Abstract
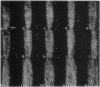
The direction in which plant tissue cells expand is reflected in the alignment of microtubules in the cortical array. When microtubules and coaligned wall microfibrils are arranged transversely around the cell, turgor pressure is chaneled into cell elongation. However, various agents (such as wounding, ethylene, abscisic acid) can cause the microtubules to reorientate by 90 degrees so that they become aligned parallel to the cell's long axis, allowing lateral expansion instead of elongation. The mechanism by which microtubules undergo rapid shifts of alignment is crucial to understanding growth control in plants, but because current models are derived from studies on fixed cells, nothing is known about the dynamics of converting one microtubule alignment to another. Cells tend to have one predominant microtubule alignment--transverse, oblique, or longitudinal--but it is not established whether each represents a stable independent set that only changes by rounds of complete de- and repolymerization, or whether reorientation is a more continuous process involving movement of stable or dynamic microtubules. By microinjecting pea (Pisum sativum) epidermal cells with rhodamine-conjugated brain tubulin and optically sectioning them by confocal laser scanning microscopy, we could follow labeled microtubules for up to 2 hr as they reorientate. Reorientation does not occur by complete depolymerization of microtubules in one orientation followed by polymerization of a new array in another orientation. Instead, increased numbers of discordant microtubules in nontransverse alignment appear in particular locations. Neighboring microtubules then adopt the new alignment, so that there is a stage during which different alignments coexist before the array on the outer tangential cell face finally adopts a uniform steeply oblique/longitudinal configuration. Rapid fluorescence recovery after photobleaching confirms that bundles of cortical microtubules are not stable but exhibit properties consistent with dynamic instability. Dynamic microtubules offer a mechanism for rapid growth responses to a range of physiological stimuli.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gotoh H., Hagihara M., Nagatsu T., Iwata H., Miura T. Activities of dipeptidyl peptidase II and dipeptidyl peptidase IV in synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Clin Chem. 1989 Jun;35(6):1016–1018. [PubMed] [Google Scholar]
- Gundersen G. G., Khawaja S., Bulinski J. C. Postpolymerization detyrosination of alpha-tubulin: a mechanism for subcellular differentiation of microtubules. J Cell Biol. 1987 Jul;105(1):251–264. doi: 10.1083/jcb.105.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A., Drechsel D., Kellogg D., Salser S., Sawin K., Steffen P., Wordeman L., Mitchison T. Preparation of modified tubulins. Methods Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Borisy G. G. Association of high-molecular-weight proteins with microtubules and their role in microtubule assembly in vitro. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2696–2700. doi: 10.1073/pnas.72.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland J., Schröder H. C., Weber K. Amino acid sequence requirements in the epitope recognized by the alpha-tubulin-specific rat monoclonal antibody YL 1/2. EMBO J. 1984 Jun;3(6):1295–1300. doi: 10.1002/j.1460-2075.1984.tb01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland J., Willingham M. C., Sandoval I. V. A rat monoclonal antibody reacting specifically with the tyrosylated form of alpha-tubulin. I. Biochemical characterization, effects on microtubule polymerization in vitro, and microtubule polymerization and organization in vivo. J Cell Biol. 1983 Nov;97(5 Pt 1):1467–1475. doi: 10.1083/jcb.97.5.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Wadsworth P., Hepler P. K. Microtubule dynamics in living dividing plant cells: confocal imaging of microinjected fluorescent brain tubulin. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8820–8824. doi: 10.1073/pnas.87.22.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]