Abstract
TNF ligand superfamily member 12, also known as TNF-related weak inducer of apoptosis (TWEAK), acts through its receptor, fibroblast growth factor-inducible 14 (Fn14), to mediate several key pathologic processes involved in tissue injury relating to lupus nephritis. To explore the potential for renal protection in lupus nephritis by targeting this pathway, we introduced the Fn14 null allele into the MRL-lpr/lpr lupus mouse strain. At 26–38 weeks of age, female Fn14-knockout MRL-lpr/lpr mice had significantly lower levels of proteinuria compared with female wild-type MRL-lpr/lpr mice. Furthermore, Fn14-knockout mice had significantly improved renal histopathology accompanied by attenuated glomerular and tubulointerstitial inflammation. There was a significant reduction in glomerular Ig deposition in Fn14-knockout mice, despite no detectable differences in either serum levels of antibodies or splenic immune cell subsets. Notably, we found that the Fn14-knockout mice displayed substantial preservation of podocytes in glomeruli and that TWEAK signaling directly damaged barrier function and increased filtration through podocyte and glomerular endothelial cell monolayers. Our results show that deficiency of the Fn14 receptor significantly improves renal disease in a spontaneous lupus nephritis model through prevention of the direct injurious effects of TWEAK on the filtration barrier and/or modulation of cytokine production by resident kidney cells. Thus, blocking the TWEAK/Fn14 axis may be a novel therapeutic intervention in immune-mediated proliferative GN.
Keywords: lupus nephritis, cytokines, clinical immunology
Lupus nephritis (LN) is one of the most common manifestations of SLE, with kidney involvement associated with significant morbidity and mortality.1 A better understanding of the pathogenesis of LN is important for identifying more targeted and less toxic therapeutic approaches. However, despite substantial progress,2,3 several recent clinical trials testing novel therapies have failed to show improvement over the existing standard of care. Thus, mechanisms of renal damage in LN are not fully elucidated, which poses a real challenge for the development of improved treatment approaches.
TNF-related weak inducer of apoptosis (TWEAK) is a multifunctional cytokine that regulates inflammation, cell survival, growth, migration, and differentiation.4 TWEAK mediates these activities by its only known signaling receptor, fibroblast growth factor-inducible 14 (Fn14), thereby activating the NF-κB, MAPK, and potentially, other signaling pathways.5,6 Fn14 is expressed at low levels in normal tissues and highly induced in diseased tissues, resulting in pathway activation. Transient TWEAK/Fn14 activation is implicated in physiologic tissue repair and regeneration; however, excessive or persistent activation drives pathologic tissue responses in contexts of chronic injury and disease and contributes to progressive tissue damage and degeneration,4,7 thereby suggesting this pathway as a potential target for intervention. Other than its known expression in endothelial cells8 and fibroblasts,9 we recently found expression of Fn14 on mesangial cells, podocytes, and tubular cells, whereas functional studies have implicated TWEAK/Fn14 signaling in the pathogenesis of renal disease.9–11
Previously, we showed that TWEAK induces human kidney mesangial cells, tubular cells, and podocytes to express multiple inflammatory mediators, including RANTES, MCP-1, IP-10, ICAM-1, and VCAM-1, thus promoting kidney infiltration of inflammatory cells and stimulating cellular proliferation.12,13 In chronic graft-versus-host disease, mice treated with an anti-TWEAK mAb had significantly diminished proteinuria and decreased kidney expression of proinflammatory cytokines.14,15 In nephrotoxic nephritis, we recently found that Fn14-knockout (KO) mice had significantly attenuated kidney disease after transfer of pathogenic antibodies compared with Fn14–wild-type (WT) mice.16 In clinical studies, significantly higher urinary TWEAK levels were present in human LN, which increased with renal flares.17–20 Interestingly, intrarenal TWEAK and Fn14 expression varied with the histologic class of LN and correlated with the activity index.21,22 Finally, elevated production of TWEAK in lupus PBMCs correlated with disease activity and proteinuria.23 Thus, both experimental and clinical studies support the hypothesis that TWEAK/Fn14 interactions play an important role in the pathogenesis of antibody-mediated nephritis, including LN. However, the relevant mechanisms have yet to be clarified. Moreover, there are significant differences in the etiology and severity of nephritis between chronic graft-versus-host disease, nephrotoxic nephritis, and spontaneous LN, the latter of which is also much more directly relevant to human disease. In this study, we evaluated the effect of Fn14 deficiency in spontaneous murine LN and explored the mechanisms relating the TWEAK/Fn14 pathway to renal damage.
Results
Fn14 Deficiency Significantly Reduces Proteinuria in MRL/lpr Mice
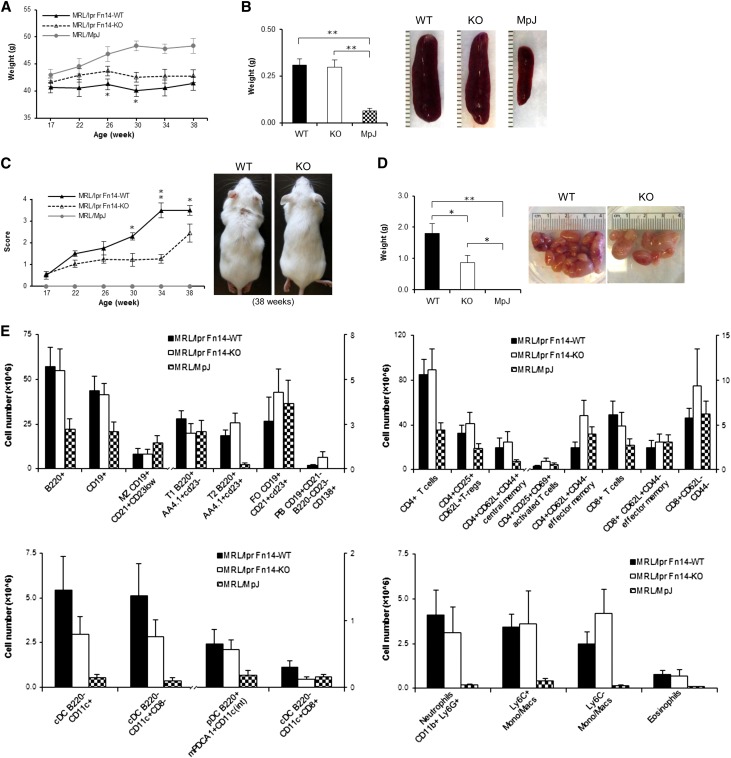
We followed the development of renal disease by measurement of urine protein starting at 12 weeks of age until euthanasia at 38 weeks. Beginning at 26 weeks of age, Fn14-KO mice displayed significantly decreased proteinuria compared with Fn14-WT mice (Figure 1A). In contrast, the Fn14-KO mice were no different than the control MRL/MpJ mice. Similarly, the percentage of mice with severe proteinuria (≥300 mg/dl) was lower in the MRL/lpr Fn14-KO strain (Figure 1B). The difference in proteinuria was confirmed quantitatively by urine albumin-to-creatinine ratios (Figure 1C). Serum creatinine (Figure 1D) and BUN (Figure 1E) further confirmed amelioration of renal disease in MRL/lpr Fn14-KO mice.
Figure 1.
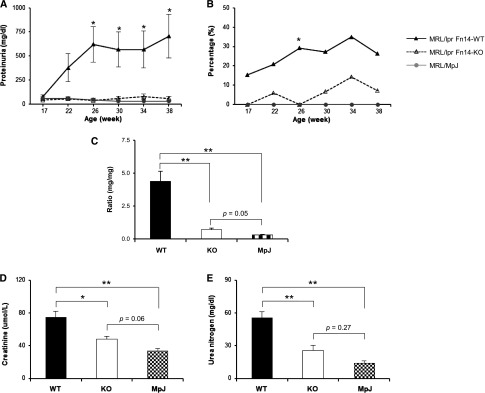
Proteinuria is attenuated in MRL/lpr Fn14-KO mice. (A) Beginning at 26 weeks until the mice were euthanized at 38 weeks, there was significantly decreased proteinuria in MRL/lpr Fn14-KO compared with MRL/lpr Fn14-WT mice. The MRL/lpr Fn14-KO strain also displayed a lower percentage of mice with (B) severe proteinuria (≥300 mg/dl at 26 weeks by chi-squared test) and (C) decreased urine albumin-to-creatinine ratio (at 38 weeks). Serum levels of (D) creatinine and (E) BUN were higher in MRL/lpr Fn14-WT compared with Fn14-KO mice. The numbers of MRL/lpr Fn14-WT, MRL/lpr Fn14-KO, and MRL/MpJ mice were 14, 11, and 5, respectively. *P<0.05; **P<0.01.
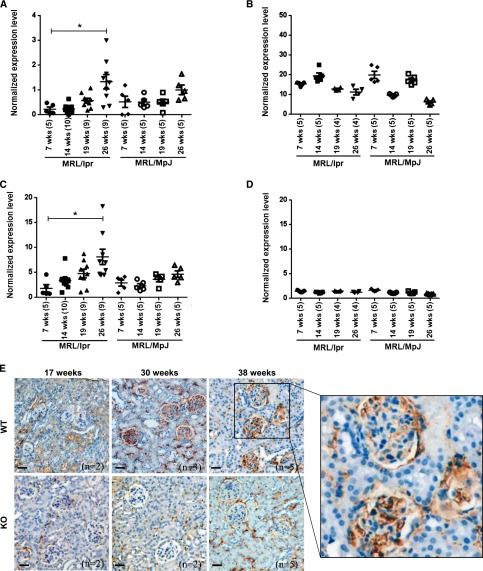
Renal Expression of TWEAK and Fn14 Increases with Age in MRL/lpr Fn14-WT Mice
To begin to understand the mechanism of renal protection in Fn14-deficient lupus mice, total kidney and spleen TWEAK and Fn14 mRNA expression levels were measured at several time points leading up to the appearance of maximal clinical disease. The comparison between renal TWEAK expression levels at 7 and 26 weeks revealed a significant increase over time in MRL/lpr Fn14-WT mice (Figure 2A). However, the expression of spleen TWEAK mRNA showed no significant increase with age (Figure 2B). A similar pattern of increases over time in kidney but not in spleen was also found in Fn14 mRNA expression (Figure 2, C and D). We stained kidney sections from 17-, 30-, and 38-week-old mice and confirmed kidney Fn14 protein expression in the MRL/lpr Fn14-WT strain (Figure 2E).
