Significance
Salmonella enterica and a number of phylogenetically distant intracellular bacterial pathogens require the MgtC virulence protein for both intraphagosomal replication and normal growth in magnesium-limited conditions. MgtC operates by interacting with and inhibiting the F1FO ATP synthase, reducing ATP levels within the bacterium. We show that by lowering ATP levels, MgtC prevents a rise in cyclic diguanylate, a second messenger that promotes biofilm formation in bacteria. We demonstrate that MgtC represses the biosynthesis of cellulose, a major structural component of Salmonella biofilms, and that cellulose interferes with replication inside macrophages and virulence in mice. Our results indicate that virulence genes can function to repress the expression of traits that interfere with virulence.
Keywords: biofilm, magnesium, ATP
Abstract
Cellulose is the most abundant organic polymer on Earth. In bacteria, cellulose confers protection against environmental insults and is a constituent of biofilms typically formed on abiotic surfaces. We report that, surprisingly, Salmonella enterica serovar Typhimurium makes cellulose when inside macrophages. We determine that preventing cellulose synthesis increases virulence, whereas stimulation of cellulose synthesis inside macrophages decreases virulence. An attenuated mutant lacking the mgtC gene exhibited increased cellulose levels due to increased expression of the cellulose synthase gene bcsA and of cyclic diguanylate, the allosteric activator of the BcsA protein. Inactivation of bcsA restored wild-type virulence to the Salmonella mgtC mutant, but not to other attenuated mutants displaying a wild-type phenotype regarding cellulose. Our findings indicate that a virulence determinant can promote pathogenicity by repressing a pathogen's antivirulence trait. Moreover, they suggest that controlling antivirulence traits increases long-term pathogen fitness by mediating a trade-off between acute virulence and transmission.
Bacterial pathogens encode genes that promote virulence. Virulence genes increase the fitness of pathogens by fostering replication at the expense of their hosts (1). Typically, virulence genes function by providing protection from host antimicrobial products, enabling the synthesis of nutrients that are limiting in host tissues and by manipulating host pathways in ways that favor pathogen survival at preferred sites. Notably, pathogens also may encode antivirulence genes, that is, genes that hamper pathogens' virulence (2–5). Here we provide a singular example of a virulence protein that promotes pathogenicity by interfering with the production of an antivirulence factor.
Cellulose is a polysaccharide composed of β(1→4)-linked d-glucose units. As a major structural component of the cell walls of plants and many eukaryotic microorganisms, cellulose accounts for ∼1.5 × 1012 tons of the annual biomass on Earth, making it the most abundant organic polymer on the planet (6). In bacteria, cellulose is an exopolysaccharide normally synthesized in the context of organized bacterial communities known as biofilms. Cellulose inhibits bacterial motility by hindering flagellar rotation (7), and provides cohesion and structural integrity to mature biofilms (8–10).
The facultative intracellular pathogen Salmonella enterica serovar Typhimurium causes gastroenteriditis in humans and a systemic infection in mice that resembles typhoid fever (11). During systemic infection, Salmonella survives and replicates in specialized membrane-bound mildly acidic vacuoles within host phagocytic cells (12, 13). Growth within these specialized compartments requires the coordinated expression of an array of virulence determinants (14), including the MgtC protein (15). MgtC is a unique virulence factor because it interacts with and inhibits the activity of Salmonella’s F1Fo ATP synthase (16), a protein complex that is responsible for synthesis of the majority of the ATP in the bacterium (17) and is also required for virulence (18). MgtC’s action prevents a nonphysiological increase in cytosolic ATP and decrease in cytosolic pH taking place during growth in mildly acidic environments, such as that experienced by Salmonella inside a macrophage phagosome (16).
In addition to its role in promoting intramacrophage survival, MgtC enables Salmonella (15, 19) and a number of phylogenetically distant intracellular bacterial pathogens (20–24) to grow normally in low-Mg2+ laboratory media. In Salmonella, growth in low-Mg2+ media also promotes mgtC expression, even when Salmonella experiences a neutral pH (15, 19). Notably, the mgtC mutant harbors higher ATP levels than the wild-type (WT) strain when grown in low-Mg2+ media, similar to what it exhibits on mild acidification of its surroundings (16). These findings suggest that a rise in ATP levels leads to physiological alterations that hinder growth in low-Mg2+ media and attenuated virulence.
We now report that, surprisingly, Salmonella produces cellulose when inside macrophages. We establish that the MgtC protein promotes Salmonella virulence by limiting cellulose production during infection. We determine that MgtC controls both expression of the cellulose synthase complex and the intracellular levels of cyclic diguanylate (c-di-GMP), the cellulose synthase’s allosteric activator. Virulence can be restored to the mgtC mutant simply by preventing cellulose biosynthesis, which does not affect ATP levels. Our findings illustrate how Salmonella uses a virulence protein to repress the expression of an antivirulence trait during infection of a mammalian host, and they define cellulose as an antivirulence determinant. Moreover, they suggest that pathogens use antivirulence traits to balance acute virulence and transmission.
Results
Growth in Low Mg2+ Requires MgtC to Reduce Intracellular ATP Levels.
