Summary
The role of YODA MITOGEN ACTIVATED PROTEIN KINASE KINASE KINASE 4 (MAPKKK4) upstream of MITOGEN ACTIVATED PROTEIN KINASE 6 (MPK6) was studied during post-embryonic root development of Arabidopsis thaliana. Loss- and gain-of-function mutants of YODA (yda1 and ΔNyda1) were characterized in terms of root patterning, endogenous auxin content and global proteomes.
We surveyed morphological and cellular phenotypes of yda1 and ΔNyda1 mutants suggesting possible involvement of auxin. Endogenous indole-3-acetic acid (IAA) levels were up-regulated in both mutants. Proteomic analysis revealed up-regulation of auxin biosynthetic enzymes tryptophan synthase and nitrilases in these mutants. The expression, abundance and phosphorylation of MPK3, MPK6 and MICROTUBULE ASSOCIATED PROTEIN 65–1 (MAP65-1) were characterized by quantitative polymerase chain reaction (PCR) and western blot analyses and interactions between MAP65-1, microtubules and MPK6 were resolved by quantitative co-localization studies and co-immunoprecipitations.
yda1 and ΔNyda1 mutants showed disoriented cell divisions in primary and lateral roots, abortive cytokinesis, and differential subcellular localization of MPK6 and MAP65-1. They also showed deregulated expression of TANGLED1 (TAN1), PHRAGMOPLAST ORIENTING KINESIN 1 (POK1), and GAMMA TUBULIN COMPLEX PROTEIN 4 (GCP4).
The findings that MPK6 localized to preprophase bands (PPBs) and phragmoplasts while the mpk6-4 mutant transformed with MPK6AEF (alanine (A)–glutamic acid (E)–phenylanine (F)) showed a root phenotype similar to that of yda1 demonstrated that MPK6 is an important player downstream of YODA. These data indicate that YODA and MPK6 are involved in post-embryonic root development through an auxin-dependent mechanism regulating cell division and mitotic microtubule (PPB and phragmoplast) organization.
Keywords: Arabidopsis, cell division plane, MAP65-1, MAPKKK, microtubules, MPK6, root, YODA
Introduction
Asymmetric cell division and differential cell growth are both fundamental mechanisms underlying plant development and morphogenesis in the absence of cell motility (Rasmussen et al., 2011a). Specific cell types (e.g. stomata; Lau & Bergmann, 2012) and entire organs (e.g. lateral roots; De Smet, 2012) originate from decisive asymmetric divisions, while specialized cell types such as Arabidopsis cotyledon and leaf pavement cells (Qian et al., 2009) and trichomes and root hair cells (Tominaga-Wada et al., 2011) are shaped by processes of spatially defined cell growth. In plants, cell division, cell growth and differentiation are affected by extracellular stimuli and intercellular interactions (reviewed in Rasmussen et al., 2012).
Following such inputs, cell division may be symmetric or asymmetric, while the plane of cell division may lie transversely, longitudinally or obliquely to the cell axis (De Smet & Beeckman, 2011; Rasmussen et al., 2011a). Auxin is the major plant hormone involved in the regulation of cell division, probably through activation of transcription factors such as AUXIN RESPONSE FACTORS (ARFs) and PLETHORA (Okushima et al., 2005; Hofhuis et al., 2013). Similarly, cell growth may be isotropic or anisotropic and diffuse or polar, yielding distinct cell types and guiding plant tissue and organ growth (Breuninger & Lenhard, 2010). All these morphogenetic processes are driven by proper, coordinated and conditioned organization of microtubules in the cell cortex (Szymanski & Cosgrove, 2009) and are consequently coupled to extracellular perception and its translation into cellular responses (Komis et al., 2011).
From the mechanistic standpoint, the positioning of the cell division plane, the directionality of cell expansion and the patterns of cytomorphogenesis are determined by cortical microtubule arrays (Lloyd & Chan, 2008; van Damme, 2009). The cell division plane is marked by a cortical microtubule annulus accompanied or preceded by the respective localization of microtubule-associated proteins (Buschmann et al., 2006; Müller et al., 2006; Walker et al., 2007), signaling proteins (Xu et al., 2008; Müller et al., 2010; Komis et al., 2011; Kirik et al., 2012), endocytotic events (Dhonukshe et al., 2005; Karahara et al., 2009) and actin filaments (Panteris, 2008) called the preprophase microtubule band (PPB; Müller et al., 2009).
The PPB marks quite accurately the future site of cell plate fusion to the parent walls by defining the plane of the centrifugal expansion of the cytokinetic phragmoplast (van Damme, 2009). However, the centrifugally expanding phragmoplast has a limited freedom of rotational motion out of the plane defined by the PPB, being continuously corrected by cortex–phragmoplast leading edge actin microfilament and microtubule attachments (Dhonukshe et al., 2005; Higaki et al., 2008).
In studies of microtubule-dependent mechanisms of plant cell division symmetry/asymmetry and cell growth directionality, mitogen-activated protein kinases (MAPKs) emerged as modal regulators (Beck et al., 2010, 2011; Kosetsu et al., 2010; Müller et al., 2010; Komis et al., 2011; Sasabe & Machida, 2012). In Arabidopsis, at least three Arabidopsis MAPKs, namely MPK4, MPK6 and MPK18, are implicated in microtubule organization and dynamics (Walia et al., 2009; Beck et al., 2010, 2011; Kosetsu et al., 2010; Müller et al., 2010). The developmental involvement of MPK4 occurs downstream of the Arabidopsis homologue of Nucleus and Phragmoplast localized kinase (ANP) family of MAPKKKs (Beck et al., 2010; Kosetsu et al., 2010) through the MAPKK MKK6 (Kosetsu et al., 2010) and targets the MAP65-1, MAP65-2 and MAP65-3 microtubule-associated proteins, controlling their interaction with interphase and mitotic/cytokinetic microtubule arrays (Kosetsu et al., 2010; Beck et al., 2011; Sasabe et al., 2011). Knockout mutants of any of the above constituents of the MPK4 pathway show incomplete cytokinesis and aberrant cytomorphogenesis as a result of disrupted phragmoplast progression and cortical array malorganization, respectively (Beck et al., 2010, 2011; Kosetsu et al., 2010).
MPK6, in contrast, seems to be involved in cell division plane determination as it interacts with the PPB and the phragmoplast, and co-fractionates and co-localizes with endocytotic markers. MPK6 depletion in mpk6 mutants causes aberrant cell file formation in the root as a result of the disturbance of the cell division plane orientation (Müller et al., 2010). MPK6 is directly phosphorylated by MKK4/5, which may be activated by alternative MAPKKKs including Mitogen-activated protein kinase kinase kinase 1 (MEKK1), ANPs and finally YODA (Colcombet & Hirt, 2008). YODA is a particularly interesting candidate in view of its developmental role in stomatal patterning. Two classes of mutants called yda (kinase inactive) and ΔNyda (a gain of function), corresponding to the same MAPKKK4, have opposite effects on stomatal development, with yda plants showing clustering of stomata and ΔNyda plants showing repression of stomatal development (Bergmann et al., 2004; Wang et al., 2007). Genetic disturbance of the components acting downstream of YODA, namely MKK4/5 and MPK3/6, leads to more exacerbated phenotypes, leading to the suggestion that stomatal development is strictly regulated by the YODA pathway (Wang et al., 2007).
As a result of the connection of MPK3/6 with YODA signaling in the control of stomatal cell fate (Bergmann et al., 2004; Wang et al., 2007; Kim et al., 2012), the embryonic development (Lukowitz et al., 2004) and the observation of similar developmental defects in primary roots of mpk6 null mutants (Müller et al., 2010), we focused here on root developmental phenotypes related to microtubule organization in allelic (loss-of-function) mutant of YODA (yda1; Lukowitz et al., 2004) and in in-frame aminoterminal deletion mutant of YODA (ΔNyda1; Lukowitz et al., 2004), representing constitutively active form of MAP-KKK4. We also studied localization of MPK6 in living cells under control of a native promoter and phenotypes of mpk6-4 mutants transformed with the kinase-dead form MPK6AEF (Bush & Krysan, 2007), which were very similar to yda.
Materials and Methods
Plant material and treatments
Sterilized seeds of Arabidopsis thaliana (L.) Heynh were imbibed and grown on Phytagel (Sigma, Prague, Czech Republic) solidified half-strength Murashige–Skoog (MS) medium, under axenic conditions as previously described (Beck et al., 2010). All the seedlings used for further experimental processing were 3–14 d old. Two loss-of-function alleles of YODA (yda; At1g63700), namely the T-DNA insertional mutants yda1 (which contains a stop codon within the catalytic kinase domain; Lukowitz et al., 2004) and yda2 (which is also kinase inactive with a proline substituted by a serine; Lukowitz et al., 2004), two gain-of-function ΔNyda alleles harboring aminoterminal deletions (ΔNyda1 and ΔNyda2; Lukowitz et al., 2004) and mpk6-4 stably transformed with the MPK6AEF construct (Bush & Krysan, 2007), as well as the wild ecotypes Landsberg erecta (Ler) and Columbia (Col-0), were used throughout.
Three-day-old plants of Ler, yda1 and ΔNyda1 growing on half-strength MS medium under standard growth conditions with dark-grown root systems were transferred to half-strength MS medium containing either 1 μM indole-3-acetic acid (IAA) or 10 μM auxinole (α-(2,4-dimethylphenylethyl-2-oxo)-IAA; auxin antagonist). Control plants were simultaneously transferred to basic half-strength MS medium. Subsequently, seedlings were cultivated under the same conditions for 5 d more. Primary root length and lateral root density were statistically evaluated using Student’s t-test.
Root whole mount immunofluorescence localization of tubulin, MAPKs and MAP65-1
Root whole mount localizations of MPK3, MPK6, MAP65-1 and microtubules by indirect immunofluorescence were carried out exactly as previously described (Beck et al., 2010) using the antibodies described in Supporting Information Methods S2. Fluorescently labeled samples (either Fei Mao (FM) 4–64 (FM 4–64), fixable FM 4–64 (FM 4–64 Fx) and 4′,6-diamidino-2-phenylindole (DAPI) stained roots or immunolabeled root whole mounts of Ler, yda1 and ΔNyda1 seedlings) were examined with a Zeiss 710 CLSM platform mounted on a Zeiss Axio Imager Z.2 upright microscope (Carl Zeiss, Jena, Germany), using excitation lines at 405, 488 and 561 nm from argon, HeNe, diode and diode pumped solid-state lasers. Images were acquired with a dry 20×/NA 0.8, an oil immersion ×40/NA 1.40 or an oil immersion ×63/NA 1.46 objective, of which the latter two were corrected for coverslip thickness (no. 1.5/0.17 mm). Differential interference contrast images were acquired by filtering transmitted polarized light through a Wollaston prism. Samples were examined by averaged eight-line scanning at a 16-bit color depth with a Nyquist-corrected planar resolution automatically set by Zeiss Zen 2012 software. Methods used for co-localization analyses are described in Supporting Information Methods S1.
Protein extraction, co-immunoprecipitation, Phos-Tag™ SDS-PAGE and western blot
Total protein extracts were obtained from the whole 14-d-old seedlings according to our previous work (Ovečka et al., 2014). For western blot detection of phospho-MAPK species and correlation with MPK3 and MPK6, the same protein sample (Ler, yda1 or ΔNyda1 extract) was loaded in three consecutive gel wells of either 8% Tris-Cl gels or 4–12% precast Tris-Cl gels (BioRad, Prague, Czech Republic). After transfer, polyvinyl difluoride (PVDF) membranes were stained with Ponceau S, allowing the visualization of the respective lanes. These were subsequently cut into strips and incubated with anti-pTEpY (anti-phospho-Threonine-Glutamic acid-phosphoTyrosine; Cell Signaling Technology, Biotech A.S., Prague, Czech Republic), anti-MPK3 or anti-MPK6 antibodies (Sigma-Aldrich). Chemiluminescence imaging of western blots was done with the ChemiDoc™ MP Imaging System (BioRad). Densitometric analyses of western blots were carried out with freeware IMAGEJ software (http://rsbweb.nih.gov/ij/).
For Phos-Tag™ (Wako Pure Chemical Industries Ltd, Osaka, Japan) phosphorylation analysis, radio-immunoprecipitation assay (RIPA) buffer (25 mM Tris-Cl, pH 7.4, 150 mM NaCl, 1mM ethylene glycol tetraacetic acid (EGTA), 1% v/v Nonidet P-40, 0.5% w/v sodium deoxycholate) was supplemented with 10 mM NaF and 100 μM Na3VO4, in order to avoid phosphate-containing phosphatase inhibitors that would interfere with the assay. For acrylamide-pendant Phos-Tag™ discrimination of phosphorylated from nonphosphorylated forms of the same protein, Phos-Tag™ and equimolar Zn2+ were co-polymerized with 7.5–10% Bis-Tris buffered sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels (Fujita et al., 2013). Proteins were resolved at 15–30 mA per gel in 100 mM 4-morpholinepropanesulfonic acid (MOPS), 100 mM Tris, 5 mM sodium bisulfite and 0.1% w/v SDS. Gels were washed with 10 mM ethylenediaminotetraacetic acid (EDTA) in Tris/glycine transfer buffer with 5% v/v methanol and subsequently in neat transfer buffer to remove Zn2+ cations before overnight wet transfer to PVDF membranes at 24 V.
For co-immunoprecipitation experiments, samples were processed exactly according to the instructions for the ChromoTek GFP-Trap®_A kit (Chromo Tek GmbH, Martinsried, Germany).
