Abstract
The Organ Procurement and Transplantation Network gives priority in kidney allocation to prior live organ donors who require a kidney transplant. In this study, we analyzed the effect of this policy on facilitating access to transplantation for prior donors who were wait-listed for kidney transplantation in the United States. Using 1:1 propensity score–matching methods, we assembled two matched cohorts. The first cohort consisted of prior organ donors and matched nondonors who were wait-listed during the years 1996–2010. The second cohort consisted of prior organ donors and matched nondonors who underwent deceased donor kidney transplantation. During the study period, there were 385,498 listings for kidney transplantation, 252 of which were prior donors. Most prior donors required dialysis by the time of listing (64% versus 69% among matched candidates; P=0.24). Compared with matched nondonors, prior donors had a higher rate of deceased donor transplant (85% versus 33%; P<0.001) and a lower median time to transplantation (145 versus 1607 days; P<0.001). Prior donors received higher-quality allografts (median kidney donor risk index 0.67 versus 0.90 for nondonors; P<0.001) and experienced lower post-transplant mortality (hazard ratio, 0.19; 95% confidence interval, 0.08 to 0.46; P<0.001) than matched nondonors. In conclusion, these data suggest that prior organ donors experience brief waiting time for kidney transplant and receive excellent-quality kidneys, but most need pretransplant dialysis. Individuals who are considering live organ donation should be provided with this information because this allocation priority will remain in place under the new US kidney allocation system.
Keywords: kidney transplantation, epidemiology, outcomes, live donors
Candidates for living organ donation in the United States typically undergo extensive medical and psychosocial evaluation in order to estimate their risks, understanding, and motivations for donating an organ.1,2 Approximately 5000–6000 adults become live kidney donors and 200–250 adults become live liver donors each year in the United States. Other forms of live organ donation, such as lung lobe donation, are rare. Research over the last 2 decades has provided detailed information about the magnitude of the diverse risks associated with living donor nephrectomy in particular. Compared with demographically matched and healthy individuals in the general population, kidney donors appear to have similar (or better) longevity and rates of cardiovascular outcomes.3–5 Most prior kidney donors endorse a good quality of life after donation.6 However, recent research has also revealed that kidney donors have an elevated relative risk for ESRD.7,8 Transplant outcomes for prior donors who became candidates for kidney transplantation have not been reported.
For kidney donation, the medical evaluation usually includes assessment of kidney function, including eGFR or measured GFR, quantification of proteinuria, imaging of the kidneys, and nephrology consultation.2 This evaluation is designed to enable transplant centers to select donors with a very low risk of ESRD after donation. A study by Ibrahim et al. of mostly Caucasian donors at a single center reported a rate of 180 cases of ESRD per million persons per year, which was lower than the rate in the general population.3 Given that kidney donors are likely to be healthier than much of the general population, studies comparing live donors with healthy matched controls have demonstrated much higher relative risks than those reported by Ibrahim et al. but have also confirmed low absolute risks of ESRD after kidney donation. In a study of 96,217 kidney donors and healthy matched controls from the National Health and Nutrition Examination Survey, Muzaale et al. reported an 8-fold higher rate of ESRD associated with kidney donation. However, the 15-year cumulative incidence was <1% in all ethnic groups.7 Among kidney donors and healthy controls in Norway, Mjøen et al. demonstrated a hazard ratio (HR) of 8.4 associated with kidney donation.8 Studies of risk factors for renal disease among kidney donors have consistently demonstrated that, similar to the general population, black race confers a risk of ESRD that is 2–5 times higher than among Caucasian donors.9–11
To recognize the contribution to organ transplantation by live donors, the Organ Procurement and Transplantation Network (OPTN) began giving priority in 1996 to prior organ donors in the allocation of deceased donor kidneys for transplantation.12 However, there are minimal data about prior organ donors who were wait-listed for kidney transplantation in the United States, including the proportion of preemptive transplants, duration of waiting time, and organ quality.13 A better understanding of these outcomes, in particular waiting time for a transplant, is salient to the patient and clinician decision-making process when considering live kidney donation. The primary aims of this study were (1) to compare time to deceased donor kidney transplantation (DDKT) between donors and nondonors, and (2) to compare the quality of kidney allografts and allograft survival among prior donors versus nondonors.