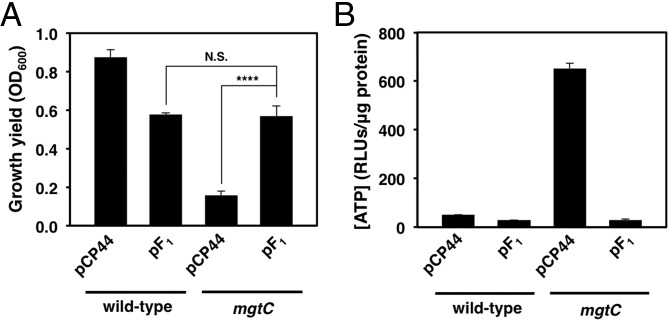
ATP exists as a Mg2+ salt in living cells (25). Therefore, when Mg2+ is limiting, bacteria may need to reduce their ATP levels. Given that the function of MgtC is to lower ATP levels (25), we reasoned that the growth defect of the mgtC mutant in low Mg2+ (15, 19) might be overcome by decreasing ATP levels in an MgtC-independent fashion. To test this notion, we examined the effect of a plasmid coexpressing the genes encoding the α, β, and γ components of the F1 subunit of the F1Fo ATP synthase, which is known to result in elevated ATPase activity (26). This plasmid restored growth of the mgtC mutant to the levels of the WT strain carrying the same plasmid (Fig. 1A). By contrast, the plasmid vector had no effect on the growth yield of the mgtC mutant (Fig. 1A), which retained elevated ATP levels (Fig. 1B). WT Salmonella carrying the plasmid with the F1 subunit genes displayed a lower growth yield than that carrying the plasmid vector (Fig. 1A), presumably because of increased ATP hydrolysis (26) (Fig. 1B). Taken together, these results indicate that Salmonella growth in low Mg2+ requires the MgtC protein to reduce the ATP concentration.
Fig. 1.
High ATP levels inhibit bacterial growth in low-Mg2+ media. (A) Growth yield of WT (14028s) and mgtC (EL4) Salmonella strains harboring a plasmid expressing the α, β, and γ components of the ATP synthase (pF1) or the plasmid vector (pCP44) after 24 h in low-Mg2+ liquid medium. Error bars represent SDs. ****P < 0.0001, not significant (N.S.), two-tailed t test. Results are representative of at least six independent experiments. (B) ATP levels of WT (14028s) and mgtC (EL4) Salmonella strains harboring pF1 or pCP44. Results are representative of at least six independent experiments.
MgtC Represses Cellulose Biosynthesis During Growth in Low Mg2+.
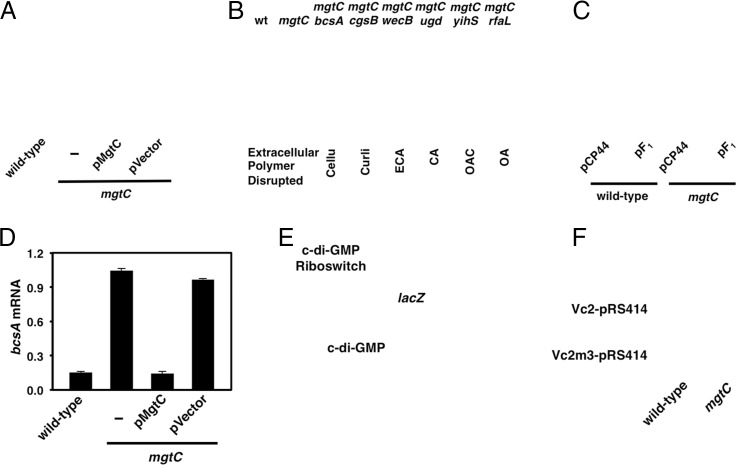
WT and mgtC Salmonella grew similarly in low-Mg2+ media during the first 4 h (Fig. S1A). However, starting at 6 h, the optical density of the WT culture increased for another 18 h, whereas that of the mgtC mutant decreased dramatically (15, 19) (Fig. S1A). The decrease in optical density of the mgtC mutant was accompanied by the formation of cellular aggregates at the liquid–air interface and at the bottom of the tube (24) (Fig. 2A). The formation of these aggregates is due to absence of MgtC protein because a plasmid expressing the mgtC gene, but not the vector control, reverted this phenotype (Fig. 2A). Given that the aggregates resembled structures deployed in Salmonella biofilms (8, 10), we wondered whether the MgtC protein curbs production of an extracellular structure(s) required for biofilm formation.
Fig. 2.
The MgtC protein represses cellulose production during growth in low-Mg2+ media. (A) Formation of cellular aggregates at the liquid–air interface in WT (14028s), mgtC (EL4), and mgtC Salmonella strains harboring the mgtC-expressing plasmid (pMgtC) or the plasmid vector (pUHE21-2-lacIq) after 24 h of growth in low-Mg2+ liquid medium. (B) Suppression analysis of the mgtC mutant (EL4) with mutations that disrupt the biosynthesis of cellulose (Cellu; MP142), curli (MP363), enterobacterial common antigen (ECA; MP118), colanic acid (CA; MP125), O-antigen capsule (OAC; MP120) or the O-antigen (OA; MP72). (C) A plasmid that expresses the F1 subunit genes (pF1), but not the plasmid vector (pCP44), suppresses the formation of cellular aggregates in the mgtC mutant (EL4), resulting in growth similar to that of the isogenic WT strain (14028s) in low-Mg2+ medium. (D) mRNA levels of the bcsA gene produced by WT (14028s), mgtC (EL4), and mgtC Salmonella strains harboring the mgtC-expressing plasmid or the plasmid vector after 8 h in low-Mg2+liquid medium. Error bars represent SDs. Values were normalized to those of the dnaK gene. (E) Schematic of reporter for c-di-GMP levels present in plasmid Vc2-pRS414 where the V. cholerae’s Vc2 riboswitch (which turns off expression of a downstream gene on c-di-GMP binding) to a promoterless lacZ gene from E. coli. (F) WT (14028s) and mgtC (EL4) Salmonella colonies harboring plasmid Vc2-pRS414 with the c-di-GMP–responding riboswitch (Upper) or a derivative with a mutant riboswitch that does not respond to c-di-GMP (Vc2m3-pRS414) (Lower) after a 48-h incubation at 37 °C on 2.5 µM Mg2+ N-minimal medium plates containing 5-bromo-4-chloro-3-indolyl-β-d- galactopyranoside (X-Gal; 80 µg/mL). The mgtC mutant harboring plasmid Vc2-pRS414 is of a lighter blue color (i.e., lower β-galactosidase) than the isogenic WT strain, reflecting the presence of higher c-di-GMP levels. By contrast, both WT and mgtC Salmonella form white colonies when harboring plasmid Vc2m3-pRS414 with a mutant riboswitch that is unresponsive to c-di-GMP. Results are representative of at least three independent experiments.
