Abstract
Background
Health outcomes throughout the life course have been linked to fetal growth restriction and low birthweight. A variety of measures exist to define low birthweight, with a lack of consensus regarding which predict adverse outcome.
Objectives
To evaluate the relationship between birthweight standards and childhood and adult outcomes in term-born infants (≥37 weeks' gestation).
Search strategy
MEDLINE (1966–January 2011), EMBASE (1980–January 2011), and the Cochrane Library (2011:1) and MEDION were included.
Selection criteria
Studies comprising live term-born infants (gestation ≥37 completed weeks), with weight or other anthropometric measurements recorded at birth along with childhood and adult outcomes.
Data collection and analysis
Data were extracted to populate 2 × 2 tables relating birthweight standard with outcome, and meta-analysis was performed where possible.
Main results
Fifty-nine articles (2 600 383 individuals) were selected. There was no significant relationship between birthweight <2.5 kg (odds ratio [OR] 0.98, 95% confidence intervals [CI] 0.87–1.10) and composite measure of childhood morbidity. Weight <10th centile on the population nomogram showed a small association (OR 1.49, 95% CI 1.02–2.19) for the same outcome. There was no significant association between either of the above measures and adult morbidity. The relationship between other measures and individual outcomes varied.
Author's conclusions
The association between low birthweight, by any definition, and childhood and adult morbidity was inconsistent. None of the current standards of low birthweight was a good predictor of adverse outcome.
Keywords: Adult morbidity, childhood morbidity, low birthweight, meta-analysis, systematic review
Introduction
The ‘fetal origins hypothesis' suggests that malnourishment in utero changes fetal programming, whereby biological pathways are altered, resulting in increased susceptibility to disease. In 1986 Barker et al.1 demonstrated an inverse relationship between birthweight and adult cardiovascular disease. Since then, numerous studies have evaluated the association between low birthweight and morbidity and mortality throughout the life course.2–10 However, the results have not always been consistent.11–14 The initial evidence for the Barker hypothesis has been criticised for failing to account for important potential confounders such as gestational age and socio-economic class, and as such is not universally accepted.15
A number of methods have been used to define low birthweight and to attempt to identify infants who may be at risk of subsequent adverse outcome, including population-based centile charts, the most commonly used threshold being the 10th centile;16 customised charts where the mother's BMI and ethnicity are used to calculate individualised growth centiles;17 and ponderal index, which takes into account the neonatal weight and length.18,19
The aim of this systematic review was to re-examine the association between low birthweight and adverse outcomes, avoiding the confounding influence of prematurity, by strictly limiting study inclusion to infants of 37 weeks' gestation or more. The findings of this review have been split into two papers. The first, focusing on neonatal outcomes (mortality and morbidity), has been published separately.20 Birthweight tests were found to be strongly associated with neonatal mortality and morbidity, especially at lower absolute birthweight threshold. The current report focuses on examining the association between low birthweight at term and morbidity and mortality during childhood and adult life.
Methods
Our methodology has been described in detail using the same data sources, search strategy and methodology as in our previous paper20 and will not be repeated here. Instead we will highlight differences from the previous paper.
Only studies including morbidity diagnosed subsequent to the neonatal period are included in this report. Where morbidity diagnosed in infancy (<1 year) was included, all conditions are permanent (e.g. cerebral palsy) and are assumed to be present through to childhood in survivors.
Meta-analysis was performed using composite and individual outcome measures. When the composite outcome measure was used, care was taken to ensure that each individual was only counted once in each analysis. Where multiple outcomes were reported, we selected the outcome most consistent with other studies; for example, in the childhood morbidity analysis, hypertension was the most commonly reported outcome therefore this was selected primarily, followed by other components of the metabolic syndrome.
Results
As shown in Supporting Information Figure S1, after an initial search of 36 956 citations, we included 92 primary articles in the overall systematic review, of which 59 contained data relating birthweight standards to childhood or adult outcomes.5,8,9,12,14,21–74 Twenty of the 59 included were added after contact with authors who provided data or information.12,21–40 2 600 383 individuals were included in the analyses reported in this manuscript. Details of the included studies are given in Supporting Information Table S1; a list of excluded studies is available from the authors on request. A total of 145 further articles were felt to contain potentially relevant data but either the authors could not be contacted or could not supply data to create 2 × 2 tables, or on clarification regarding the population the study was excluded. If the population was the same but the measure of growth restriction or adverse outcome differed, both studies were included, but care was taken not to include multiple studies reporting from the same population within a single meta-analysis, or within the overall count of the number of individuals included in the review.39,40,43,44,48,69
The majority of studies used a population growth chart <10th percentile (n = 21) or birthweight <2.5 kg (n = 23) as the index test. A wide variety of outcome measures including mortality and morbidity (e.g. hypertension, diabetes mellitus, learning difficulties, cerebral palsy) were reported. For comparison, we grouped outcomes according to age, that is, childhood and adolescent (12 months to 18 years) and adult (>18 years).
Childhood and adolescent outcomes
A Forest plot for the association of measures of low birthweight with childhood and adolescent outcomes is given in Supporting Information Figure S2. Meta-analysis was performed to assess the association of birthweight <2.5 kg with a composite group of adverse outcomes reported in primary studies (including obesity, hypertension, type 1 diabetes mellitus, asthma, hypercholesterolaemia, learning difficulties and strabismus). There was no significant association present (odds ratio [OR] 0.98, 95% confidence interval [CI] 0.87–1.10). A meta-analysis for birthweight <10th centile on the population chart showed a small association that was just significant (OR 1.49, 95% CI 1.02–2.19); however, there was significant heterogeneity present. When limiting the composite outcome analysis to conditions associated with the metabolic syndrome (obesity, hypertension, hypercholesterolaemia) for birthweight <2.5 kg the association remained non-significant (nine studies OR 0.97, 95% CI 0.84–1.18, I2 = 0) and for the population chart <10th centile the association became non-significant (four studies OR 1.01, 95% CI 0.64–1.58, I2 = 54). When the analysis was restricted to learning difficulties or mental handicap, birthweight <3rd centile on the population chart and <10th centile both showed a weak but significant association. When individual outcomes were considered, there was no significant association between any measure of low birthweight and childhood obesity, hypertension, asthma, visual impairment or psychiatric diagnosis.