Results
From January 1, 1996 to December 31, 2010, a total of 239 kidney transplant candidates were identified as having been prior live organ donors. Because some prior donors received a transplant, experienced transplant failure, and were added again to the waiting list, the waiting-list cohort comprised 252 listings.
Table 1 compares the demographic characteristics of the 239 transplant candidates (95% of the total of 252) who were prior donors and 239 nondonor matched pairs. Table 1 also shows characteristics of all kidney transplant listings during the study period. Among prior donors, the median age at the time of listing was 52 years and 57% of were men. Forty-three percent of prior organ donors listed for a transplant were black, versus only 28% of all listed candidates. Compared with all listed candidates, a higher percentage of prior organ donors had college education (46% of prior donors versus 39% of candidates overall). Notably, among the 239 wait-listings by prior donors, 7% had previously received a kidney transplant. The most common cause of ESRD listed for prior donors was hypertension, followed by GN and diabetes.
Table 1.
Demographic and clinical characteristics of candidates listed for a kidney transplant, stratified by prior organ donor status (matched)
| Variable | Prior Donors (n=239) | Matched Nondonors (n=239) | P Value | Standard Difference | All Candidates (n=385,246) |
|---|---|---|---|---|---|
| Age (yr)a | 52 (44, 60) | 52 (41, 61) | 0.54 | 0.056 | 50 |
| Mena | 137 (57) | 146 (61) | 0.40 | −0.077 | 229,831 (60) |
| Black racea | 103 (43) | 98 (41) | 0.64 | 0.042 | 107,121 (28) |
| Blood typea | 0.97 | −0.043 | |||
| A | 46 (19) | 42 (18) | 128,872 (34) | ||
| AB | 2 (1) | 2 (1) | 14,659 (4) | ||
| B | 31 (13) | 31 (13) | 55,289 (14) | ||
| O | 160 (67) | 164 (69) | 186,426 (48) | ||
| Diabetesa | 44 (18) | 42 (18) | 0.81 | 0.022 | 154,583 (40) |
| Private insurancea | 133 (56) | 130 (54) | 0.78 | 0.025 | 171,213 (44) |
| College educationa | 109 (46) | 111 (46) | 0.85 | −0.017 | 149,597 (39) |
| SES index category (quartile)a | 0.88 | 0.037 | |||
| First (lowest) | 66 (28) | 71 (30) | 96,635 (28) | ||
| Second | 46 (19) | 47 (20) | 67,225 (19) | ||
| Third | 40 (17) | 34 (14) | 71,464 (20) | ||
| Fourth (highest) | 87 (36) | 87 (36) | 117,225 (33) | ||
| Previous transplanta | 16 (7) | 19 (8) | 0.60 | −0.048 | 58,557 (15) |
| PRA (%)a | 0.41 | 0.104 | |||
| <20 | 128 (54) | 144 (60) | 200,547 (52) | ||
| 20–80 | 9 (4) | 5 (2) | 23,156 (6) | ||
| >80 | 5 (2) | 5 (2) | 12,841 (3) | ||
| Missing | 97 (40) | 85 (36) | 148,702 (39) | ||
| BMI | 28.6 | 26.3 | <0.001 | 26.6 | |
| Cause of ESRD | 0.21 | ||||
| Diabetes | 29 (12) | 32 (13) | 124,847 (32) | ||
| Hypertension | 77 (32) | 84 (35) | 84,576 (22) | ||
| Immune mediated | 70 (29) | 50 (21) | 82,808 (22) | ||
| Other | 63 (27) | 73 (31) | 93,015 (24) | ||
| Dialysis at listing | 154 (64) | 166 (69) | 0.24 | 279,905 (73) | |
| Type of transplant | <0.001 | ||||
| DDKT | 203 (85) | 78 (33) | 139,701 (36) | ||
| LDKT | 4 (2) | 38 (16) | 53,611 (14) | ||
| Not transplanted | 32 (13) | 123 (51) | 191,934 (50) |
Data are presented as n (%) or median (interquartile range). P values are related to the comparison of prior donors to matched pairs. SES, socioeconomic status; LDKT, live donor kidney transplant; BMI, body mass index.