To identify the nature of the cellular aggregates produced by the mgtC mutant, we carried out a suppression analysis by inactivating genes involved in the biosynthesis of extracellular polymers that are implicated in biofilm formation or that have the potential to contribute to biofilm development (8, 10). Inactivation of the bcsA gene, which encodes a catalytic subunit of the cellulose synthase complex (27), suppressed aggregation (Fig. 2B) and partially rescued the growth yield of the mgtC mutant (Fig. S1B). By contrast, inactivation of the genes required for the biosynthesis of curli, enterobacterial common antigen, colanic acid, O-antigen capsule, or the O-antigen had no effect on the aggregation behavior (Fig. 2B) or growth yield (Fig. S1B) of the mgtC mutant grown in low-Mg2+ liquid medium.
That the aggregates formed by the mgtC mutant contained cellulose was further supported by three independent approaches. First, the cellulose-hydrolyzing enzyme cellulase dissolved the aggregates (Fig. S2A); second, the cellulose-binding dye calcofluor (28) stained the aggregates (Fig. S2B); and third, when grown on low-Mg2+ solid medium, the seemingly asymptomatic mgtC mutant (19) (Fig. S2C) accumulated calcofluor and the cellulose-binding dye Congo red (28) in a bcsA-dependent manner (Fig. S2D). These data indicate that the MgtC protein prevents cellulose biosynthesis and/or its surface deployment.
MgtC Represses Cellulose Biosynthesis by Lowering ATP levels.
The increased cellulose levels displayed by the mgtC mutant are a downstream effect of ATP accumulation because expression of the α, β, and γ components of the F1 subunit of the ATP synthase prevented cellulose production in the mgtC mutant (Fig. 2C). These results suggest that ATP alters the levels and/or activity of Salmonella’s cellulose synthase complex. As proposed, inactivation of the mgtC gene resulted in a sevenfold increase in the amount of bcsA mRNA (Fig. 2D), a phenotype that was corrected to WT by the plasmid expressing the mgtC gene, but not by the plasmid vector (Fig. 2D). The increase in bcsA mRNA is due to heightened cytosolic ATP because bcsA mRNA levels were lowered on introduction of the plasmid expressing the F1 subunit genes, but not the vector control, into the mgtC mutant (Fig. S3).
The bacterial cellulose synthase complex requires allosteric activation by the second messenger c-di-GMP (27, 29). To explore whether inactivation of the mgtC gene alters c-di-GMP levels, we used as a reporter a plasmid harboring the c-di-GMP–responding Vc2 riboswitch from Vibrio cholerae (30) fused to a promoterless lacZ gene from Escherichia coli. Because c-di-GMP binding to this riboswitch turns off lacZ expression, the higher the intracellular c-di-GMP concentration, the lower the β-galactosidase activity (30) (Fig. 2E). We determined that β-galactosidase activity was lower in the mgtC mutant harboring the reporter plasmid than in the isogenic WT strain (Fig. 2F), indicative of higher c-di-GMP levels in the mgtC mutant than in WT Salmonella. Control experiments demonstrated similar β-galactosidase activity in WT and mgtC Salmonella harboring a plasmid with a mutant riboswitch derivative unable to respond to c-di-GMP (30) (Fig. 2F). Taken together, these data indicate that the higher ATP concentration present in the mgtC mutant promotes cellulose production by enhancing the levels of active cellulose synthase complex.
MgtC Represses Cellulose Biosynthesis Within Phagocytic Cells.
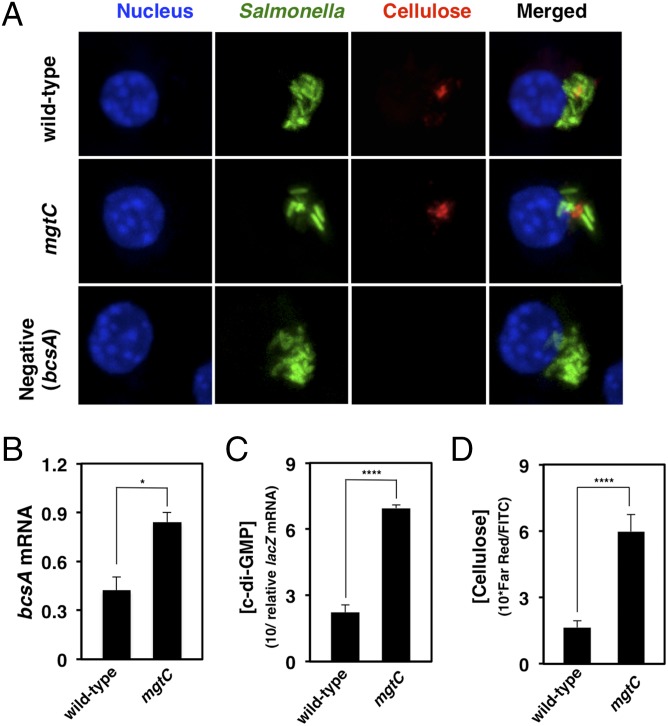
The mgtC gene is one of the most highly induced genes when Salmonella is inside a macrophage (14). We thus wondered whether Salmonella produces cellulose when inside a host cell and whether MgtC exerts a regulatory effect on cellulose production in this environment, similar to what was observed during growth in low-Mg2+ laboratory media (Fig. 2 and Fig. S2). To look specifically for cellulose inside host cells, we carried out fluorescent microscopy of macrophages infected with Salmonella following permeabilization and incubation with a recombinant peptide consisting of a cellulose-binding module fused to a 6-His tag (31) and an anti–6-His antibody conjugated to a fluorescent probe. We detected cellulose in macrophages containing WT and mgtC Salmonella but not in a mutant that cannot make cellulose due to inactivation of the bcsA gene (Fig. 3A). The mgtC mutant had higher levels of bcsA mRNA (Fig. 3B), c-di-GMP (Fig. 3C), and cellulose (Fig. 3D) compared with WT Salmonella during growth within macrophages. Taken together, these data indicate that Salmonella makes cellulose inside host cells, and that MgtC controls this process by dictating the amount of active cellulose synthase.