Adult outcomes
A Forest plot of odds ratios for the association of measures of fetal growth restriction and adult outcomes is given in Figure 1. A meta-analysis was performed for the association of birthweight <2.5 kg with a composite measure of adult morbidity (including obesity, hypertension, hypercholesterolaemia, type 2 diabetes mellitus, coronary heart disease, and polycystic ovarian syndrome). There was no significant association between birthweight <2.5 kg or birthweight <10th centile on the population chart with this composite outcome. Limiting the composite outcome analysis to conditions associated with the metabolic syndrome (obesity, hypertension, hypercholesterolaemia, coronary heart disease, type 2 diabetes) did not change the results. When individual morbidities were considered, birthweight <10th centile according to the population chart was significantly associated with adult obesity in a single study (OR 1.86, 95% CI 1.20–2.88). Birthweight <2.5 kg showed a weak association with hypertension, diabetes mellitus or impaired glucose tolerance and cardiovascular mortality. Ponderal index (kg/m3) <24 was also weakly associated with mortality from cardiovascular disease. Childhood or adulthood end stage renal disease showed a significant association with birthweight <10th centile on the population chart.
Figure 1.
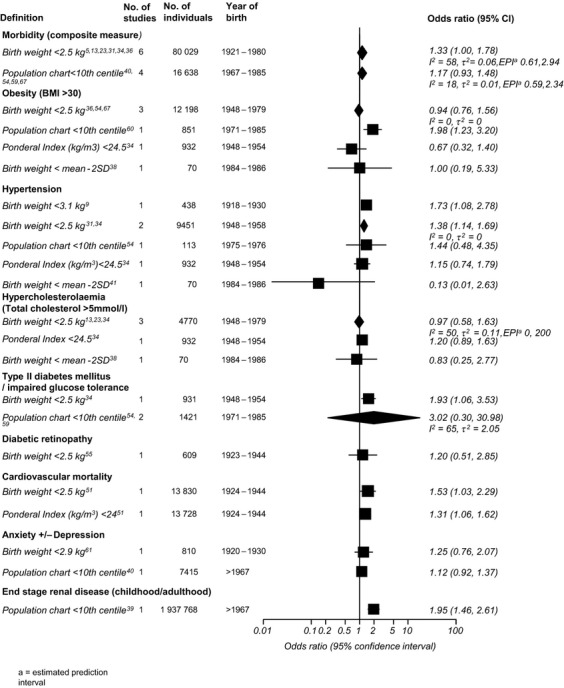
Forest plot of odds ratios for the association between birthweight standards and adult outcomes.
Quality assessment
The results for the quality assessment are presented in Table1. The majority of included studies were of cohort design (88%) and most were retrospective studies (58%). Most studies were of high or moderate quality according to our pre-specified criteria. Studies often failed to describe adequately the test or outcome in a way that would make them reproducible, and very few studies described any interventions that were performed between the time of the birthweight measurement and the outcome test. Where possible, a subgroup analysis using only high quality studies was performed and the results are presented in Table2.
Table 1.
Methodological quality of studies included in systematic review of birthweight standards for childhood and adult outcomes
| Quality item | No. (%) of studies, n = 59 | ||
|---|---|---|---|
| Yes | No | Unclear | |
| Cohort study design | 52 (88) | 7 (12) | 0 |
| Population adequately described | 59 (100) | 0 | 0 |
| Consecutive recruitment | 21 (36) | 11 (18) | 27 (46) |
| Prospective recruitment | 21 (36) | 34 (58) | 4 (7) |
| Appropriate outcome measure | 59 (100) | 0 | 0 |
| Outcome measure blinded | 10 (17) | 1 (2) | 48 (81) |
| >90% of individuals had outcome measure | 14 (24) | 40 (68) | 5 (8) |
| Index test and outcome measure described | 34 (57) | 4 (7) | 21 (36) |
| Intervention between index test and outcome | 4 (7) | 0 | 55 (93) |
| Quality classification | |||
| High | 28 (46) | – | – |
| Medium | 24 (41) | – | – |
| Low | 8 (13) | – | – |
Table 2.