Figure 2.
Renal TWEAK/Fn14 expression increases with age in MRL/lpr mice. (A) mRNA expression level of TWEAK in kidneys from MRL/lpr Fn14-WT mice was higher at 26 than 7 weeks of age but revealed no difference between those two time points in MRL/MpJ mice (P>0.05). (B) mRNA expression level of TWEAK in spleens did not increase with age in either MRL/lpr or MRL/MpJ mice. (C and D) The expression levels of Fn14 mRNA in (C) kidneys and (D) spleens displayed similar trends as TWEAK in the two strains. In A–D, the numbers of mice are provided in parentheses after the age on the x axis. The experiments depicted in A–D were repeated two times with similar results. *P<0.05. (E) IHC staining verified Fn14 protein expression in the kidneys from MRL/lpr Fn14-WT mice at ages of 17, 30, and 38 weeks. MRL/lpr Fn14-KO mice at 38 weeks (n=5) show minimal background staining. Representative images are shown. Scale bar, 40 μm.
Fn14 Deficiency Attenuates Glomerular, Tubular, and Interstitial Injury in MRL/lpr Mice
Histologic analysis at 38 weeks of age revealed marked glomerular injury in the MRL/lpr Fn14-WT mice that was significantly attenuated in MRL/lpr Fn14-KO mice (Figure 3A), particularly in mesangial proliferation and endocapillary hypercellularity (Figure 3B). Moreover, Ki-67 staining showed decreased cellular proliferation in both glomerular and tubular areas in MRL/lpr Fn14-KO mice (Figure 3, C and D). Apoptosis measured by caspase-3 expression was similar in all mice groups (data not shown).
Figure 3.
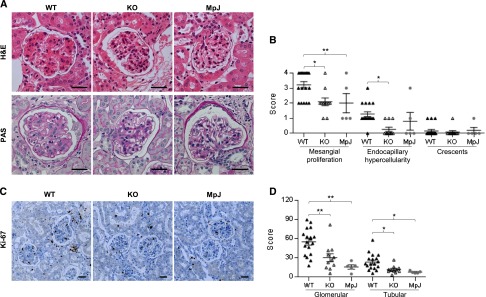
Kidney proliferative changes are attenuated in MRL/lpr Fn14-KO mice. (A) Representative kidney sections (stained with H&E or periodic acid–Schiff [PAS]) of three mouse strains are shown that display reduced glomerular damage in MRL/lpr Fn14-KO mice compared with MRL/lpr Fn14-WT mice. H&E, hematoxylin and eosin. (B) Semiquantitative scoring results on the basis of H&E and PAS staining revealed significant differences in histopathological features of LN between the MRL/lpr Fn14-WT and Fn14-KO mice. H&E, hematoxylin and eosin. (C) Representative images of Ki-67 staining are shown. (D) Ki-67–stained sections were scored and exhibited fewer Ki-67–positive cells in both glomerular and tubular areas in kidneys of Fn14-KO mice compared with the MRL/lpr Fn14-WT strain. There were no differences between the MRL/lpr Fn14-KO mice and MRL/MpJ mice (P>0.05). The numbers of MRL/lpr Fn14-WT, MRL/lpr Fn14-KO, and MRL/MpJ mice (all at 38 weeks of age) were 14, 11, and 5, respectively. Scale bar, 25 μm. *P<0.05; **P<0.01.
Analysis of kidney histology showed modest tubulointerstitial disease in this model. Nevertheless, tubular injury was decreased in Fn14-KO mice compared with MRL/lpr Fn14-WT mice, which was shown by significantly less tubular atrophy, fewer casts, and less dilation (Figure 4, A and B). Notably, the expression of the kidney injury molecule-1 (KIM-1)/T cell Ig and mucin domain-1 (TIM-1) was significantly reduced in MRL/lpr Fn14-KO mice (Figure 4, C and D). No difference in interstitial fibrosis was observed between Fn14-KO mice and either MRL/lpr Fn14-WT or MRL/MpJ controls (Figure 4B).
Figure 4.
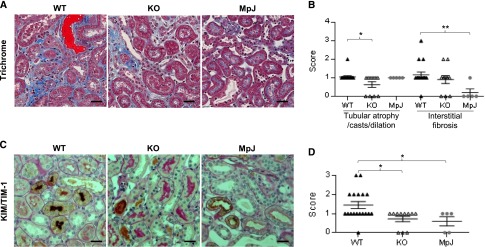
Kidney tubulointerestitial changes are decreased in MRL/lpr Fn14-KO mice. (A) By trichrome staining, tubular casts (red) and interstitial fibrosis (blue) could be seen in MRL/lpr Fn14-WT mice but were relatively rare in the MRL/MpJ mice. (B) Semiquantitative scoring results on the basis of trichrome staining revealed significant difference in tubular injury but not in interstitial fibrosis between the MRL/lpr Fn14-WT and Fn14-KO mice. (C) Representative images of KIM-1/TIM-1 staining are shown, displaying diffuse staining in proximal tubules in kidneys of MRL/lpr Fn14-WT mice, patchy staining in Fn14-KO mice, and slight staining in MRL/MpJ mice. (D) Semiquantitative scoring showed reduced KIM-1/TIM-1 staining in Fn14-KO mice compared with MRL/lpr Fn14-WT mice, but it was not different from MRL/MpJ mice (P>0.05). The numbers of MRL/lpr Fn14-WT, MRL/lpr Fn14-KO, and MRL/MpJ mice (all at 38 weeks of age) were 14, 11, and 5, respectively. Scale bar, 25 μm. *P<0.05; **P<0.01.
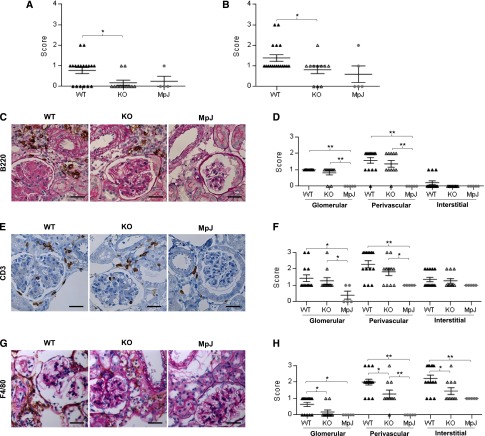
Leukocyte Infiltration Is Decreased in MRL/lpr Fn14-KO Kidneys
TWEAK stimulation of kidney resident cells promotes PBMC chemotaxis in vitro, particularly of macrophages.12 B and T cells and F4/80+ macrophages were all prominent in glomerular and perivascular areas in the MRL/lpr Fn14-WT kidneys, whereas macrophages were also increased in the interstitium. In contrast, infiltrating leukocytes were significantly decreased in cortical and perivascular areas in Fn14-KO mice (Figure 5, A and B). Although there was no significant difference in B (Figure 5, C and D) or T cell (Figure 5, E and F) staining, we found significantly fewer macrophages in the glomerular, tubular, and interstitial regions of MRL/lpr Fn14-KO mouse kidneys (Figure 5, G and H).
Figure 5.
Kidney cellular infiltration is attenuated in MRL/lpr Fn14-KO mice. (A and B) Semiquantitative scoring results on the basis of H&E and periodic acid–Schiff (PAS) staining revealed significant reduction of both (A) cortical (nonperivascular) and (B) perivascular inflammation in MRL/lpr Fn14-KO mice compared with MRL/lpr Fn14-WT mice. H&E, hematoxylin and eosin. (C) Representative images of B220 staining are shown, displaying intense infiltration in the perivascular areas of kidneys from MRL/lpr mice but no infiltration in MRL/MpJ mice, which was also confirmed by (D) semiquantitative scoring. (E) Representative images of CD3 staining are shown, reflecting (F) higher scores of glomerular and perivascular infiltration in MRL/lpr mice compared with MRL/MpJ mice. There were no differences in both B220 and CD3 staining between the MRL/lpr Fn14-WT and Fn14-KO mice (P>0.05). (G) Representative images of F4/80 staining are shown. (H) Sections of F4/80 staining were scored separately in the glomerular, perivascular, and interstitial areas, showing significant reduction in MRL/lpr Fn14-KO mice in all of these regions compared with MRL/lpr Fn14-WT mice. The MRL/lpr Fn14-KO mice differed from MRL/MpJ mice in F4/80 infiltration in the perivascular but not glomerular or interstitial areas. The numbers of MRL/lpr Fn14-WT, MRL/lpr Fn14-KO, and MRL/MpJ mice (all at 38 weeks of age) were 14, 11, and 5, respectively. Scale bar, 25 μm. *P<0.05; **P<0.01.