Fig. 3.
Salmonella produces cellulose inside macrophages, and the MgtC protein reduces the levels of active cellulose synthase inside macrophages. (A) Fluorescent microscopy images of J774A.1 macrophages at 9 h postinfection with WT (14028s), mgtC (EL4), or bcsA (MP140) Salmonella strains harboring a plasmid (pFPV25.1) that expresses GFP constitutively. Representative images depict signals derived from the nucleic acid stain DAPI (blue), GFP- expressing Salmonella strains (green), and cellulose–CBM3a peptide–anti-His Alexa Fluor 647 complex (red). (B) bcsA mRNA levels in WT (14028s) and mgtC (EL4) Salmonella strains harvested from macrophages at 9 h postinfection. (C) Relative c-di-GMP concentrations in WT (14028s) and mgtC (EL4) Salmonella strains harboring the plasmid reporter (Vc2-pRS414) harvested from macrophages at 9 h postinfection. The concentration of c-di-GMP is expressed as 10/lacZ mRNA level. (D) Cellulose concentration in WT (14028s) and mgtC (EL4) Salmonella strains in macrophages at 9 h postinfection. The concentration of cellulose is expressed as 10 ⋅ [Far Red signal]/[FITC signal]; n = 9. Error bars represent SDs. *P < 0.05, ****P < 0.0001, two-tailed t test. Results are representative of three independent experiments.
Cellulose Hinders Salmonella Replication Inside Macrophages.
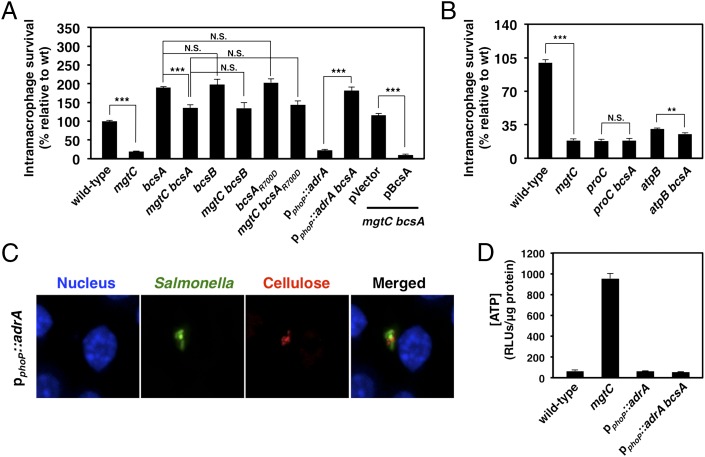
If the virulence attenuation of the mgtC mutant results from enhanced cellulose levels, then inactivation of the cellulose synthase bcsA gene should rescue the mgtC mutant. As predicted, deletion of the bcsA gene increased replication of the mgtC mutant inside the macrophage-like cell line J774A.1 (Fig. 4A). Complementation experiments demonstrated that a plasmid expressing the bcsA gene from a heterologous promoter recovered cellulose production (Fig. S2E) and suppressed intramacrophage survival in the mgtC bcsA double mutant (Fig. 4A), whereas the plasmid vector had no effect (Fig. 4A and Fig. S2E). The bcsA mutation appears to suppress the replication defect of the mgtC mutant specifically because mutations in proC and atpB, which do not promote the cellulose-mediated aggregation displayed by the mgtC mutant (Fig. S4) but hinder intramacrophage replication due to an inability to synthesize proline (32) and to carry out oxidative phosphorylation (18), respectively, were not corrected by the bcsA mutation (Fig. 4B).
Fig. 4.
Prevention of cellulose production restores intramacrophage replication to an mgtC null mutant. (A) Replication within J774A.1 macrophages of WT (14028s), mgtC (EL4), bcsA (MP140), bcsA mgtC (MP142), bcsB (MP654), bcsB mgtC (MP655), bcsAR700D (MP518), bcsAR700D mgtC (MP520), pphoP::adrA attTn7 (MP269), and pphoP::adrA attTn7 bcsA (MP291) Salmonella strains and of the bcsA mgtC (MP142) mutant harboring a plasmid expressing the bcsA gene (pBcsA) or the vector control (pUHE21-2lacIq) at 18 h postinfection. Error bars represent SDs. ***P < 0.001, ****P < 0.0001, two-tailed t test. Results are representative of at least four independent experiments. (B) Replication within J774A.1 macrophages of WT (14028s), mgtC (EL4), proC (EL605), proC bcsA (MP358), atpB (EL515), and atpB bcsA (MP715) at 18 h postinfection. Error bars represent SDs. ***P < 0.001, ****P < 0.0001, two-tailed t test. Results are representative of two independent experiments. (C) Cellulose biosynthesis by strain pphoP::adrA attTn7 (MP269) inside macrophages. (D) ATP levels of WT (14028s), mgtC (EL4), pphoP::adrA attTn7 (MP269), and bcsA pphoP::adrA attTn7 (MP291) Salmonella strains after 8 h of growth in low-Mg2+ medium. Results are representative of two independent experiments.
