Abstract
A congenital myasthenic syndrome caused by endplate acetylcholinesterase deficiency is a rare autosomal recessive disease. We report a child who had early onset ptosis, complete ophthalmoplegia, facial and proximal muscle weakness, easy fatigability, a decremental electromyographic response, and a repetitive compound muscle action potential not improved by anti-acetylcholinesterase medication. Mutation analysis of collagenic tail of endplate acetylcholinesterase (COLQ) that encodes the collagenic structural subunit of acetylcholinesterase revealed two canonical splice-site mutations: a previously identified IVS15+1G > A mutation and a novel IVS2-1G > A mutation. Treatment with albuterol resulted in progressive improvement of muscle strength, exercise tolerance, and of the ophthalmoplegia. Further clinical studies are needed on the efficacy of albuterol in different types of congenital myasthenic syndrome and on the physiologic basis of its beneficial effects.
Keywords: Congenital myasthenic syndrome, endplate acetylcholinesterase deficiency, COLQ mutation, Albuterol
Introduction
Congenital myasthenia syndromes are a group of heterogeneous disorder caused by defects in proteins residing in the presynaptic, synaptic basal lamina, or postsynaptic regions of the motor endplate. The clinical, electromyographic and pathologic features of acetylcholinesterase deficiency were first described in 1977 in a patient presenting in the neonatal period with fatigable ptosis, generalized weakness increased by exertion, hyporeflexia, and refractoriness to anti-acetylcholinesterase drugs. Repetitive stimulation of motor nerves revealed a decremental response and single nerve stimuli evoked a repetitive compound muscle action potential. Acetylcholinesterase was absent from the motor end-plates by histochemical and electron cytochemical criteria, and density gradient centrifugation studies of muscle extracts pointed to ColQ, the collagenic tail subunit of Acetylcholinesterase, as the disease protein [1]. In 1998, COLQ was mapped to chromosome 3p25 and the basis of endplate acetylcholinesterase deficiency was traced to mutations in COLQ [2, 3].