Cloning of MPK6 under a native promoter and transformation of mpk6-2 mutants
Both the C-terminal and N-terminal fusions of GREEN FLUORESCENT PROTEIN (GFP) with AtMPK6 driven by its own promoter were prepared using genomic DNA from leaf tissue. The 1784-bp AtMPK6 promoter upstream of the ATG start codon was amplified using respective primers Mpk6npF and Mpk6npR (Table S1). The AtMPK6 genomic sequence for C-terminal fusion (GFP is fused with the C-terminus of MPK6) was amplified using primers Mpk6ctF and Mpk6ctR (Table S1) and the AtMPK6 genomic sequence for N-terminal fusion was amplified using primers Mpk6ntF and Mpk6ntR (Table S1). The amplified promoter, AtMPK6 genomic DNA and enhanced GFP (eGFP) were assembled using a recombination reaction according to instructions included in the MultiSite Gateway® Three-Fragment Vector Construction Kit (Life Technologies Czech Republic s.r.o., Prague, Czech Republic) and cloned into pB7m34GW.0, which was then used for Agrobacterium tumefaciens-mediated floral dip transformation of Arabidopsis mpk6-2−/− mutant plants. Transgenic progeny was selected using 20 μM phosphinotricin or 250 mg l−1 BASTA®. Selected 5-d-old T2 plants were used for live imaging experiments. Localization of GFP-tagged MPK6 was determined with a Zeiss 710 CLSM platform or Lightsheet Z1 platform (Carl Zeiss, Jena, Germany). Roots were counterstained with FM4-64. Some images were post-acquisition processed using the Decon-volutionLab plugin (Biomedical Imaging Group, EPFL, Switzerland; http://bigwww.epfl.ch/) of IMAGEJ.
Measurements of endogenous auxin
Endogenous concentrations of free IAA and its catabolites/conjugates were determined using the LC-MS/MS (liquid chromatography–tandem mass spectrometry) method (Novak et al., 2012). Briefly, 20 mg fresh weight of 14-d-old Arabidopsis seedlings of the control and mutant lines was collected, extracted in ice-cold 50 mM sodium phosphate buffer (pH 7) and purified by solid phase extraction (SPE) on hydrophilic-lipophilic balance reversed-phase sorbent columns (OasisR HLB; 1 cc/30 mg; Waters, Milford, MA, USA). To each extract, 5 pmol of [13C6]-IAA, [13C6]-oxIAA, [13C6]-IAAsp and [13C6]-IAGlu was added as internal standards to validate the quantification. Purified samples were analyzed using the LC-MS/MS system consisting of an ACQUITY UPLCR System (Waters) and XevoT TQ-S (Waters) triple quadrupole mass spectrometer. Quantification was obtained using a multiple reaction monitoring (MRM) mode of selected precursor ions and the appropriate product ion. For the Ler control and each mutant, four independent biological replicates were performed.
Chemicals, root morphometry and phenotyping, quantitative PCR and proteomic analyses are described in Methods S2–S6. All protein and peptide pertinent mass spectrometry data are available in Table S2.
Results
Root phenotypes of yda and ΔNyda mutants
Previous studies showed that loss-or gain-of-function mutants of YODA affect stomatal fate as early as during the protodermal asymmetric definition of guard mother cell (GMC) precursors giving rise to GMCs (Bergmann et al., 2004). However, yda mutants show also defects in embryonic and seedling development, suggesting a general role of the YODA pathway in cell fate determination (Lukowitz et al., 2004). As previously described, yda allelic mutants develop rosette leaves and shoot development is nearly abrogated (Lukowitz et al., 2004). ΔNyda mutants, in contrast, show severe defects in the aerial organs, including fusion of cotyledons (Lukowitz et al., 2004). In both cases, the two loss-of-function alleles (yda1 and yda2) and the two gain-of-function alleles (ΔNyda1 and ΔNyda2) were similar in terms of plant phenotypes, and therefore yda1 and ΔNyda1 were selected for detailed experiments. Cellular root phenotypes were evaluated exclusively in yda1 and mpk6-4 + MPK6AEF mutants showing stomatal clusters as well as in ΔNyda1 mutants showing no stomata (Fig. S1).
An overview of yda and ΔNyda seedlings showed obvious changes in root patterning and development when compared with the Ler wild-type (Fig. 1). Young 4-d-old seedlings of yda mutants showed short roots or in some exceptional cases no roots (Fig. 1b–d). Seedlings of ΔNyda at this stage showed shorter, agravitropic and twisted roots (Fig. 1e,f). Differences in root patterning such as primary root lengths and lateral root density were still obvious (Fig. 1g–i) at later stages of root development (8 d after germination) as evidenced also by quantitative evaluation (Fig. 1j,k). Both yda1 and ΔNyda1 mutants showed shorter primary roots and a higher density of lateral roots when compared with the Ler control (Fig. 1g–k). A similar trend was found in 14-d-old seedlings (Fig. S2).
Fig. 1.
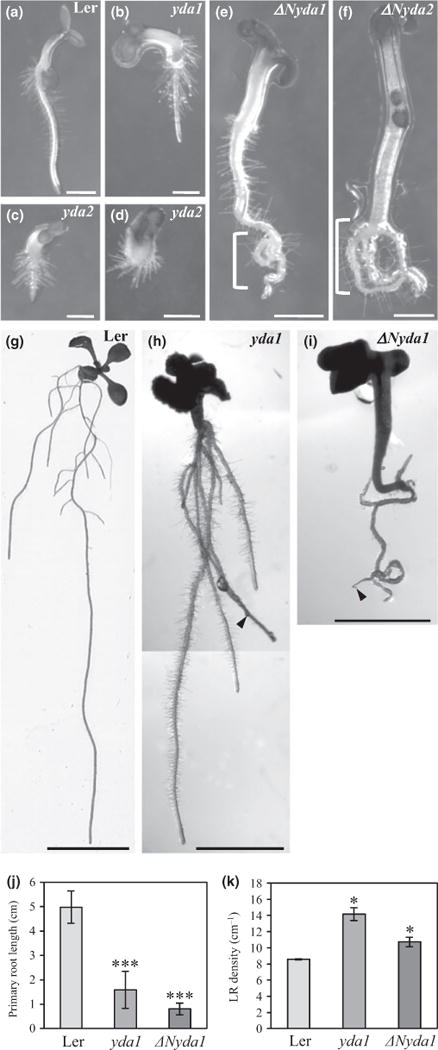
Phenotypes of primary roots, lateral roots and root hairs in the yda1 and ΔNyda1 mutants. (a–f) Primary root development of 4-d-old Arabidopsis seedlings of Landsberg erecta (Ler) (a), yda1 (b), yda2 (c, d), ΔNyda1 (e) and ΔNyda2 (f). yda mutants show disturbed primary root growth resulting in thickened, short roots compared with Ler (b–d; cf. a). By contrast, ΔNyda1 mutants show promoted root elongation and in some cases curling of primary roots (e, f, brackets). (g–i) Overview of lateral and adventitious roots of 8-d-old seedlings of Ler (g), yda1 (h) and ΔNyda1 (i). Both yda1 and ΔNyda1 mutants showed shorter primary roots in comparison to Ler (g; cf. h, i). (j, k) Quantitative evaluation of primary root lengths (j) and lateral root (LR) density (k) of 8-d-old Ler, yda1 and ΔNyda1 seedlings. Lateral root density was obtained from the root branching zone of the wild-type and both mutants. Bar charts represent the mean ± SD for Ler (N = 21), yda1 (N = 12) and ΔNyda1 (N = 19). *, P<0.05; ***, P< 0.001. Arrowheads (h, i) mark the apex of the primary root. Bars: (a–d) 0.5 mm; (e, f) 1 mm; (g) 1 cm; (h, i) 2 mm.
Distances from root tips to the first visible root hair encompassing meristematic and early elongation zones were much shorter in mutant plants (Fig. S3). One possible explanation for such root phenotypes is termination of the primary root meristem and changes in the concentrations of endogenous auxin in these two mutants. Therefore, we compared endogenous concentrations of IAA in both mutants and Ler controls. Measurements showed significantly elevated IAA concentrations in both mutants as compared with the Ler control (Fig. 2a). At the tissue level, yda1 showed a higher density of lateral root primordia and ectopic cell divisions in the pericycle (Fig. 2d), while ΔNyda1 showed ectopic cell divisions in the central cylinder and pericycle (Fig. 2f) but also vigorous twisting of all root tissues (Fig. 2g).
Fig. 2.
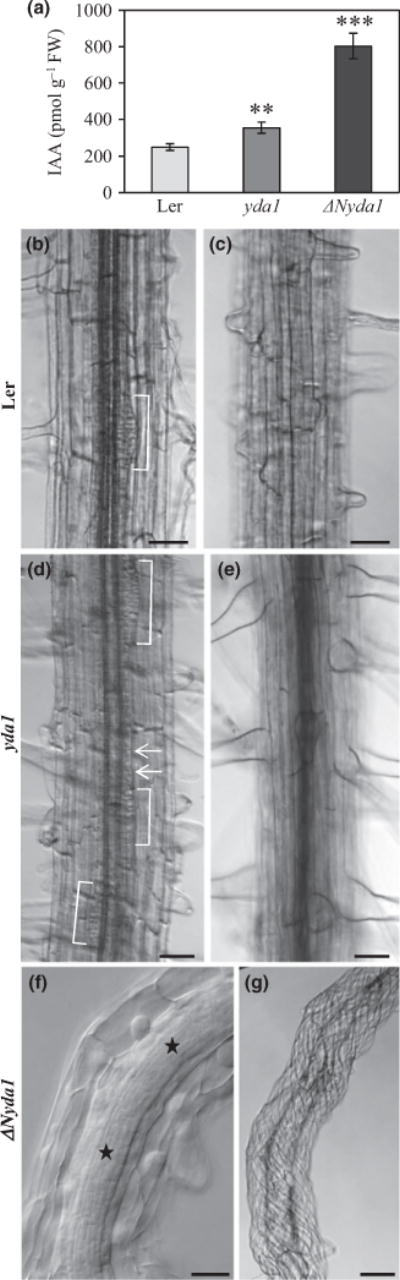
Endogenous indole-3-acetic acid (IAA) content and primary root phenotype of Landsberg erecta (Ler), yda1 and ΔNyda1 plants. (a) Quantitative determination (mean ± SD, N = 4 for all cases examined) of endogenous IAA concentration in whole plants of Ler, yda1 and ΔNyda1. Fourteen-day-old Arabidopsis plants cultivated in standard light conditions, but with dark-grown roots, were analyzed in two independent experiments with four biological replicates. **, P<0.01; ***, P< 0.001. (b–g) Root morphology of 14-d-old Ler, yda1 and ΔNyda1 plants. Median (b, d, f) and surface (c, e, g) views of a mature part of the primary root are shown. In Ler, lateral root primordial emerged at regular intervals (b). In yda1, lateral root primordia developed at a higher density (d, brackets), and some additional ectopic cell divisions in the pericycle were observed (d, arrows). In both Ler and yda1, cell files were arranged parallel to the longitudinal axis of the root (c, e). In ΔNyda1, ectopic cell divisions were frequent in both the central cylinder and the pericycle (f, asterisks) and cell files were extensively twisted (g). Bars: (b–e, g) 50 μm; (f) 20 μm.
Because twisting is a typical feature of some microtubule-defective mutants (Furutani et al., 2000; Sedbrook et al., 2004; Ishida et al., 2007; Buschmann et al., 2009), the organization of cortical microtubules was studied in epidermal cells of the ΔNyda1 mutant and compared with that of the Ler control (Fig. 3). However, no significant differences in microtubule orientation and organization between the mutant and control were detected. The only visible difference was the formation of oblique cell walls between neighbor cells in the ΔNyda1 mutant (Fig. 3c). Oblique cell divisions were further studied and quantitatively evaluated after vital staining of roots with FM4-64 and by microtubule immunolabeling as described in the following sections in relation to auxin.
Fig. 3.
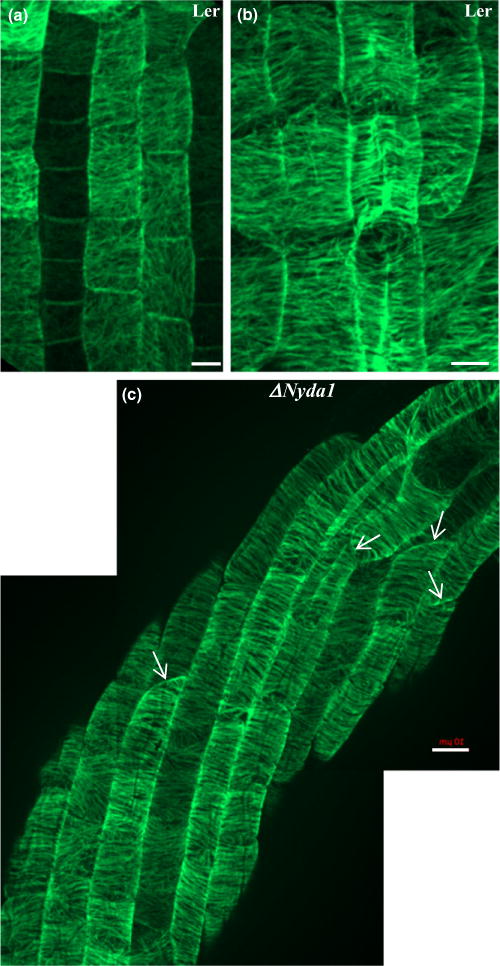
Organization of cortical microtubules in interphase epidermal cells of the primary roots of Landsberg erecta (Ler) and ΔNyda1 Arabidopsis plants after tubulin immunolocalization in root wholemounts and CLSM. (a, b) Transition and elongation zones in roots of Ler. In the transition zone of the root (a), cells in the rootward direction had cortical microtubules randomly oriented, while in cells in the shootward direction, cortical microtubules were gradually rearranged into a loosely transversal orientation. In the elongation zone (b), cortical microtubules in all cells had a transversal orientation. (c) Cortical microtubules in root cells of the ΔNyda1 mutant. In contrast to the extensive twisting of elongating root epidermal cells, cortical microtubules kept a normal transversal orientation. Oblique cross cell walls between neighboring cells in epidermal cell files are indicated by arrows (c). The root tip direction is at the bottom of the figures. Bars, 10 μm.