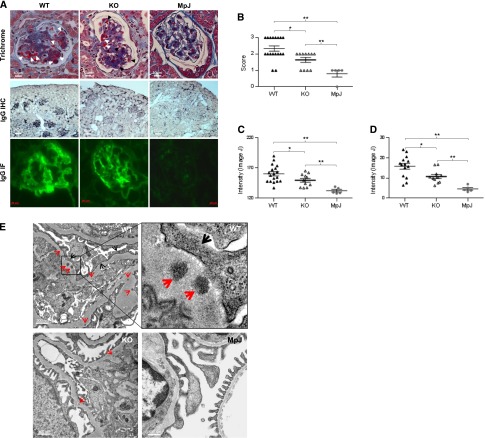
Glomerular Deposition of IgG Is Decreased in MRL/lpr Fn14-KO Mice
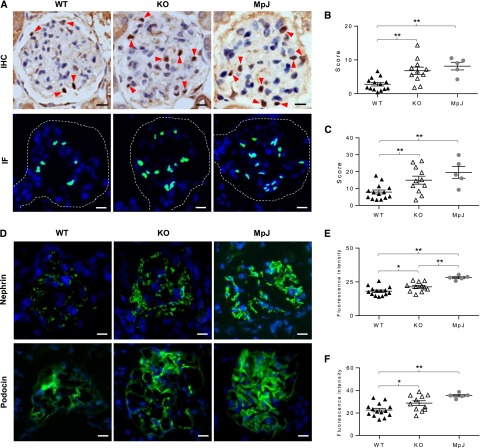
Deposition of autoantibodies is a pivotal initiating event in the pathogenesis of LN. We found a significant reduction in immune complex (IC) deposition in MRL/lpr Fn14-KO mice by light microscopy (Figure 6, A and B), immunohistochemistry (IHC) (Figure 6, A and C), and immunofluorescence (IF) (Figure 6, A and D). The difference between MRL/lpr Fn14-WT and Fn14-KO mice was also reflected by transmission electron microscopy (TEM), which displayed more prominent electron-dense deposits and fusion of podocyte foot processes in MRL/lpr Fn14-WT mice than in Fn14-KO mice (Figure 6E).
Figure 6.
Glomerular IC deposition is reduced in MRL/lpr Fn14-KO mice. (A) Representative images of mouse kidneys are shown for (top panel) trichrome staining, (middle panel) IgG staining by IHC, and (bottom panel) IF. In trichrome staining, white arrows indicate IC, and black arrows indicate red blood cells. (B) Slides stained by trichrome or periodic acid–Schiff (images not shown) were used for scoring immune deposits, with higher scores in the MRL/lpr Fn14-WT than the Fn14-KO strain. (C and D) IgG deposition detected by (C) IHC and (D) IF was quantitated by ImageJ, showing a significant reduction in Fn14-KO mice compared with MRL/lpr Fn14-WT mice, although still higher than MRL/MpJ controls. (E) Representative TEM images showed that, compared with MRL/lpr Fn14-KO mice (n=3), electron-dense deposits (red arrows) were more prominent in the glomerular basement membrane of MRL/lpr Fn14-WT mice (n=2). Fusion of podocyte foot processes (black arrows) was also more severe in MRL/lpr Fn14-WT mice. Neither electron-dense deposit nor foot process fusion was seen in glomeruli in MRL/MpJ mice (n=3). For A–D, the numbers of mice in each of the MRL/lpr Fn14-WT, MRL/lpr Fn14-KO, and MRL/MpJ strains were 14, 11, and 5, respectively. For A–E, age = 38 weeks. *P<0.05; **P<0.01.
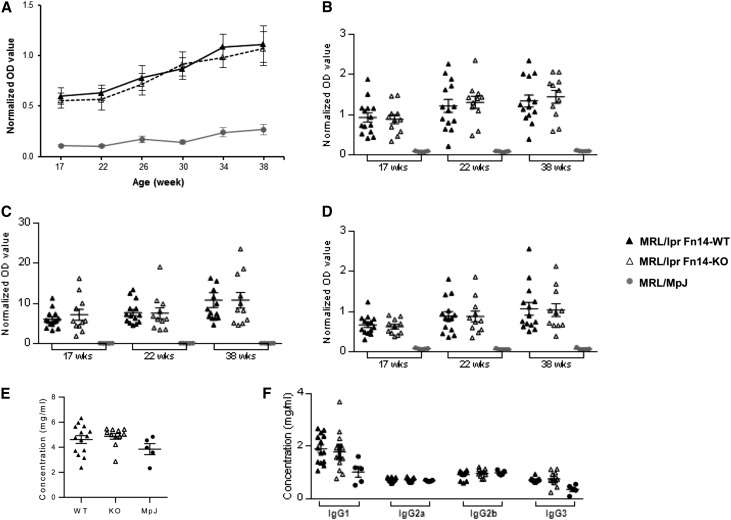
Circulating Levels of Antibodies, Splenocyte Numbers, and Distribution Are Not Affected in MRL/lpr Fn14-KO Mice
To assess the effect of the Fn14 deficiency on the systemic immune response and clarify whether the decreased glomerular immune deposition observed in MRL/lpr Fn14-KO mice was a function of reduced circulating titers of pathogenic antibodies, serum autoantibodies were determined at multiple time points. We found no detectable difference in autoantibody titers in sera from MRL/lpr Fn14-KO mice compared with MRL/lpr Fn14-WT mice between 17 and 38 weeks of age (Figure 7, A–D). Furthermore, there were no significant differences between MRL/lpr Fn14-KO and MRL/lpr Fn14-WT mice in total serum IgG concentrations or the levels of specific serum IgG isotypes (Figure 7, E and F).
Figure 7.
Serum antibody levels are not altered with Fn14 deficiency in MRL/lpr mice. (A) There were no significant differences in the titers of IgG anti-dsDNA antibodies between MRL/lpr Fn14-WT and Fn14-KO mice at any time point (P>0.05). (B–D) There were no differences between MRL/lpr Fn14-WT and Fn14-KO mice in (B) anti-chromatin, (C) anti-ribosomal P, and (D) anti-cardiolipin autoantibodies (P>0.05) at any of the time points. MRL/MpJ mice had significantly lower serum levels of these autoantibodies than the MRL/lpr strains at all time points. The experiments depicted in A–D were repeated two times with similar results. (E and F) There were no significant differences in the serum levels of (E) total IgG or (F) specific IgG isotypes between MRL/lpr Fn14-WT and Fn14-KO mice at 38 weeks of age (P>0.05). The numbers of MRL/lpr Fn14-WT, MRL/lpr Fn14-KO, and MRL/MpJ mice were 14, 11, and 5, respectively. dsDNA, double-stranded DNA.
Other systemic effects of MRL/lpr Fn14 deficiency were also investigated. At weeks 26 and 30, the Fn14-KO mice exhibited higher body weight than MRL/lpr Fn14-WT mice (Figure 8A). In addition, at 38 weeks, there was no effect of Fn14 deficiency on spleen size (Figure 8B). Lymph node scores (30–38 weeks) and weights (38 weeks) of MRL/lpr Fn14-KO mice were intermediate, significantly lower than MRL/lpr Fn14-WT, and significantly higher than MRL/MpJ mice (Figure 8, C and D). There were no differences in the absolute numbers of splenic B, T, dendritic, or myeloid cells between MRL/lpr Fn14-WT and Fn14-KO mice (Figure 8E).
Figure 8.
Fn14 deficiency affects lymphadenopathy but not splenocyte weight or cellular composition in MRL/lpr mice. (A) MRL/lpr Fn14-WT and Fn14-KO mice exhibited less weight gain compared with MRL/MpJ mice between 26 and 38 weeks of age. A significant difference between MRL/lpr Fn14-WT and Fn14-KO mice was seen at 26 and 30 weeks. (B) At euthanasia (38 weeks of age), there were no differences in spleen weights between the MRL/lpr Fn14-KO and Fn14-WT mice. Representative images of spleens from mice at age of 38 weeks are shown. MRL/lpr Fn14-WT mice differed from Fn14-KO mice in (C) lymphadenopathy scores beginning at 30 weeks of age and (D) lymph node weight at 38 weeks. Representative images of (C) lymphadenopathy and (D) lymph nodes from MRL/lpr Fn14-WT and Fn14-KO mice at the age of 38 weeks are shown. No visibly enlarged lymph nodes were present in MRL/MpJ mice. For A–D, the numbers of MRL/lpr Fn14-WT, MRL/lpr Fn14-KO, and MRL/MpJ mice were 14, 11, and 5, respectively. (E) FACS analysis showed that, in four major subpopulations of spleen cells (T, B, dendritic, and myeloid cells), there were no significant differences between MRL/lpr Fn14-WT (n=8) and Fn14-KO (n=6) mice at week 38. Five MRL/MpJ mice were studied in the cytometric analysis. *P<0.05; **P<0.01.
Fn14 Deficiency Protects the Integrity of the Renal Filtration Barrier
The significant reduction in glomerular immune deposits in MRL/lpr Fn14-KO mice, despite similar circulating autoantibody titers, suggested that the decrease in deposits was potentially because of an effect of TWEAK signaling on the integrity of the glomerular filtration unit. This was analyzed by evaluating kidney podocytes in situ as well as assessing the function of podocytes and glomerular endothelial cells in vitro. Wilms’ tumor 1 (WT1) staining revealed a significant decrease in the number of WT1-stained cells in MRL/lpr Fn14-WT mice compared with the MRL/lpr Fn14-KO or MRL/MpJ strains (Figure 9, A–C). Furthermore, both nephrin and podocin displayed significantly reduced expression in MRL/lpr Fn14-WT mice (Figure 9, D–F).
Figure 9.