Subgroup analysis according to birthweight standard and outcome, where possible, for study quality, ethnicity, year of birth of study population and singleton population
| Birthweight standard | No. of studies | Subgroup | Odds ratio (95% CI) | Estimated prediction interval (EPI) | I2, τ2 |
|---|---|---|---|---|---|
| Childhood morbidity | |||||
| Birthweight <2.5 kg | 512,22,24,25,28 | Singleton | 0.95 (0.63–1.44) | – | I2 = 0, τ2 = 0 |
| Birthweight <2.5 kg | 523,25,28,32,33 | High quality studies | 0.82 (0.63–1.07) | – | I2 = 0, τ2 = 0 |
| Birthweight <2.5 kg | 712,24–26,28,32,33 | Ethnicity >90% White European | 0.99 (0.68–1.44) | – | I2 = 0, τ2 = 0 |
| Population chart <10th centile | 48,22,63,74 | Singleton | 1.35 (0.82–2.24) | 0.15–12.24 | I2 = 90, τ2 = 0.20 |
| Population chart <10th centile | 822,30,45,56,62,63,66,74 | High quality studies | 1.65 (0.96–2.83) | 0.31–8.86 | I2 = 89, τ2 = 0.40 |
| Population chart <10th centile | 222,30 | Year of birth ≥1990 | 0.67 (0.35–1.31) | – | I2 = 76, τ2 = 0.19 |
| Population chart <10th centile | 358,63,73 | Ethnicity White European | 1.67 (1.40–1.98) | – | I2 = 0, τ2 = 0 |
| Adult morbidity | |||||
| Birthweight <2.5 kg | 45,23,31,36 | Singleton | 1.41 (0.80–2.47) | 0.14, 13.82 | I2 = 70, τ2 = 0.20 |
| Birthweight <2.5 kg | 231,34 | High-quality studies | 1.39 (1.14–1.69) | – | I2 = 0, τ2 = 0 |
Subgroup analyses
The results for subgroup analyses within the meta-analysis groups for each age group and birthweight standard are presented in Table2. When childhood morbidity was considered, there was no significant association between birthweight <2.5 kg or the population chart <10th centile in any of the subgroups analysed. There was a significant association between birthweight <2.5 kg and adult morbidity when high-quality studies were considered (OR 1.39, 95% CI 1.14–1.69); however, only two studies were included in this analysis.
Predictive ability of standards of low birthweight to predict childhood and adult outcomes
Only two measures of low birthweight met our pre-specified criteria for calculation of predictive values for childhood morbidity. Customised chart <1st centile had a high specificity (0.99; 95% CI 0.97–1.00) but poor sensitivity (0.06; 95% CI 0.04–0.11) for childhood cerebral palsy.49 A customised chart <5th centile had a specificity of 0.90 (95% CI 0.87–0.93) and a sensitivity of 0.25 (95% CI 0.19–0.31) for the same outcome.49 The positive likelihood ratio was 5.6 (95% CI 2.04–15.34) for <1st centile and 2.57 (95% CI 1.78–3.72) for <5th centile. The corresponding negative likelihood ratios were 0.95 (95% CI 0.91–0.98) and 0.83 (95% CI 0.77–0.90).
Birthweight as a continuous variable
Seven papers reported regression analysis using birthweight as a continuous outcome.9,12–14,26,28,47 These studies looked at adult hypertension (age 50 and 60 years) and hypercholesterolaemia, childhood obesity and hypertension, and composite childhood metabolic risk index. Only one found a significant association. Andersson et al.9 performed logistic regression to examine the association between birthweight and hypertension (defined as treatment for hypertension and/or systolic BP ≥160 mmHg and/or diastolic BP >95 mmHg). At age 60, the OR was 0.96 (95% CI 0.92–0.99, P = 0.028 for change in risk of hypertension per 100 g birthweight).
Direct comparison of absolute versus population centiles
Only one study compared two birthweight standards in the same population. For type 1 diabetes in childhood, birthweight <2.5 kg had an OR of 0.68 (95% CI 0.22–2.12), and a population chart <10th centile an OR of 0.46 (95% CI 0.26–0.82).22
Publication bias
The Peters test was performed where there were ten or more studies included in the meta-analysis (population <10th centile and childhood morbidity and birthweight <2.5 kg for the same outcome). There was no statistically significant small study effect in either of the groups analysed (P-value 0.326–0.996).
Discussion
Main findings
For outcomes in childhood, there was a significant association between birthweight <10th centile according to population chart and a composite measure of morbidity. However, when this analysis was restricted to a singleton population, or for metabolic outcomes, it became non-significant. When individual measures were considered, there was no significant association between any measure of low birthweight and childhood obesity, hypertension or asthma. For adult outcomes, there was no consistent association seen between birthweight standards and adult health, although individual studies showed a weak association between birthweight <2.5 kg and hypertension, cardiovascular mortality and diabetes. The predictive ability of customised centile charts (<1st centile and <10th centile) for childhood cerebral palsy was calculated. The likelihood ratios indicated that both were poor tests.75
Our analysis for the relationship between birthweight standards and neonatal outcomes has already been published.20
Strengths and limitations
This review provides the best available evidence, at the time of writing, regarding the association between different measures of low birthweight at term and adverse outcomes. No other review has attempted to compare different definitions of low birthweight to inform clinical practice. The strength of our review and the validity of our inferences lie in the methodology used. We have complied with existing guidelines for the reporting of systematic reviews of diagnostic and observational studies.76,77 We have used the most up-to-date techniques for performing and interpreting meta-analysis.78–80 Every effort was made to obtain the most complete data set possible through contact with authors and experts in the field.
There are several limitations to our review. Although every effort was made to control for potential confounding factors through subgroup analysis, due to the quality and reporting of the primary studies this was not always possible. We strictly limited our review to infants born at 37 weeks' gestation or more; however, the method of estimating gestation in the primary studies was often inaccurate. Very few studies used ultrasound measurement of crown–rump length at 10–13 weeks' gestation, which is the most accurate method.81 Due to poor reporting in the primary studies, our ability to perform subgroup analysis according to ethnicity was limited. Although our results did not differ much when limited to a White European population, it is known that Black African or Caribbean and Asian populations have smaller babies, and therefore it is likely that the same thresholds would not give the same results in all ethnic backgrounds.82 We did not analyse according to social class; however, previous epidemiological studies that have accounted for this have found that the association between birthweight and cardiovascular risk factors persisted across social groups, suggesting that known and unknown confounding variables do not affect this relationship.83
Comparing different standards of birthweight through analyses using different populations may not give a true result. However, no studies reported more than two standards in the same population, and only one study compared absolute birthweight and population centile charts, in which neither showed a significant association, limiting our ability to deal with this issue. Unfortunately, no meta-analysis was possible for certain birthweight standards or outcomes, for example, the ponderal index, or customised centile charts.