Root phenotypes of yda1 and ΔNyda1 mutants after treatment with exogenous auxin and auxinole
We exogenously applied 1 μM IAA to the half-strength MS medium and observed the phenotypes of the mutant plants and Ler wild-types. Exogenously applied auxin reduced the length of the primary roots in Ler as well as in both mutants; however, differences between the auxin-treated wild-type and mutant seedlings were less pronounced than those between the wild-type and mutant seedlings in the absence of exogenous auxin (Fig. 4a–k). However, lateral root density after auxin treatment was similar in the control and in both mutants (Fig. 4l).
Fig. 4.
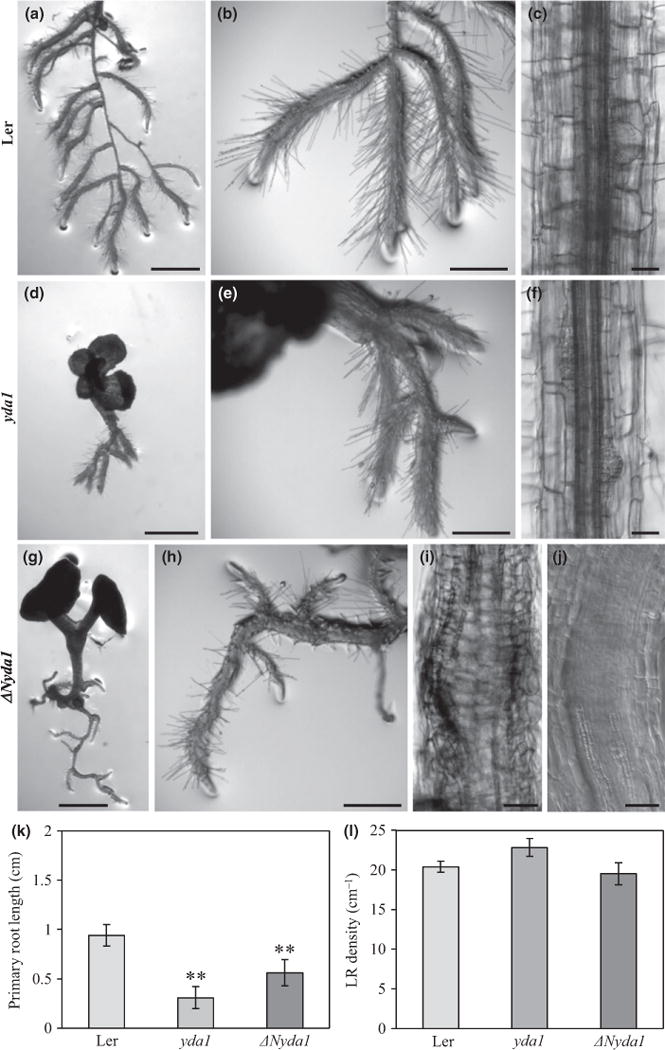
Effects of external indole-3-acetic acid (IAA) on the root phenotype of Landsberg erecta (Ler), yda1 and ΔNyda1 Arabidopsis plants. (a–j) Root morphology of plants grown on medium containing 1 μM IAA. In Ler, the root system consisted of excessively branched primary roots with lateral roots of equal length (a, b). IAA reduced the elongation of root cells and induced the formation of lateral root primordia (c). Roots of yda1 were short (d) and formed lateral roots (e) and lateral root primordia (f) close to each other. ΔNyda1 plants developed a root system with regularly spaced lateral roots (g, h). External IAA did not induce enhanced formation of lateral root primordia in ΔNyda1 plants. Instead, massive ectopic cell division was locally induced in the central cylinder, which even interfered with regular differentiation of vascular cell files (i, j). Plants 3-d-old were transferred to medium containing 1 μM IAA and examined 5 d after transfer (a, b, d, e, g, h) and 11 d after transfer (c, f, i, j). (k, l) Quantitative evaluation of primary root lengths (k) and lateral root density (l) of 8-d-old Ler, yda1 and ΔNyda1 seedlings. Lateral root density was obtained from the root branching zone of the wild-type and both mutants. Bar charts represent the mean ± SD for Ler (N = 21), yda1 (N = 14) and ΔNyda1 (N= 14).**, P < 0.01. Bars: (a, d, g) 1 mm; (b, e, h) 500 μm; (c, f, i) 50 μm; (j) 20 μm.
Next, we observed the effects of the auxin antagonist auxinole, which competes with auxin for binding sites on the auxin receptor TRANSPORT INHIBITOR RESPONSE 1 (TIR1). Auxinole reduced primary root length in Ler but not in yda1 and ΔNyda1 by comparison to mock treatment (Fig. 5a–j). The total number of lateral roots was lower in both mutants treated with auxinole (Fig. 5k). Interestingly, auxinole caused moderate twisting of control Ler roots and strong twisting of yda1 roots, while twisting of ΔNyda1 roots was not significantly changed.
Fig. 5.
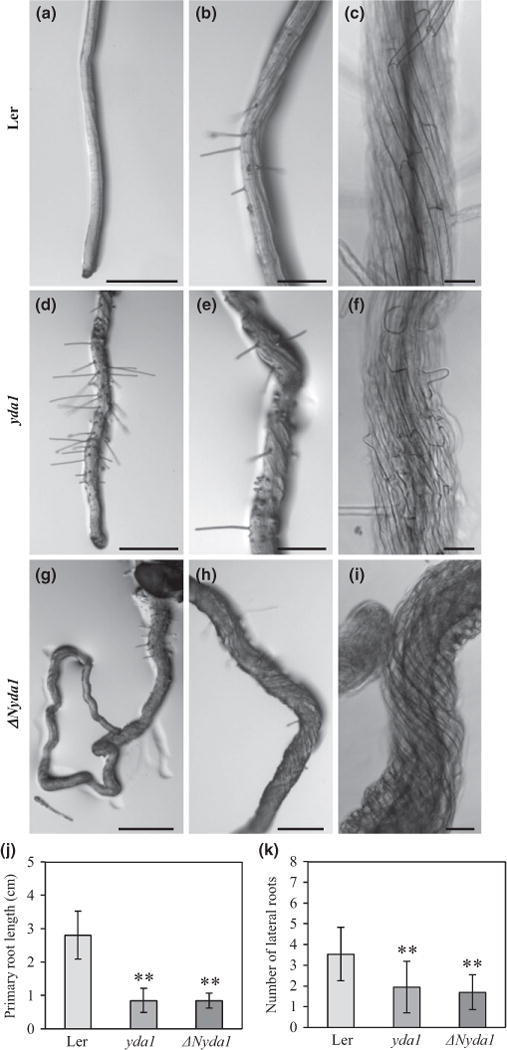
Effects of auxinole (α-(2,4-dimethylphenylethyl-2-oxo)-IAA) on the root phenotype of Landsberg erecta (Ler), yda1 and ΔNyda1 Arabidopsis plants. (a–i) Primary root morphology of plants grown on medium containing 10μM auxinole. Roots of all three lines exhibited agravitropic growth, reduced formation of root hairs and reduced branching. In Ler, moderate twisting of the root was induced (a–c). Root twisting in yda1 plants was more pronounced (d–f) and excessive twisting of ΔNyda1 plants was not affected by auxinole (g–i). Plants 3-d-old were transferred to medium containing 10μM auxinole and examined 5 d after transfer (a, b, d, e, g, h) and 11 d after transfer (c, f, i). (j, k) Quantitative evaluation of primary root lengths (j) and number of lateral roots (k) of 8-d-old Ler, yda1 and ΔNyda1 seedlings. Bar charts represent the mean ± SD for Ler (N= 18), yda1 (N = 14) and ΔNyda1 (N = 20). **, P<0.01. Bars: (a, d, g) 500 μm; (b, e, h) 200 μm; (c, f, i) 50 μm.
Proteomic analysis revealed up-regulation of auxin biosynthetic enzymes in yda1 and ΔNyda1 mutants
To characterize the proteomes of Ler, yda1 and ΔNyda1, a shot-gun proteomic analysis was performed employing two-dimensional nano-LC-MS/MS. Identification and quantification details for differentially abundant proteins identified in yda1 and ΔNyda1 are provided in Tables S3 and S4.
The comparative analysis detected 92 up-regulated proteins (with at least 1.5-fold increased abundance) and 10 unique proteins in the yda1 mutant. In contrast, 20 proteins were less abundant and an additional seven proteins were not detected in the yda1 mutant. The same analysis of ΔNyda1 resulted in 52 up-regulated and 23 ΔNyda1 unique proteins, as well as 15 down-regulated and 18 wild-type unique proteins.
The classification of differentially regulated proteins using Kyoto Encyclopedia of Genes and Genomes (KEGG) annotations generated by the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) web-based application (Jensen et al., 2009) is presented in Figures S4–S6.
Notably, several proteins involved in auxin biosynthesis were up-regulated in both mutants. Tryptophan synthase alpha chain, an enzyme involved in the synthesis of the auxin (IAA) precursor tryptophan, was detected in ΔNyda1 seedlings but not in Ler. Nitrilase 3 was identified as a unique protein in ΔNyda1, while nitrilase 2 was identified as a unique protein in yda1. In addition, nitrilase 1 was 3.7-fold up-regulated in yda1. Nitrilases are enzymes that convert indole-3-acetonitrile to IAA in the indole-3-acet-aldoxime pathway (Bartel & Fink, 1994; Mashiguchi et al., 2011).
Overall, these data indicate accelerated IAA synthesis in both the yda1 and ΔNyda1 mutants, which exhibited higher concentrations of endogenous IAA. The increased levels of auxin biosynthetic enzymes help to explain the higher content of endogenous IAA in both mutants. This also suggests that the nitrilase pathway of auxin biosynthesis was probably up-regulated in the yda1 and ΔNyda1 mutants.
Disturbance of cell division plane determination in yda1 and ΔNyda1 mutants
The arrangement and patterning of cells and tissues in the yda1 and ΔNyda1 mutants were surveyed in living roots by vital staining of cell membranes with the styryl dye FM4-64.
Cells in the root epidermis of Ler primary roots showed the normal succession from formative periclinal to proliferative anticlinal cell divisions, generating an ordered arrangement of cells into linear files (Fig. 6a,c). Also, the linear files of the inner root tissues were well organized (i.e. the cortex, the endodermis and the pericycle). Quiescent central cells were typically shaped, ordered and surrounded by stem cell initials (Figs 6b,d, S7).
Fig. 6.

Primary root cell architecture in Landsberg erecta (Ler), yda1 and ΔNyda1 Arabidopsis plants after staining of living roots with membrane marker FM4-64 and CLSM. (a–d) Surface (a, c) and central (b, d) optical sections of a Ler root. (a) Overview and (c) higher magnification of the epidermis showing the orderly arrangement of epidermal cells in linear files resulting from symmetric periclinal and anticlinal divisions in Ler. (b) Overview and (d) higher magnification of an orderly structured stem cell niche region in Ler. (e–h) Surface (e, g) and central (f, h) optical sections of a yda1 root at similar planes as shown in (a)–(d). (e) Overview and (g) higher magnification of the epidermis showing disturbed cell division planes. Similarly, in central sections (f, h) the stem cell niche is disordered with ectopic cell divisions and displaced cell division planes. (i–l) Surface (i, k) and central (j, l) sections of a ΔNyda1 root. (i, k) Epidermal cell divisions are severely disordered with aberrant, oblique cell division planes. The stem cell niche (j, l) shows similarly defective organization with ectopic cell divisions and altered cell division planes as in yda1. (m) Quantification of oblique cell walls graphically depicting mean ± SD for Ler (N = 610), yda1 (N = 645) and ΔNyda1 (N = 596). Cell walls were evaluated in 10 independent plants in each case. White asterisks in (b, f, j) mark root cap layers. ***, P < 0.001. Bars: (a, b, i, j) 50 μm; (e, f) 20 μm; (c, d, g, h, k, l) 10 μm.
In yda1 primary roots, the cell division patterns in the meristematic zone were disturbed. Many daughter cross-walls in epidermal and central confocal sections appeared oblique (Fig. 6e–h, m), while divisions of the meristem initials also appeared disordered (Figs 6h, S7). Similar root phenotypes and disturbances in cell division orientation were regularly recorded in mpk6-4 mutants transformed with the kinase-dead form MPK6AEF (Bush & Krysan, 2007; Fig. S8). This suggests that MPK6 is a major player regulating cell division plane orientation downstream of YODA.
Finally, c. 7% of newly divided root cells showed cytokinetic defects resulting in binucleate or multinucleate daughter cells with cell wall stubs (Fig. S9; 94 out of 1340 late cytokinetic or post-cytokinetic cells examined in total; N = 37 roots).
In ΔNyda1 primary roots, cell division planes were also visibly affected in the root epidermis (Fig. 6i,k,m), the internal root tissues and the stem cell niche (Figs 6j,l, S7). In contrast to yda1 primary roots, ΔNyda1 never exhibited incomplete cytokinesis and binucleate cells (60/60 late cytokinetic and 44/44 post-cyto-kinetic cells).
Subsequently, we examined cell arrangements in lateral roots of Ler, yda1 and ΔNyda1 plants. In contrast to Ler lateral roots (Fig. 7a,b), those of yda1 exhibited a severely distorted outline, due to the occurrence of bulged epidermal cells (Figs 7c,d,f, S9). Furthermore, many lateral root epidermal cells showed incomplete cytokinesis as evidenced by the observation of cell wall stubs (Fig. 7e). Incomplete cytokinesis in the case of yda1 lateral roots was significantly more pronounced than in the primary root, as it affected nearly 17% of the total number of cells examined (159 cases out of 937 examined in total; N = 47 lateral roots).
Fig. 7.