Podocytes are protected by Fn14 deficiency in MRL/lpr mice. (A) Representative images of WT1 staining by IHC and IF are shown. (Upper panel) The brown nuclei indicated by red arrows represent WT1-positive cells. (Lower panel) By IF, WT1-positive nuclei (green) were overlapped by 4′,6-diamidino-2-phenylindole staining (blue). Single glomeruli are outlined by dashed lines. The sections were scored by counting the number of WT1-positive nuclei per glomerulus (10 random glomeruli per section). Compared with MRL/lpr Fn14-WT mice, the Fn14-KO mice had increased scores by both (B) IHC and (C) IF staining and were not significantly different from MRL/MpJ mice. (D–F) Representative images are shown (IF staining) for nephrin and podocin expressions (green), which were the strongest in MRL/MpJ mice followed by MRL/lpr Fn14-KO and Fn14-WT mice. The experiments depicted in D–F were repeated three times with similar results. The numbers of MRL/lpr Fn14-WT, MRL/lpr Fn14-KO, and MRL/MpJ mice (all at 38 weeks of age) were 14, 11, and 5, respectively. Scale bar, 10 μm. *P<0.05; **P<0.01.
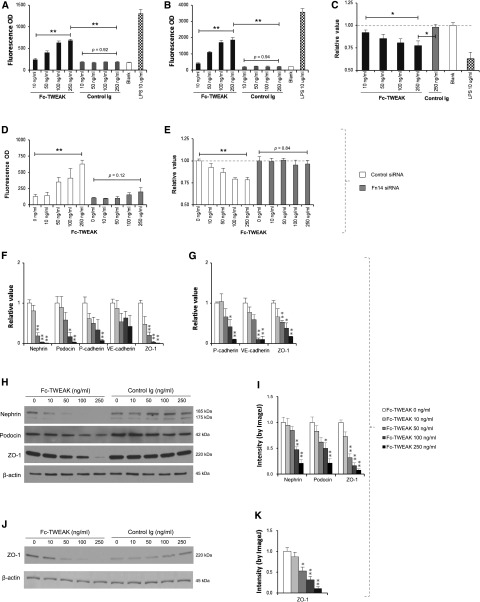
To directly assess the effect of the TWEAK/Fn14 pathway on the integrity of podocytes and endothelial cells, in vitro permeability was assessed by separately culturing podocytes and glomerular endothelial cells on tissue inserts and quantifying the permeability for BSA (67 kD) and dextran (150 kD) through the cellular barrier. Both FITC-labeled BSA and dextran progressively transversed through the podocyte monolayer barrier with increasing concentrations of Fc-TWEAK (Figure 10, A and B). Furthermore, the viability of podocytes decreased on exposure to Fc-TWEAK (Figure 10C). To confirm that this action of TWEAK is specific and Fn14-mediated, we used an siRNA approach. The siRNA treatment silenced the expression of Fn14 (data not shown) and abrogated the increase in permeability and decrease in viability of podocytes induced by Fc-TWEAK (Figure 10, D and E). The addition of a caspase inhibitor (z-VAD-fmk) partially inhibited the effects of Fc-TWEAK in both permeability and viability assays (Supplemental Figure 1, A and B). Glomerular endothelial cells exhibited similar responses to Fc-TWEAK stimulation in both permeability and viability assays, with a similar dependency on Fn14 (data not shown).
Figure 10.
Activation of the TWEAK/Fn14 pathway modulates the integrity of the renal filtration barrier in vitro. (A and B) Fc-TWEAK but not control Ig stimulation increased permeability for both (A) BSA-FITC and (B) dextran-FITC through podocyte monolayers in a dose-dependent manner (by Spearman rank correlation; P<0.01). LPS treatment was used as a positive control. (C) The viability assay for podocytes showed significant differences between the Fc-TWEAK 250 ng/ml group and the Fc-TWEAK 10 ng/ml or control Ig 250 ng/ml group. All values were normalized to the untreated blank group (as a baseline of one). (D and E) Fn14 siRNA but not control siRNA blocked the effects of Fc-TWEAK on both (D) permeability and (E) death of podocytes. (F) mRNA levels of nephrin, podocin, P-cadherin, and ZO-1 decreased with increasing concentrations of Fc-TWEAK in the culture media. (G) A similar finding was seen with P-cadherin, VE-cadherin, and ZO-1 mRNA expressions by glomerular endothelial cells. (H) Decreasing expressions of nephrin, podocin, and ZO-1 by podocytes with increasing concentration of Fc-TWEAK but not the control Ig are observed by Western blot. (I) The intensity of bands of Western blot were quantitated by ImageJ, showing significant decrease of nephrin, podocin, and ZO-1 by podocytes with Fc-TWEAK. (J) Glomerular endothelial cells expressed less ZO-1 protein after Fc-TWEAK stimulation, which was shown by (K) ImageJ quantitation of Western bot bands. Data were representative of three to five independent experiments. For F–K, comparisons are to media alone (Fc-TWEAK=0 ng/ml). *P<0.05; **P<0.01.
In further delineating the molecular mechanisms where TWEAK/Fn14 treatment of glomerular cells promotes their permeability, we found that mRNA levels of junction proteins expressed by podocytes (nephrin, podocin, P-cadherin, and ZO-1) and glomerular endothelial cells (P-cadherin, VE-cadherin, and ZO-1) were significantly reduced with increasing concentrations of Fc-TWEAK (Figure 10, F and G). We confirmed this observation by Western blot analysis (Figure 10, H–K). The diminished expression of podocyte junction proteins in response to TWEAK was also restored in Fn14 siRNA-transfected cells (Supplemental Figure 1, C–E). Thus, TWEAK signaling by Fn14 directly affects the expression of junction proteins in podocytes and glomerular endothelial cells.
TWEAK Acts Directly on Kidney Cells
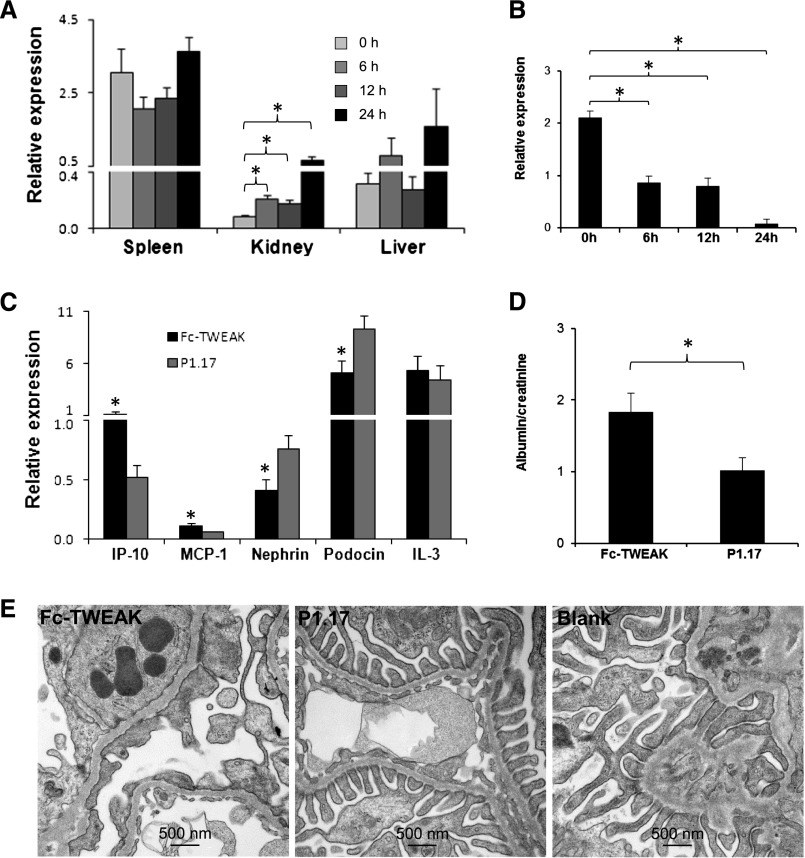
The difference in kidney phenotype between MRL/lpr Fn14-sufficient and -KO mice may be because of a direct effect of TWEAK on glomerular cells and the filtration barrier or an indirect result mediated by systemic proinflammatory effects of this cytokine or glomerular antibody deposition. To better differentiate between these two possibilities, we injected TWEAK intravenously into MRL/MpJ mice and measured the levels of the TWEAK-responsive chemokines IP-10 and MCP-1 in spleen, liver, and kidney at 0, 6, 12, and 24 hours. We found that the kidney was the most sensitive tissue to the effects of TWEAK, with significant increases in renal IP-10 expression obvious by 6 hours postinjection (Figure 11A), whereas kidney levels of cytokines not reported to be TWEAK-regulated, IL-3 and IL-7, were not significantly affected (data not shown). Notably, podocin mRNA levels were also significantly decreased as early as 6 hours (Figure 11B). In contrast, liver and spleen IP-10 (Figure 11A) and MCP-1 (data not shown) expressions were not increased versus baseline, at least until the 24-hour time point. Moreover, there were no significant differences in serum levels of IP-10 and MCP-1 between baseline and any of the subsequent time points (data not shown).
Figure 11.
Exogenous TWEAK has direct kidney effects. (A and B) Female MRL/MpJ mice at 8 weeks of age (n=3 in each group) received a single intravenous injection of 200 µg Fc-TWEAK or the P1.17 isotype control, and the kidneys were obtained at euthanasia. Fc-TWEAK–injected mice displayed (A) a significant increase in kidney mRNA expression of IP-10 and (B) decreased expression of podocin at 6, 12, and 24 hours compared with baseline. There were no differences in IP-10 levels in the spleen or liver at any of these time points (P>0.05). (C–E) Predisease female MRL/lpr mice (8 weeks old) were injected with 200 µg Fc-TWEAK or P1.17 intravenously and euthanized 12 hours later. Fc-TWEAK–injected mice (n=4) had significantly higher renal mRNA expression of IP-10 and MCP-1 and lower nephrin and podocin expressions than P1.17-injected mice (n=4). (C) Levels of IL-3, a cytokine not regulated by TWEAK, were unchanged. Furthermore, TWEAK-injected mice exhibited (D) increased proteinuria and (E) podocyte foot process effacement. Representative TEM images are shown. Blank is a representative kidney section from a noninjected age- and sex-matched MRL/lpr mouse. In A–C, relative values of mRNA expression were multiplied by 102. *P<0.05.
