Abstract
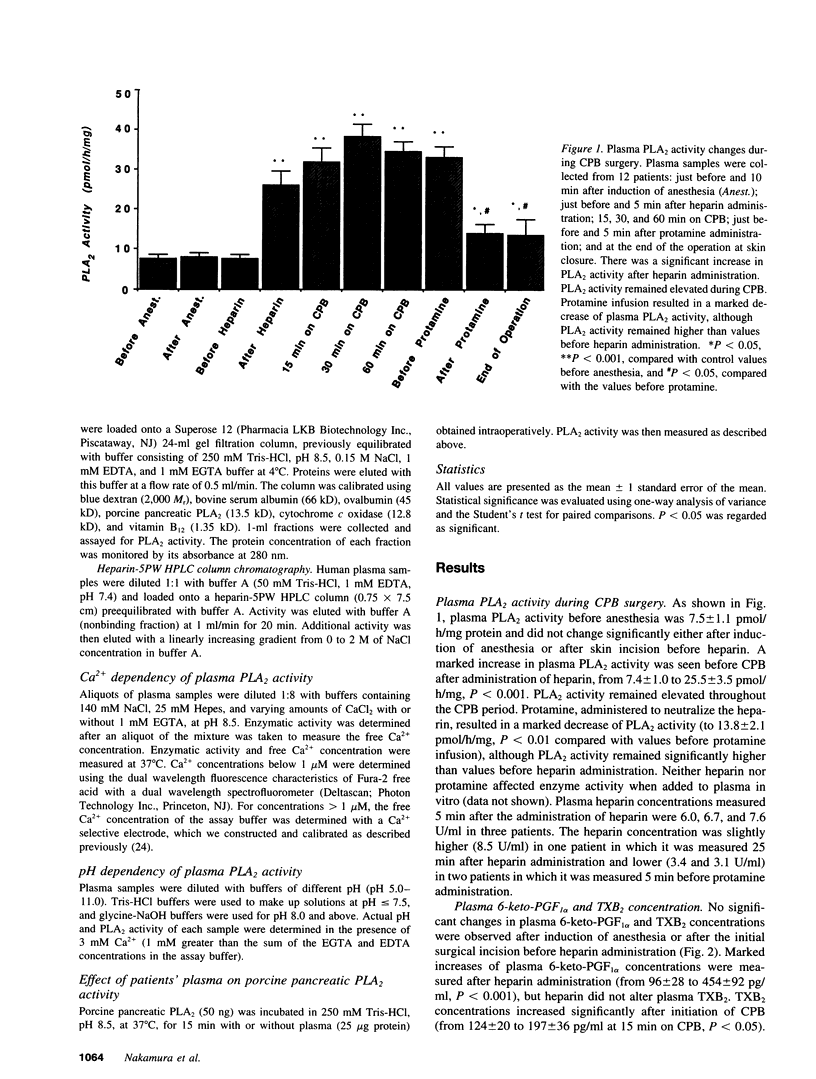
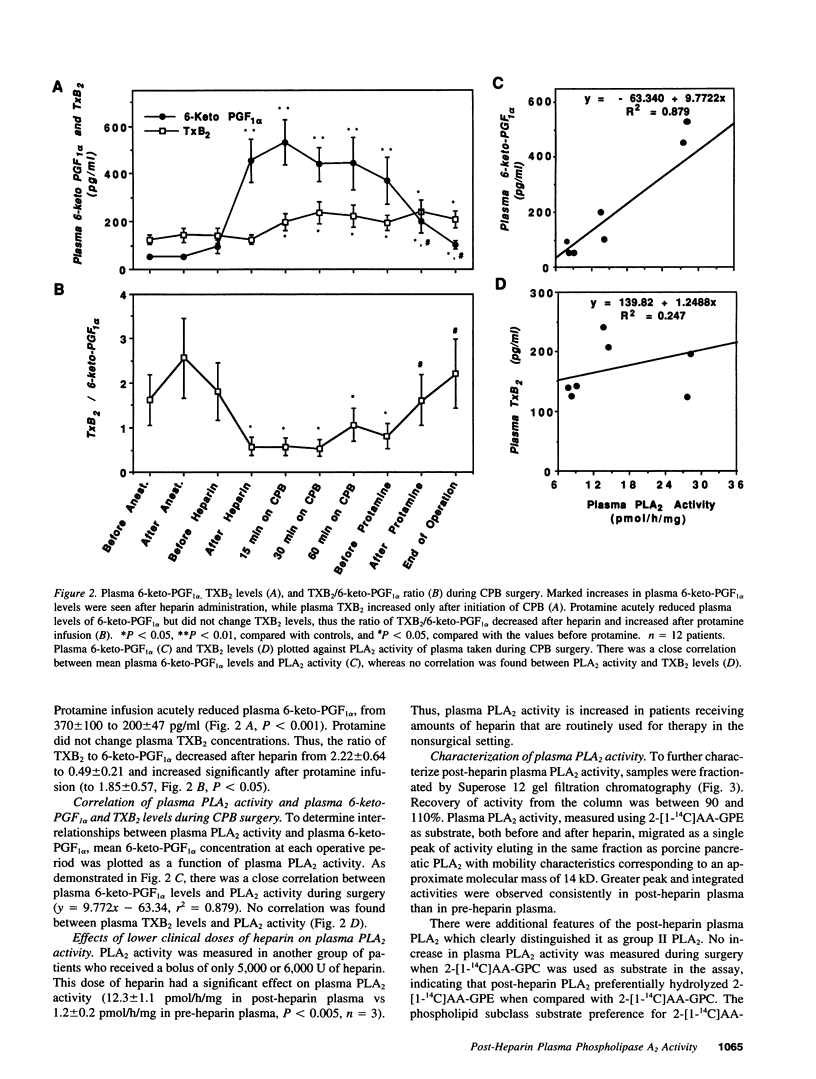
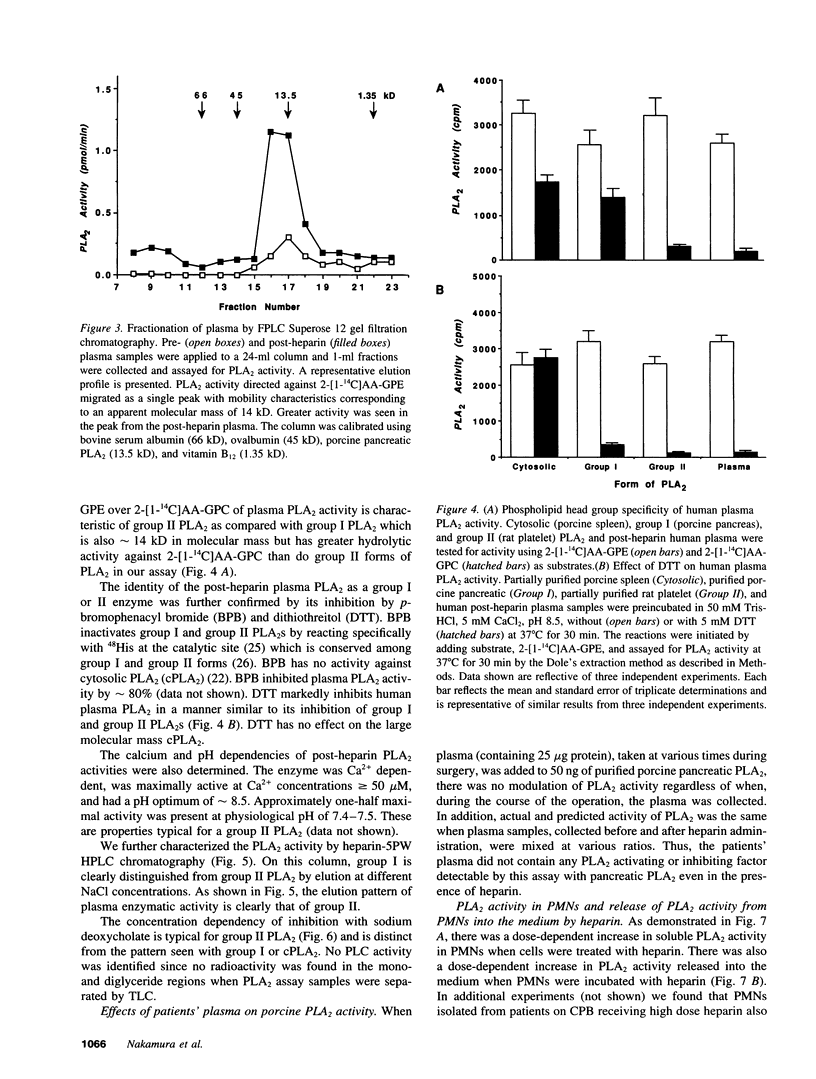
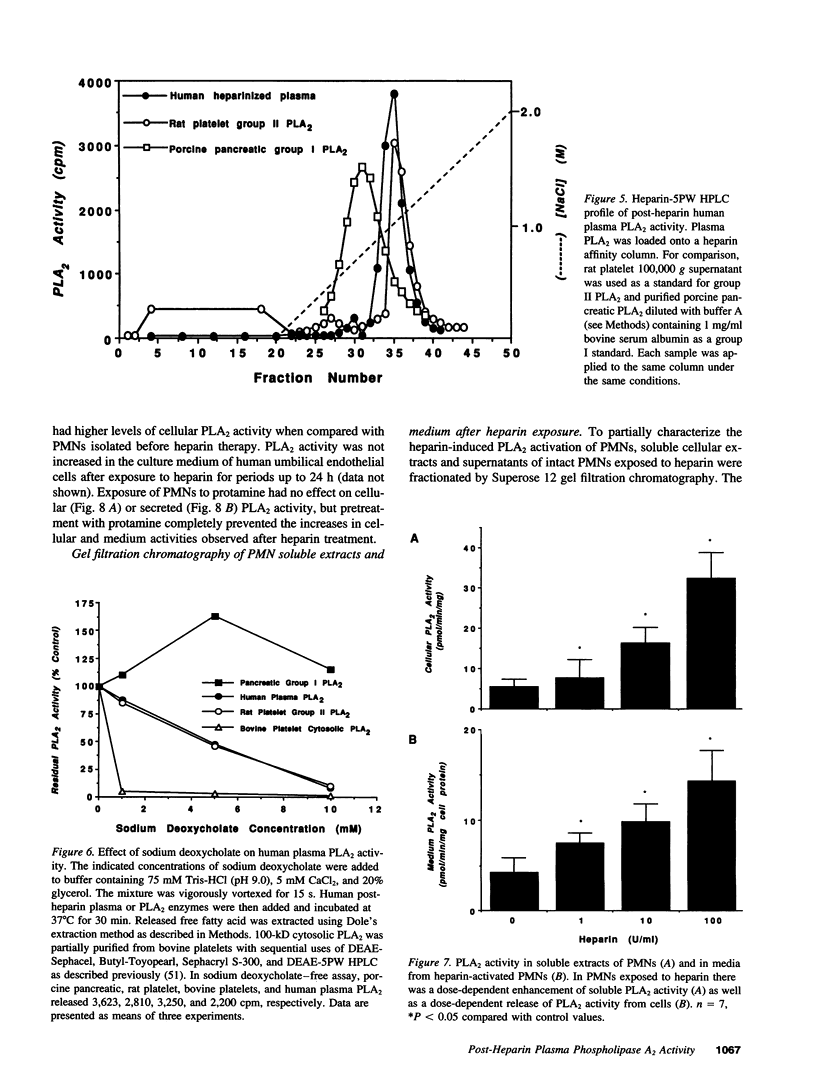
Although eicosanoid production contributes to physiological and pathophysiological consequences of cardiopulmonary bypass (CPB), the mechanisms accounting for the enhanced eicosanoid production have not been defined. Plasma phospholipase A2 (PLA2) activity, 6-keto-prostaglandin F1 alpha (6-keto-PGF1 alpha), and thromboxane B2 (TXB2) levels were measured at various times during cardiac surgery. Plasma PLA2 activity increased after systemic heparinization, before CPB. This was highly correlated with concurrent increases in plasma 6-keto-PGF1 alpha, TXB2 concentrations did not increase with heparin administration but did increase significantly after initiation of CPB. High plasma PLA2 activity, 6-keto-PGF1 alpha, and TXB2 concentrations were measured throughout the CPB period. Protamine, administered to neutralize the heparin, caused an acute reduction of both plasma PLA2 activity and plasma 6-keto-PGF1 alpha, but no change in plasma TXB2 concentrations. Thus the ratio of TXB2 to 6-keto-PGF1 alpha increased significantly after protamine administration. Enhanced plasma PLA2 activity was also measured in patients with lower doses of heparin used clinically for nonsurgical applications. Human plasma PLA2 was identified as group II PLA2 by its sensitivity to deoxycholate and dithiothreitol, its substrate specificity, and its elution characteristics on heparin affinity chromatography. Heparin addition to PMNs in vitro resulted in dose-dependent increases in cellular PLA2 activity and release of PLA2. The PLA2 released from the PMN had characteristics similar to those of post-heparin plasma PLA2. In conclusion, plasma PLA2 activity and 6-keto-PGF1 alpha concentrations are markedly enhanced with systemic heparinization. Part of the anticoagulant