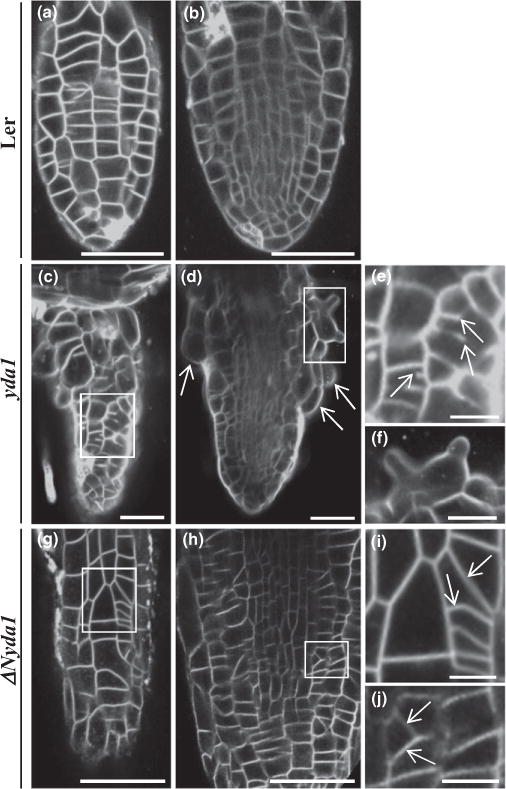
Cell division patterns as visualized in young emerging lateral roots of Arabidopsis Landsberg erecta (Ler), yda1 and ΔNyda1 stained with membrane marker FM4-64 and imaged with CLSM. (a, b) Epidermal (a) and central (b) optical sections of a lateral root of Ler showing the fairly ordered cell arrangement of epidermal and central lateral root tissues. (c, d) Epidermal (c) and central (d) optical sections of a yda1 lateral root exhibiting abnormal cell patterning (c) and bulging of epidermal cells (d, arrows). (e) Higher magnification of the outlined area in (c) showing incomplete cell plate formation in two cells (arrows). (f) Higher magnification of the outlined area in (d) showing a radially expanded trichoblast with two emerging bulges. (g, h) Epidermal (g) and central (h) optical sections of the same ΔNyda1 lateral root showing variably disturbed cell division planes (outlined areas). (i, j) Magnified views of the respective outlined areas in (g) and (h) showing oblique cell plates (arrows). Bars: (a–d, g, h) 50 μm; (e, f, i, j) 10 μm.
In lateral roots of ΔNyda1 plants (Fig. 7g–j), cell division plane orientation was again affected, as was the case in primary roots in both epidermal and internal root tissues (Fig. 6i–l). In contrast to yda1 lateral roots, however, incomplete cytokinesis was never observed.
Visualization of mitotic and cytokinetic microtubule arrays in the root meristem of yda1 and ΔNyda1 mutants
Cell division plane determination (which was presumably affected in yda1 and ΔNyda1 primary and lateral roots) but also cytokinetic progression (which was probably affected in yda1 lateral roots) are functions of specialized microtubule arrays such as the PPB and the phragmoplast. We therefore employed tubulin immunofluorescence labeling to follow microtubule organization in dividing cells of primary and lateral roots of yda1 and ΔNyda1 plants, comparing it with the microtubule organization in Ler root cells (Fig. 8).
Fig. 8.
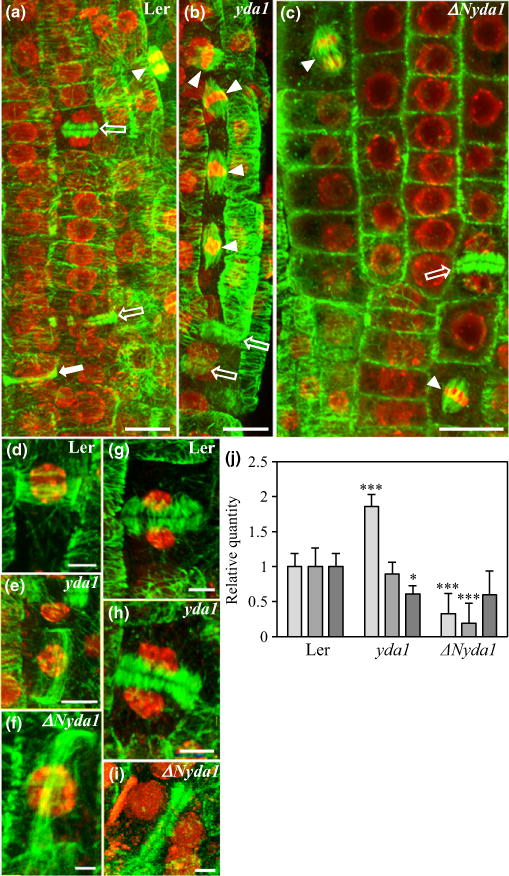
Overview of mitotic and cytokinetic microtubule arrays of Arabidopsis Landsberg erecta (Ler), yda1 and ΔNyda1 root whole mounts immunostained for tubulin (green) and counterstained with the DNA stain DAPI (red pseudocolor). (a) Epidermal meristematic region of Ler root showing two cytokinetic cells with phragmoplasts (outlined white arrows), one preprophase cell with preprophase band (PPB; full white arrow) and an anaphase cell with a spindle (arrowhead). (b) An array of synchronously dividing yda1 root epidermal cells with metaphase spindles (arrowheads) the top one of which is tilted. Outlined white arrows show two cytokinetic cells with phragmoplasts. (c) Part of the meristematic root epidermal area in ΔNyda1. Arrowheads denote two metaphase spindles, one of which is transversely oriented. The outlined arrow shows a phragmoplast. (d–f) Typical, transversely oriented PPB of a Ler preprophase epidermal root cell as compared with oblique PPB formation in yda1 (e) and ΔNyda1 (f). (g–i) Perpendicular phragmoplast in a Ler cytokinetic epidermal cell (g) and oblique phragmoplasts in yda1 (h) and ΔNyda1 (i). (j) Expression levels of TAN1 (light gray bars), POK1 (mid gray bars) and GCP4 (dark gray bars) genes in extracts from Ler, yda1 and ΔNyda1 plants. The graph shows TAN1, POK1 and GCP4 transcript levels (mean ± SD) quantified by qPCR and normalized against EF1a transcripts. *, P<0.05; ***, P<0.001. Bars: (a–c) 10 μm; (e) 5 μm; (d, g, h) 3.3 μm; (f) 1.5 μm; (i) 2.5 μm.
In Ler root cells, most PPBs and phragmoplasts were perpendicular to the root axis (Fig. 8a,d,g), with only a few being obliquely arranged (5% of PPBs (N = 23) and 7% of phragmop-lasts (N = 30)).
In primary and lateral roots of yda1, the numbers of oblique PPBs and phragmoplasts (Fig. 8b,e,h) were significantly increased (13% of PPBs (N = 22) and 51% of phragmoplasts (N = 35)) compared with Ler (Fig. 8a). Clusters of synchronously dividing cells were routinely observed (Figs 8b, S10). In ΔNyda1, the percentages of oblique PPBs and phragmoplasts (Fig. 8f,i; 27% of PPBs (N = 35) and 59% of phragmoplasts (N = 59)) were substantially increased when compared with Ler. In addition, spindles were tilted in both mutants (Fig. 8b,c; 46% of spindles (N = 31)).
The cell division plane is controlled by several proteins including TAN1, POK1 and GCP4 (Müller et al., 2006; Kong et al., 2010; Rasmussen et al., 2011b). Therefore, genes encoding these proteins were chosen for quantitative PCR analysis in both mutants and control plants. Results from qPCR analysis revealed that TAN1 was more strongly expressed in yda1 while it was less strongly expressed in ΔNyda1 (Fig. 8j). Also, POK1 was less strongly expressed in ΔNyda1 while GCP4 was less strongly expressed in yda1 (Fig. 8j). These expression changes in these genes might contribute to misaligned cell division planes in both mutants.
In yda1 main roots, daughter cells with fully reconstituted nuclei able to organize radial perinuclear microtubules exhibited residual young phragmoplasts, suggesting a delay in phragmoplast expansion (Fig. 9a). This accounted for the majority of cytokinetic figures taken into account (24 out of 36 phragmoplasts from two different main and two lateral roots). In this respect, we also observed post-cytokinetic binucleate cells with incomplete daughter cell walls, suggesting a defect in cell plate formation during cytokinesis (Figs 9b–d, S9). In some instances, micronuclei were observed (e.g. Fig. 9c), probably arising from aberrant mitoses where individual chromosomes fail to congress with the rest (Fig. 9e).
Fig. 9.
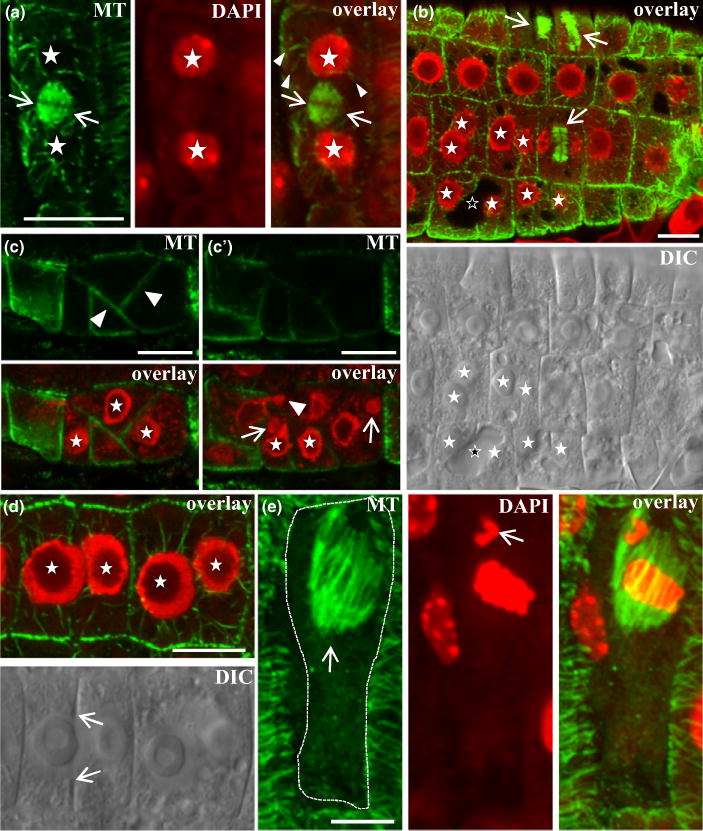
Cell division defects in Arabidopsis yda1 root epidermal cells immunostained for tubulin, counterstained for DNA with DAPI (red pseudocolor), or visualized with differential interference contrast (DIC) optics. (a) Magnification of an epidermal cell showing reconstituted daughter nuclei (asterisks) with perinuclear microtubules (arrowheads) separated by a young phragmoplast (arrows). (b) Immunofluorescent and DIC identification of binucleate cells (nuclei marked with full white asterisks) with incomplete cell plate formation. Arrows point to phragmoplasts. The outlined asterisk marks a vacuole in a binucleate cell which failed to form a cell plate. (c, c′) Different focal planes of an abnormal epidermal cell cluster showing oblique crosswall formation (arrowheads in c) and incomplete cytokinesis. (c′) One nucleus forms a protrusion from one cell compartment to the next (arrowhead) while two cells contain micronuclei (arrows). (d) Immunofluorescent and DIC images of two adjacent binucleate root epidermal cells. Asterisks mark nuclei while arrows point to the margins of an incomplete cell plate. (e) Elongated epidermal cell with ectopically positioned early anaphase spindle (arrow in e). The dashed line delineates the borders of the cell. The arrow in the middle panel (DAPI channel) shows a misplaced chromosome. Bars: (a, b, c, c′, d) 10 μm; (e) 5 μm.
Phosphorylation levels and abundance of MPK3 and MPK6 in yda1 and ΔNyda1 mutants
The developmental roles of YODA involve signal transduction to MPK3 and MPK6 via MAPKKs such as MKK4, MKK5, MKK7 and MKK9 (Wang et al., 2007). Here, we examined the abundance of MPK3 and MPK6 at the transcript and protein levels and the phosphorylation status of MPK3 and MPK6, as complete experimental data on the activity status of MPK3 and MPK6 are lacking in both mutants (Wang et al., 2007).
First, we quantified transcripts of MPK3 and MPK6 in root extracts of Ler, yda1 and ΔNyda1 by real-time qPCR using the ELONGATION FACTOR 1a (EF1a) gene as a normalization standard. Transcripts of MPK3 and MPK6 were decreased in the yda1 mutant (Fig. S11). By contrast, in the ΔNyda1 mutant MPK3 transcripts were slightly but nonsignificantly increased, while MPK6 transcript levels were similar to those of the Ler wild-type (Fig. S11). YODA transcripts were down-regulated in yda1 and strongly up-regulated in ΔNyda1 (Fig. S12), which confirms previously published results (Bergmann et al., 2004).
At the protein level, MPK6 was more abundant than MPK3 in the roots of Ler (Fig. S13). Next, levels of phospho-MPK3 and phospho-MPK6 were investigated using a commercial anti-pTEpY antibody in comparison to specific anti-MPK3 and anti-MPK6 antibodies. Immunoblots revealed that levels of both phosphorylated MPK3 and MPK6 proteins were significantly increased in extracts of ΔNyda1, but surprisingly they were also increased in extracts of yda1 (Fig. 10a,c,d). The over-activation of MPK3 and MPK6 in yda1 mutants was also mentioned previously (Lampard et al., 2009) but it has never before been demonstrated experimentally.
Fig. 10.