Because prominent effects on the kidney in response to TWEAK were seen at 6 and 12 hours postinjection, at which time there were not yet any measurable systemic effects, we injected predisease MRL/lpr mice with Fc-TWEAK and euthanized the mice 12 hours later. Once again, kidney but not liver and spleen chemokine expression was significantly upregulated in mice injected with Fc-TWEAK but not an isotype control. Moreover, nephrin and podocin mRNA were also significantly decreased (Figure 11C). Finally, we determined whether the net effect of TWEAK on kidney gene expression has functional and structural consequences. Importantly, we found that a single injection of TWEAK significantly increased proteinuria by 12 hours (at which time, no systemic effect was discernible, and antibody deposition was not a confounding factor) compared with control-injected mice (Figure 11D). Finally, we showed a marked increase in podocyte foot process effacement in TWEAK-injected mice by electron microscopy (Figure 11E). These results together with the in vitro studies conclusively indicate that the renal phenotype in MRL/lpr Fn14-WT mice is most likely mediated by a kidney-specific effect of TWEAK acting locally.
Discussion
This study shows the pivotal role of TWEAK/Fn14 signaling in spontaneous murine LN, which strongly resembles the proliferative nephritis in human SLE.24,25 We show that Fn14 deficiency ameliorates renal injury, which was reflected by reduced proteinuria and markedly attenuated histopathology. Novel mechanistic analysis also elucidated that MRL/lpr Fn14-KO mice have a significantly preserved glomerular filtration barrier. This is most likely through a direct effect of TWEAK/Fn14 signaling, because silencing of the pathway protects podocytes and endothelial cells, two key components of the glomerular filtration unit. These effects of TWEAK on podocytes and the structure/function of the kidney filtration barrier had not been previously appreciated, and they illuminate a novel contribution of the TWEAK/Fn14 pathway to kidney pathophysiology.
Compared with other lupus models in common use, MRL/lpr mice have higher levels of autoantibodies as well as earlier and more rapidly progressive nephritis.26–28 MRL/lpr mice also exhibit more prominent involvement of lymphoid tissue29 and similar expression patterns of chemokines and chemokine receptors in inflamed organs as patients with LN.25 Our findings showing upregulation of renal TWEAK and Fn14 expression in MRL/lpr mice and protection from LN in Fn14-deficient animals strongly indicate that TWEAK/Fn14 activation contributes to the pathogenesis of murine LN, driving renal damage and dysfunction.
Renal histologic changes are important as indicators of severity and prognosis of LN.30,31 Our results revealed notable improvement in renal histology in MRL/lpr Fn14-KO mice, especially in reduction of mesangial proliferation.32,33 Ki-67 is a nuclear protein associated with and maybe necessary for cellular proliferation,34 whereas KIM-1/TIM-1 is an apoptotic cell phagocytosis and scavenger receptor that is most highly upregulated in proximal tubular epithelium in kidney injury.35 MRL/lpr Fn14-KO mice had significantly decreased Ki-67 (both glomerular and tubular) and KIM-1/TIM-1 (tubular) staining. Of special note is the increasing recognition of tubulointerstitial disease as one of the most robust prognostic factors in LN.36 Although tubulointerstitial disease was not particularly severe in the MRL/lpr mice, it was reduced by Fn14 deficiency. The decreases in tubular Ki-67 and KIM-1/TIM-1 staining are additional indicators of a salutatory effect of Fn14 deficiency on the tubulointerstitial compartment in LN.
TWEAK-stimulated chemokines induce migration of local macrophages,12,15 and kidney infiltration of macrophages is one of the inflammatory responses resulting from the engagement of Fn14 by TWEAK.14,37 The significant decrease in kidney infiltration of macrophages in MRL/lpr Fn14-KO mice confirms local modulation of inflammation mediated by activation of this pathway.
Interestingly, we did not detect any difference in serum antibody or autoantibody titers between the MRL/lpr Fn14-WT and Fn14-KO mice at any time point tested, although glomerular Ig deposition was reduced and noticeable improvement in renal damage was observed. In a previous study, we showed no significant differences in autoantibody titers between these strains up to 22 weeks of age38; here, we confirm and further extend this observation up until 38 weeks of age. Both anti–double-stranded DNA and antichromatin antibodies are closely associated with nephritogenicity.2,39 Anticardiolipin IgG is commonly found in the MRL/lpr strain40 and has been linked with proteinuria.41 Antiribosomal P antibodies are highly specific for SLE42 and share antigenic epitopes on the ribosome with anti-DNA antibodies.43 Furthermore, we found no change in total IgG levels or specific isotypes. Therefore, the most likely conclusion from the unchanged levels of nephritogenic antibodies in MRL/lpr Fn14-KO mice is that preventing TWEAK/Fn14 signaling thwarts the development of renal disease by mechanisms distinct from those modulating the humoral immune response.
Splenomegaly is commonly seen in sick MRL/lpr mice and can diminish with treatments that ameliorate disease severity and LN.44,45 Spleen weights were, however, not affected in MRL/lpr Fn14-KO mice. Moreover, there were no significant differences in splenic immune cell subpopulations, indicating little to no influence of Fn14 deficiency on the systemic immune response. However, lymphadenopathy in MRL/lpr Fn14-KO mice was less severe than in Fn14-WT mice. This discrepancy between lymph nodes and spleen is currently unexplained and deserves additional study.
It is highly notable in our study that IC deposition in glomeruli was significantly reduced in MRL/lpr Fn14-KO mice. The explanation of this observation is not immediately apparent, because serum titers of autoantibodies typically associated with LN were not altered. Although the degree of deposits in MRL/lpr Fn14-WT mice seen by electron microscopy was not severe, several mice with accelerated nephritis already had died by 38 weeks of age. Therefore, if not a sampling issue caused by the number of mice examined, it is likely that more prominent deposits (and other lupus-related features, such as even more marked splenomegaly) would be present at a later time point had the mice been euthanized later.
Glomerular endothelial cells, the glomerular basement membrane, and podocytes are important components of the filtration barrier. Previous studies on the effects of TWEAK/Fn14 interactions on podocytes are very limited.12,46 We previously reported that podocytes express Fn14 and that TWEAK stimulation of podocytes promotes an inflammatory response.12 Moreover, Fn14 expression in podocytes is elevated in proteinuric diseases.46 In MRL/lpr Fn14-WT mice, we detected in individual cells decreased podocyte expression of nephrin and podocin, both important structural components of the slit diaphragm that are decreased in LN.47,48 Another striking finding was the decline in the absolute number of podocytes.49 Although TWEAK is proapoptotic, this effect was originally described as weak (hence, the unabridged name of this cytokine). Indeed, in many cell types, TWEAK promotes apoptosis only in conjunction with other cytokines,50 whereas we found a marked effect on podocytes even with TWEAK used alone. Thus, our results showed a major protective effect of Fn14 deficiency that not only maintains the integrity of the structure of slit diaphragm but also, potentially inhibits podocyte cell death triggered by TWEAK activation. Other than contributing to increased IC deposition, the decrease in podocyte numbers and the expressions of nephrin, podocin, and other junction proteins in MRL/lpr Fn14-WT compared with KO glomeruli may directly cause albuminuria.51 Hence, the relatively higher number of WT1-positive cells and the preserved expression of key structural proteins of the renal filtration barrier in MRL/lpr Fn14-KO mice reflect a protective effect of inhibiting the TWEAK/Fn14 pathway on resident podocytes.
Direct evidence for a detrimental effect of the TWEAK/Fn14 pathway on the functional integrity of the glomerular filtration barrier is provided by our in vitro studies. We show a prominent increase in the permeability for FITC-labeled BSA and dextran through podocyte or endothelial cell monolayers in response to Fc-TWEAK stimulation; moreover, TWEAK/Fn14 activation induces the death of podocytes and glomerular endothelial cells. The silencing of Fn14 by siRNA treatment protected against the increases in barrier permeability and death of Fc-TWEAK–stimulated cells, providing additional support that TWEAK/Fn14 signaling is responsible for injury to the glomerular filtration barrier. Furthermore, the damage to the filtration barrier induced by TWEAK/Fn14 signaling seems to be mediated by decreasing expression of nephrin, podocin, and ZO-1, which are important in maintaining the function of slit diaphragms.52 Indeed, previous studies showed that changes in junction proteins may cause death of podocytes directly in addition to affecting podocyte function.53 Moreover, TWEAK/Fn14 activation downregulates ZO-1 expression by endothelial cells.54 Nevertheless, this latter study was performed in cells derived from human brain microvascular endothelium rather than the physiologically distinct murine glomerular endothelial cells studied here. Furthermore, in this study, we expand on our previous results by (1) describing an effect on additional junctional proteins in endothelial cells, (2) discovering a parallel effect in podocytes, and (3) proving that this effect is Fn14-mediated. We found that a caspase inhibitor incompletely reversed the changes in viability and permeability of podocytes and glomerular endothelial cells after Fc-TWEAK stimulation. Because TWEAK/Fn14-induced cell death may be caspase-dependent55 or -independent,56,57 we cannot presently rule out other forms of TWEAK-induced cell death. Regardless, it seems that augmented TWEAK/Fn14 signaling may be responsible for the dysfunction of glomerular filtration barrier through both decreasing cell numbers and impairing expression of junctional proteins. The effects of TWEAK on podocytes and barrier structure and function provide novel and heretofore unrecognized insights into the contribution of this cytokine ligand/receptor system to the pathogenesis of renal disease. Furthermore, decreased extravasation of antibodies, despite serum autoantibody levels similar to the MRL/lpr Fn14-WT mice, presents an elegant explanation as to why the Fn14-KO mice exhibited reduced immune deposits. Finally, inhibiting TWEAK/Fn14 interactions in LN may be beneficial by limiting the pathologic effects of the pathway on glomerular resident cells as well as reducing the influx of macrophages, which would be a rich source of TWEAK in the inflamed lupus kidney.