The endplate species of acetylcholinesterase is an asymmetric enzyme composed of globular forms of type T catalytic subunits (ACHET) encoded by ACHET , and a collagenic ColQ protein encoded by COLQ . The ColQ protein anchors the complex in the synaptic basal lamina and is composed of three identical strands. The N-terminal proline-rich domain of each strand binds a catalytic homotetramer. The collagenic central domain of ColQ confers rigidity on the molecule and binds the enzyme to the synaptic basal lamina by electrostatic bonds; the C-terminal globular domain is required for assembly of the triple-helical rod domain and contributes to binding the enzyme to the synaptic basal lamina by covalent bonds (reviewed in [2, 4]).
Case report
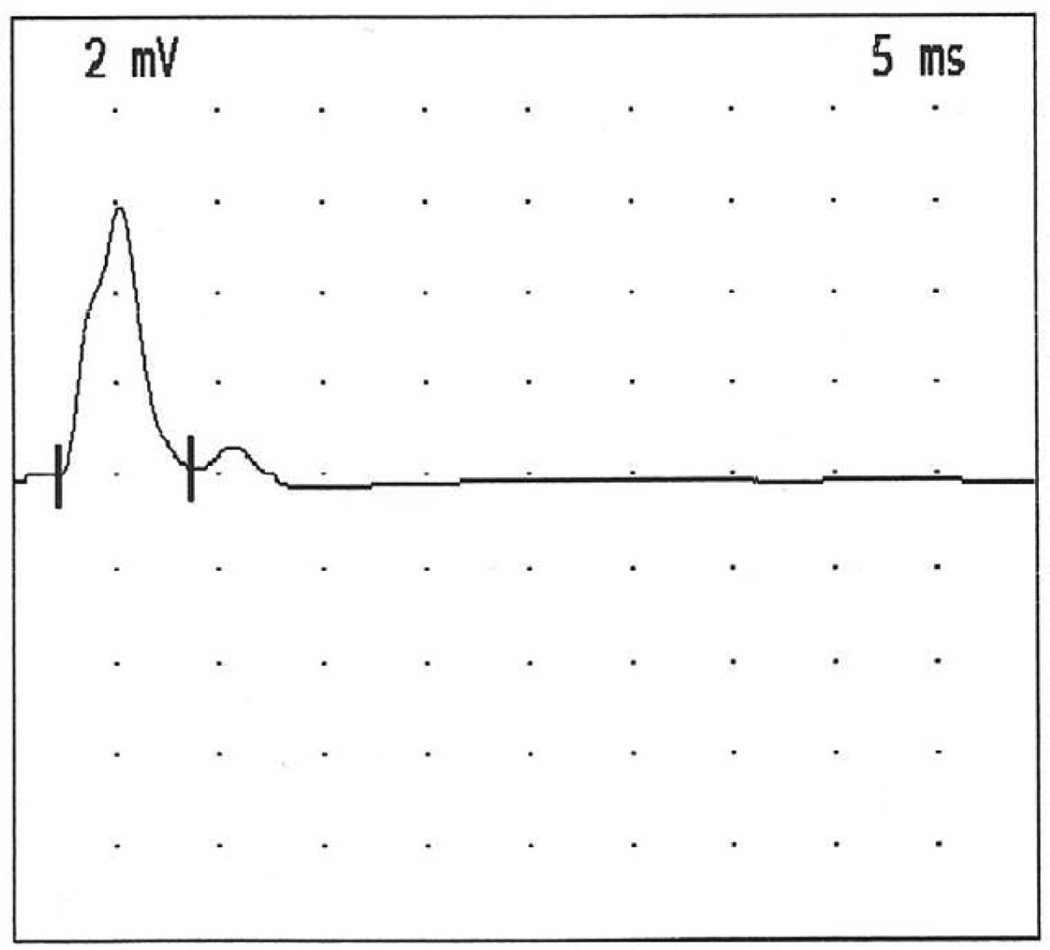
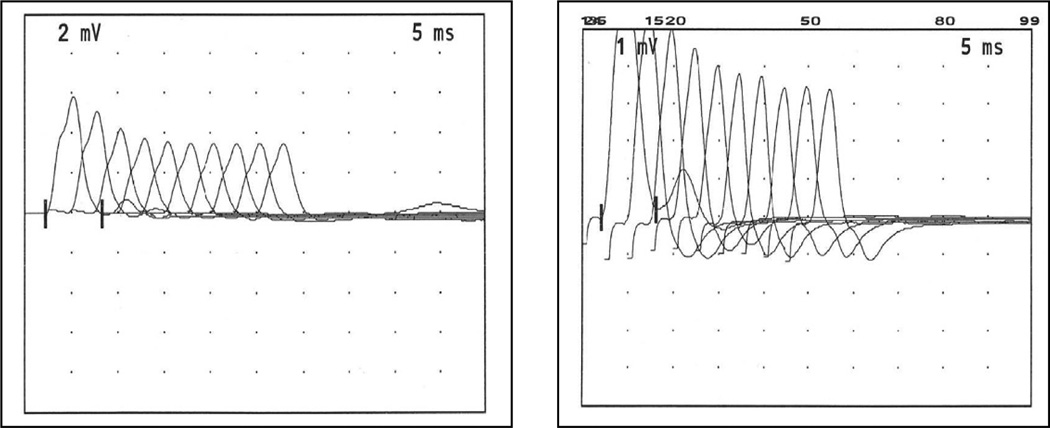
The patient, now 8 years of age, is the only child of a Philippine mother and a Chinese father. He was born at full term after an uneventful pregnancy and had a normal delivery. The mother had a seizure at age 4 years; the father enjoyed good health. The patient had a generalized seizure on day 7 and again at age 2 months. In the first year of life, he was mildly hypotonic, had progressive bilateral ptosis and progressive limitation of his eye movements in both horizontal and vertical direction, but had no feeding or respiratory problems. Investigations included metabolic workup, spinal fluid studies, EEG, brain MRI, brainstem auditory and visual evoked potential and nerve conduction studies without repetitive stimulation gave normal results. He stood up at age 18 months, walked independently at age 2 years, and fatigued easily with these antigravity activities on mild exertion. At age 5 years, he had persistent bilateral ptosis, complete ophthalmoplegia, mild facial weakness, and Medical Research Council grade 4/5 weakness and abnormal fatigability of the proximal limb muscles. When tired he refused to walk, and could not be tested for the Gowers sign. The tendon reflexes were normal. The serum creatine kinase level was normal and tests for anti-acetylcholine receptor antibodies were negative. Edrophonium tests were repeatedly negative. Single stimuli applied to the median, ulnar, tibial and peroneal nerves evoked a repetitive compound muscle action potential (Figure 1) and stimulation at 3 and 20 Hz elicited a marked decremental response (Figure 2). Repeated edrophonium tests revealed no effect on the decremental repetitive motor nerve stimulation response. Needle electromyographic study of the left biceps muscle was interpreted as normal.