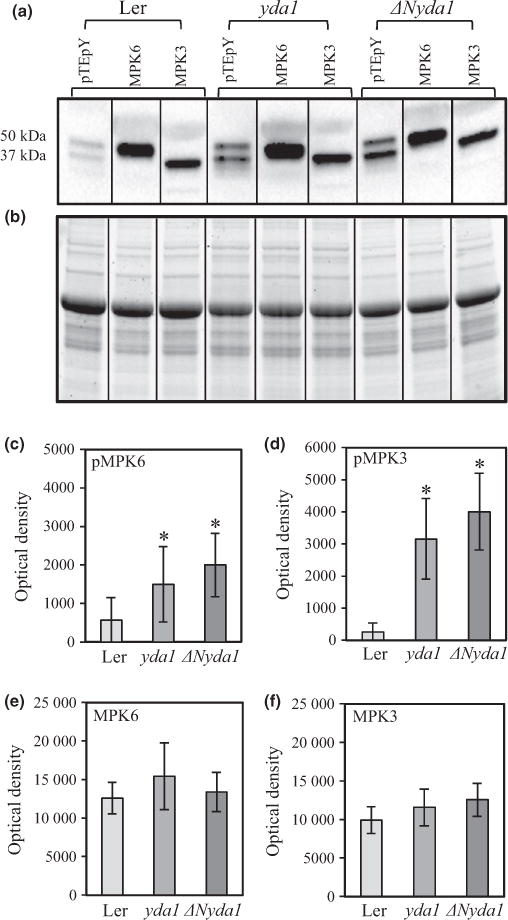
Quantitative analysis of protein levels and phosphorylation of MPK3 and MPK6 in extracts from Landsberg erecta (Ler), yda1 and ΔNyda1 Arabidopsis plants. (a, b) Representative western blots from Ler, yda1 and ΔNyda1 probed with antibodies against the pTEpY motif, MPK6 and MPK3 (a) with respective loading control using stain-free gel (b). Vertical bars in (a) and (b) denote that the blot is assembled from strips as described in the Materials and Methods section. (c–f) Graphic depiction of band optical densities (mean ± SD; averaged from three biological repeats) from three independent western blots. Band optical densities correspond to pMPK6(c), pMPK3 (d), total MPK6(e) and total MPK3 (f). *, P<0.05
Partial co-localization of MPK6 and microtubules in root tip cells of yda1 and ΔNyda1 mutants
The previous study showed that MPK6 partially co-localized with microtubule arrays and particularly with PPBs and phragmop-lasts (Müller et al., 2010). Furthermore, the root phenotype described for yda1 in the preceding sections showed a resemblance to the previously described root phenotypes of mpk6 knockout mutants (Müller et al., 2010). In the context of the possibly differential regulation of MPK6 in yda1 and ΔNyda1 and the phenotypic similarities between mpk6 and yda mutants, the subcellular localization of MPK6 in dividing cells of Ler, yda1 and ΔNyda1 roots was investigated. MPK6 immunoreactive spots co-localized with both PPBs and phragmoplasts in root cells of Ler (Fig. 11a,b; 8/8 PPBs and 11/11 phragmoplasts from five roots in total). These spots were not exactly following the filamentous microtubule pattern within such arrays, but they were rather preferentially clustered in PPBs and phragmoplasts (Fig. 11a,b).
Fig. 11.
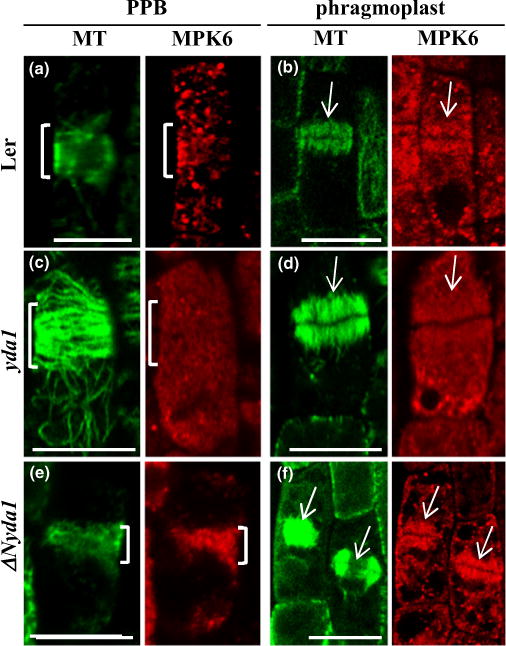
Immunofluorescent co-localization of MPK6 and microtubules in preprophase and cytokinetic cells of Arabidopsis Landsberg erecta (Ler), yda1 and ΔNyda1. (a) Spotlike accumulation of MPK6 fluorescence in the vicinity of the preprophase band (PPB) in a Ler epidermal preprophase cell (brackets in both panels). (b) Accumulation of MPK6 signal within a phragmoplast of a Ler cytokinetic cell (arrows). (c) Absence of MPK6 localization from the PPB (brackets) of a yda1 epidermal preprophase cell. (d) The phragmoplast of a yda1 cytokinetic cell is devoid of MPK6 immunofluorescence (arrows). (e) Spot-like decoration of PPB (brackets) with MPK6 in a ΔNyda1 root epidermal preprophase cell. (f) MPK6 labeling of two phragmoplasts in epidermal cytokinetic cells of ΔNyda1. Bars, 10 μm.
In yda1, both PPBs (Fig. 11c) and phragmoplasts (Fig. 11d) were consistently devoid of MPK6 immunostaining (17/17 PPBs from four roots and 33/33 phragmoplasts from 11 roots). Conversely, both PPBs (Fig. 11e) and phragmoplasts (Fig. 11f) were intensely labeled with anti-MPK6 antibody (18/18 phragmoplasts from seven roots and 9/9 PPBs from five roots) in the ΔNyda1 mutant.
Specific associations of GFP-tagged MPK6 with PPBs, phragmoplasts and cell plates (Fig. 12) were documented also in the mpk6-2 mutant plants which were phenotypically rescued by proMPK6::GFP:MPK6 and proMPK6::MPK6:GFP constructs (Fig. S14).
Fig. 12.
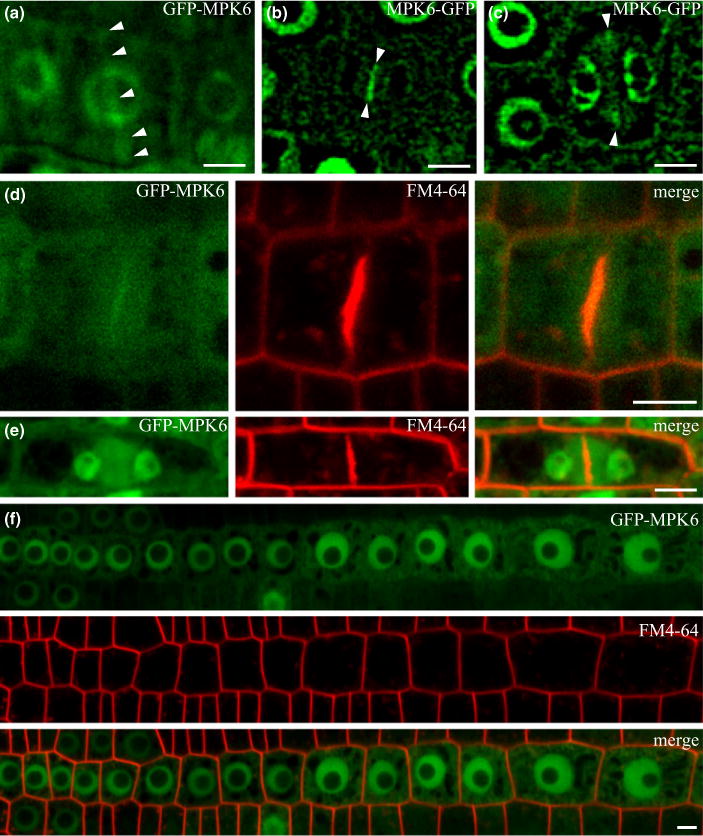
Localization of MPK6 in Arabidopsis mpk6-2 mutants expressing C-and N-terminal GFP fusion of MPK6 under control of MPK6 promoter. GFP-tagged MPK6 was associated with preprophase band (PPB) (a), early phragmoplast (b), late phragmoplast (c, e), phragmoplast and cell plate (b, d). In interphase root cells (f), MPK6 was localized in nuclei and the cytoplasm. Arrowheads indicate PPB (a) and the middle plane of the phragmoplast (b, c). Live cell imaging of MPK6 in lightsheet microscopy (a) and confocal microscopy (b–f) in cells counterstained with FM4-64. Bars, 5 μm.
Western blot analyses with anti-pTEpY antibody showed that MPK3 was also robustly phosphorylated. Thus, as for MPK6, the probable differential localization of MPK3 in Ler, yda1 and ΔNyda1 and its probable relationship to microtubule arrays were studied by co-immunolocalizations in root whole mounts. In all cases, the MPK3 signal exhibited a speckled cytoplasmic pattern but it was not related to microtubular structures such as PPBs and phragmoplasts (Fig. S15).
Differential distribution and altered co-localization of MAP65-1 and microtubules in yda1 and ΔNyda1 mutants
Microtubule-associated protein MAP65-1 is a known target of MAPK signaling along with MAP65-2 and MAP65-3 (Beck et al., 2010; Sasabe et al., 2011) and it was identified as a target of MPK6 (Smertenko et al., 2006; Popescu et al., 2009; Hoehenwarter et al., 2013). In this context, we next examined whether aberrant MPK6 signaling as a result of eliminated (yda1) or increased (ΔNyda1) signaling by the YODA pathway would affect the subcellular localization of MAP65-1 in relation to the respective interphase and mitotic microtubule organization.
Furthermore, we quantified the co-localization of MAP65-1 with microtubules in cortical arrays, PPBs and phragmoplasts of Ler, yda1 and ΔNyda1. In this case, co-localization of MAP65-1 with microtubules may be related to: the abundance of MAP65-1 protein (Mao et al., 2005), and its affinity for the microtubule lattice as this is down-regulated by phosphorylation (Mao et al., 2005; Smertenko et al., 2006).
Immunofluorescent co-localization of MAP65-1 and microtubules in roots of Ler showed that MAP65-1 consistently co-localized with cortical interphase microtubule bundles, but also with PPBs and phragmoplasts in Ler (Figs 13a–c,j–l, S16–S18).
Fig. 13.
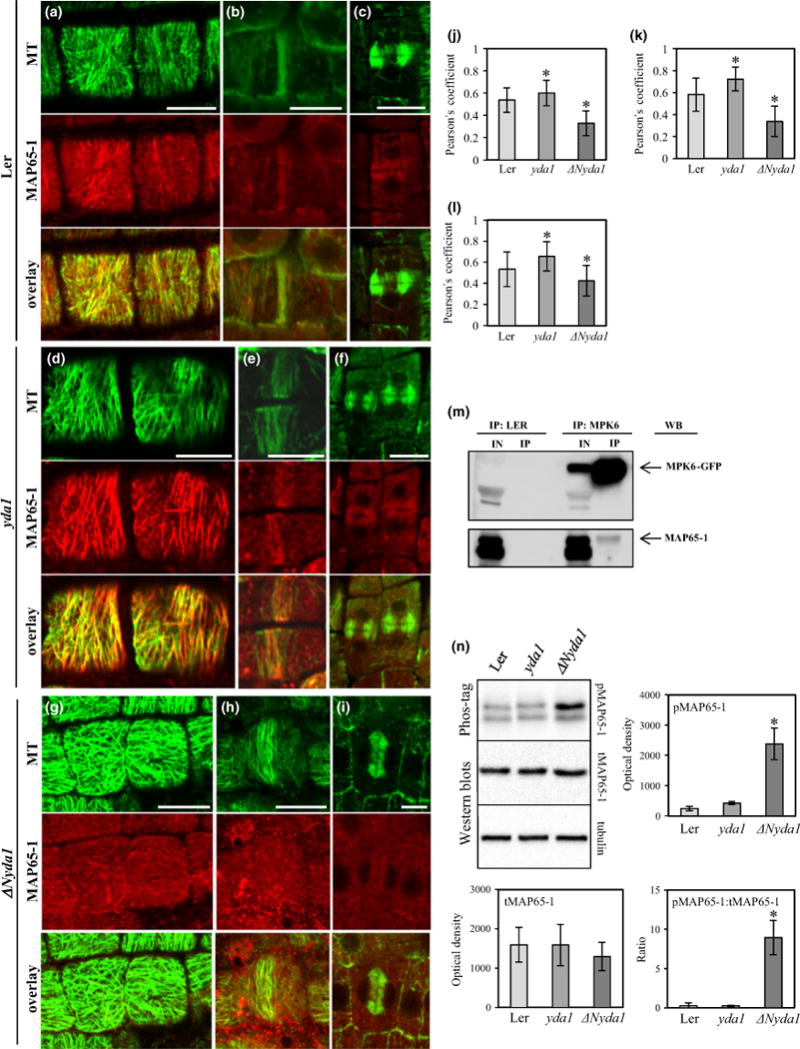
Qualitative and quantitative immunofluorescent co-localization of MAP65-1 with microtubules, and abundance and phosphorylation levels of MAP65-1 in Arabidopsis Landsberg erecta (Ler), yda1 and ΔNyda1 plants. (a–c) Co-localization of MAP65-1 with cortical microtubules (a), preprophase bands (PPBs; b) and phragmoplasts (c) in Ler. (d–f) Co-localization of MAP65-1 with cortical microtubules (d), PPBs (e) and phragmoplasts (f) in yda1. (g–i) Co-localization of MAP65-1 with cortical microtubules (g), PPBs (h) and phragmoplasts (i) in ΔNyda1. (j–l) Averaged Pearson’s coefficients (mean ± SD) from quantitative MAP65-1/microtubule co-localizations in cortical arrays (j; N = 38, N = 38, and N = 31 for Ler, yda1 and ΔNyda1, respectively), PPBs (k; N= 10, N= 21, and N = 19 or Ler, yda1 and ΔNyda1, respectively) and phragmoplasts (l; N= 17, N= 21, and N = 13 for Ler, yda1 and ΔNyda1, respectively). *, P<0.05. (m) GFP-Trap_Aco-immunoprecipitation of MPK6 and MAP65-1. The upper panel shows pulled down MPK6-GFP from the crude extract from plants expressing MPK6-GFP. The bottom panel shows a faint band corresponding to MAP65-1 after western blot probing with anti-MAP65-1 antibody. IN, input; IP, (co)-immunoprecipitated proteins; WB, western blot. (n) Representative Phos-Tag™ (top panel) and western blots of MAP65-1 (middle panel) against tubulin loading control (bottom panel) and graphic depiction (mean ± SD) of phospho-MAP65-1 (pMAP65-1; top panel) total MAP65-1 (tMAP65-1; middle panel) and ratio of pMAP65-1 to tMAP65-1 (bottom panel). *, P<0.05. Bars, 10 μm.