With regard to the outcomes examined, we recognise that our age categories were very broad and that the risks and severity of the conditions differ across the life course. However, due to the nature of the reporting in the primary studies it was not possible to examine this further with the data available. We did not restrict the outcomes included, but we found that some health outcomes, such as cancer, were poorly represented in the included studies. However, we are confident that our searches were robust and that nothing further could have been done to address this.
Interpretation
There is a vast literature exploring the relationship between low birthweight and adverse outcomes, using different methodologies to do so. Other systematic reviews performed in this field using birthweight as a continuous variable have shown mixed results. Owen et al.35 examined the association between birthweight and blood cholesterol level, and found a weak association; however, this analysis did not exclude pre-term infants. Huxley et al.2 found an inverse association between birthweight and systolic blood pressure in children, adolescents and adults, but again did not exclude pre-term infants from the analysis. Whincup et al. found mixed results in the relationship between type II diabetes mellitus and birthweight. Nine of 31 studies included in their systematic review showed a significant inverse relationship between birthweight and this outcome but again, prematurity was not excluded.84
The original literature published in support of the Barker hypothesis has been criticised for failing to control for potential confounding factors within their analysis, including prematurity.15 We have made every effort to consider these, and the findings with regard to childhood and adult health outcomes linked with the metabolic syndrome have been inconsistent. Where a composite outcome was used, no significant association with childhood or adult morbidity was seen. No significant association was present for childhood diabetes, hypertension or obesity. Weak associations were seen between birthweight and adult hypertension, diabetes and cardiovascular mortality, but the results are based on one or two studies.
While low birthweight is significantly associated with neonatal mortality and morbidity,20 the associations between all measures of low birthweight assessed and childhood and adult health outcomes were inconsistent. Where a significant association was present, no single measure of low birthweight appeared superior to the others examined to recommend their use, and for the two standards where sensitivity, specificity and likelihood ratios were calculated, the predictive value was low. This highlights that current birthweight standards are poor predictors of adverse childhood and adult outcomes. Considering childhood cerebral palsy as an example: the prevalence of this condition is 1–2.4 per 1000 in children born at or near term.85 Using the positive likelihood ratio 5.6 (for birthweight on customized chart <1st centile to predict this outcome), the odds of a baby with a birthweight under this centile developing cerebral palsy are 0.0024 × 5.6 = 0.013, that is, 1%. The negative likelihood ratio is 0.95, therefore being born above this centile does not significantly change the risk in comparison with the background prevalence.
Future research is necessary to identify a birthweight standard which can predict adverse health outcomes. First, it is important to compare the different standards across the same population to enable an unbiased comparison, and to further explore the standards which were less frequently reported. This could be performed through individual patient data meta-analysis, where multiple definitions of fetal growth restriction could be compared across the same population, and factors such as ethnicity more adequately assessed.86 Another option would be to perform further analysis on the large Scandinavian birth registries, which record a variety of birth anthropometry that can be linked to health outcomes.87 If a standard with high predictive ability is not identified, then birthweight in combination with other factors should be explored to predict adverse outcome in clinical practice. Future research in this field should consider and adequately report potential confounding factors, including prematurity. The importance of improving the quality of prognosis research has recently been highlighted.88
Conclusion
None of the current definitions of low birthweight has a good enough predictive ability for adverse outcome to recommend their superiority in clinical practice. Although the association between low birthweight and neonatal mortality is strong, the association between low birthweight and childhood and adult morbidity is inconsistent. Further research, as outlined above, is required to identify the optimum definition of low birthweight that can predict adverse outcomes.
Disclosure of interests
There are no competing interests to declare.
Contribution of authorship
GL Malin designed the review, carried out data extraction, analysis and interpretation of data and drafted the article, and is responsible for the integrity of the work as a whole. RK Morris carried out data extraction and interpretation of data, revised the article critically for intellectual content and approved the final draft for publication. RD Riley carried out statistical analysis and interpretation of the data, revised the article critically for intellectual content, and approved the final draft for publication. MJ Teune assisted with interpretation of the data, revised the article critically for intellectual content and approved the final draft for publication. KS Khan conceived the review. He also assisted with analysis and interpretation of data, revised the article critically for intellectual content, and approved the final draft for publication.
Funding
Dr Gemma Malin was funded by the Mary Crosse Fellowship, Birmingham Womens' Hospital. Dr Katie Morris is an NIHR Clinical Lecturer. The funding bodies had no role in the conduct or reporting of this review or the decision to submit the manuscript for publication.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Characteristics of studies included in systematic review of low birthweight standards and childhood and adult outcomes.
Figure S1. Study selection process for systematic review of the prognostic and predictive ability of current birthweight standards for short and long term outcomes.
Figure S2. Forest plot of odds ratios for the association between birthweight standards and childhood outcomes.