It is important to consider whether the less severe renal disease observed in the MRL/lpr Fn14-KO mice is because of effects of TWEAK locally in the kidney or rather, a result of the systemic effects of this cytokine. In vitro, we conclusively showed that TWEAK, through Fn14, directly affects the viability of podocytes and glomerular endothelial cells and increases the flux through layers of these cell types. Furthermore, we showed that the expression of junction proteins in both podocytes and glomerular endothelial cells was decreased after TWEAK stimulation. The results from in vivo studies also convincingly point to a direct effect model. We found that TWEAK specifically upregulates kidney chemokine expression before the onset of systemic inflammatory changes. Furthermore, the injection of TWEAK replicated the decrease in kidney nephrin and podocin expression found in vitro, induced proteinuria, and resulted in podocyte foot process effacement. The in vitro and in vivo studies are highly consistent and strongly support our conclusion that TWEAK signaling is mediating pathologic processes locally in the kidneys of Fn14-sufficient lupus mice. Although not technically feasible at present, whether there is any additional contribution of systemic activation of TWEAK/Fn14 signaling and which cell type is the primary target for TWEAK in the kidney can be addressed in the future by studying the effects of kidney resident cell-specific Fn14-KO strains.
The results of our in vivo experiments involving Fc-TWEAK injection are, indeed, consistent with a direct effect of TWEAK on renal cells. However, because both IP-10 upregulation and decreased podocin expression were seen at the 6-hour time point postinjection, an inflammatory response induced by TWEAK that contributes to the observed effects on podocytes and the induction of proteinuria cannot be completely excluded. Therefore, in vivo, TWEAK may be contributing to renal injury by both inflammation-independent effects on barrier integrity as well as induction of inflammatory mediators. Which, if any, of these podocyte-specific pathways is dominant in contributing to renal disease in MRL/lpr mice remains to be determined.
In conclusion, the TWEAK/Fn14 pathway is instrumental in the pathogenesis of spontaneous LN in MRL/lpr mice. Elimination of Fn14 signaling significantly lessens kidney disease, most likely by directly preserving the integrity of the glomerular filtration barrier, reducing renal damage, and attenuating inflammation locally in the kidney rather than suppressing systemic immune responses. Targeting the TWEAK/Fn14 pathway may be a promising new renal-protective approach for the treatment of LN.
Concise Methods
Animal Models
Female MRL/MpJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and housed in the animal facility of the Albert Einstein College of Medicine. Fn14+/+ (WT) and Fn14−/− (KO) mice on the MRL-lpr/lpr (MRL/lpr) background were generated (backcross generation 9) at Biogen Idec under Biogen Idec Institutional Animal Care and Use Committee Protocol 339–1038 and transferred to Albert Einstein College of Medicine at 8–10 weeks of age. Mice for the study were generated by crossing of heterozygous mice; thus, littermates were used for the comparisons between MRL/lpr Fn14-WT and -KO mice. The Fn14-KO was introduced into the 000485 MRL/lpr strain (The Jackson Laboratory) in which the lupus-related phenotype is preserved. SNP analysis at the time of derivation revealed homozygosity for MRL origin at all known lupus susceptibility loci.
For the in vivo studies, groups of female MRL/MpJ or MRL/lpr mice at 8 weeks of age received a single intravenous injection of 200 µg Fc-TWEAK or the P1.17 isotype control; 6, 12, or 24 hours later, the mice were euthanized, and blood, urine, kidney, liver, and spleen were collected for additional analysis. All animal study protocols (20080701 and 20110703) were approved by the Albert Einstein College of Medicine Institutional Animal Care and Use Committee.
Measurement of Proteinuria and Biochemical Parameters
Body weight of mice was assessed biweekly. Proteinuria was monitored by Uristix Test Strips (Bayer), where gradations in color correspond to urine protein concentrations of 30, 100, 300, and >2000 mg/dl. Urine albumin (Bethyl Labs), BUN (BioAssay Systems), and urine and serum creatinine (BioAssay Systems) were determined according to the manufacturer’s instructions.
Real-Time PCR
Total RNA extraction, reverse transcription, and real-time PCR (in quadruplicate) were performed using methods previously described.58 The final expression levels of target genes were normalized to glyceraldehyde-3-phosphate dehydrogenase values, and the values were multiplied by 102 or 103 for display purposes. Primer sequences are listed in Supplemental Table 1.
IHC and Histologic Evaluation
Hematoxylin and eosin, periodic acid–Schiff, and trichrome staining of paraffin sections was performed by standard methods. The detection of glomerular IgG deposition was performed using biotinylated goat anti-mouse IgG (Vector Laboratories) and an NBT-BCIP solution (Roche Diagnostics) as described previously.2 For Fn14 staining, rabbit anti-mouse Fn14 mAb (Epitomics) was used followed by applying horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and then DAB-chromogen (Dako). The WT1 staining protocol was similar to that for Fn14 expression but with a primary rabbit antibody to mouse WT1 (Santa Cruz). IHC staining for CD3 (Thermo Fisher Scientific), Ki-67 (Vector Laboratories), and caspase-3 (Cell Signaling Technology) was performed using the Discovery XT Automated IHC Platform (Ventana Medical Systems), which includes CC1 mild antigen retrieval. The Bond Rx Automated IHC Platform (Leica Microsystems) was used for the staining of F4/80 (AbD Serotec, Raleigh, NC), B220 (BD Biosciences), and KIM-1/TIM-1 (BioExpress). ER1 (pH 6.0) or ER2 (pH 8.0) antigen retrieval was carried out. The refined HRP-polymer detection method and DAB detection system were used according to the manufacturers’ instructions. The stains were scored by a renal pathologist blinded to the mice grouping. The scoring systems used for quantitation are detailed in Supplemental Table 2.
TEM and IF Staining
As described previously,2 TEM and IF staining was performed for the detection of IgG deposition in glomeruli. The expressions of nephrin (rabbit antinephrin; Thermo Fisher Scientific) and podocin (rabbit antipodocin; Immuno-Biological Laboratories) were also determined by IF. FITC-labeled IgGs targeting goat or rabbit IgG were used as secondary antibodies. The intensities were quantitated by ImageJ (National Institutes of Health). WT1 was also detected by IF with positive nuclei (overlapped by 4′,6-diamidino-2-phenylindole staining) counted per glomerulus. Ten random glomeruli in each section were counted for the numbers of WT1-positive nuclei or measured for fluorescence intensity of nephrin and podocin expressions.
Determination of Serum Antibody Concentrations
Total IgG and IgG isotype concentrations were measured by ELISA in serum samples diluted 1:200 as described.16 Anti–double-stranded DNA, chromatin, and cardiolipin ELISAs were performed as previously described.2,40 Antibodies against ribosomal P (P0, P1, and P2) were measured using a commercially available kit from Aesku Diagnostics (Wendelsheim, Germany).38 Sera were diluted to 1:100, and OD values were normalized to the average absorbance of sera from two sick control MRL/lpr mice.
Scoring of Lymphadenopathy
As described previously,59 a lymphadenopathy score was assigned using a scale of 0–4, with 0=none, 1=a single palpable lymph node anywhere, 2=bilateral axillary, femoral, or cervical nodes, 3=generalized palpable femoral, axillary, and cervical nodes, and 4=massive generalized adenopathy.
FACS of Splenocytes
Lymph nodes and spleens were harvested and weighed at euthanasia. Fresh spleen tissues were digested using a mixture of collagenase D (Roche Diagnostics) and DNase I (Roche Diagnostics) in HBSS followed by filtration through a 40-μm nylon mesh. Erythrocytes were lysed using BD Pharm Lyse (BD Biosciences), and cells were counted using the Countess Automated Cell Counter (Invitrogen). Cells were stained with live/dead Aqua viability dyes (Invitrogen), incubated with Fc-block (anti-CD16/CD32; BD Biosciences), and stained with fluorochrome-conjugated antibodies as listed: antibodies for B220, CD3, CD4, CD11c, CD19, CD23, CD25, CD40, CD44, CD62L, CD69, CD86, CD93, CD138, IgM, F4/80, MHC II, and NK1.1 from BD Biosciences; antibodies for CD8, CD11b, CD21/35, Gr-1, IgD, TLR2, and TLR4 from eBioscience; and an antibody for PDCA-1 from Miltenyi. Data were acquired on an LSR II Flow Cytometer (BD Biosciences). Compensation and analysis of the FACS data were performed using FlowJo software (TreeStar, Inc.).