Characteristics used to match donors and nondonors. Year of listing was also used in matching.
Most prior organ donors were receiving dialysis by the time of wait-listing. The rate of dialysis at the time of kidney transplant listing was not significantly different between prior donors and matched pairs (64% versus 69%; P=0.24). We also divided the 15-year study period into four intervals (1996–1999, 2000–2003, 2004–2007, and 2008–2010) and examined the percentage of prior donors on dialysis at listing. The percentages of donors on dialysis at listing was 72%, 72%, 65%, and 56%, respectively, during these time intervals (P=0.19 for comparison across intervals). These results are presented in Supplemental Appendix 1.
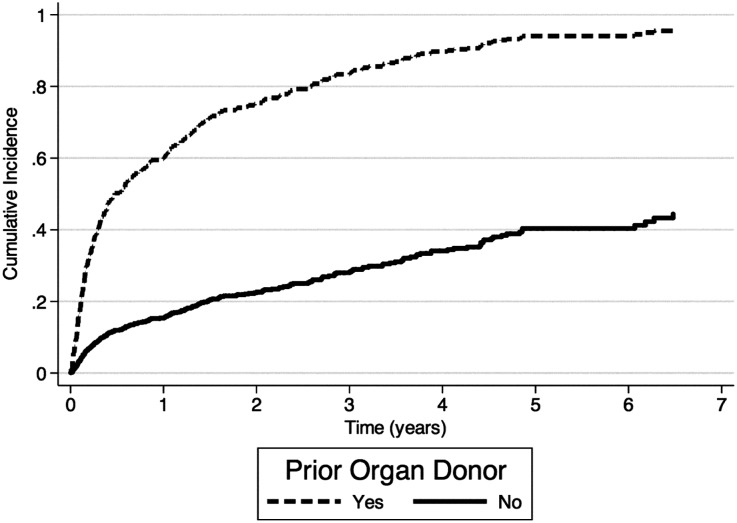
However, prior donors had brief waiting times to DDKT. The median time to DDKT was 145 days for prior organ donors, compared with 1607 days for matched candidates. Using a competing-risks approach, Figure 1 illustrates the much shorter waiting time for DDKT for prior donors compared with nondonor candidates. Similarly, using a conventional Kaplan–Meier approach, Supplemental Appendix 2 shows shorter waiting time for prior donors.
Figure 1.
Cumulative incidence of DDKT among prior organ donors and matched nondonor candidates, treating death and live donor transplantation as competing risks.
Prior organ donors were more likely than matched candidates to receive a deceased donor kidney transplant in 3 years (80% versus 21% of matched pairs; P<0.001). Prior donors were much less likely to receive a live donor kidney transplant (2% versus 16% of matched pairs) during the study period. Prior organ donors were less likely to die before transplantation (5% versus 13%; HR, 0.36; 95% confidence interval [95% CI], 0.18 to 0.71; P=0.003). Median follow-up time from the date of wait-listing was 1766 days in the matched wait-listed cohort.