References
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–81. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–31. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 3.Leon DA, Lithell HO, Vagero D, Koupilova I, Mohsen R, Berglund L, et al. Reduced fetal growth rate and increased risk of death from ischaemic heart disease: cohort study of 15 000 Swedish men and women born 1915–29. BMJ. 1998;317:241–5. doi: 10.1136/bmj.317.7153.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martyn CN, Barker DJP, Osmond C. Mothers' pelvic size, fetal growth, and death from stroke and coronary heart disease in men in the UK. Lancet. 1996;348:1264–8. doi: 10.1016/s0140-6736(96)04257-2. [DOI] [PubMed] [Google Scholar]
- 5.Rich-Edwards J, Stampfer MJ, Manson J, Rosner B, Hankinson SE, Colditz GA, et al. Birthweight and risk of cardiovascular disease in a cohort of women followed up since 1976. BMJ. 1997;315:396. doi: 10.1136/bmj.315.7105.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law CM, Shiell AW. Is blood pressure inversely related to birthweight? The strength of evidence from a systematic review of the literature. J Hypertens. 1996;14:935–41. [PubMed] [Google Scholar]
- 7.Nobili VA. Low birthweight and catch-up-growth associated with metabolic syndrome: a ten year systematic review. Pediatr Endocrinol Rev. 2008;6:241–7. [PubMed] [Google Scholar]
- 8.Jelliffe-Pawlowski LLH. Neurodevelopmental outcome at 8 months and 4 years among infants born full-term small-for-gestational-age. J Perinatol. 2004;24:505–14. doi: 10.1038/sj.jp.7211111. [DOI] [PubMed] [Google Scholar]
- 9.Andersson SWL. Blood pressure and hypertension in middle-aged women in relation to weight and length at birth: a follow-up study. J Hypertens. 2000;18:1753–61. doi: 10.1097/00004872-200018120-00008. [DOI] [PubMed] [Google Scholar]
- 10.Da Silveira VMF, Horta BL. Birthweight and metabolic syndrome in adults: meta-analysis. Rev Saude Publica. 2008;42:10–8. doi: 10.1590/s0034-89102008000100002. [DOI] [PubMed] [Google Scholar]
- 11.Newsome CA, Shiell AW, Fall CH, Phillips DI, Shier R, Law CM, et al. Is birthweight related to later glucose and insulin metabolism? A systematic review. Diabet Med. 2003;20:339–48. doi: 10.1046/j.1464-5491.2003.00871.x. [DOI] [PubMed] [Google Scholar]
- 12.Catalano PM, Farrell K, Thomas A, Huston-Presley L, Mencin P, de Mouzon SH, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–13. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Libby G, McEwan SR, Morris AD, Belch JJF. No difference in the association between birthweight and total cholesterol for males and females. A SHARP (Scottish Heart and Arterial Disease Risk Prevention) study. Vasc Med. 2008;13:271–4. doi: 10.1177/1358863X08093465. [DOI] [PubMed] [Google Scholar]
- 14.Libby GM. Birthweight does not predict blood pressure in a young working population: a SHARP (Scottish Heart and Arterial Disease Risk Prevention) Study. Ann Epidemiol. 2008;18:298–301. doi: 10.1016/j.annepidem.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Wilson J. The Barker hypothesis – an analysis. Aust N Z J Obstet Gynaecol. 1999;39:1–7. doi: 10.1111/j.1479-828x.1999.tb03432.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferdynus C, Quantin C, Abrahamowicz M, Platt R, Burguet A, Sagot P, et al. Can birthweight standards based on healthy populations improve the identification of small-for-gestational-age newborns at risk of adverse neonatal outcomes? Pediatrics. 2009;123:723–30. doi: 10.1542/peds.2007-2564. [DOI] [PubMed] [Google Scholar]
- 17.Gardosi J. Intrauterine growth restriction: new standards for assessing adverse outcome. Best Pract Res Clin Obstet Gynaecol. 2009;23:741–9. doi: 10.1016/j.bpobgyn.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Walther FJ, Ramaekers LH. The ponderal index as a measure of the nutritional status at birth and its relation to some aspects of neonatal morbidity. J Perinat Med. 1982;10:42–7. doi: 10.1515/jpme.1982.10.1.42. [DOI] [PubMed] [Google Scholar]
- 19.Botero D, Lifshitz F. Intrauterine growth retardation and long-term effects on growth. Curr Opin Pediatr. 1999;11:340–7. doi: 10.1097/00008480-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Malin GL, Morris RK, Riley RD, Teune M, Khan KS. When is birthweight at term abnormally low? A systematic review and meta-analysis of the association and predictive ability of current birthweight standards for neonatal outcomes. BJOG. 2014;121:515–26. doi: 10.1111/1471-0528.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamb MM, Dabelea D, Yin X, Ogden LG, Klingensmith GJ, Rewers M, et al. Early-life predictors of higher body mass index in healthy children. Ann Nutr Metab. 2010;56:16–22. doi: 10.1159/000261899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Algert CS, McElduff A, Morris JM, Roberts CL. Perinatal risk factors for early onset of Type 1 diabetes in a 2000–2005 birth cohort. Diabet Med. 2009;26:1193–7. doi: 10.1111/j.1464-5491.2009.02878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amigo H, Bustos P, Alvarado ME, Barbieri M, Bettiol H, da Silva AAM, et al. Size at birth and lipoprotein concentrations in adulthood: two prospective studies in Latin American cities. J Epidemiol Community Health. 2010;64:855–9. doi: 10.1136/jech.2008.078345. [DOI] [PubMed] [Google Scholar]