Cell Culture
Conditionally immortalized podocytes (MPC5; murine cell line) were grown at 33°C as described previously.60 The RPMI 1640 medium was supplemented with 10% FBS and 50 IU/ml IFN-γ (eBioscience), which allows MPC5 cells to proliferate (permissive). After being switched to 37°C and deprived of IFN-γ (nonpermissive), cells cease proliferation and differentiate along the podocyte lineage. Podocyte media were refreshed daily to minimize the residual concentration of IFN-γ before TWEAK stimulation, which was started 10–14 days after switching to IFN-γ–deficient media. Murine glomerular endothelial cells were cultured in RPMI 1640 media.61
In Vitro Permeability and Viability Assays
Podocytes and endothelial cells were separately seeded at 1×106 cells/ml in culture inserts placed in a 24-well plate.54 Cells were starved before a 48-hour stimulation with Fc-TWEAK (Biogen Idec), the control Ig P1.17 (Biogen Idec), or LPS (Sigma-Aldrich). FITC-labeled BSA (US Biologic) or dextran (150 kD; Sigma-Aldrich) was added to the media inside the insert at 50 µg/ml for 1 hour, after which time the media inside the wells were transferred to a black 96-well plate (Whatman). The fluorescein density was tested with a 1420 Multilabel Counter (PerkinElmer).61
For the viability assay, podocytes or glomerular endothelial cells were cultured in 96-well plates followed by stimulation as above. CellTiter 96 Solution (Promega) was used for the detection of cell viability.62 In some of the permeability and viability experiments, the caspase inhibitor z-VAD-fmk (G-Biosciences) was added to the media at a concentration of 20 µM during Fc-TWEAK stimulation.
siRNA Transfection
Fn14 and control siRNA and Lipofectamine 2000 reagent were purchased from Life Technologies (Supplemental Table 1). Glomerular endothelial cells were plated in growth medium without antibiotics, such that they would be 50% confluent at transfection. Oligomer-Lipofectamine 2000 complexes (15 pmol RNA:1.5 µl reagent) were prepared and then added to the culture wells. For podocytes, the transfection was performed 7 days after MPC5 cells were deprived of IFN-γ and moved to grow at 37°C.63 Cells were used for assays 3 days after transfection. The efficiency of transfection was determined by quantitative RT-PCR for Fn14 mRNA expression.
Western Blot Analyses
Lysates were extracted from cell cultures by RIPA lysis buffer (Thermo Fisher Scientific) with the addition of the protease inhibitor cocktail (Thermo Fisher Scientific). The 4%–15% Mini-proTEAN Precast Gel (Bio-Rad) was used to run the lysate samples at denatured and reduced conditions followed by protein transfer to a PVDF membrane (EMD Millipore). Rabbit antinephrin, antipodocin, or anti–ZO-1 (EMD Millipore) was applied initially with goat anti-rabbit HRP (Southern Biotech) as the secondary antibody. β-Actin was detected using an HRP-conjugated rabbit antibody (Cell Signaling Technology). The ImageJ quantitated intensity of bands was normalized to β-actin.
Statistical Methods
Data were expressed as means±SEMs. Statistical analysis was performed using the JMP (SAS). An unpaired t test (two tailed) was used to compare the differences between two groups after significant effects were observed in an ANOVA. Chi-squared test was used for the ratio comparison. Spearman rank correlation coefficients were used to analyze the relationship between the doses of Fc-TWEAK (and other reagents used for stimulation) and the permeability/viability indexes. Differences were considered significant at P<0.05.
Disclosures
L.W., P.W., and L.C.B. are full-time employees and stockholders of Biogen Idec. These studies were supported by a research grant from Biogen Idec (to C.P.) and National Institutes of Health Grant R01-DK090319 (to C.P.).
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant R01-DK090319 (to C.P.) and a research grant from Biogen Idec (to C.P.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014030233/-/DCSupplemental.
References
- 1.Borchers AT, Leibushor N, Naguwa SM, Cheema GS, Shoenfeld Y, Gershwin ME: Lupus nephritis: A critical review. Autoimmun Rev 12: 174–194, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Xia Y, Pawar RD, Nakouzi AS, Herlitz L, Broder A, Liu K, Goilav B, Fan M, Wang L, Li QZ, Casadevall A, Putterman C: The constant region contributes to the antigenic specificity and renal pathogenicity of murine anti-DNA antibodies. J Autoimmun 39: 398–411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lech M, Anders HJ: The pathogenesis of lupus nephritis. J Am Soc Nephrol 24: 1357–1366, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burkly LC, Michaelson JS, Zheng TS: TWEAK/Fn14 pathway: An immunological switch for shaping tissue responses. Immunol Rev 244: 99–114, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Brown SA, Cheng E, Williams MS, Winkles JA: TWEAK-independent Fn14 self-association and NF-κB activation is mediated by the C-terminal region of the Fn14 cytoplasmic domain. PLoS ONE 8: e65248, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruiz-Ortega M, Ortiz A, Ramos AM: Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) and kidney disease. Curr Opin Nephrol Hypertens 23: 93–100, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Burkly LC, Dohi T: The TWEAK/Fn14 pathway in tissue remodeling: For better or for worse. Adv Exp Med Biol 691: 305–322, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Lynch CN, Wang YC, Lund JK, Chen YW, Leal JA, Wiley SR: TWEAK induces angiogenesis and proliferation of endothelial cells. J Biol Chem 274: 8455–8459, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Ucero AC, Benito-Martin A, Fuentes-Calvo I, Santamaria B, Blanco J, Lopez-Novoa JM, Ruiz-Ortega M, Egido J, Burkly LC, Martinez-Salgado C, Ortiz A: TNF-related weak inducer of apoptosis (TWEAK) promotes kidney fibrosis and Ras-dependent proliferation of cultured renal fibroblast. Biochim Biophys Acta 1832: 1744–1755, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Sanz AB, Sanchez-Niño MD, Ortiz A: TWEAK, a multifunctional cytokine in kidney injury. Kidney Int 80: 708–718, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Michaelson JS, Wisniacki N, Burkly LC, Putterman C: Role of TWEAK in lupus nephritis: A bench-to-bedside review. J Autoimmun 39: 130–142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao HX, Campbell SR, Burkly LC, Jakubowski A, Jarchum I, Banas B, Saleem MA, Mathieson PW, Berman JW, Michaelson JS, Putterman C: TNF-like weak inducer of apoptosis (TWEAK) induces inflammatory and proliferative effects in human kidney cells. Cytokine 46: 24–35, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Campbell S, Burkly LC, Gao HX, Berman JW, Su L, Browning B, Zheng T, Schiffer L, Michaelson JS, Putterman C: Proinflammatory effects of TWEAK/Fn14 interactions in glomerular mesangial cells. J Immunol 176: 1889–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Molano A, Lakhani P, Aran A, Burkly LC, Michaelson JS, Putterman C: TWEAK stimulation of kidney resident cells in the pathogenesis of graft versus host induced lupus nephritis. Immunol Lett 125: 119–128, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Zhao Z, Burkly LC, Campbell S, Schwartz N, Molano A, Choudhury A, Eisenberg RA, Michaelson JS, Putterman C: TWEAK/Fn14 interactions are instrumental in the pathogenesis of nephritis in the chronic graft-versus-host model of systemic lupus erythematosus. J Immunol 179: 7949–7958, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Xia Y, Campbell SR, Broder A, Herlitz L, Abadi M, Wu P, Michaelson JS, Burkly LC, Putterman C: Inhibition of the TWEAK/Fn14 pathway attenuates renal disease in nephrotoxic serum nephritis. Clin Immunol 145: 108–121, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz N, Rubinstein T, Burkly LC, Collins CE, Blanco I, Su L, Hojaili B, Mackay M, Aranow C, Stohl W, Rovin BH, Michaelson JS, Putterman C: Urinary TWEAK as a biomarker of lupus nephritis: A multicenter cohort study. Arthritis Res Ther 11: R143, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz N, Su L, Burkly LC, Mackay M, Aranow C, Kollaros M, Michaelson JS, Rovin B, Putterman C: Urinary TWEAK and the activity of lupus nephritis. J Autoimmun 27: 242–250, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Xuejing Z, Jiazhen T, Jun L, Xiangqing X, Shuguang Y, Fuyou L: Urinary TWEAK level as a marker of lupus nephritis activity in 46 cases. J Biomed Biotechnol 2012: 359647, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Shehaby A, Darweesh H, El-Khatib M, Momtaz M, Marzouk S, El-Shaarawy N, Emad Y: Correlations of urinary biomarkers, TNF-like weak inducer of apoptosis (TWEAK), osteoprotegerin (OPG), monocyte chemoattractant protein-1 (MCP-1), and IL-8 with lupus nephritis. J Clin Immunol 31: 848–856, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Lu J, Szeto CC, Tam LS, Lai FM, Li EK, Chow KM, Li PK, Kwan BC: Relationship of intrarenal gene expression and the histological class of lupus nephritis — a study on repeat renal biopsy. J Rheumatol 39: 1942–1947, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Lu J, Kwan BC, Lai FM, Choi PC, Tam LS, Li EK, Chow KM, Wang G, Li PK, Szeto CC: Gene expression of TWEAK/Fn14 and IP-10/CXCR3 in glomerulus and tubulointerstitium of patients with lupus nephritis. Nephrology (Carlton) 16: 426–432, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Liu ZC, Zhou QL, Li XZ, Yang JH, Ao X, Veeraragoo P, Zuo XX: Elevation of human tumor necrosis factor-like weak inducer of apoptosis in peripheral blood mononuclear cells is correlated with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Cytokine 53: 295–300, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Lee TP, Chiang BL: Sex differences in spontaneous versus induced animal models of autoimmunity. Autoimmun Rev 11: A422–A429, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa H: Chemokine blockade for lupus model mice. Front Biosci 13: 2900–2908, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Li L, Nukala S, Du Y, Han J, Liu K, Hutcheson J, Pathak S, Li Q, Mohan C: Murine lupus strains differentially model unique facets of human lupus serology. Clin Exp Immunol 168: 178–185, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinjoh K, Kyogoku M, Good RA: Genetic selection for crescent formation yields mouse strain with rapidly progressive glomerulonephritis and small vessel vasculitis. Proc Natl Acad Sci U S A 90: 3413–3417, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kyogoku M, Nose M, Sawai T, Miyazawa M, Tachiwaki O, Kawashima M: Immunopathology of murine lupus-overview, SL/Ni and MRL/Mp-lpr/lpr-. Prog Clin Biol Res 229: 95–130, 1987 [PubMed] [Google Scholar]