The enhanced virulence resulting from deletion of the bcsA gene is related to the inability to synthesize cellulose, as demonstrated by several findings. First, replacement of the WT copy of the bcsA gene by a variant specifying a BcsA protein with a single amino acid substitution in the c-di-GMP–binding site, which renders BcsA unable to synthesize cellulose (7), was sufficient to increase replication of the mgtC mutant inside macrophages (Fig. 4A). Second, deletion of the bcsB gene, which encodes the second catalytic subunit of the cellulose synthase complex (27), abolished cellulose production (Fig. S2F) and enhanced intramacrophage replication of the mgtC mutant (Fig. 4A). And third, the bcsA mutation did not restore the ATP concentration of the mgtC mutant to WT levels (Fig. S1C), and the same was true for mutations that prevented the biosynthesis of other extracellular polymers (Fig. S1C). These results demonstrate that excess cellulose, as opposed to the accumulation of ATP per se, is responsible for the attenuated virulence of the mgtC mutant.
To further examine the hypothesis that excess cellulose results in virulence attenuation, we investigated the behavior of an engineered strain with the macrophage-activated phoP promoter (33) fused to a promoterless adrA gene, which encodes a c-di-GMP synthase (34) (Fig. S5 A and B). The goal was to create a Salmonella strain displaying increased c-di-GMP levels and resulting elevated cellulose content, but normal ATP levels, inside macrophages. The engineered strain produced cellulose in response to the low Mg2+ signal activating the phoP promoter (Fig. S5C) in a bcsA-dependent manner (Fig. S5D) and inside macrophages (Fig. 4C). The strain was defective for replication inside macrophages (Fig. 4A), and this defect was overcome on deletion of the bcsA gene (Fig. 4A). Notably, the engineered strain displayed similar ATP levels (Fig. 4D) and was phagocytosed at similar rates (Fig. S5E) as its isogenic bcsA counterpart, indicating that its intramacrophage growth defect is related to excessive cellulose production as opposed to aberrant cytosolic ATP levels and/or the efficiency with which Salmonella is phagocytized by macrophages. Taken together, these results demonstrate that MgtC promotes intramacrophage replication by limiting cellulose biosynthesis.
Inactivation of Cellulose Biosynthesis Renders Salmonella Hypervirulent in Mice.
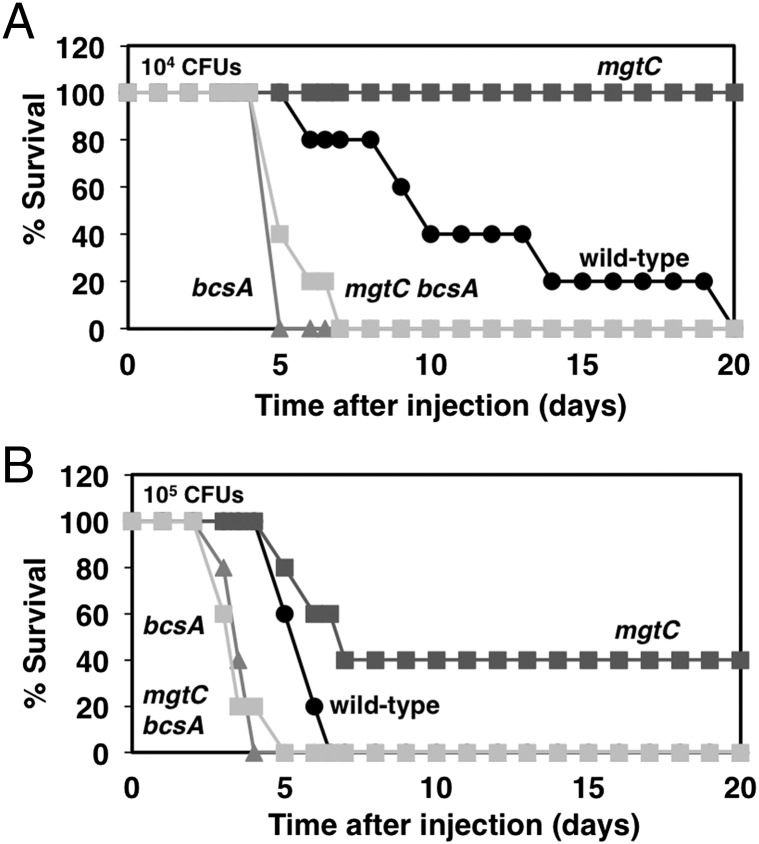
Deletion of the bcsA gene renders WT Salmonella hypervirulent. This is because (i) there were twice as many bcsA mutant bacteria as WT Salmonella inside macrophages at 18 h postinfection (Fig. 4A), and (ii) C3H/HeN mice inoculated intraperitoneally with 104 or 105 colony-forming units (CFU) of the bcsA mutant died within 5 d, whereas those that received WT Salmonella died within 20 d and 7 d, respectively (Fig. 5). The bcsA mgtC double mutant behaved like the bcsA single mutant: inoculation with 104 or 105 CFU of either strain resulted in death of the animals within 5–7 d postinoculation (Fig. 5). This behavior is in contrast to that exhibited by the mgtC mutant, which was highly attenuated at the same doses (Fig. 5). We conclude that WT Salmonella curtails its virulence potential by producing some cellulose during infection.