- 24.Burke V, Beilin LJ, Blake KV, Doherty D, Kendall GE, Newnham JP, et al. Indicators of fetal growth do not independently predict blood pressure in 8-year-old Australians – a prospective cohort study. Hypertension. 2004;43:208–13. doi: 10.1161/01.HYP.0000113296.77924.28. [DOI] [PubMed] [Google Scholar]
- 25.Cornelius MD, Goldschmidt L, Willford JA, Leech SL, Larkby C, Day NL. Body Size and Intelligence in 6-year-olds: are offspring of teenage mothers at risk? Matern Child Health J. 2009;13:847–56. doi: 10.1007/s10995-008-0399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardner DS, Hosking J, Metcalf BS, Jeffery AN, Voss LD, Wilkin TJ. Contribution of early weight gain to childhood overweight and metabolic health: a longitudinal study (EarlyBird 36) Pediatrics. 2009;123:e67–73. doi: 10.1542/peds.2008-1292. [DOI] [PubMed] [Google Scholar]
- 27.Hands B, Kendall G, Larkin D, Parker H. Perinatal risk factors for mild motor disability. Intl J Disabil Dev Educ. 2009;56:317–31. [Google Scholar]
- 28.Hindmarsh PC, Bryan S, Geary MPP, Cole TJ. Effects of current size, postnatal growth, and birth size on blood pressure in early childhood. Pediatrics. 2010;126:e1507–13. doi: 10.1542/peds.2010-0358. [DOI] [PubMed] [Google Scholar]
- 29.Kindlund K, Thomsen SF, Stensballe LG, Skytthe A, Kyvik KO, Backer V, et al. Birthweight and risk of asthma in 3–9-year-old twins: exploring the fetal origins hypothesis. Thorax. 2010;65:146–9. doi: 10.1136/thx.2009.117101. [DOI] [PubMed] [Google Scholar]
- 30.Kuhle S, Allen AC, Veugelers PJ. Perinatal and childhood risk factors for overweight in a provincial sample of Canadian Grade 5 students. Int J Pediatr Obes. 2010;5:88–96. doi: 10.3109/17477160903060028. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Law C, Power C. Body mass index throughout the life-course and blood pressure in mid-adult life: a birth cohort study. J Hypertens. 2007;25:1215–23. doi: 10.1097/HJH.0b013e3280f3c01a. [DOI] [PubMed] [Google Scholar]
- 32.McKinney PA, Parslow R, Gurney KA, Law GR, Bodansky HJ, Williams R. Perinatal and neonatal determinants of childhood type 1 diabetes: a case-control study in Yorkshire, U.K. Diabetes Care. 1999;22:928–32. doi: 10.2337/diacare.22.6.928. [DOI] [PubMed] [Google Scholar]
- 33.Menezes AMB, Hallal PC, Horta BL, Araujo CLP, Vieira MD, Neutzling M, et al. Size at birth and blood pressure in early adolescence: a prospective birth cohort study. Am J Epidemiol. 2007;165:611–6. doi: 10.1093/aje/kwk031. [DOI] [PubMed] [Google Scholar]
- 34.Mi JC, Cheng H, Zhao X-Y, Hou D-Q, Chen F-F, Zhang K-L. Developmental origin of metabolic syndrome: interaction of thinness at birth and overweight during adult life in Chinese population. Obes Rev. 2008;9(Suppl 1):91–4. doi: 10.1111/j.1467-789X.2007.00446.x. [DOI] [PubMed] [Google Scholar]
- 35.Owen CG, Whincup PH, Odoki K, Gilg JA, Cook DG. Birthweight and blood cholesterol level: a study in adolescents and systematic review. Pediatrics. 2003;111:1081–9. doi: 10.1542/peds.111.5.1081. [DOI] [PubMed] [Google Scholar]
- 36.Pandolfi C, Zugaro A, Lattanzio F, Necozione S, Barbonetti A, Colangeli MS. Low birthweight and later development of insulin resistance and biochemincal/clinical features of polycystic ovarian syndrome. Metab, Clin Exp. 2008;57:999–1004. doi: 10.1016/j.metabol.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 37.Pearce MS, O'Sullivan JJ. Relationship between birthweight and blood pressure variability in children. J Hum Hypertens. 2003;17:677–80. doi: 10.1038/sj.jhh.1001595. [DOI] [PubMed] [Google Scholar]
- 38.Salonen MK. Tracking serum lipid levels and the association of cholesterol conceentrations, blood pressure and cigarette smoking with carotid artery intima-media thickness in young adults born small for gestational age. Circ J. 2010;74:2419–25. doi: 10.1253/circj.cj-10-0398. [DOI] [PubMed] [Google Scholar]
- 39.Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birthweight increases risk for end-stage renal disease. J Am Soc Nephrol. 2008;19:151–7. doi: 10.1681/ASN.2007020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berle JO, Mykletun A, Daltveit AK, Rasmussen S, Dahl AA. Outcomes in adulthood for children with foetal growth retardation. A linkage study from the Nord-Trondelag Health Study (HUNT) and the Medical Birth Registry of Norway. Acta Psychiatr Scand. 2006;113:501–9. doi: 10.1111/j.1600-0447.2005.00704.x. [DOI] [PubMed] [Google Scholar]
- 41.Bilge I, Poyrazoglu S, Bas F, Emre S, Sirin A, Gokalp S, et al. Ambulatory blood pressure monitoring and renal functions in term small-for-gestational age children. Pediatr Nephrol. 2011;26:119–26. doi: 10.1007/s00467-010-1646-3. [DOI] [PubMed] [Google Scholar]
- 42.Chaudhari S, Kulkarni S, Barve S, Pandit AN, Sonak U, Sarpotdar N, et al. Neurologic sequelae in high risk infants – a three year follow up. Indian Pediatr. 1996;33:645–53. [PubMed] [Google Scholar]