In yda1 root cells, all the above-mentioned microtubule arrays were more strongly decorated with MAP65-1 (Fig. 13d–f). This was shown to be statistically significant when Pearson’s coefficient for co-localization was taken into account (Figs 13j–l, S19–S21).
By contrast, cortical microtubules (Fig. 13g), PPBs (Fig. 13h) and phragmoplasts (Fig. 13i) of ΔNyda1 were nearly completely devoid of MAP65-1 as quantitatively demonstrated in co-localization analyses (Figs 13j–l, S22–S24).
Abundance and phosphorylation of MAP65-1 in yda1 and ΔNyda1 mutants and interaction between MAP65-1 and MPK6
Quantitative fluorescence colocalization of MAP65-1 and microtubules showed increased amounts of microtubule-bound MAP65-1 in yda1 and the near-depletion of microtubule-associated MAP65-1 in ΔNyda1, probably owing to differential regulation of MPK6 in the respective mutants. This prompted us to examine whether MAP65-1 and MPK6 physically interact by means of co-immunoprecipitation. Results showed that MAP65-1 was pulled down, albeit quite weakly, with MPK6 in such co-immunoprecipitation assays (Fig. 13m), perhaps as expected for transient interactions of these two proteins (also mentioned in Popescu et al., 2009). In Ler, yda1 and ΔNyda1, MAP65-1 total protein levels (tMAP65-1) analyzed by western blot were conserved against the alpha-tubulin loading control (Fig. 13n). The phosphorylated form of MAP65-1 (pMAP65-1) remained at moderate levels in Ler and yda1 root extracts, but was significantly elevated in extracts from ΔNyda1 (Fig. 13n).
Discussion
Major phenotypic discrimination during stomatal ontogenesis shows that yda1 mutants bear stomatal clusters while ΔNyda1 mutants form no stomata at all (Nadeau, 2009). Additionally, both mutants exhibit disturbed embryogenesis, which can be traced to the first asymmetric division of the zygote resulting in aberrant basal daughter cell formation (Lukowitz et al., 2004). The embryonic YODA pathway seems to be triggered by the paternally inherited interleukin-1 receptor-associated kinase SHORT SUSPENSOR, which is N-myristoylated and S-palmitoylated, suggesting stable anchorage to the plasma membrane (Bayer et al., 2009). Here, we experimentally investigated the orientations of cell divisions and cytokinesis in post-embryonic primary and lateral roots of yda1 and ΔNyda1 mutants in relation to auxin. Genetic perturbation of YODA consistently affected cell division plane orientation and cytokinesis in the root meristems and affected primary and lateral root morphogenesis.
yda1 and ΔNyda1 mutants show auxin-related phenotypes
Phenotypic features of yda1 and ΔNyda1, such as shorter primary roots, a higher density of lateral roots and agravitropic and twisted growth of ΔNyda1, suggested possible involvement of auxin (Tian et al., 2004; Goh et al., 2014). In addition, root twisting similar to that in ΔNyda1 was induced by auxinole, a TIR1 auxin receptor inhibitor (Hayashi et al., 2008, 2012). Measurements of endogenous IAA showed a moderately increased concentration of IAA in the yda1 mutant and a strongly up-regulated concentration of IAA in the ΔNyda1 mutant. Additionally, the ΔNyda1 mutant was at least partially insensitive to exogenously applied IAA. These findings were further strengthened by proteomic analysis, which revealed an overabundance of auxin biosynthetic enzymes such as tryptophan synthase and nitrilases in both mutants, which was again more pronounced in the ΔNyda1 mutant.
Previously it was reported that the auxin precursor tryptophan can induce more lateral roots (Dubrovsky et al., 2008), which is consistent with the ΔNyda1 mutant showing up-regulated tryptophan synthase. At the subcellular level, auxin is the major regulator of cell division (De Smet & Beeckman, 2011), while cell division orientation and progression were significantly affected in both yda1 and ΔNyda1 mutants.
Post-embryonic cell division defects in roots of yda1 and ΔNyda1 mutants
It was suggested that YODA is somehow involved in asymmetric division in early embryo development (Lukowitz et al., 2004) and that defects originating from loss of function may be carried over to future vegetative development. The proposed carryover may affect developmental aspects of root proliferation such as those including deregulated cell divisions in the stem cell niche. However, cytokinetic progression and cell division plane determination are general cellular mechanisms that are established in meristematic cells and are probably not embryo-specific. This is in accordance with the high expression levels of both MPK6 (Bush & Krysan, 2007; Müller et al., 2010) and YODA (Bergmann et al., 2004) in tissues such as the root tip and lateral root primordia. Lateral root primordia and roots are produced by the mature pericycle and this is clearly a post-embryogenic aspect of root development (Benkova & Bielach, 2010). Our studies on lateral roots revealed higher lateral root density and cell division defects in both yda1 and ΔNyda1 mutants.
Exclusively in yda1 and not in ΔNyda1 mutants, we observed that phragmoplasts show delayed expansion. In such cases, daughter nuclei that were already reconstituted and showed perinuclear microtubules were separated by young phragmoplasts. Furthermore, we observed a failure of cell plate completion, resulting in binucleate cells.
Specification of MPK6 downstream of YODA in the regulation of cell division
MPK6 and MPK3 can redundantly regulate stomatal ontogenesis downstream of YODA (Wang et al., 2007). However, MPK6 alone suffices to affect root division patterns similarly to YODA, as mpk6-2 and yda1 mutants as well as the mpk6-4 mutant transformed with MPK6AEF show similar cell division plane misorientations (this study and Müller et al., 2010). This suggests that MPK6 alone may be generally involved in central mechanisms regulating cell division. The matter of developmental redundancy of MPK3 and MPK6 was elegantly addressed by Bush & Krysan (2007), who studied a kinase-dead version of MPK6 (an MPK6 with a TEY motif substituted for AEF) transformed into the mpk6-4 mutant background. This kinase-dead MPK6 was unable to be phosphorylated and activated, but otherwise capable of undergoing interactions with upstream effectors (Bush & Krysan, 2007). The resulting transgenic line showed stomatal clustering similar to that in yda. In this case, although MPK3 is functional, it seems to be displaced by MPK6AEF (Bush & Krysan, 2007).
When the phosphorylation status of MPK3 and MPK6 was investigated using the anti-pTEpY antibody, phospho-MPK3 and phospho-MPK6 levels were found to be not only increased in ΔNyda1 but quite unexpectedly also in yda1. Although this was previously implied (Lampard et al., 2009), it is shown here for the first time. MPK6 recruitment to definite microtubular structures pertinent to cell division plane orientation, such as the PPB and the phragmoplast, seems to be differentially regulated upon YODA perturbation. Thus, in ΔNyda1 seedlings, a fraction of MPK6 becomes activated and shows increased association with microtubular structures such as the PPB and the phragmoplast, suggesting that co-localization of MPK6 with PPBs and phragmoplast is probably based on specific YODA-dependent MPK6 activation. By contrast, although yda1 plants also showed increased levels of phospho-MPK3 and phospho-MPK6, they were notably devoid of MPK6 accumulation in PPBs and phragmoplasts. Perhaps it is related to differential subcellular localization and it will probably require discrimination between nonactive and active pools of MPK3 and MPK6 using monospecific phospho-MAPK antibodies, as was recently described for Medicago sativa L. STRESS-INDUCED MITOGEN ACTIVATED PROTEIN KINASE (SIMK) (Ovečka et al., 2014). Unfortunately, commercially available pERK antibodies recognize both activated MPK3 and MPK6.
Cytoskeletal proteins downstream of YODA
Cell division planes are defined by intercellular interactions, hormonal signaling and ultimately coordination of the cortical microtubule to PPB transition (Ten Hove & Heidstra, 2008; van Damme, 2009; Müller et al., 2009; Rasmussen et al., 2011b). To date, definite early markers of PPB definition such as TAN1, POK1 and GCP4 have been described (Panteris, 2008; Müller et al., 2009; Rasmussen et al., 2011b). Here, we showed that TAN1, POK1 and GCP4 were differentially expressed in yda1 and ΔNyda1, which might contribute to deregulated cell division planes in these mutants. Further, nitrilases were overabundant in both mutants and they might provide another link between auxin and cytoskeletal microtubular structures, as NITRILASE1 was shown to localize to the phragmoplast (Doskočilová et al., 2013). In addition, nitrilases were also implicated as playing a role in lateral root development (Kutz et al., 2002).
Microtubule-associated proteins (MAPs) have been confirmed to be tunable regulators of division processes, being phosphorylated by either cyclin-dependent kinases or MAPKs (Binarová et al., 1998; Calderini et al., 1998; Bogre et al., 1999; Smertenko et al., 2006). Arabidopsis MAP65 proteins are ubiquitous MAPs localizing to interphase and mitotic microtubule arrays (Müller et al., 2004; Lucas & Shaw 2012). While single map65-1 and map65-2 mutants show no discernible phenotypes, the map65-1map65-2 double mutant displays cell proliferation defects (Lucas & Shaw, 2012). In the MAP65 family, MAP65-1, MAP65-2 and MAP65-3 were identified as targets of MPK4 (Beck et al., 2010; Sasabe et al., 2011), whereas MAP65-1 was identified as an MPK6-phosphorylatable protein (Smertenko et al., 2006; Popescu et al., 2009) or substrate (Hoehenwarter et al., 2013). Here we provide co-immunoprecipitation data showing the MPK6 and MAP65-1 interaction (Fig. 13). While total levels of MAP65-1 protein were roughly maintained in Ler, yda1 and ΔNyda1, the extent of co-localization between MAP65-1 and microtubules was markedly different. Principally, yda1 mutants showed a stronger association of MAP65-1 with cortical microtubules, PPBs and phragmoplasts, while the respective microtubule arrays in ΔNyda1 were poorly decorated with MAP65-1. Considering the proposed role of MAP65-1 phosphorylation in the speed of phragmoplast expansion (Sasabe et al., 2006, Murata et al., 2013), we reason that MAP65-1 overaccumulation in yda1 phragmoplasts may prohibit the disassembly of lagging microtubule bundles and cause the observed delay in phragmoplast expansion and the failure of complete cell plate formation. In contrast, the depletion of MAP65-1 from phragmoplast microtubules in the ΔNyda1 mutant may accelerate phragmoplast expansion. As phragmoplast expansion is corrected by actin/microtubule attachments with cortical sites of final cell plate fusion, its probably faster expansion in ΔNyda1 may lead to failure of such adaptive movements and fusion of cell plates to sites outside the PPB region. This extensive co-localization of MAP65-1 with microtubules in yda1 and the decreased co-localization of MAP65-1 with microtubules in ΔNyda1 can be related to differential, YODA-dependent phosphorylation of MAP65-1 by MPK6.
Concluding remarks
There is accumulating evidence that auxin and MAPKs play a decisive role in developmental fate and tissue patterning by spatially defining symmetric and asymmetric divisions (De Smet & Beeckman, 2011; Lau & Bergmann, 2012; Šamajová et al., 2013). Auxin and MAPKs such as MPK4 (Beck et al., 2010, 2011; Sasabe et al., 2011) and MPK6 (Müller et al., 2010) seem to define the spatial orientation of cell division planes, allowing pattern formation of higher plant vegetative organs. Microtubule-associated proteins currently represented by members of the plant MAP65 family are verified MAPK targets (Sasabe et al., 2011). However, more studies are necessary to address the overall cytoskeletal implications of MAPK signaling, as regulatory proteins such as katanin, END BINDING PROTEIN 1 (EB1) and Rho of plants (Rop) are predicted MAPK targets (Šamajová et al., 2013). Here, we showed that genes regulating cell division orientation, such as TAN1, POK1 and GCP4, were differentially expressed while nitrilases (including phragmoplast-associated NI-TRILASE1) were up-regulated in the yda1 and ΔNyda1 mutants. Finally, the results presented imply a role of MPK6 downstream of the YODA in the determination of cell division orientation and cytokinesis in Arabidopsis primary and lateral roots. A definite and complete pathway of MAPKs and MAPs, dedicated to the spatial determination of cell division plane merits the attention of future studies.
Supplementary Material
Fig. S1 Stomatal pattern in Ler, yda1, ΔNyda1 and mpk6- 4 + MPK6AEF.
Fig. S2 Representative phenotype of 14-d-old plants of Ler, yda1 and ΔNyda1 used for IAA content analysis.
Fig. S3 Root hair emergence with respect to root tip in Ler, yda1 and ΔNyda1.
Fig. S4 Classification of differentially regulated proteins detected by comparative proteomic analysis of Arabidopsis yda1 mutant and wild-type (Ler) according to KEGG annotations.
Fig. S5 Classification of differentially regulated proteins detected by comparative proteomic analysis of Arabidopsis ΔNyda1 mutant and wild-type (Ler) according to KEGG annotations.
Fig. S6 Classification of differentially regulated proteins detected by comparative proteomic analysis of Arabidopsis yda1 and ΔNyda1 mutants according to KEGG annotations.
Fig. S7 Semithin sections stained with toluidine blue showing root tips of wild-type Ler, yda1 and ΔNyda1 mutant roots.
Fig. S8 Phenotype of 4-d-old seedlings and root cell architecture in mpk6-4 + MPK6AEF seedlings stained with FM4-64 styryl dye.
Fig. S9 Identification of multinucleate and binucleate cells in yda1 main root epidermis.
Fig. S10 Overview of mitotic and cytokinetic microtubule arrays of yda1 primary root whole mounts immunostained for tubulin and counterstained with the DNA stain DAPI.