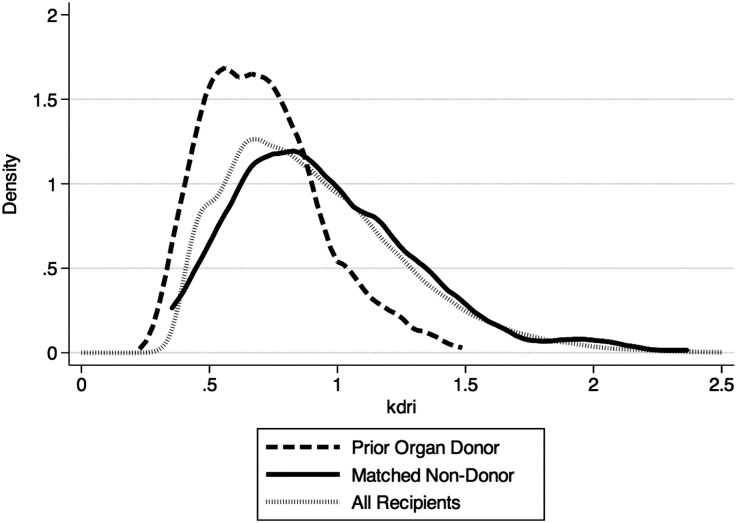
Table 2 shows characteristics of the 208 DDKT recipients who were prior donors and their nondonor matched pairs. At transplantation, 74% of recipients had received chronic dialysis, whereas 7% were hepatitis C seropositive. Figure 2 demonstrates that prior organ donors received higher-quality kidneys as measured by the kidney donor risk index (KDRI) (donor versus nondonor KDRI scores of 0.67 versus 0.90; P<0.001).
Table 2.
Demographic and clinical characteristics of DDKT recipients, stratified by prior organ donor status (matched)
| Variable | Prior Donors (n=208) | Matched Nondonor (n=208) | P Value | Standard Difference | All Recipients (n=121,308) |
|---|---|---|---|---|---|
| Age (yr)a | 52 (44,61) | 52 (44,61) | <0.001 | −0.050 | 52 (42, 61) |
| Mena | 121 (58) | 115 (55) | 0.55 | 0.084 | 73,585 (61) |
| Black racea | 95 (46) | 104 (50) | 0.38 | −0.086 | 36,796 (30) |
| Previous transplanta | 12 (6) | 8 (4) | 0.36 | 0.090 | 16,431 (14) |
| College educationa | 97 (47) | 96 (46) | 0.92 | 0.010 | 44,346 (37) |
| Blood typea | 0.30 | <0.001 | |||
| A | 37 (18) | 32 (15) | 46, 536 (38) | ||
| AB | 2 (1) | 7 (3) | 6695 (6) | ||
| B | 28 (14) | 33 (16) | 14,906 (12) | ||
| O | 141 (68) | 136 (66) | 53,171 (44) | ||
| Pretransplant dialysis | 153 (74) | 154 (74) | 0.91 | −0.011 | 109,377 (90) |
| Hepatitis C seropositivea | 15 (7) | 15 (7) | 1 | <0.001 | 6911 (6) |
| Diabetesa | 36 (17) | 38 (18) | 0.80 | −0.025 | 39,316 (32) |
| PRAa | 0.65 | 0.059 | |||
| <20 | 113 (54) | 120 (58) | 76,297 (63) | ||
| 20–80 | 8 (4) | 5 (2) | 7726 (6) | ||
| >80 | 3 (1) | 5 (2) | 3457 (3) | ||
| Missing | 84 (40) | 78 (38) | 33,828 (28) | ||
| SES index category (quartile)a | 0.88 | 0.016 | |||
| First (lowest) | 60 (29) | 60 (29) | 33,240 (28) | ||
| Second | 41 (20) | 40 (19) | 23,884 (20) | ||
| Third | 36 (17) | 42 (20) | 25,222 (21) | ||
| Fourth (highest) | 71 (34) | 66 (32) | 36,216 (31) | ||
| BMI | 28.9 | 27 | 0.002 | 26.9 | |
| Cause of ESRD | 0.28 | ||||
| Diabetes | 26 (12) | 32 (15) | 30,195 (25) | ||
| Hypertension | 74 (36) | 79 (38) | 32,603 (27) | ||
| Immune mediated | 63 (30) | 46 (22) | 30,491 (25) | ||
| Other | 45 (22) | 51 (25) | 28,019 (23) | ||
| KDRI score | 0.67 (0.54, 0.83) | 0.90 (0.71, 1.21) | <0.001 | 0.79 (0.53, 1.12) |
Data are presented as n (%) or median (interquartile range). P values are related to the comparison of prior donors to matched pairs. SES, socioeconomic status; BMI, body mass index.
Characteristics used to match donors and nondonors. Year of transplant was also used in matching.
Figure 2.