and vasodilating effects of heparin may be due to increased plasma prostacyclin (PGI2) levels. In addition the pulmonary vasoconstriction sometimes associated with protamine infusion during cardiac surgery might be due to decreased plasma PLA2 activity, with an associated increased TXB2/6-keto-PGF1 alpha ratio.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addonizio V. P., Jr, Smith J. B., Strauss J. F., 3rd, Colman R. W., Edmunds L. H., Jr Thromboxane synthesis and platelet secretion during cardiopulmonary bypass with bubble oxygenator. J Thorac Cardiovasc Surg. 1980 Jan;79(1):91–96. [PubMed] [Google Scholar]
- Alonso F., Henson P. M., Leslie C. C. A cytosolic phospholipase in human neutrophils that hydrolyzes arachidonoyl-containing phosphatidylcholine. Biochim Biophys Acta. 1986 Sep 12;878(2):273–280. doi: 10.1016/0005-2760(86)90156-6. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Nemenoff R. Renal tubular arachidonic acid metabolism. Kidney Int. 1991 Mar;39(3):438–449. doi: 10.1038/ki.1991.55. [DOI] [PubMed] [Google Scholar]
- Bøyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976 Jun;Suppl 5:9–15. [PubMed] [Google Scholar]
- Chenoweth D. E., Cooper S. W., Hugli T. E., Stewart R. W., Blackstone E. H., Kirklin J. W. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5a anaphylatoxins. N Engl J Med. 1981 Feb 26;304(9):497–503. doi: 10.1056/NEJM198102263040901. [DOI] [PubMed] [Google Scholar]
- Colman R. W. Humoral mediators of catastrophic reactions associated with protamine neutralization. Anesthesiology. 1987 May;66(5):595–596. [PubMed] [Google Scholar]
- Conzen P. F., Habazettl H., Gutmann R., Hobbhahn J., Goetz A. E., Peter K., Brendel W. Thromboxane mediation of pulmonary hemodynamic responses after neutralization of heparin by protamine in pigs. Anesth Analg. 1989 Jan;68(1):25–31. [PubMed] [Google Scholar]
