Biophys Acta. 1964 Feb 17;80:279–293. doi: 10.1016/0926-6550(64)90100-8. [DOI] [PubMed] [Google Scholar]
- VAN ITERSON, LEENE W. A CYTOCHEMICAL LOCALIZATION OF REDUCTIVE SITES IN A GRAM-NEGATIVE BACTERIUM. TELLURITE REDUCTION IN PROTEUS VULGARIS. J Cell Biol. 1964 Mar;20:377–387. doi: 10.1083/jcb.20.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON, LEENE W. A CYTOCHEMICAL LOCALIZATION OF REDUCTIVE SITES IN A GRAM-POSITIVE BACTERIUM. TELLURITE REDUCTION IN BACILLUS SUBTILIS. J Cell Biol. 1964 Mar;20:361–375. doi: 10.1083/jcb.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ITERSON W. Some features of a remarkable organelle in Bacillus subtilis. J Biophys Biochem Cytol. 1961 Jan;9:183–192. doi: 10.1083/jcb.9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDERWINKEL E., MURRAY R. G. [Bacterial intracytoplasmic organelles and the site of oxidation-reduction activity]. J Ultrastruct Res. 1962 Aug;7:185–199. doi: 10.1016/s0022-5320(62)80035-5. [DOI] [PubMed] [Google Scholar]
- WADDINGTON C. H., PERRY M. M. Helical arrangement of ribosomes in differentiating muscle cells. Exp Cell Res. 1963 May;30:599–600. doi: 10.1016/0014-4827(63)90339-2. [DOI] [PubMed] [Google Scholar]
- WARNER J. R., KNOPF P. M., RICH A. A multiple ribosomal structure in protein synthesis. Proc Natl Acad Sci U S A. 1963 Jan 15;49:122–129. doi: 10.1073/pnas.49.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIBULL C., BECKMAN H., BERGSTROM L. Localization of enzymes in Bacillus megaterium, strain M. J Gen Microbiol. 1959 Jun;20(3):519–531. doi: 10.1099/00221287-20-3-519. [DOI] [PubMed] [Google Scholar]
- WEIBULL C. Characterization of the protoplasmic constituents of bacillus megaterium. J Bacteriol. 1953 Dec;66(6):696–702. doi: 10.1128/jb.66.6.696-702.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]
- WINKLER A. Feinstruktur des Bakterienzellinhaltes. Zentralbl Bakteriol Orig. 1958 Dec;173(5-6):369–375. [PubMed] [Google Scholar]
- Warner J. R., Rich A., Hall C. E. Electron Microscope Studies of Ribosomal Clusters Synthesizing Hemoglobin. Science. 1962 Dec 28;138(3548):1399–1403. doi: 10.1126/science.138.3548.1399. [DOI] [PubMed] [Google Scholar]