Distribution of KDRI scores among prior donor versus nondonor kidney transplant recipients.
Table 3 shows the results of Cox proportional hazards analysis of the outcomes of mortality and all-cause allograft failure. Prior live organ donors had lower mortality than matched recipients (HR, 0.19; 95% CI, 0.08 to 0.46; P<0.001). However, all-cause graft failure did not vary significantly between the two groups (HR, 0.84; 95% CI, 0.47 to 1.50; P=0.56). Median follow-up time, calculated from the date of transplant, for prior donors and matched pairs in the recipient cohort was 1634 days.
Table 3.
Wait-list and kidney transplant outcomes among prior live donors and matched nondonor controls with Cox regression analysis
| Outcome | HR (95% CI) | P Value |
|---|---|---|
| Candidate cohort | ||
| Pretransplant mortality | 0.36 (0.18 to 0.71) | 0.003 |
| Transplant | 7.35 (4.70 to 11.4) | <0.001 |
| Kidney transplant recipient cohort | ||
| Mortality | 0.19 (0.08 to 0.46) | <0.001 |
| All-cause allograft failure | 0.84 (0.47 to 1.50) | 0.56 |
Sensitivity analysis confirmed that for the outcome of all-cause post-transplant mortality, the finding of lower mortality among prior kidney donors was robust across a variety of distributions of an unmeasured confounder with a strong association with mortality. For example, using a baseline prevalence of 10% in the donor population of a confounder that confers an increased hazard of death of 5.0, an unmeasured confounder would need to have a prevalence of 40% among nondonors (four times the prevalence among donors) to make the association between prior donor status and improved survival nonsignificant. These results are presented in Supplemental Appendix 3.
We performed secondary analyses in which we used propensity scores to match each prior kidney donor 1:3 to nondonors. Results were very similar to those found using 1:1 matching and are therefore not shown. We also performed secondary analyses in which we included only those donors (n=224) who were added to the waiting list once during the observation period. The results did not deviate from results using 1:1 matching and are therefore not shown.
Discussion
Prior living organ donors that subsequently developed ESRD constitute a small proportion of the candidates waiting for a kidney transplant. In this study, we analyzed the effect of the OPTN policy that assigns priority for prior organ donors on the kidney transplant waiting list. The results show that the median time to kidney transplantation is significantly lower among prior organ donors compared with matched controls (145 versus 1607 days). Prior organ donors also had significantly better post-transplant survival compared with matched controls. However, most prior organ donors required dialysis before transplant.
As per OPTN policy 12.9.3, prior organ donors (defined as having donated a kidney or a segment of lung, liver, or small bowel) receive four additional points for organ allocation if they require organ transplantation after donation.14 Our study shows that this policy appears to be effective at minimizing waiting time and allowing transplant centers to select very high-quality transplants for prior donors. Although recent studies demonstrate that progression to ESRD is a rare outcome among live organ donors, live kidney donation increases the relative risk of ESRD and this risk should be addressed during the processes of informed consent.8,15 Our results will provide more specific and valuable information to potential organ donors about waiting times and outcomes if they require a kidney transplant in the future.
Under the new kidney allocation system, prior live organ donors will continue to receive four points in kidney allocation and local priority for kidneys that are not first shared for allocation to 99% panel reactive antibody (PRA) candidates, to recipients of multiorgan transplants, or to recipients of zero-antigen mismatched candidates. Therefore, the new kidney allocation policy is expected to have minimal effect on prior donors because their priority will supersede the priority given to candidates based on longevity matching (in which the top 20% of kidneys by the kidney donor profile index are allocated to the top 20% of candidates by the estimated post-transplant survival score). In addition, we found that 7% of prior live donors had already undergone transplantation before listing for kidney transplantation during the period of study. The OPTN policy of providing priority in kidney allocation for prior organ donors will apply to these prior donors at every listing for kidney transplantation, regardless of prior transplant status.