- Cooper J. D., McDonald J. W., Ali M., Menkes E., Masterson J., Klement P. Prostaglandin production associated with the pulmonary vascular response to complement activation. Surgery. 1980 Aug;88(2):215–221. [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Davies G. C., Sobel M., Salzman E. W. Elevated plasma fibrinopeptide A and thromboxane B2 levels during cardiopulmonary bypass. Circulation. 1980 Apr;61(4):808–814. doi: 10.1161/01.cir.61.4.808. [DOI] [PubMed] [Google Scholar]
- Feddersen K., Aurell M., Delin K., Häggendal J., Arén C., Rådegran K. Effects of cardiopulmonary bypass and prostacyclin on plasma catecholamines, angiotensin II and arginine-vasopressin. Acta Anaesthesiol Scand. 1985 Feb;29(2):224–230. doi: 10.1111/j.1399-6576.1985.tb02190.x. [DOI] [PubMed] [Google Scholar]
- Ghirardi P., Marzo A., Rossi C., Respighi E., Brusoni B. Plasma lipids during extracorporeal circulation. J Thorac Cardiovasc Surg. 1975 Oct;70(4):661–665. [PubMed] [Google Scholar]
- Greeley W. J., Bushman G. A., Kong D. L., Oldham H. N., Peterson M. B. Effects of cardiopulmonary bypass on eicosanoid metabolism during pediatric cardiovascular surgery. J Thorac Cardiovasc Surg. 1988 May;95(5):842–849. [PubMed] [Google Scholar]
- Gronich J. H., Bonventre J. V., Nemenoff R. A. Identification and characterization of a hormonally regulated form of phospholipase A2 in rat renal mesangial cells. J Biol Chem. 1988 Nov 15;263(32):16645–16651. [PubMed] [Google Scholar]
- Henderson L. M., Chappell J. B., Jones O. T. Superoxide generation is inhibited by phospholipase A2 inhibitors. Role for phospholipase A2 in the activation of the NADPH oxidase. Biochem J. 1989 Nov 15;264(1):249–255. doi: 10.1042/bj2640249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh P. D., Campbell W. B., Willerson J. T., Hillis L. D. Prostaglandins and ischemic heart disease. Am J Med. 1981 Dec;71(6):1009–1026. doi: 10.1016/0002-9343(81)90335-1. [DOI] [PubMed] [Google Scholar]