Fig. S11 Quantitative analysis of transcript levels of MPK3 and MPK6 genes in extracts from Ler, yda1 and ΔNyda1 plants.
Fig. S12 Quantitative analysis of transcript level of YODA gene in extracts from 14-d-old plants of Ler, yda1 and ΔNyda1.
Fig. S13 Quantitative analysis of MPK3 and MPK6 protein levels of 14-d-old Ler plants.
Fig. S14 Phenotype of the mpk6-2 mutant, the mpk6-2 mutant rescued with the proMPK6::GFP:MPK6 construct (mpk6-2 + proMPK6::GFP:MPK6) and wild-type (Col-0).
Fig. S15 Immunofluorescent co-localization of MPK3 and microtubules in preprophase bands (PPBs) and phragmoplasts of Ler, yda1 and ΔNyda1 cells.
Fig. S16 Scatter plot demonstrating co-localization between cortical microtubules and MAP65-1 in a root epidermal cell of Ler.
Fig. S17 Scatter plot demonstrating co-localization between PPB and MAP65-1 in a root epidermal preprophase cell of Ler.
Fig. S18 Scatter plot demonstrating co-localization between microtubules and MAP65-1 in the phragmoplast of a root epidermal cytokinetic cell of Ler.
Fig. S19 Scatter plot demonstrating co-localization between cortical microtubules and MAP65-1 in the outlined root epidermal cell of yda1.
Fig. S20 Scatter plot demonstrating co-localization between microtubules and MAP65-1 in the PPB of a root epidermal preprophase cell of yda1.
Fig. S21 Scatter plot demonstrating co-localization between microtubules and MAP65-1 in the phragmoplast of a root epidermal cytokinetic cell of yda1.
Fig. S22 Scatter plot demonstrating co-localization between cortical microtubules and MAP65-1 in a root epidermal cell of ΔNyda1.
Fig. S23 Scatter plot demonstrating co-localization between microtubules and MAP65-1 in the PPB of a root epidermal preprophase cell of ΔNyda1.
Fig. S24 Scatter plot demonstrating co-localization between microtubules and MAP65-1 in the phragmoplast of a root epidermal cell of ΔNyda1.
Table S1 List of forward and reverse primers used for qRT-PCR quantification and amplification of MPK6 native promoter and MPK6 genomic DNA.
Table S2 Protein identification details for two-dimensional LC-MS/MS analysis of wild-type Ler and the yda1 and ΔNyda1 mutants.
Table S3 List of differentially regulated proteins in yda1 mutant seedlings as identified by shot-gun differential proteomic analysis.
Table S4 List of differentially regulated proteins in ΔNyda1 mutant seedlings as identified by shot-gun differential proteomic analysis.
Methods S1 Quantitative co-localizations.
Methods S2 Chemicals.
Methods S3 Root morphometry and phenotyping.
Methods S4 Visualization of stomata.
Methods S5 Quantitative analysis of transcript levels by quantitative PCR.
Methods S6 Proteomic analysis.
Acknowledgments
We thank Ulla Mettbach, Jens Muller, Nils Böhm, Martina Beck and Diedrik Menzel for their help in the initial stages of this project. Seeds of yda and ΔNyda mutants were kindly provided by Wolfgang Lukowitz. We thank Andrei Smertenko for providing rabbit polyclonal anti-MAP65-1 serum, Ken-ichiro Hayashi for auxinole, Eva Hirnerová for her help with auxin analyses, and Xuan Ding and Tony Arick for their assistance with mass spectrometry data collection and export, respectively. This research was supported by grant no. P501/11/1764 from the Czech Science Foundation GAČR, by the National Program for Sustainability I (grant no. LO1204) provided by the Czech Ministry of Education, and by student grant IGA PrF UP 2014–033, and P.V. was supported by grant CZ.1.07/2.3.00/30.0041 POSTUP II at Palacký University, Olomouc, Czech Republic. MS-based proteomics analysis was performed at and funded by the Institute for Genomics, Biocomputing and Biotechnology, Mississippi State University.
Footnotes
Additional supporting information may be found in the online version of this article.
Please note: Wiley Blackwell are not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
References
- Bartel B, Fink GR. Differential regulation of an auxin-producing nitrilase gene family. Arabidopsis thaliana Proceedings of the National Academy of Sciences, USA. 1994;91:6649–6653. doi: 10.1073/pnas.91.14.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M, Nawy T, Giglione C, Galli M, Meinnel T, Lukowitz W. Paternal control of embryonic patterning in Arabidopsis thaliana. Science. 2009;323:1485–1488. doi: 10.1126/science.1167784. [DOI] [PubMed] [Google Scholar]
- Beck M, Komis G, Müller J, Menzel D, Šamaj J. Arabidopsis homologs of nucleus-and phragmoplast-localized kinase 2 and 3 and mitogen-activated protein kinase 4 are essential for microtubule organization. Plant Cell. 2010;22:755–771. doi: 10.1105/tpc.109.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck M, Komis G, Ziemann A, Menzel D, Šamaj J. Mitogen-activated protein kinase 4 is involved in the regulation of mitotic and cytokinetic microtubule transitions in Arabidopsis thaliana. New Phytologist. 2011;189:1069–1083. doi: 10.1111/j.1469-8137.2010.03565.x. [DOI] [PubMed] [Google Scholar]
- Benková E, Bielach A. Lateral root organogenesis – from cell to organ. Current Opinion Plant Biology. 2010;13:677–683. doi: 10.1016/j.pbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Lukowitz W, Somerville CR. Stomatal development and pattern controlled by a MAPKK kinase. Science. 2004;304:1494–1497. doi: 10.1126/science.1096014. [DOI] [PubMed] [Google Scholar]
- Binarová P, Dolezel J, Draber P, Heberle-Bors E, Strnad M, Bögre L. Treatment of Vicia faba root tip cells with specific inhibitors to cyclin-dependent kinases leads to abnormal spindle formation. Plant Journal. 1998;16:697–707. doi: 10.1046/j.1365-313x.1998.00340.x. [DOI] [PubMed] [Google Scholar]
- Bögre L, Calderini O, Binarova P, Mattauch M, Till S, Kiegerl S, Jonak C, Pollaschek C, Barker P, Huskisson NS, et al. A MAP kinase is activated late in plant mitosis and becomes localized to the plane of cell division. Plant Cell. 1999;11:101–113. [PMC free article] [PubMed] [Google Scholar]
- Breuninger H, Lenhard M. Control of tissue and organ growth in plants. Current Topics in Developmental Biology. 2010;91:185–220. doi: 10.1016/S0070-2153(10)91007-7. [DOI] [PubMed] [Google Scholar]
- Buschmann H, Chan J, Sanchez-Pulido L, Andrade-Navarro MA, Doonan JH, Lloyd CW. Microtubule-associated AIR9 recognizes the cortical division site at preprophase and cell-plate insertion. Current Biology. 2006;16:1938–1943. doi: 10.1016/j.cub.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Buschmann H, Hauptmann M, Niessing D, Lloyd CW, Schäffner AR. Helical growth of the Arabidopsis mutant tortifolia2 does not depend on cell division patterns but involves handed twisting of isolated cells. Plant Cell. 2009;21:2090–2106. doi: 10.1105/tpc.108.061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush SM, Krysan PJ. Mutational evidence that the Arabidopsis MAP kinase MPK6 is involved in anther, inflorescence, and embryo development. Journal of Experimental Botany. 2007;58:2181–2191. doi: 10.1093/jxb/erm092. [DOI] [PubMed] [Google Scholar]
- Calderini O, Bögre L, Vicente O, Binarova P, Heberle-Bors E, Wilson C. A cell cycle regulated MAP kinase with a possible role in cytokinesis in tobacco cells. Journal of Cell Science. 1998;111:3091–3100. doi: 10.1242/jcs.111.20.3091. [DOI] [PubMed] [Google Scholar]
- Colcombet J, Hirt H. Arabidopsis MAPKs: a complex signalling network involved in multiple biological processes. BiochemicalJournal. 2008;413:217–226. doi: 10.1042/BJ20080625. [DOI] [PubMed] [Google Scholar]
- van Damme D. Division plane determination during plant somatic cytokinesis. Current Opinion in Plant Biology. 2009;12:745–751. doi: 10.1016/j.pbi.2009.09.014. [DOI] [PubMed] [Google Scholar]
- De Smet I. Lateral root initiation: one step at a time. New Phytologist. 2012;193:867–873. doi: 10.1111/j.1469-8137.2011.03996.x. [DOI] [PubMed] [Google Scholar]
- De Smet I, Beeckman T. Asymmetric cell division in land plants and algae: the driving force for differentiation. Nature Reviews Molecular Cell Biology. 2011;12:177–188. doi: 10.1038/nrm3064. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Mathur J, Hülskamp M, Gadella TW., Jr Microtubule plus-ends reveal essential links between intracellular polarization and localized modulation of endocytosis during division-plane establishment in plant cells. BMC Biology. 2005;3:11. doi: 10.1186/1741-7007-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doskočilová A, Kohoutová L, Volc J, Kourová H, Benada O, Chumová J, Plihal O, Petrovská B, Halada P, Bögre L, et al. NITRILASE1 regulates the exit from proliferation, genome stability and plant development. New Phytologist. 2013;198:685–698. doi: 10.1111/nph.12185. [DOI] [PubMed] [Google Scholar]
- Dubrovsky J, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benkova E. Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proceedings of the National Academy of Sciences, USA. 2008;105:8790–8794. doi: 10.1073/pnas.0712307105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Pytela J, Hotta T, Kato T, Hamada T, Akamatsu R, Ishida Y, Kutsuna N, Hasezawa S, Nomura Y, et al. An atypical tubulin kinase mediates stress-induced microtubule depolymerization in Arabidopsis. Current Biology. 2013;23:1969–1978. doi: 10.1016/j.cub.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Furutani I, Watanabe Y, Prieto R, Masukawa M, Suzuki K, Naoi K, Thitamadee S, Shikanai T, Hashimoto T. The SPIRAL genes are required for directional control of cell elongation in Arabidopsis thaliana. Development. 2000;127:4443–4453. doi: 10.1242/dev.127.20.4443. [DOI] [PubMed] [Google Scholar]
- Goh T, Voβ U, Farcot E, Bennett MJ, Bishopp A. Systems biology approaches to understand the role of auxin in root growth and development. Physiologia Plantarum. 2014;151:73–78. doi: 10.1111/ppl.12162. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Nave J, Zheng N, Hirose M, Kuboki A, Shimada Y, Kepinski S, Nozaki H. Rational design of an auxin antagonist of the SCFTIR1 auxin receptor complex. ACS Chemical Biology. 2012;7:590–598. doi: 10.1021/cb200404c. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Tan X, Zheng N, Hatate T, Kimura Y, Kepinski S, Nozaki H. Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling. Proceedings of the National Academy of Sciences, USA. 2008;105:5632–5637. doi: 10.1073/pnas.0711146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higaki T, Kutsuna N, Sano T, Hasezawa S. Quantitative analysis of changes in actin microfilament contribution to cell plate development in plant cytokinesis. BMC Plant Biology. 2008;8:80. doi: 10.1186/1471-2229-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehenwarter W, Thomas M, Nukarinen E, Egelhofer V, Röhrig H, Weckwerth W, Conrath U, Beckers GJ. Identification of novel in vivo MAP kinase substrates in Arabidopsis thaliana through use of tandem metal oxide affinity chromatography. Molecular & Cell Proteomics. 2013;12:369–380. doi: 10.1074/mcp.M112.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofhuis H, Laskowski M, Du Y, Prasad K, Grigg S, Pinon V, Scheres B. Phyllotaxis and rhizotaxis in Arabidopsis are modified by three PLETHORA transcription factors. Current Biology. 2013;23:956–962. doi: 10.1016/j.cub.2013.04.048. [DOI] [PubMed] [Google Scholar]
- Ishida T, Kaneko Y, Iwano M, Hashimoto T. Helical microtubule arrays in a collection of twisting tubulin mutants of Arabidopsis thaliana. Proceeding of the National Academy of Sciences, USA. 2007;104:8544–8549. doi: 10.1073/pnas.0701224104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen LJ, Kuhn M, Stark M, Chaffron S, Creevey C, Muller J, Doerks T, Julien P, Roth A, Simonovic M, et al. STRING 8-a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Research. 2009;37:D412–D416. doi: 10.1093/nar/gkn760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karahara I, Suda J, Tahara H, Yokota E, Shimmen T, Misaki K, Yonemura S, Staehelin LA, Mineyuki Y. The preprophase band is a localized center of clathrin-mediated endocytosis in late prophase cells of the onion cotyledon epidermis. Plant Journal. 2009;57:819–831. doi: 10.1111/j.1365-313X.2008.03725.x. [DOI] [PubMed] [Google Scholar]
- Kim TW, Michniewicz M, Bergmann DC, Wang ZY. Brassinosteroid regulates stomatal development by GSK3-mediated inhibition of a MAPK pathway. Nature. 2012;482:419–422. doi: 10.1038/nature10794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik A, Ehrhardt DW, Kirik V. TONNEAU2/FASS regulates the geometry of microtubule nucleation and cortical array organization in interphase Arabidopsis cells. Plant Cell. 2012;24:1158–1170. doi: 10.1105/tpc.111.094367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komis G, Illés P, Beck M, Šamaj J. Microtubules and mitogen-activated protein kinase signalling. Current Opinion in Plant Biology. 2011;14:650–657. doi: 10.1016/j.pbi.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Kong Z, Hotta T, Lee YR, Horio T, Liu B. The γ-tubulin complex protein GCP4 is required for organizing functional microtubule arrays in Arabidopsis thaliana. Plant Cell. 2010;22:191–204. doi: 10.1105/tpc.109.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosetsu K, Matsunaga S, Nakagami H, Colcombet J, Sasabe M, Soyano T, Takahashi Y, Hirt H, Machida Y. The MAP kinase MPK4 is required for cytokinesis in Arabidopsis thaliana. Plant Cell. 2010;22:3778–3790. doi: 10.1105/tpc.110.077164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz A, Müller A, Hennig P, Kaiser WM, Piotrowski M, Weiler EW. A role for nitrilase 3 in the regulation of root morphology in sulphur-starving Arabidopsis thaliana. Plant Journal. 2002;30:95–106. doi: 10.1046/j.1365-313x.2002.01271.x. [DOI] [PubMed] [Google Scholar]