Several different reasons may account for the reduced post-transplant mortality observed among prior donors. Although we matched donors to nondonors on a series of important characteristics, there are plausible residual confounders that are distinct to the organ donor population. For example, organ donors undergo extensive psychosocial evaluation.2 As a result, donors may have greater social resources that help improve their outcomes after kidney transplant. The observed mortality benefit may reflect better medication adherence or enhanced access to health care among prior donors. As a second example, transplant centers may provide more attentive care to prior donors, due to a sense of responsibility for their outcomes. In addition, prior organ donors had much shorter waiting-list time, which may have allowed them to avoid health deterioration related to CKD. However, if access to health care is better among prior organ donors, it is perhaps surprising that the need for dialysis at the time of listing did not vary significantly between prior organ donors and matched controls. It is possible that many prior kidney donors were not referred in a timely way to nephrology, or that some had unexpectedly rapid progression to ESRD. Notably, the percentage of prior live organ donors who were on dialysis at the time of listing declined from 72% to 56% during the study period, although this difference was not statistically significant (P=0.19).
Our study is subject to certain limitations, in addition to the possibility of unmeasured confounding. Although the OPTN maintains records about individuals who received elevated waiting-list priority due to prior organ donor status, important historical information about these donors (e.g., date of donation and type of organ) was not recorded in the OPTN database for individuals whose organ donation took place before 1987.16 The OPTN contract was first awarded in 1986. However, the type of organ previously donated is not relevant to the main outcomes from our study related to waiting time and allograft quality at transplant. Moreover, it is likely that all or most of the prior organ donors in this study were kidney donors, because 95% of all live donors in the United States are kidney donors.17 The primary strength of the study is the use of national registry data with excellent ascertainment of waiting time, allograft quality, and post-transplant death and graft failure events. The ascertainment of the main outcomes of allograft quality and waiting time are reported uniformly to the OPTN for all transplant candidates, minimizing the potential for bias.
In conclusion, current OPTN policies enable brief waiting times to kidney transplantation for living organ donors who progressed to ESRD, and these prior donors have favorable post-transplant outcomes as well. However, almost 60% of prior donors required dialysis by the time of listing. Early identification of CKD and early nephrology referral may lead to improved rates of preemptive kidney transplantation among prior donors.
Concise Methods
We performed a retrospective cohort study of adult (aged ≥18 years at listing) kidney transplant candidates using data provided by the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, wait-listed candidates, and transplant recipients in the United States submitted by the members of the OPTN, and has been described elsewhere.18 The US Department of Health and Human Services Health Resources and Services Administration provides oversight to the activities of the OPTN and SRTR contractors. This study was approved by the University of Pennsylvania Institutional Review Board. Deaths were ascertained through center reports and linkage to the Social Security Death Master File.
Generation of Cohorts
We assembled two cohorts: a waiting-list cohort and a kidney transplant recipient cohort. For each cohort, the primary exposure was prior live organ donor status as determined by the United Network for Organ Sharing (UNOS) and reported to the SRTR. Since 1996, UNOS has maintained records about prior organ donor status among kidney transplant candidates; this status is confirmed by UNOS staff for each prior donor because the status provides additional priority in organ allocation.
For the waiting-list cohort, individuals who were wait-listed for a kidney transplant between January 1, 1996, and December 31, 2010 were included and followed until transplant, delisting, or the end of the study period (March 2, 2012, the last recorded date in the data set). We excluded pediatric candidates (aged <18 years), candidates who were never active on the waiting list, and candidates who were transplanted on the same day as the listing date. A small number of candidates received a transplant that failed and were listed again during the study period. For these individuals, the subsequent listing was analyzed as a distinct event.
The primary outcome for the waiting-list cohort was median time to DDKT. The secondary outcomes for the waiting-list cohort were rate of transplantation and percent on dialysis at the time of listing.
The kidney transplant recipient cohort consisted of individuals within the waiting-list cohort who subsequently underwent kidney transplantation. Individuals in the cohort were followed until death or the end of the study period for the analysis of patient survival, and were followed until death, allograft failure, or end of the study period for the analysis of allograft survival. The primary outcome for the recipient cohort was kidney allograft quality, as measured by the KDRI. The KDRI is a composite risk index that was developed to estimate the risk of deceased donor allograft failure. The KDRI includes donor attributes of age, race, donation after cardiac death status, height, weight, serum creatinine, and hepatitis C virus serostatus.19 Because the KDRI can only be calculated for DDKT candidates, this analysis was limited to recipients who received a DDKT. Secondary outcomes included post-transplant mortality and allograft failure.