Figure 1.
Repetitive CMAP evoked from left abductor digiti minimi by a single supramaximal stimulus applied to the left ulnar nerve.
Figure 2.
Decremental EMG responses on stimulating the left ulnar nerve and recording from left abductor digiti minimi muscle. Three Hz simulation elicits a 41% decrement (left panel) and 20 Hz stimulation elicits a 59% decrement (right panel) of the amplitude of the fourth CMAP compared to that of the first CMAP.
The negative edrophonium test and the repetitive compound muscle action potentials pointed to COLQ as the disease gene. Mutation analysis of COLQ by Sanger sequencing revealed two splice-site mutations: a previously identified IVS15+1G > A mutation that predicts skipping of exon 15 and production of a stop codon after 64 missense codons [4], and a novel IVS2-1G > A in the intron of the exon coding for the N-terminal proline-rich domain that predicts skipping of 13 nucleotides followed by a threonine missense codon and a stop codon [5]. The father is heterozygous for IVS15+1G > A and the mother is heterozygous for IVS2-1G > A.
Albuterol therapy was initiated at 1 mg three times daily at age of 6 years and 1 month and was increased to 2 mg three times daily after 3 weeks. The patient’s response was monitored by clinical examination, the Quantitative Myasthenia Gravis tests [6] and by monitoring his ptosis and ocular ductions. During the next 36 months, the Quantitative Myasthenic Gravis score improved continuously (Please see table) as did the patient’s strength and exercise tolerance. Importantly, the patient also regained partial ability to move his eyes horizontally and downward but the bilateral ptosis did not improve, and repeated repetitive motor nerve stimulation studies revealed no improvement of the decremental response. Albuterol therapy had no major side effects. When not taking the medication during a holiday, the patient complained of increased weakness and fatigability.
Table.
Serial monitoring using Quantitative Myasthema Gravis (QMG) test. Performance at pre-salbutamol was compared to 4 months, 16 months, 29 months, 40 months post salbutamol, showing definite improvement post treatment and also progressive improvement over 3 years of treatment.
|
TEST ITEMS WEAKNESS |
NONE | MILD | MODERATE | SEVERE |
DATE 17-4-08 Pre- albuterol |
DATE 17-11-08 4 months albuterol |
DATE 13-11-09 16 months albuterol |
DATE 23-12-10 29 months albuterol |
DATE 11-11-11 40 months albuterol |
|---|---|---|---|---|---|---|---|---|---|
| GRADE | 0 | 1 | 2 | 3 | SCORE | SCORE | SCORE | SCORE | SCORE |
| Double vision (lateral gaze) Sec. |
60 | 11–59 | 1–10 | Spontaneous | 2 (10s) | 0 | 0 | 0 | 0 |
| Ptosis (upward gaze) Sec. | 60 | 11–59 | 1–10 | Spontaneous | 1 (15s) | 1 (40s) | 1 (32s) | 1 (60s) | 1 (60s) |
| Facial Muscles | Normal lid closure | Complete, weak, some resistance | Complete, without resistance | Incomplete | 1 | 0 | 0 | 0 | 0 |
| Swallowing 4 oz. Water (1/2 cup) |
Normal | Minimal coughing or throat clearing | Severe coughing, choking or nasal regurgitation | Cannot swallow (test not attempted) | 0 | 0 | 0 | 0 | 0 |
| Speech following counting aloud from 1–50 (onset of dysarthria) |
None at 50 | Dysarthria at 30–49 | Dysarthria at 10–29 | Dysarthria at 9 | 0 | 0 | 0 | 0 | 0 |
| Right arm outstretched (90°, sitting) Sec. |
240 | 90–239 | 10–89 | 0–9 | 3 (5s) | 2 (17s) | 2 (50s) | 2 (75s) | 1 (128s) |
| Left arm outstretched (90°, sitting) Sec. |
240 | 90–239 | 10–89 | 0–9 | 3 (5s) | 2 (17s) | 2 (50s) | 1 (95s) | 1 (128s) |
| Forced vital capacity | ≧80% | 65–79% | 50–64% | <50% | 0 | 0 | 0 | 0 | 0 |