- Hobbhahn J., Conzen P. F., Zenker B., Goetz A. E., Peter K., Brendel W. Beneficial effect of cyclooxygenase inhibition on adverse hemodynamic responses after protamine. Anesth Analg. 1988 Mar;67(3):253–260. [PubMed] [Google Scholar]
- Horrow J. C. Protamine: a review of its toxicity. Anesth Analg. 1985 Mar;64(3):348–361. [PubMed] [Google Scholar]
- Ikeda Y., Takagi A., Yamamoto A. Purification and characterization of lipoprotein lipase and hepatic triglyceride lipase from human postheparin plasma: production of monospecific antibody to the individual lipase. Biochim Biophys Acta. 1989 Jun 28;1003(3):254–269. doi: 10.1016/0005-2760(89)90231-2. [DOI] [PubMed] [Google Scholar]
- Itoh F., Kaji T., Hayakawa Y., Oguma Y., Sakuragawa N. Heparin enhances thrombin-stimulated prostaglandin I2 production by cultured endothelial cells. Thromb Res. 1990 Feb 15;57(4):481–488. doi: 10.1016/0049-3848(90)90065-k. [DOI] [PubMed] [Google Scholar]
- KORN E. D. Clearing factor, a heparin-activated lipoprotein lipase. I. Isolation and characterization of the enzyme from normal rat heart. J Biol Chem. 1955 Jul;215(1):1–14. [PubMed] [Google Scholar]
- Kim D. K., Bonventre J. V. Purification of a 100 kDa phospholipase A2 from spleen, lung and kidney: antiserum raised to pig spleen phospholipase A2 recognizes a similar form in bovine lung, kidney and platelets, and immunoprecipitates phospholipase A2 activity. Biochem J. 1993 Aug 15;294(Pt 1):261–270. doi: 10.1042/bj2940261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. K., Kudo I., Inoue K. Purification and characterization of rabbit platelet cytosolic phospholipase A2. Biochim Biophys Acta. 1991 Apr 24;1083(1):80–88. doi: 10.1016/0005-2760(91)90127-4. [DOI] [PubMed] [Google Scholar]
- Kramer R. M., Johansen B., Hession C., Pepinsky R. B. Characterization of a secretable phospholipase A2 from human platelets. Adv Prostaglandin Thromboxane Leukot Res. 1990;20:79–86. [PubMed] [Google Scholar]