Fig. 5.
Inactivation of the cellulose synthase gene bcsA restores virulence to an mgtC null mutant and increases virulence of WT Salmonella. Shown is survival of C3H/HeN mice injected intraperitonally with WT (14028s), mgtC (EL4), bcsA (MP140), or bcsA mgtC (MP142) Salmonella strains at doses of 1 × 104 CFU (A) and 1 × 105 CFU (B). WT vs. mgtC, P < 0.01; WT vs. bcsA, P < 0.01; bcsA vs. mgtC bcsA, not significant (N.S.), Mantel–Cox test. Results are representative of two independent experiments.
Discussion
Bacterial cellulose has been traditionally associated with the formation of biofilms (8, 10, 29, 35, 36). Even though biofilm formation is antithetical to acute virulence (37), we have now established that Salmonella produces cellulose inside macrophages and that one of Salmonella’s virulence proteins prevents the synthesis of excessive levels of cellulose during infection (Fig. 3). By reducing the intracellular concentrations of ATP, the MgtC virulence protein prevents the onset of a c-di-GMP signaling network that activates cellulose biosynthesis. In spite of the pleiotropic effects resulting from increased ATP levels displayed by the attenuated mgtC mutant (16), inactivation of the cellulose synthase gene bcsA was sufficient to restore virulence to the mgtC mutant (Figs. 4 and 5) without correcting ATP levels (Fig. S1C). The idea that cellulose levels must be tightly controlled during infection is further supported by the attenuation displayed by a strain designed to overproduce cellulose inside macrophages without altering ATP levels (Fig. 4 A, C, and D).
The second messenger c-di-GMP is used by a large number of bacterial species to regulate a wide range of physiological processes, including the transition from a motile planktonic state to a sessile existence in biofilms (37). The repression of c-di-GMP signaling pathways is critical during the establishment of acute infections, when bacteria must often silence traits associated with biofilm formation and environmental persistence and display those that promote virulence (37). In agreement with this notion, artificial induction of c-di-GMP signaling via the biofilm regulator CsgD impairs Salmonella’s ability to invade epithelial cells during the initial stages of colonization of the mammalian host (38, 39). Our findings now challenge this paradigm by establishing that Salmonella engages in c-di-GMP signaling that feeds into cellulose biosynthesis when the bacterium is within host phagocytic cells, and that the MgtC protein regulates one of the outputs of this signaling system (i.e., cellulose production) by reducing ATP levels within the bacterium (Fig. 1B and Fig. 2C).
The findings that the MgtC protein decreases transcription of the cellulose synthase gene bcsA (Fig. 2D) and lowers c-di-GMP levels (Fig. 2F) help explain why inactivation of the phoP gene results in heightened biofilm formation through a pathway that requires the ability of cells to produce cellulose (40). This is because the PhoP protein, a major regulator of Salmonella virulence (41), activates mgtC transcription directly by binding to the mgtC promoter and recruiting RNA polymerase (42).
The bcsA mgtC double mutant replicated better than WT and mgtC Salmonella inside macrophages but slightly less than the bcsA mutant (Fig. 4A). Moreover, during growth in low-Mg2+ liquid medium, the bcsA mgtC double mutant grew better than the mgtC mutant but not as well as the WT and bcsA Salmonella (Fig. S6). Taken together, these results suggest that MgtC controls an additional cellular function(s) that contributes to intramacrophage replication and growth in low-Mg2+ media.
An increase in virulence is expected to maximize the short-term rate of pathogen reproduction within the host. However, our results demonstrate that Salmonella reduces its virulence by expressing cellulose within host tissues. Because pathogens increase their overall reproductive rate by increasing their transmission (1), expression of cellulose, and potentially of other antivirulence traits (2–5), might allow Salmonella to exploit host resources slowly, which could prolong infection and increase the chance of transmission to new hosts. This hypothesis is in agreement with evolutionary models predicting that pathogens evolve levels of virulence that maximize a trade-off between exploitation of host resources (short-term fitness) and transmission (long-term fitness) (1).
Our results identify two ways in which pathogens can achieve a successful infection. First, they provide a singular example of a virulence protein (i.e., MgtC) that, rather than targeting the host, operates within the bacterium to repress production of a factor (i.e., cellulose) that antagonizes acute virulence. Second, they demonstrate that the dichotomy between virulence and biofilm formation can be implemented by a virulence protein as part of the virulence program of a pathogen.
Materials and Methods
Bacterial Strains, Plasmid Constructs, Primers, and Growth Conditions.
The bacterial strains and plasmids used in this study are listed in Table S1, and oligonucleotide sequences are presented in Table S2. Single gene knockouts and deletions were carried out as described (43). Mutations generated via this method were subsequently moved into clean genetic backgrounds via phage P22-mediated transduction as described (44). Details of strain constructions are presented in SI Materials and Methods. Bacterial strains used in recombination and transduction experiments were grown in LB medium at 30 °C or 37 °C (43, 44). When required, the LB medium was supplemented with ampicillin (100 µg/mL), chloramphenicol (20 µg/mL), kanamycin (50 µg/mL), erythromycin (200 µg/mL), and/or l-arabinose (0.2% wt/vol). Growth under conditions of defined Mg2+ concentration was carried out in N-minimal medium (16). For strains containing mutations in F1Fo ATP synthase, 30 mM of d-glucose was used as the carbon source in N-minimal medium. When required, N-minimal medium was supplemented with ampicillin (25 µg/mL), erythromycin (200 µg/mL), isopropyl β-d-1-thiogalactopyranoside (IPTG) (250 µM), and either 10 mM (high) or 10 µM (low) MgCl2.