| Rt hand grip: male (Kg): female |
≧45 ≧30 |
15–44 10–29 |
5–14 5–9 |
0–4 0–4 |
3 (1–2kg) | 3 (3kg) | 2 (8 kg) | 2 (11 kg) | 2 (7 kg) |
| Left hand grip: male (Kg): female |
≧35 ≧25 |
15–34 10–24 |
5–14 5–9 |
0–4 0–4 |
3 (1–2kg) | 3 (2.5 kg) | 2 (7 kg) | 2 (9 kg) | 2 (8 kg) |
| Head, lifted (45%, supine) Sec. |
120 | 30–119 | 1–29 | 0 | 3 (0s) | 2 (5s) | 2 (7s) | 2 (15s) | 1 (41s) |
| Right leg outstretched (45–50%, supine) Sec. |
100 | 31–99 | 1–30 | 0 | 2 (13s) | 2 (20s) | 2 (27s) | 1 (60s) | 1 (60s) |
| Left leg outstretched (45–50%, supine) Sec. |
100 | 31–99 | 1–30 | 0 | 2 (13s) | 2 (19s) | 2 (27s) | 1 (60s) | 1 (58s) |
| Total MG Score | 23 | 17 | 15 | 12 | 10 |
Thirty months after the start of therapy, the dose of Albuterol was increased to 2.5 mg three times daily. On this regimen, the patient is now able to run around when playing and enjoys full participation with his peers in daily school activities. He has normal intelligence and performs well in school.
Discussion
Since 1998, more than 30 different COLQ mutations have been identified. The mutations appear in all three domains of COLQ and include missense, nonsense, splice-site, frameshift, and in-frame deletions, with most producing a truncated and insertion incompetent ColQ protein. In a series of 22 patients, 11 had a slowly or subacutely progressive course [7]. As more patients harboring COLQ mutations were identified, the clinical spectrum of the ColQ-associated congenital myasthenic syndrome became wider [7–11]. Our patient who carries the two splice-site mutation that result in a truncated transcript became symptomatic during the first year of life with ptosis, progressive ophthalmoplegia, hypotonia, weakness of the facial, cervical and proximal limb muscles and poor exercise tolerance, but had no respiratory or feeding problems. Other patients harboring mutations in the C-terminal domain of the ColQ protein have had a milder course [3, 12]. Albuterol and ephedrine are both adrenergic agonist used for treatment of acute asthma for relieving bronchospasm. Albuterol is a β2-adrenergic agonist, while ephedrine acts on both α-and β-adrenergic receptors. The congenital myasthenic syndrome caused by defects in DOK7 [13] and in COLQ have benefited from ephedrine but the reported number of patients is small [7, 12]. Recently, albuterol was also found to be beneficial in 15 patients with DOK7 myasthenia, in 3 patients with ColQ deficiency [14], and in two patients with low-expressor mutations in the acetylcholine receptor episilon subunit [15]. Most observed responses reflected increased muscle strength and endurance, but the weakness of the external ocular muscles, when present, did not improve. Patients exposed to ephedrine or albuterol often experience adrenergic side effects but our patient had no tremor, nervousness, hypertension, palpitation or arrhythmia during 3 years of therapy. Further studies on a larger number of congenital myasthenic syndrome patients are required to determine the types of congenital myasthenic syndrome benefited by albuterol, and more basic science studies are needed to elucidate the physiologic basis of the beneficial response.
Acknowledgements
The work done by Andrew G. Engel M.D. was supported by NIH Grants NS6277 and by a Grant from the Muscular Dystrophy Association. We thank Mr. Anderson Tam optometrist, for help monitoring the patient’s ocular ductions, Ms. Susanna Choi, Msc and Ms. Connie CK Hui, physiotherapists, for performing the Quantitative Myasthenia Gravis tests before and during albuterol therapy.