- Lowenstein E., Johnston W. E., Lappas D. G., D'Ambra M. N., Schneider R. C., Daggett W. M., Akins C. W., Philbin D. M. Catastrophic pulmonary vasoconstriction associated with protamine reversal of heparin. Anesthesiology. 1983 Nov;59(5):470–473. doi: 10.1097/00000542-198311000-00022. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y., Hostetler K. Y. Properties of phospholipase C isolated from rat liver lysosomes. J Biol Chem. 1980 Jan 25;255(2):646–652. [PubMed] [Google Scholar]
- McIntyre R. W., Flezzani P., Knopes K. D., Reves J. G., Watkins W. D. Pulmonary hypertension and prostaglandins after protamine. Am J Cardiol. 1986 Oct 1;58(9):857–858. doi: 10.1016/0002-9149(86)90371-1. [DOI] [PubMed] [Google Scholar]
- Morel D. R., Lowenstein E., Nguyenduy T., Robinson D. R., Repine J. E., Chenoweth D. E., Zapol W. M. Acute pulmonary vasoconstriction and thromboxane release during protamine reversal of heparin anticoagulation in awake sheep. Evidence for the role of reactive oxygen metabolites following nonimmunological complement activation. Circ Res. 1988 May;62(5):905–915. doi: 10.1161/01.res.62.5.905. [DOI] [PubMed] [Google Scholar]
- Morel D. R., Zapol W. M., Thomas S. J., Kitain E. M., Robinson D. R., Moss J., Chenoweth D. E., Lowenstein E. C5a and thromboxane generation associated with pulmonary vaso- and broncho-constriction during protamine reversal of heparin. Anesthesiology. 1987 May;66(5):597–604. doi: 10.1097/00000542-198705000-00002. [DOI] [PubMed] [Google Scholar]
- Murakami M., Kudo I., Inoue K. Molecular nature of phospholipases A2 involved in prostaglandin I2 synthesis in human umbilical vein endothelial cells. Possible participation of cytosolic and extracellular type II phospholipases A2. J Biol Chem. 1993 Jan 15;268(2):839–844. [PubMed] [Google Scholar]
- Olthoff D., Kunze D., Rüstow B. Phospholipase A activation following extracorporeal circulation and its blockade by procaine. Thorac Cardiovasc Surg. 1983 Aug;31(4):230–233. doi: 10.1055/s-2007-1021985. [DOI] [PubMed] [Google Scholar]