- Lampard GR, Lukowitz W, Ellis BE, Bergmann DC. Novel and expanded roles for MAPK signaling in Arabidopsis stomatal cell fate revealed by cell type-specific manipulations. Plant Cell. 2009;21:3506–3517. doi: 10.1105/tpc.109.070110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, Bergmann DC. Stomatal development: a plant’s perspective on cell polarity, cell fate transitions and intercellular communication. Development. 2012;139:3683–3692. doi: 10.1242/dev.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C, Chan J. The parallel lives of microtubules and cellulose microfibrils. Current Opinion in Plant Biology. 2008;11:641–646. doi: 10.1016/j.pbi.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Shaw SL. MAP65-1 and MAP65-2 promote cell proliferation and axial growth in Arabidopsis roots. Plant Journal. 2012;71:454–463. doi: 10.1111/j.1365-313X.2012.05002.x. [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Roeder A, Parmenter D, Somerville C. A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell. 2004;116:109–119. doi: 10.1016/s0092-8674(03)01067-5. [DOI] [PubMed] [Google Scholar]
- Mao G, Chan J, Calder G, Doonan JH, Lloyd CW. Modulated targeting of GFP-AtMAP65-1 to central spindle microtubules during division. Plant Journal. 2005;43:469–478. doi: 10.1111/j.1365-313X.2005.02464.x. [DOI] [PubMed] [Google Scholar]
- Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H, et al. The main auxin biosynthesis pathway in Arabidopsis. Proceedings of the National Academy of Sciences, USA. 2011;108:18512–18517. doi: 10.1073/pnas.1108434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J, Beck M, Mettbach U, Komis G, Hause G, Menzel D, Šamaj J. Arabidopsis MPK6 is involved in cell division plane control during early root development, and localizes to the pre-prophase band, phragmoplast, trans-Golgi network and plasma membrane. Plant Journal. 2010;61:234–248. doi: 10.1111/j.1365-313X.2009.04046.x. [DOI] [PubMed] [Google Scholar]
- Müller S, Han S, Smith LG. Two kinesins are involved in the spatial control of cytokinesis in Arabidopsis thaliana. Current Biology. 2006;16:888–894. doi: 10.1016/j.cub.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Müller S, Smertenko A, Wagner V, Heinrich M, Hussey PJ, Hauser MT. The plant microtubule-associated protein AtMAP65–3/PLE is essential for cytokinetic phragmoplast function. Current Biology. 2004;14:412–417. doi: 10.1016/j.cub.2004.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller S, Wright AJ, Smith LG. Division plane control in plants: new players in the band. Trends in Cell Biology. 2009;19:180–188. doi: 10.1016/j.tcb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Murata T, Sano T, Sasabe M, Nonaka S, Higashiyama T, Hasezawa S, Machida Y, Hasebe M. Mechanism of microtubule array expansion in the cytokinetic phragmoplast. Nature Communications. 2013;4:1967. doi: 10.1038/ncomms2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau JA. Stomatal development: new signals and fate determinants. Current Opinion in Plant Biology. 2009;12:29–35. doi: 10.1016/j.pbi.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák O, Hényková E, Sairanen I, Kowalczyk M, Pospíšil T, Ljung K. Tissue-specific profiling of the Arabidopsis thaliana auxin metabolome. Plant Journal. 2012;72:523–536. doi: 10.1111/j.1365-313X.2012.05085.x. [DOI] [PubMed] [Google Scholar]
- Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, et al. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell. 2005;17:444–463. doi: 10.1105/tpc.104.028316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovečka M, Takáč T, Komis G, Vadovič P, Bekešová S, Doskočilová A, Smékalova V, Luptovčiak I, Šamajová O, Schweighofer A, et al. Salt-induced subcellular kinase relocation and seeding susceptibility caused by overexpression of Medicago SIMKK in Arabidopsis. Journal of Experimental Botany. 2014;65:2335–2350. doi: 10.1093/jxb/eru115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panteris E. Cortical actin filaments at the division site of mitotic plant cells: a reconsideration of the ‘actin-depleted zone’. New Phytologist. 2008;179:334–341. doi: 10.1111/j.1469-8137.2008.02474.x. [DOI] [PubMed] [Google Scholar]
- Popescu SC, Popescu GV, Bachan S, Zhang Z, Gerstein M, Snyder M, Dinesh-Kumar SP. MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes & Development. 2009;23:80–92. doi: 10.1101/gad.1740009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian P, Hou S, Guo G. Molecular mechanisms controlling pavement cell shape in Arabidopsis leaves. Plant Cell Reports. 2009;28:1147–1157. doi: 10.1007/s00299-009-0729-8. [DOI] [PubMed] [Google Scholar]
- Rasmussen CG, Humphries JA, Smith LG. Determination of symmetric and asymmetric division planes in plant cells. Annual Review of Plant Biology. 2011a;62:387–409. doi: 10.1146/annurev-arplant-042110-103802. [DOI] [PubMed] [Google Scholar]
- Rasmussen CG, Sun B, Smith LG. Tangled localization at the cortical division site of plant cells occurs by several mechanisms. Journal of Cell Science. 2011b;124:270–279. doi: 10.1242/jcs.073676. [DOI] [PubMed] [Google Scholar]
- Rasmussen MW, Roux M, Petersen M, Mundy J. MAP kinase cascades in Arabidopsis innate immunity. Frontiers in Plant Sciences. 2012;3:169. doi: 10.3389/fpls.2012.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šamajová O, Komis G, Šamaj J. Emerging topics in the cell biology of mitogen-activated protein kinases. Trends in Plant Science. 2013;18:40–48. doi: 10.1016/j.tplants.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Sasabe M, Kosetsu K, Hidaka M, Murase A, Machida Y. Arabidopsis thaliana MAP65-1 and MAP65-2 function redundantly with MAP65-3/PLEIADE in cytokinesis downstream of MPK4. Plant Signaling and Behavior. 2011;6:743–747. doi: 10.4161/psb.6.5.15146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe M, Machida Y. Regulation of organization and function of microtubules by the mitogen-activated protein kinase cascade during plant cytokinesis. Cytoskeleton (Hoboken) 2012;69:913–918. doi: 10.1002/cm.21072. [DOI] [PubMed] [Google Scholar]
- Sasabe M, Soyano T, Takahashi Y, Sonobe S, Igarashi H, Itoh TJ, Hidaka M, Machida Y. Phosphorylation of NtMAP65-1 by a MAP kinase down-regulates its activity of microtubule bundling and stimulates progression of cytokinesis of tobacco cells. Genes & Development. 2006;20:1004–1014. doi: 10.1101/gad.1408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook JC, Ehrhardt DW, Fisher SE, Scheible WR, Somerville CR. The Arabidopsis SKU6/SPIRAL1 gene encodes a plus end-localized microtubule-interacting protein involved in directional cell expansion. Plant Cell. 2004;16:1506–1520. doi: 10.1105/tpc.020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko AP, Chang HY, Sonobe S, Fenyk SI, Weingartner M, Bögre L, Hussey PJ. Control of the AtMAP65-1 interaction with microtubules through the cell cycle. Journal of Cell Science. 2006;119:3227–3237. doi: 10.1242/jcs.03051. [DOI] [PubMed] [Google Scholar]
- Szymanski DB, Cosgrove DJ. Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Current Opinion in Plant Biology. 2009;19:R800–R811. doi: 10.1016/j.cub.2009.07.056. [DOI] [PubMed] [Google Scholar]
- Ten Hove CA, Heidstra R. Who begets whom? Plant cell fate determination by asymmetric cell division. Current Opinion in Plant Biology. 2008;11:34–41. doi: 10.1016/j.pbi.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Tian C, Muto H, Higuchi K, Matamura T, Tatematsu K, Koshiba T, Yamamoto KT. Disruption and overexpression of auxin response factor 8 gene of Arabidopsis affect hypocotyl elongation and root growth habit, indicating its possible involvement in auxin homeostasis in light condition. Plant Journal. 2004;40:333–343. doi: 10.1111/j.1365-313X.2004.02220.x. [DOI] [PubMed] [Google Scholar]
- Tominaga-Wada R, Ishida T, Wada T. New insights into the mechanism of development of Arabidopsis root hairs and trichomes. International Review of Cell and Molecular Biology. 2011;286:67–106. doi: 10.1016/B978-0-12-385859-7.00002-1. [DOI] [PubMed] [Google Scholar]
- Walia A, Lee JS, Wasteneys G, Ellis B. Arabidopsis mitogen-activated protein kinase MPK18 mediates cortical microtubule functions in plant cells. Plant Journal. 2009;59:565–575. doi: 10.1111/j.1365-313X.2009.03895.x. [DOI] [PubMed] [Google Scholar]
- Walker KL, Müller S, Moss D, Ehrhardt DW, Smith LG. Arabidopsis TANGLED identifies the division plane throughout mitosis and cytokinesis. Current Biology. 2007;17:1827–1836. doi: 10.1016/j.cub.2007.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 2007;19:63–73. doi: 10.1105/tpc.106.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Zhao Q, Rodrigo-Peiris T, Brkljacic J, He CS, Müller S, Meier I. RanGAP1 is a continuous marker of the Arabidopsis cell division plane. Proceedings of the National Academy of Sciences, USA. 2008;105:18637–18642. doi: 10.1073/pnas.0806157105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Stomatal pattern in Ler, yda1, ΔNyda1 and mpk6- 4 + MPK6AEF.
Fig. S2 Representative phenotype of 14-d-old plants of Ler, yda1 and ΔNyda1 used for IAA content analysis.
Fig. S3 Root hair emergence with respect to root tip in Ler, yda1 and ΔNyda1.
Fig. S4 Classification of differentially regulated proteins detected by comparative proteomic analysis of Arabidopsis yda1 mutant and wild-type (Ler) according to KEGG annotations.
Fig. S5 Classification of differentially regulated proteins detected by comparative proteomic analysis of Arabidopsis ΔNyda1 mutant and wild-type (Ler) according to KEGG annotations.
Fig. S6 Classification of differentially regulated proteins detected by comparative proteomic analysis of Arabidopsis yda1 and ΔNyda1 mutants according to KEGG annotations.
Fig. S7 Semithin sections stained with toluidine blue showing root tips of wild-type Ler, yda1 and ΔNyda1 mutant roots.
Fig. S8 Phenotype of 4-d-old seedlings and root cell architecture in mpk6-4 + MPK6AEF seedlings stained with FM4-64 styryl dye.
Fig. S9 Identification of multinucleate and binucleate cells in yda1 main root epidermis.
Fig. S10 Overview of mitotic and cytokinetic microtubule arrays of yda1 primary root whole mounts immunostained for tubulin and counterstained with the DNA stain DAPI.
Fig. S11 Quantitative analysis of transcript levels of MPK3 and MPK6 genes in extracts from Ler, yda1 and ΔNyda1 plants.
Fig. S12 Quantitative analysis of transcript level of YODA gene in extracts from 14-d-old plants of Ler, yda1 and ΔNyda1.
Fig. S13 Quantitative analysis of MPK3 and MPK6 protein levels of 14-d-old Ler plants.
Fig. S14 Phenotype of the mpk6-2 mutant, the mpk6-2 mutant rescued with the proMPK6::GFP:MPK6 construct (mpk6-2 + proMPK6::GFP:MPK6) and wild-type (Col-0).
Fig. S15 Immunofluorescent co-localization of MPK3 and microtubules in preprophase bands (PPBs) and phragmoplasts of Ler, yda1 and ΔNyda1 cells.
Fig. S16 Scatter plot demonstrating co-localization between cortical microtubules and MAP65-1 in a root epidermal cell of Ler.
Fig. S17 Scatter plot demonstrating co-localization between PPB and MAP65-1 in a root epidermal preprophase cell of Ler.
Fig. S18 Scatter plot demonstrating co-localization between microtubules and MAP65-1 in the phragmoplast of a root epidermal cytokinetic cell of Ler.
Fig. S19 Scatter plot demonstrating co-localization between cortical microtubules and MAP65-1 in the outlined root epidermal cell of yda1.
Fig. S20 Scatter plot demonstrating co-localization between microtubules and MAP65-1 in the PPB of a root epidermal preprophase cell of yda1.
Fig. S21 Scatter plot demonstrating co-localization between microtubules and MAP65-1 in the phragmoplast of a root epidermal cytokinetic cell of yda1.
Fig. S22 Scatter plot demonstrating co-localization between cortical microtubules and MAP65-1 in a root epidermal cell of ΔNyda1.
Fig. S23 Scatter plot demonstrating co-localization between microtubules and MAP65-1 in the PPB of a root epidermal preprophase cell of ΔNyda1.
Fig. S24 Scatter plot demonstrating co-localization between microtubules and MAP65-1 in the phragmoplast of a root epidermal cell of ΔNyda1.
Table S1 List of forward and reverse primers used for qRT-PCR quantification and amplification of MPK6 native promoter and MPK6 genomic DNA.
Table S2 Protein identification details for two-dimensional LC-MS/MS analysis of wild-type Ler and the yda1 and ΔNyda1 mutants.
Table S3 List of differentially regulated proteins in yda1 mutant seedlings as identified by shot-gun differential proteomic analysis.
Table S4 List of differentially regulated proteins in ΔNyda1 mutant seedlings as identified by shot-gun differential proteomic analysis.
Methods S1 Quantitative co-localizations.
Methods S2 Chemicals.
Methods S3 Root morphometry and phenotyping.
Methods S4 Visualization of stomata.
Methods S5 Quantitative analysis of transcript levels by quantitative PCR.
Methods S6 Proteomic analysis.