For physiological experiments, after overnight growth in high-MgCl2 liquid medium, cells were washed three times in Mg2+-free medium and inoculated (1:50) into low-MgCl2 liquid medium.
Estimation of ATP Levels from Bacterial Samples.
ATP measurements were carried out with heat-inactivated cells (70 °C for 20 min) after 8 h of growth in low-Mg2+ medium. Measurements were performed in a Synergy H1 Reader (BioTek) with a BacTiter-Glo Microbial Cell Viability Assay Kit (Promega) according to the manufacturers’ instructions. Protein concentration in cell samples were estimated from heat-lysed cells (98 °C for 10 min) using a BCA Protein Assay Kit (Pierce). ATP measurements were normalized by the protein content of the samples.
Macrophage Survival Assay.
Macrophage survival assays were conducted with the macrophage-like cell line J774A.1 as described previously (15), except that the macrophage medium was supplemented with IPTG (1 mM) when these cells were infected with Salmonella strains harboring plasmid pUHE-21–2lacIq or its derivatives.
Mouse Virulence Assays.
Mice virulence assays were carried out with 5- to 6-wk-old C3H/HeN female mice as described previously (5). Experiments were performed according to protocols approved by the Yale University Institutional Animal Care & Use Committee.
Supplementary Material
Acknowledgments
We thank Diego Serra for his suggestion to use the cellulose-binding protein, Ronald Breaker for the plasmids harboring the c-di-GMP riboswitches, Peter R. Jensen for the plasmids expressing the F1 subunit of the ATP synthase, and members of the Groisman laboratory for productive discussions. This work was supported in part by National Institutes of Health Grant AI49561 (to E.A.G.). E.A.G. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1500989112/-/DCSupplemental.
References
- 1.Frank SA. Models of parasite virulence. Q Rev Biol. 1996;71(1):37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- 2.Foreman-Wykert AK, Miller JF. Hypervirulence and pathogen fitness. Trends Microbiol. 2003;11(3):105–108. doi: 10.1016/s0966-842x(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 3.Parsons DA, Heffron F. sciS, an icmF homolog in Salmonella enterica serovar Typhimurium, limits intracellular replication and decreases virulence. Infect Immun. 2005;73(7):4338–4345. doi: 10.1128/IAI.73.7.4338-4345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mouslim C, Hilbert F, Huang H, Groisman EA. Conflicting needs for a Salmonella hypervirulence gene in host and non-host environments. Mol Microbiol. 2002;45(4):1019–1027. doi: 10.1046/j.1365-2958.2002.03070.x. [DOI] [PubMed] [Google Scholar]
- 5.Choi J, Groisman EA. The lipopolysaccharide modification regulator PmrA limits Salmonella virulence by repressing the type three-secretion system Spi/Ssa. Proc Natl Acad Sci USA. 2013;110(23):9499–9504. doi: 10.1073/pnas.1303420110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klemm D, Heublein B, Fink HP, Bohn A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew Chem Int Ed Engl. 2005;44(22):3358–3393. doi: 10.1002/anie.200460587. [DOI] [PubMed] [Google Scholar]
- 7.Zorraquino V, et al. Coordinated cyclic-di-GMP repression of Salmonella motility through YcgR and cellulose. J Bacteriol. 2013;195(3):417–428. doi: 10.1128/JB.01789-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solano C, et al. Genetic analysis of Salmonella enteritidis biofilm formation: critical role of cellulose. Mol Microbiol. 2002;43(3):793–808. doi: 10.1046/j.1365-2958.2002.02802.x. [DOI] [PubMed] [Google Scholar]
- 9.Serra DO, Richter AM, Hengge R. Cellulose as an architectural element in spatially structured Escherichia coli biofilms. J Bacteriol. 2013;195(24):5540–5554. doi: 10.1128/JB.00946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zogaj X, Nimtz M, Rohde M, Bokranz W, Römling U. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol. 2001;39(6):1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]
- 11.Coburn B, Grassl GA, Finlay BB. Salmonella, the host and disease: A brief review. Immunol Cell Biol. 2007;85(2):112–118. doi: 10.1038/sj.icb.7100007. [DOI] [PubMed] [Google Scholar]
- 12.Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchmeier NA, Heffron F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect Immun. 1991;59(7):2232–2238. doi: 10.1128/iai.59.7.2232-2238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksson S, Lucchini S, Thompson A, Rhen M, Hinton JC. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol Microbiol. 2003;47(1):103–118. doi: 10.1046/j.1365-2958.2003.03313.x. [DOI] [PubMed] [Google Scholar]
- 15.Blanc-Potard AB, Groisman EA. The Salmonella selC locus contains a pathogenicity island mediating intramacrophage survival. EMBO J. 1997;16(17):5376–5385. doi: 10.1093/emboj/16.17.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EJ, Pontes MH, Groisman EA. A bacterial virulence protein promotes pathogenicity by inhibiting the bacterium’s own F1Fo ATP synthase. Cell. 2013;154(1):146–156. doi: 10.1016/j.cell.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okuno D, Iino R, Noji H. Rotation and structure of FoF1-ATP synthase. J Biochem. 2011;149(6):655–664. doi: 10.1093/jb/mvr049. [DOI] [PubMed] [Google Scholar]
- 18.Turner AK, et al. Contribution of proton-translocating proteins to the virulence of Salmonella enterica serovars Typhimurium, Gallinarum, and Dublin in chickens and mice. Infect Immun. 2003;71(6):3392–3401. doi: 10.1128/IAI.71.6.3392-3401.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soncini FC, García Véscovi E, Solomon F, Groisman EA. Molecular basis of the magnesium-deprivation response in Salmonella typhimurium: identification of PhoP-regulated genes. J Bacteriol. 1996;178(17):5092–5099. doi: 10.1128/jb.178.17.5092-5099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchmeier N, et al. A parallel intraphagosomal survival strategy shared by Mycobacterium tuberculosis and Salmonella enterica. Mol Microbiol. 2000;35(6):1375–1382. doi: 10.1046/j.1365-2958.2000.01797.x. [DOI] [PubMed] [Google Scholar]