- Patelski J., Waligora Z., Howard A. N. Effects of heparin on lipolytic enzyme activities in vivo and in vitro. Enzyme. 1976;21(1):21–29. doi: 10.1159/000458838. [DOI] [PubMed] [Google Scholar]
- Plachetka J. R., Salomon N. W., Larson D. F., Copeland J. G. Platelet loss during experimental cardiopulmonary bypass and its prevention with prostacyclin. Ann Thorac Surg. 1980 Jul;30(1):58–63. doi: 10.1016/s0003-4975(10)61203-9. [DOI] [PubMed] [Google Scholar]
- Reves J. G., Karp R. B., Buttner E. E., Tosone S., Smith L. R., Samuelson P. N., Kreusch G. R., Oparil S. Neuronal and adrenomedullary catecholamine release in response to cardiopulmonary bypass in man. Circulation. 1982 Jul;66(1):49–55. doi: 10.1161/01.cir.66.1.49. [DOI] [PubMed] [Google Scholar]
- Rådegran K., McAslan C. Circulatory and ventilatory effects of induced platelet aggregation and their inhibition by acetylsalicylic acid. Acta Anaesthesiol Scand. 1972;16(2):76–84. doi: 10.1111/j.1399-6576.1972.tb00573.x. [DOI] [PubMed] [Google Scholar]
- Shakir K. M. Phospholipase A2 activity of post-heparin plasma: a rapid and sensitive assay and partial characterization. Anal Biochem. 1981 Jun;114(1):64–70. doi: 10.1016/0003-2697(81)90452-8. [DOI] [PubMed] [Google Scholar]
- Shaw W. A., Harlan W. R. Simple assay for glycerophospholipid hydrolase activity of postheparin plasma. J Lipid Res. 1977 Jan;18(1):123–127. [PubMed] [Google Scholar]
- Stanley T. H., Berman L., Green O., Robertson D. Plasma catecholamine and cortisol responses to fentanyl--oxygen anesthesia for coronary-artery operations. Anesthesiology. 1980 Sep;53(3):250–253. doi: 10.1097/00000542-198009000-00016. [DOI] [PubMed] [Google Scholar]
- Swerdlow B. N., Mihm F. G., Goetzl E. J., Matthay M. A. Leukotrienes in pulmonary edema fluid after cardiopulmonary bypass. Anesth Analg. 1986 Mar;65(3):306–308. [PubMed] [Google Scholar]