- 29.Brookoff D, Weiss L: Ultrastructure of the bone marrow in three murine strains with non-malignant lymphoproliferative syndromes NZBxW, BXSB, and MRL/lpr. Anat Rec 207: 451–459, 1983 [DOI] [PubMed] [Google Scholar]
- 30.Ortega LM, Schultz DR, Lenz O, Pardo V, Contreras GN: Review: Lupus nephritis: Pathologic features, epidemiology and a guide to therapeutic decisions. Lupus 19: 557–574, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Jónsdóttir T, Zickert A, Sundelin B, Henriksson EW, van Vollenhoven RF, Gunnarsson I: Long-term follow-up in lupus nephritis patients treated with rituximab—clinical and histopathological response. Rheumatology (Oxford) 52: 847–855, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Koh AE, Njoroge SW, Feliu M, Cook A, Selig MK, Latchman YE, Sharpe AH, Colvin RB, Paul E: The SLAM family member CD48 (Slamf2) protects lupus-prone mice from autoimmune nephritis. J Autoimmun 37: 48–57, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Racz Z, Nagy E, Rosivall L, Szebeni J, Hamar P: Sugar-free, glycine-stabilized intravenous immunoglobulin prevents skin but not renal disease in the MRL/lpr mouse model of systemic lupus. Lupus 19: 599–612, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Jonat W, Arnold N: Is the Ki-67 labelling index ready for clinical use? Ann Oncol 22: 500–502, 2011 [DOI] [PubMed] [Google Scholar]
- 35.Ichimura T, Brooks CR, Bonventre JV: Kim-1/Tim-1 and immune cells: Shifting sands. Kidney Int 81: 809–811, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh C, Chang A, Brandt D, Guttikonda R, Utset TO, Clark MR: Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res (Hoboken) 63: 865–874, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hotta K, Sho M, Yamato I, Shimada K, Harada H, Akahori T, Nakamura S, Konishi N, Yagita H, Nonomura K, Nakajima Y: Direct targeting of fibroblast growth factor-inducible 14 protein protects against renal ischemia reperfusion injury. Kidney Int 79: 179–188, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Wen J, Xia Y, Stock A, Michaelson JS, Burkly LC, Gulinello M, Putterman C: Neuropsychiatric disease in murine lupus is dependent on the TWEAK/Fn14 pathway. J Autoimmun 43: 44–54, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bizzaro N, Villalta D, Giavarina D, Tozzoli R: Are anti-nucleosome antibodies a better diagnostic marker than anti-dsDNA antibodies for systemic lupus erythematosus? A systematic review and a study of metanalysis. Autoimmun Rev 12: 97–106, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Gharavi AE, Mellors RC, Elkon KB: IgG anti-cardiolipin antibodies in murine lupus. Clin Exp Immunol 78: 233–238, 1989 [PMC free article] [PubMed] [Google Scholar]
- 41.Moisini I, Huang W, Bethunaickan R, Sahu R, Ricketts PG, Akerman M, Marion T, Lesser M, Davidson A: The Yaa locus and IFN-α fine-tune germinal center B cell selection in murine systemic lupus erythematosus. J Immunol 189: 4305–4312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanly JG, Thompson K, McCurdy G, Fougere L, Theriault C, Wilton K: Measurement of autoantibodies using multiplex methodology in patients with systemic lupus erythematosus. J Immunol Methods 352: 147–152, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Sun KH, Liu WT, Tang SJ, Tsai CY, Hsieh SC, Wu TH, Han SH, Yu CL: The expression of acidic ribosomal phosphoproteins on the surface membrane of different tissues in autoimmune and normal mice which are the target molecules for anti-double-stranded DNA antibodies. Immunology 87: 362–371, 1996 [PMC free article] [PubMed] [Google Scholar]
- 44.Sun L, Zhou L, Chen M, Zhong R, Liu J: Amelioration of systemic lupus erythematosus by Withangulatin A in MRL/lpr mice. J Cell Biochem 112: 2376–2382, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Lu LD, Stump KL, Wallace NH, Dobrzanski P, Serdikoff C, Gingrich DE, Dugan BJ, Angeles TS, Albom MS, Mason JL, Ator MA, Dorsey BD, Ruggeri BA, Seavey MM: Depletion of autoreactive plasma cells and treatment of lupus nephritis in mice using CEP-33779, a novel, orally active, selective inhibitor of JAK2. J Immunol 187: 3840–3853, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Sanchez-Niño MD, Poveda J, Sanz AB, Mezzano S, Carrasco S, Fernandez-Fernandez B, Burkly LC, Nair V, Kretzler M, Hodgin JB, Ruiz-Ortega M, Selgas R, Egido J, Ortiz A: Fn14 in podocytes and proteinuric kidney disease. Biochim Biophys Acta 1832: 2232–2243, 2013 [DOI] [PubMed] [Google Scholar]
- 47.Perysinaki GS, Moysiadis DK, Bertsias G, Giannopoulou I, Kyriacou K, Nakopoulou L, Boumpas DT, Daphnis E: Podocyte main slit diaphragm proteins, nephrin and podocin, are affected at early stages of lupus nephritis and correlate with disease histology. Lupus 20: 781–791, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Moysiadis DK, Perysinaki GS, Bertsias G, Stratakis S, Kyriacou K, Nakopoulou L, Boumpas DT, Daphnis E: Early treatment with glucocorticoids or cyclophosphamide retains the slit diaphragm proteins nephrin and podocin in experimental lupus nephritis. Lupus 21: 1196–1207, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Wagner N, Wagner KD, Xing Y, Scholz H, Schedl A: The major podocyte protein nephrin is transcriptionally activated by the Wilms’ tumor suppressor WT1. J Am Soc Nephrol 15: 3044–3051, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Chicheportiche Y, Bourdon PR, Xu H, Hsu YM, Scott H, Hession C, Garcia I, Browning JL: TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J Biol Chem 272: 32401–32410, 1997 [DOI] [PubMed] [Google Scholar]
- 51.Kato T, Mizuno S, Kamimoto M: The decreases of nephrin and nuclear WT1 in podocytes may cause albuminuria during the experimental sepsis in mice. Biomed Res 31: 363–369, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Grahammer F, Schell C, Huber TB: The podocyte slit diaphragm—from a thin grey line to a complex signalling hub. Nat Rev Nephrol 9: 587–598, 2013 [DOI] [PubMed] [Google Scholar]
- 53.Goldwich A, Steinkasserer A, Gessner A, Amann K: Impairment of podocyte function by diphtheria toxin—a new reversible proteinuria model in mice. Lab Invest 92: 1674–1685, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Stephan D, Sbai O, Wen J, Couraud PO, Putterman C, Khrestchatisky M, Desplat-Jégo S: TWEAK/Fn14 pathway modulates properties of a human microvascular endothelial cell model of blood brain barrier. J Neuroinflammation 10: 9, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikner A, Ashkenazi A: TWEAK induces apoptosis through a death-signaling complex comprising receptor-interacting protein 1 (RIP1), Fas-associated death domain (FADD), and caspase-8. J Biol Chem 286: 21546–21554, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabour Alaoui S, Dessirier V, de Araujo E, Alexaki VI, Pelekanou V, Lkhider M, Stathopoulos EN, Castanas E, Bagot M, Bensussan A, Tsapis A: TWEAK affects keratinocyte G2/M growth arrest and induces apoptosis through the translocation of the AIF protein to the nucleus. PLoS ONE 7: e33609, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Justo P, Sanz AB, Sanchez-Niño MD, Winkles JA, Lorz C, Egido J, Ortiz A: Cytokine cooperation in renal tubular cell injury: The role of TWEAK. Kidney Int 70: 1750–1758, 2006 [DOI] [PubMed] [Google Scholar]
- 58.Alexaki VI, Notas G, Pelekanou V, Kampa M, Valkanou M, Theodoropoulos P, Stathopoulos EN, Tsapis A, Castanas E: Adipocytes as immune cells: Differential expression of TWEAK, BAFF, and APRIL and their receptors (Fn14, BAFF-R, TACI, and BCMA) at different stages of normal and pathological adipose tissue development. J Immunol 183: 5948–5956, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Jevnikar AM, Grusby MJ, Glimcher LH: Prevention of nephritis in major histocompatibility complex class II-deficient MRL-lpr mice. J Exp Med 179: 1137–1143, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 61.Pawar RD, Castrezana-Lopez L, Allam R, Kulkarni OP, Segerer S, Radomska E, Meyer TN, Schwesinger CM, Akis N, Gröne HJ, Anders HJ: Bacterial lipopeptide triggers massive albuminuria in murine lupus nephritis by activating Toll-like receptor 2 at the glomerular filtration barrier. Immunology 128[Suppl]: e206–e221, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pawar RD, Pitashny M, Gindea S, Tieng AT, Levine B, Goilav B, Campbell SR, Xia Y, Qing X, Thomas DB, Herlitz L, Berger T, Mak TW, Putterman C: Neutrophil gelatinase-associated lipocalin is instrumental in the pathogenesis of antibody-mediated nephritis in mice. Arthritis Rheum 64: 1620–1631, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim EY, Choi KJ, Dryer SE: Nephrin binds to the COOH terminus of a large-conductance Ca2+-activated K+ channel isoform and regulates its expression on the cell surface. Am J Physiol Renal Physiol 295: F235–F246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.