- 43.Evensen KA, Vik T, Helbostad J, Indredavik MS, Kulseng S, Brubakk AM, et al. Motor skills in adolescents with low birthweight. Arch Dis Child Fetal Neonatal Ed. 2004;89:F451–5. doi: 10.1136/adc.2003.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evensen KA, Lindqvist S, Indredavik MS, Skranes J, Brubakk AM, Vik T, et al. Do visual impairments affect risk of motor problems in preterm and term low birthweight adolescents? Eur J Paediatr Neurol. 2009;13:47–56. doi: 10.1016/j.ejpn.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Evensen KAI, Steinshamn S, Tjonna AE, Stolen T, Hoydal MA, Wisloff U, et al. Effects of preterm birth and fetal growth retardation on cardiovascular risk factors in young adulthood. Early Hum Dev. 2009;85:239–45. doi: 10.1016/j.earlhumdev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Fitzhardinge PM, Steven EM. The small-for-date infant. II. Neurological and intellectual sequelae II. Neurological and intellectual sequelae. Pediatrics. 1972;50:50–7. [PubMed] [Google Scholar]
- 47.Hemachandra AHK. The association between intrauterine growth restriction in the full-term infant and high blood pressure at age 7 years: results from the Collaborative Perinatal Project. Int J Epidemiol. 2006;35:871–7. doi: 10.1093/ije/dyl080. [DOI] [PubMed] [Google Scholar]
- 48.Indredavik MS, Vik T, Evensen KAI, Skranes J, Taraldsen G, Brubakk AM. perinatal risk and psychiatric outcome in adolescents born preterm with very low birthweight or term small for gestational age. J Dev Behav Pediatr. 2010;31:286–94. doi: 10.1097/DBP.0b013e3181d7b1d3. [DOI] [PubMed] [Google Scholar]
- 49.Jacobsson BA. Cerebral palsy and restricted growth status at birth: population-based case-control study. BJOG. 2008;115:1250–5. doi: 10.1111/j.1471-0528.2008.01827.x. [DOI] [PubMed] [Google Scholar]
- 50.Johnson CC. Nueroblastoma: case control analysis of birth characteristics. J Natl Cancer Inst. 1985;74:789–92. [PubMed] [Google Scholar]
- 51.Kajantie E, Osmond C, Barker DJP, Forsen T, Phillips DIW, Eriksson JG. Size at birth as a predictor of mortality in adulthood: a follow-up of 350 000 person-years. Int J Epidemiol. 2005;34:655–63. doi: 10.1093/ije/dyi048. [DOI] [PubMed] [Google Scholar]
- 52.Kotecha SJ, Watkins WJ, Heron J, Henderson J, Dunstan FD, Kotecha S. spirometric lung function in school-age children: effect of intrauterine growth retardation and catch-up growth. Am J Respir Crit Care Med. 2010;181:969–74. doi: 10.1164/rccm.200906-0897OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larroque B. School difficulties in 20 year olds who were born small for gestational age at term in a regional cohort study. Pediatrics. 2001;108:111–5. doi: 10.1542/peds.108.1.111. [DOI] [PubMed] [Google Scholar]
- 54.Levitt NSL. Impaired glucose tolerance and elevated blood pressure in low birth rate weight, nonobese, young South African adults: early programming of cortisol axis. J Clin Endocrinol Metab. 2000;85:4611–8. doi: 10.1210/jcem.85.12.7039. [DOI] [PubMed] [Google Scholar]
- 55.Liew G, Wang JJ, Klein R, Duncan BB, Yeh HC, Brancati FL, et al. Birthweight is not related to risk of diabetic retinopathy in type 2 diabetes: the atherosclerosis risk in communities study. Curr Eye Res. 2008;33:193–8. doi: 10.1080/02713680701855044. [DOI] [PubMed] [Google Scholar]
- 56.Low JA, Handley-Derry MH, Burke SO, Peters RD, Pater EA, Killen HL, et al. Association of intrauterine fetal growth retardation and learning deficits at age 9 to 11 years. Am J Obstet Gynecol. 1992;167:1499–505. doi: 10.1016/0002-9378(92)91727-r. [DOI] [PubMed] [Google Scholar]
- 57.Low JA, Galbraith RS, Muir D, Killen H, Pater B, Karchmar J, et al. Intrauterine growth retardation: a study of long-term morbidity. Am J Obstet Gynecol. 1982;142:670–7. doi: 10.1016/s0002-9378(16)32439-5. [DOI] [PubMed] [Google Scholar]
- 58.Lurbe E, Carvajal E, Torro I, Aguilar F, Alvarez J, Redon J. Influence of concurrent obesity and low birthweight on blood pressure phenotype in youth. Hypertension. 2009;53:912–7. doi: 10.1161/HYPERTENSIONAHA.109.129155. [DOI] [PubMed] [Google Scholar]
- 59.Meas T, Deghmoun S, Alberti C, Carreira E, Armoogum P, Chevenne D, et al. Independent effects of weight gain and fetal programming on metabolic complications in adults born small for gestational age. Diabetologia. 2010;53:907–13. doi: 10.1007/s00125-009-1650-y. [DOI] [PubMed] [Google Scholar]
- 60.Meas T, Deghmoun S, Armoogum P, Alberti C, Levy-Marchal C. Consequences of being born small for gestational age on body composition: an 8-year follow-up study. J Clin Endocrinol Metab. 2008;93:3804–9. doi: 10.1210/jc.2008-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nelson KB, Broman SH. Perinatal risk factors in children with serious motor and mental handicaps. Ann Neurol. 1977;2:371–7. doi: 10.1002/ana.410020505. [DOI] [PubMed] [Google Scholar]
- 62.Nobili VM, Marcelli M, Marchesini G, Vanni E, Manco M, Villani A, et al. Intrauterine growth retardation, insulin resistance, and nonalcoholic fatty liver disease in children. Diabetes Care. 2007;30:2638–40. doi: 10.2337/dc07-0281. [DOI] [PubMed] [Google Scholar]
- 63.O'Keeffe MJ, O'Callaghan M, Williams GM, Najman JM, Bor W, O'Keeffe MJ, et al. Learning, cognitive, and attentional problems in adolescents born small for gestational age. Pediatrics. 2003;112:301–7. doi: 10.1542/peds.112.2.301. [DOI] [PubMed] [Google Scholar]