- VOGEL W. C., ZIEVE L. POST-HEPARIN PHOSPHOLIPASE. J Lipid Res. 1964 Apr;5:177–183. [PubMed] [Google Scholar]
- Vadas P. Elevated plasma phospholipase A2 levels: correlation with the hemodynamic and pulmonary changes in gram-negative septic shock. J Lab Clin Med. 1984 Dec;104(6):873–881. [PubMed] [Google Scholar]
- Vadas P., Hay J. B. Involvement of circulating phospholipase A2 in the pathogenesis of the hemodynamic changes in endotoxin shock. Can J Physiol Pharmacol. 1983 Jun;61(6):561–566. doi: 10.1139/y83-086. [DOI] [PubMed] [Google Scholar]
- Watkins W. D., Peterson M. B., Kong D. L., Kono K., Buckley M. J., Levine F. H., Philbin D. M. Thromboxane and prostacyclin changes during cardiopulmonary bypass with and without pulsatile flow. J Thorac Cardiovasc Surg. 1982 Aug;84(2):250–256. [PubMed] [Google Scholar]
- Wright G. W., Ooi C. E., Weiss J., Elsbach P. Purification of a cellular (granulocyte) and an extracellular (serum) phospholipase A2 that participate in the destruction of Escherichia coli in a rabbit inflammatory exudate. J Biol Chem. 1990 Apr 25;265(12):6675–6681. [PubMed] [Google Scholar]
- Ylikorkala O., Saarela E., Viinikka L. Increased prostacyclin and thromboxane production in man during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1981 Aug;82(2):245–247. [PubMed] [Google Scholar]
- Zhou J. A., Fang W. Y., Wang C., Zhou J. L., Guan H. P. The effects of dauricine and verapamil as calcium channel blockers on the plasma concentration of 6-keto-prostaglandin F1 alpha and thromboxane B2 during cardiopulmonary bypass. Thorac Cardiovasc Surg. 1987 Jun;35(3):172–175. [PubMed] [Google Scholar]
- de Haas G. H., Postema N. M., Nieuwenhuizen W., van Deenen L. L. Purification and properties of phospholipase A from porcine pancreas. Biochim Biophys Acta. 1968 Apr 24;159(1):103–117. doi: 10.1016/0005-2744(68)90248-9. [DOI] [PubMed] [Google Scholar]