- 21.Grabenstein JP, Fukuto HS, Palmer LE, Bliska JB. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect Immun. 2006;74(7):3727–3741. doi: 10.1128/IAI.00255-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lavigne JP, O’callaghan D, Blanc-Potard AB. Requirement of MgtC for Brucella suis intramacrophage growth: A potential mechanism shared by Salmonella enterica and Mycobacterium tuberculosis for adaptation to a low-Mg2+ environment. Infect Immun. 2005;73(5):3160–3163. doi: 10.1128/IAI.73.5.3160-3163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maloney KE, Valvano MA. The mgtC gene of Burkholderia cenocepacia is required for growth under magnesium limitation conditions and intracellular survival in macrophages. Infect Immun. 2006;74(10):5477–5486. doi: 10.1128/IAI.00798-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rang C, et al. Dual role of the MgtC virulence factor in host and non-host environments. Mol Microbiol. 2007;63(2):605–622. doi: 10.1111/j.1365-2958.2006.05542.x. [DOI] [PubMed] [Google Scholar]
- 25.Storer AC, Cornish-Bowden A. Concentration of MgATP2- and other ions in solution: Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koebmann BJ, Westerhoff HV, Snoep JL, Nilsson D, Jensen PR. The glycolytic flux in Escherichia coli is controlled by the demand for ATP. J Bacteriol. 2002;184(14):3909–3916. doi: 10.1128/JB.184.14.3909-3916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Omadjela O, et al. BcsA and BcsB form the catalytically active core of bacterial cellulose synthase sufficient for in vitro cellulose synthesis. Proc Natl Acad Sci USA. 2013;110(44):17856–17861. doi: 10.1073/pnas.1314063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown RM, et al. The biosynthesis and degradation of cellulose. J Appl Polym Sci. 1983;37:33–78. [Google Scholar]
- 29.Ross P, et al. Regulation of cellulose synthesis in Acetobacter xylinum by cyclic diguanylic acid. Nature. 1987;325(6101):279–281. doi: 10.1038/325279a0. [DOI] [PubMed] [Google Scholar]
- 30.Sudarsan N, et al. Riboswitches in eubacteria sense the second messenger cyclic di-GMP. Science. 2008;321(5887):411–413. doi: 10.1126/science.1159519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blake AW, et al. Understanding the biological rationale for the diversity of cellulose-directed carbohydrate-binding modules in prokaryotic enzymes. J Biol Chem. 2006;281(39):29321–29329. doi: 10.1074/jbc.M605903200. [DOI] [PubMed] [Google Scholar]
- 32.Lee EJ, Choi J, Groisman EA. Control of a Salmonella virulence operon by proline-charged tRNA(Pro) Proc Natl Acad Sci USA. 2014;111(8):3140–3145. doi: 10.1073/pnas.1316209111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heithoff DM, et al. Coordinate intracellular expression of Salmonella genes induced during infection. J Bacteriol. 1999;181(3):799–807. doi: 10.1128/jb.181.3.799-807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simm R, Morr M, Kader A, Nimtz M, Römling U. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Mol Microbiol. 2004;53(4):1123–1134. doi: 10.1111/j.1365-2958.2004.04206.x. [DOI] [PubMed] [Google Scholar]
- 35.Matthysse AG, et al. The effect of cellulose overproduction on binding and biofilm formation on roots by Agrobacterium tumefaciens. Mol Plant Microbe Interact. 2005;18(9):1002–1010. doi: 10.1094/MPMI-18-1002. [DOI] [PubMed] [Google Scholar]
- 36.Bassis CM, Visick KL. The cyclic-di-GMP phosphodiesterase BinA negatively regulates cellulose-containing biofilms in Vibrio fischeri. J Bacteriol. 2010;192(5):1269–1278. doi: 10.1128/JB.01048-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamayo R, Pratt JT, Camilli A. Roles of cyclic diguanylate in the regulation of bacterial pathogenesis. Annu Rev Microbiol. 2007;61:131–148. doi: 10.1146/annurev.micro.61.080706.093426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamprokostopoulou A, Monteiro C, Rhen M, Römling U. Cyclic di-GMP signalling controls virulence properties of Salmonella enterica serovar Typhimurium at the mucosal lining. Environ Microbiol. 2010;12(1):40–53. doi: 10.1111/j.1462-2920.2009.02032.x. [DOI] [PubMed] [Google Scholar]
- 39.Ahmad I, et al. Complex c-di-GMP signaling networks mediate transition between virulence properties and biofilm formation in Salmonella enterica serovar Typhimurium. PLoS ONE. 2011;6(12):e28351. doi: 10.1371/journal.pone.0028351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prouty AM, Gunn JS. Comparative analysis of Salmonella enterica serovar Typhimurium biofilm formation on gallstones and on glass. Infect Immun. 2003;71(12):7154–7158. doi: 10.1128/IAI.71.12.7154-7158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol. 2001;183(6):1835–1842. doi: 10.1128/JB.183.6.1835-1842.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zwir I, Latifi T, Perez JC, Huang H, Groisman EA. The promoter architectural landscape of the Salmonella PhoP regulon. Mol Microbiol. 2012;84(3):463–485. doi: 10.1111/j.1365-2958.2012.08036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97(12):6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis RW, Bolstein D, Roth JR. Advanced Bacterial Genetics. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 1980. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.