Statistical Analyses
Analyses were conducted using Stata software (version 13.1; StataCorp., College Station, TX). Two-sided tests of hypotheses were conducted. A P value <0.05 was the criterion for statistical significance. We used the t test to compare means of normally distributed variables between prior donors and nondonors. We used Fisher’s exact or the chi-squared test to compare categorical variables between prior donors and nondonors, as appropriate.
For the waiting-list cohort and the recipient cohort, we generated propensity score–matched groups of donors and nondonors.20 Donors for both cohorts were exactly matched on listing donor service area. We excluded donors in donor service areas in which there had been <2 organ donors listed in the past 10 years (n=5). Nearest-neighbor (1:1) matching methods were then implemented to choose nondonor controls. We used logistic regression to generate a propensity score for the outcome of being a prior kidney donor. Covariates used in the waiting-list cohort model included age at listing, sex, race (black race/nonblack), year of wait-listing, PRA (defined by UNOS convention as either <20%, 20%–80%, or >80%), blood group (type O, A, B, or AB), diabetes, private insurance, education (college/not), and socioeconomic status quartile (as derived using zip code with the Agency for Healthcare Research and Quality’s socioeconomic status index).12 Prior donors were matched 1:1 using a greedy, nearest-neighbor approach. For the waiting-list cohort, we fit a matched-pair stratified Cox proportional hazards model for the outcome of DDKT, censoring for death and live kidney donor transplantation. As a secondary analysis, we examined time to transplantation using a competing-risks regression model, in which death and live donor transplantation were identified as competing risks. We also fit matched-pair stratified Cox models for the outcome of death, censoring for transplantation.
The 1:1 propensity score–matched cohort for the recipient cohort included age, sex, race, year of transplant, PRA category, diabetes, hepatitis C serostatus, dialysis at listing, socioeconomic status, and history of prior transplantation. We did not match on (or adjust for) waiting time or allograft quality because shorter waiting time and better allograft quality could be plausibly considered to be mediators between prior donor status and post-transplant outcomes. We fit matched-pair stratified Cox proportional hazards models for the outcomes of mortality and all-cause allograft failure.
The proportional hazards assumption for these models was confirmed through inspection of Kaplan–Meier curves and log–log plots. We also performed sensitivity analyses of our propensity score–matched models to estimate the potential effect of unobserved confounders on the outcome of mortality (Supplemental Appendix 3).21,22
Missing Data
A small minority of candidates and recipients had any missing data on relevant covariates, except for PRA, which was missing among 40% of prior donors in the waiting list and recipient cohorts. In primary analyses, for categorical values with >1% missing data, we created a separate category for missingness.
Disclosures
None.
Supplementary Material
Acknowledgments
The data reported here were supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government. P.P.R. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This research was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (F32-DK096758-01 to M.N.H. and DK097201 to F.P.W.).
Preliminary results were presented at the American Society of Nephrology Kidney Week Meeting on November 7, 2013, in Atlanta, Georgia.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014030302/-/DCSupplemental.