Abbreviations
- ColQ
collagenic tail of endplate acetylcholinesterase
- COLQ
gene encoding ColQ
- DOK7
gene encoding docking protein 7
- EEG
electroencephalogram
- MRI
magnetic resonance imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engel AG, Lambert EH, Gomez MR. A new myasthenic syndrome with end-plate acetylcholinesterase deficiency, small nerve terminals, and reduced acetylcholine release. Ann Neurol. 1977;1:315–330. doi: 10.1002/ana.410010403. [DOI] [PubMed] [Google Scholar]
- 2.Ohno K, Brengman JM, Tsujino A, Engel AG. Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc Natl Acad Sci USA. 1998;95:9654–9659. doi: 10.1073/pnas.95.16.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donger C, Krejci E, Serradell AP, Eymard B, Bon S, Nicole S, Chateau D, Gary F, Fardeau M, Massoulié J, Guicheney P. Mutation in the human acetylcholinesterase-associated gene, COLQ, is responsible for congenital myasthenic syndrome with endplate acetylcholinesterase deficiency. Am J Hum Genet. 1998;63:967–975. doi: 10.1086/302059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohno K, Engel AG, Brengman JM, Shen XM, Heidenreich F, Vincent A, Milone M, Tan E, Demirci M, Walsh P, Nakano S, Akiguchi I. The spectrum of mutations causing endplate acetylcholinesterase deficiency. Ann Neurol. 2000;47:162–170. [PubMed] [Google Scholar]
- 5.Wang M, Marin A. Characterization and prediction of alternative splice sites. Gene. 2006;366:219–227. doi: 10.1016/j.gene.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Jaretzki A, Barohn RJ, Ernstoff RM, Kaminski HJ, Keesey JC, Penn AS, Sanders DB. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55:16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- 7.Mihaylova V, Muller JS, Vilchez JJ, Salih MA, Kabiraj MM, D'Amico A, Bertini E, Wölfle Schreiner F, Kurlemann G, Rasic VM, Siskova D, Colomer J, Herczegfalvi A, Fabriciova K, Weschke B, Scola R, Hoellen F, Schara U, Abicht A, Lochmüller H. Clinical and molecular genetic findings in COLQ-mutant congenital myasthenic syndromes. Brain. 2008;131:747–759. doi: 10.1093/brain/awm325. [DOI] [PubMed] [Google Scholar]
- 8.Shapira YA, Sadeh ME, Bergtraum MP, Tsujino A, Ohno K, Shen XM, Brengman BS, Edwardson S, Matoth I. Engel AG Three novel COLQ mutations and variation of phenotypic expressivity due to G240X. Neurology. 2002;58:603–609. doi: 10.1212/wnl.58.4.603. [DOI] [PubMed] [Google Scholar]
- 9.Ishigaki K, Nicolle D, Krejci E, Leroy JP, Koenig J, Fardeau M, Eymard B, Hantaï D. Two novel mutations in the COLQ gene causing endplate acetylcholinesterase deficiency. Neuromuscul Disord. 2003;13:236–244. doi: 10.1016/s0960-8966(02)00243-2. [DOI] [PubMed] [Google Scholar]
- 10.Müller JS, Petrova S, Kiefer R, Stucka R, König C, Baumeister SK, Huebner A, Lochmüller H, Abicht A. Synaptic congenital myasthenic syndrome in three patients due to a novel missense mutation (T441A) of the COLQ gene. Neuropediatrics. 2004;35:183–189. doi: 10.1055/s-2004-820996. [DOI] [PubMed] [Google Scholar]
- 11.Schreiner F, Hoppenz M, Klaeren R, Reimann J, Woelfle J. Novel COLQ mutation 950delC in synaptic congenital myasthenic syndrome and symptomatic heterozygous relatives. Neuromuscul Disord. 2007 Mar;17:262–265. doi: 10.1016/j.nmd.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Bestue-Cardiel M, de-Cabazon-Alvarez AS, Capablo-Liesa JL, López-Pisón J, Peña-Segura JL, Martin-Martinez J, Engel AG. Congenital endplate acetylcholinesterase deficiency responsive to ephedrine. Neurology. 2005;65:144–146. doi: 10.1212/01.wnl.0000167132.35865.31. [DOI] [PubMed] [Google Scholar]
- 13.Lashley D, Palace J, Jayawant S, Robb S, Beeson D. Ephedrine treatment in congenital myasthenic syndrome due to mutations in DOK7. Neurology. 2010;74:1517–1523. doi: 10.1212/WNL.0b013e3181dd43bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liewluck T, Selcen D, Engel AG. Beneficial effects of albuterol in congenital endplate acetylcholinesterase deficiency and DOK-7 myasthenia. Muscle Nerve. 2011;44:789–794. doi: 10.1002/mus.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadeh M, Shen X-M, Engel AG. Beneficial effect of albuterol in congenital myasthenic syndrome with ε subunit mutations. Muscle Nerve. 2011;44:289–291. doi: 10.1002/mus.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]