- 64.Pathai S, Cumberland PM, Rahi JS. Prevalence of and early-life influences on childhood strabismus findings from the Millennium Cohort Study. Arch Pediatr Adolesc Med. 2010;164:250–7. doi: 10.1001/archpediatrics.2009.297. [DOI] [PubMed] [Google Scholar]
- 65.Paz I, Gale R, Laor A, Danon YL, Stevenson DK, Seidman DS. The cognitive outcome of full-term small for gestational age infants at late adolescence. Obstet Gynecol. 1995;85:452–6. doi: 10.1016/0029-7844(94)00430-l. [DOI] [PubMed] [Google Scholar]
- 66.Peng Y. Outcome of low birthweight in China: a 16 year longitudinal study. Acta Paediatr. 2005;94:843–9. doi: 10.1111/j.1651-2227.2005.tb01999.x. [DOI] [PubMed] [Google Scholar]
- 67.Plante LA. Small size at birth and later diabetic pregnancy. Obstet Gynecol. 1998;92:781–4. doi: 10.1016/s0029-7844(98)00302-0. [DOI] [PubMed] [Google Scholar]
- 68.Rahiala E, Tenhola S, Vanninen E. Ambulatory blood pressure in 12 year old children born small for gestational age. Hypertension. 2002;39:909–13. doi: 10.1161/01.hyp.0000013864.24138.a5. [DOI] [PubMed] [Google Scholar]
- 69.Sommerfelt KA, Andersson HW, Sonnander K, Ahlsten G, Ellersten B, Markestad T, et al. Behavior in term, small for gestational age preschoolers. Early Hum Dev. 2001;65:107–21. doi: 10.1016/s0378-3782(01)00200-6. [DOI] [PubMed] [Google Scholar]
- 70.Strauss RS. Adult functional outcome of those born small for gestational age: twenty-six year follow up of the 1970 British birth cohort. JAMA. 2000;283:625–32. doi: 10.1001/jama.283.5.625. [DOI] [PubMed] [Google Scholar]
- 71.Thompson C, Syddall H, Rodin I, Osmond C, Barker DJP. Birthweight and the risk of depressive disorder in late life. Br J Psychiatry. 2001;179:450–5. doi: 10.1192/bjp.179.5.450. [DOI] [PubMed] [Google Scholar]
- 72.Uvebrant P, Hagberg G. Intrauterine growth in children with cerebral palsy. Acta Paediatr. 1992;81:407–12. doi: 10.1111/j.1651-2227.1992.tb12259.x. [DOI] [PubMed] [Google Scholar]
- 73.Walther FJ, Ramaekers LH. Language development at the age of 3 years of infants malnourished in utero. Neuropediatrics. 1982;13:77–81. doi: 10.1055/s-2008-1059601. [DOI] [PubMed] [Google Scholar]
- 74.Wei JN, Li HY, Chang CH, Sung FC, Li CY, Lin CC, et al. Birthweight and type 1 diabetes among schoolchildren in Taiwan – a population-based case-controlled study. Diabetes Res Clin Pract. 2006;74:309–15. doi: 10.1016/j.diabres.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 75.Deeks JJ, Altman DJ. Diagnostic tests 4. Likelihood ratios. BMJ. 2004;329:168–9. doi: 10.1136/bmj.329.7458.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khan KS, Dinnes J, Kleijnen J. Systematic reviews to evaluate diagnostic tests. Eur J Obstet Gynecol Reprod Biol. 2001;95:6–11. doi: 10.1016/s0301-2115(00)00463-2. [DOI] [PubMed] [Google Scholar]
- 77.Stroup FD, Berlin JA, Morton SC, Olkin I, Williamson D, Drummond R, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 78.Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58:982–90. doi: 10.1016/j.jclinepi.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 79.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration; 2008. [Google Scholar]
- 80.Macaskill P, Gatsonis C, Deeks J, Harbord RM, Takwoingi Y. Analysing and presenting results. In: Deeks J, Bossuyt P, Gatsonis C, editors. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy, Version 1.0. London: The Cochrane Collaboration; 2010. [ http://srdta.cochrane.org/]. Accessed 20 October 2014. [Google Scholar]
- 81.NCC-WCH. Antenatal Care: Routine Care for the Healthy Pregnant Woman. 2nd edn. 2008. [ http://guidance.nice.org.uk/CG62/NICEGuidance/pdf/English]. Accessed 3 February 2014. [PubMed] [Google Scholar]
- 82.Gardosi J. Ethnic differences in fetal growth. Ultrasound Obstet Gynecol. 1995;6:73–4. doi: 10.1046/j.1469-0705.1995.06020073.x. [DOI] [PubMed] [Google Scholar]
- 83.Phillips DIW. Birthweight and adulthood disease and the controversies. Fetal Matern Med Rev. 2006;17:205–27. [Google Scholar]
- 84.Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa SK, et al. Birthweight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–97. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 85.Nelson KB. The epidemiology of cerebral palsy in term infants. Ment Retard Dev Disabil Res Rev. 2002;8:146–50. doi: 10.1002/mrdd.10037. [DOI] [PubMed] [Google Scholar]
- 86.Khan KS, Bachmann LM, ter Riet G. Systematic reviews with individual patient data meta-analysis to evaluate diagnostic tests. Eur J Obstet Gynecol Reprod Biol. 2003;108:121–5. doi: 10.1016/s0301-2115(03)00098-8. [DOI] [PubMed] [Google Scholar]
- 87.Knudsen LB, Olsen J. The Danish medical birth registry. Dan Med Bull. 1998;45:320–3. [PubMed] [Google Scholar]
- 88.Riley RD, Hayden JA, Steyerberg EW, Moons KGM, Abrams K, Kyzas PA, et al. Prognostic research strategy (PROGRESS) 2: prognostic factor research. PLoS Med. 2013;10:e1001380. doi: 10.1371/journal.pmed.1001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of studies included in systematic review of low birthweight standards and childhood and adult outcomes.
Figure S1. Study selection process for systematic review of the prognostic and predictive ability of current birthweight standards for short and long term outcomes.
Figure S2. Forest plot of odds ratios for the association between birthweight standards and childhood outcomes.