References
- 1.Delmonico F, Council of the Transplantation Society : A Report of the Amsterdam Forum on the Care of the Live Kidney Donor: Data and medical guidelines. Transplantation 79[Suppl]: S53–S66, 2005 [PubMed] [Google Scholar]
- 2.Davis CL, Delmonico FL: Living-donor kidney transplantation: A review of the current practices for the live donor. J Am Soc Nephrol 16: 2098–2110, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, Gross CR, Matas AJ: Long-term consequences of kidney donation. N Engl J Med 360: 459–469, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garg AX, Meirambayeva A, Huang A, Kim J, Prasad GV, Knoll G, Boudville N, Lok C, McFarlane P, Karpinski M, Storsley L, Klarenbach S, Lam N, Thomas SM, Dipchand C, Reese P, Doshi M, Gibney E, Taub K, Young A, Donor Nephrectomy Outcomes Research Network : Cardiovascular disease in kidney donors: Matched cohort study. BMJ 344: e1203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, Taranto SE, McBride MA, Montgomery RA: Perioperative mortality and long-term survival following live kidney donation. JAMA 303: 959–966, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Gross CR, Messersmith EE, Hong BA, Jowsey SG, Jacobs C, Gillespie BW, Taler SJ, Matas AJ, Leichtman A, Merion RM, Ibrahim HN, RELIVE Study Group : Health-related quality of life in kidney donors from the last five decades: Results from the RELIVE study. Am J Transplant 13: 2924–2934, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, Segev DL: Risk of end-stage renal disease following live kidney donation. JAMA 311: 579–586, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mjøen G, Hallan S, Hartmann A, Foss A, Midtvedt K, Øyen O, Reisæter A, Pfeffer P, Jenssen T, Leivestad T, Line PD, Øvrehus M, Dale DO, Pihlstrøm H, Holme I, Dekker FW, Holdaas H: Long-term risks for kidney donors. Kidney Int 86: 162–167, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Lentine KL, Schnitzler MA, Xiao H, Saab G, Salvalaggio PR, Axelrod D, Davis CL, Abbott KC, Brennan DC: Racial variation in medical outcomes among living kidney donors. N Engl J Med 363: 724–732, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherikh WS, Young CJ, Kramer BF, Taranto SE, Randall HB, Fan PY: Ethnic and gender related differences in the risk of end-stage renal disease after living kidney donation. Am J Transplant 11: 1650–1655, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Gibney EM, King AL, Maluf DG, Garg AX, Parikh CR: Living kidney donors requiring transplantation: Focus on African Americans. Transplantation 84: 647–649, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Smith JM, Biggins SW, Haselby DG, Kim WR, Wedd J, Lamb K, Thompson B, Segev DL, Gustafson S, Kandaswamy R, Stock PG, Matas AJ, Samana CJ, Sleeman EF, Stewart D, Harper A, Edwards E, Snyder JJ, Kasiske BL, Israni AK: Kidney, pancreas and liver allocation and distribution in the United States. Am J Transplant 12: 3191–3212, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellison MD, McBride MA, Taranto SE, Delmonico FL, Kauffman HM: Living kidney donors in need of kidney transplants: A report from the organ procurement and transplantation network. Transplantation 74: 1349–1351, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Organ Procurement and Transplantation Network: Policy 12.9.3 entitled “Priority on the Waitlist.” Available at http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_172.pdf. Accessed February 17, 2014
- 15.Muzaale AD, Massie AB, Wainwright J, McBride MA, Wang M, Segev DL: Long-term risk of ESRD attributable to live kidney donation: Matching with healthy non-donors [Abstract]. Am J Transplant 13: 204–205, 2013 [Google Scholar]
- 16.Davis CL, Cooper M: The state of U.S. living kidney donors. Clin J Am Soc Nephrol 5: 1873–1880, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.United Network for Organ Sharing: Available at http://optn.transplant.hrsa.gov. Accessed May 22, 2013
- 18.Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, Snyder JJ, Kasiske BL: Scientific Registry of Transplant Recipients: Collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando) 27: 50–56, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS: A comprehensive risk quantification score for deceased donor kidneys: The kidney donor risk index. Transplantation 88: 231–236, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Rosenbaum PR: Design of Observational Studies, New York, Springer, 2010 [Google Scholar]
- 21.Wong YN, Mitra N, Hudes G, Localio R, Schwartz JS, Wan F, Montagnet C, Armstrong K: Survival associated with treatment vs observation of localized prostate cancer in elderly men. JAMA 296: 2683–2693, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Weinhandl ED, Liu J, Gilbertson DT, Arneson TJ, Collins AJ: Survival in daily home hemodialysis and matched thrice-weekly in-center hemodialysis patients. J Am Soc Nephrol 23: 895–904, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.