Abstract
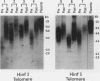
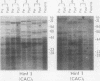
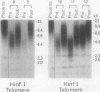
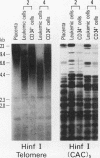
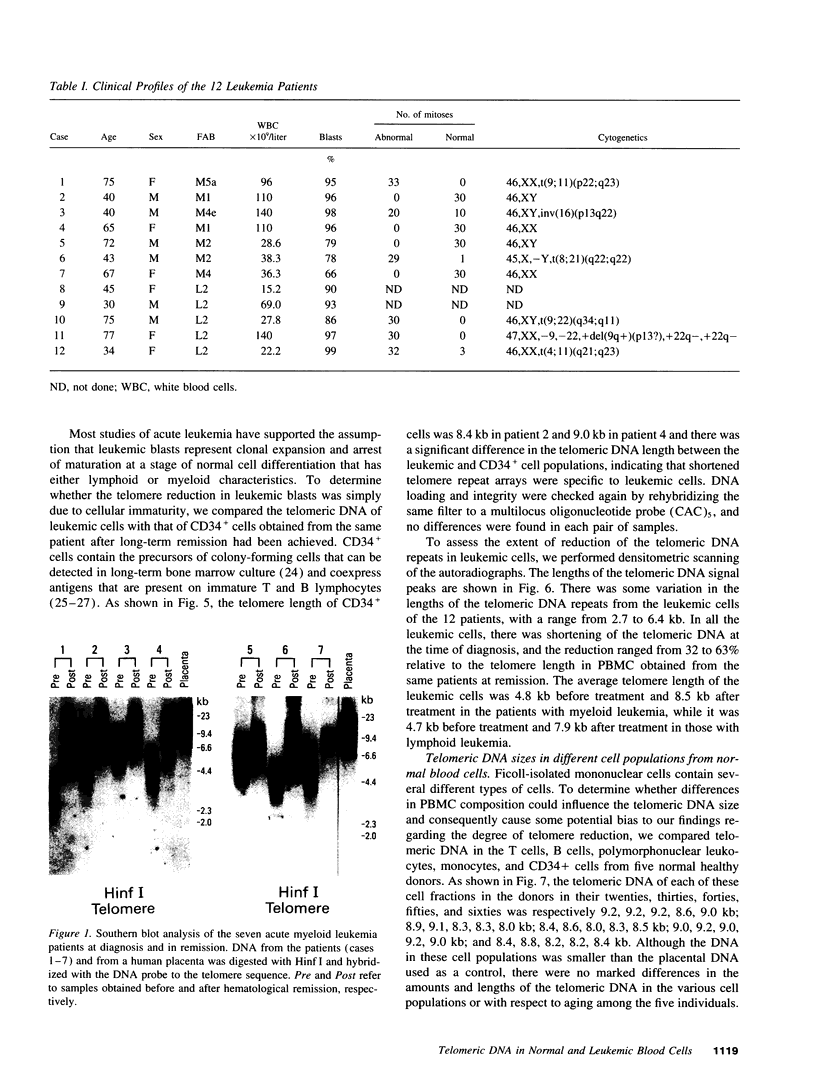
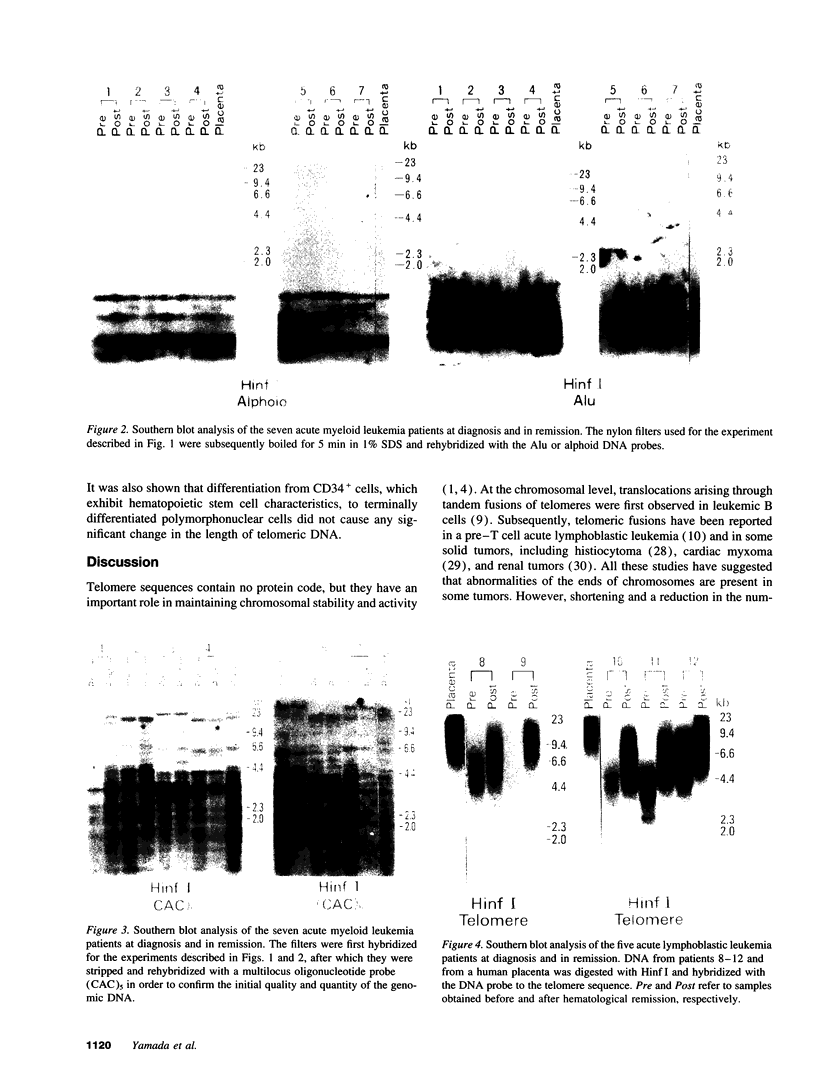
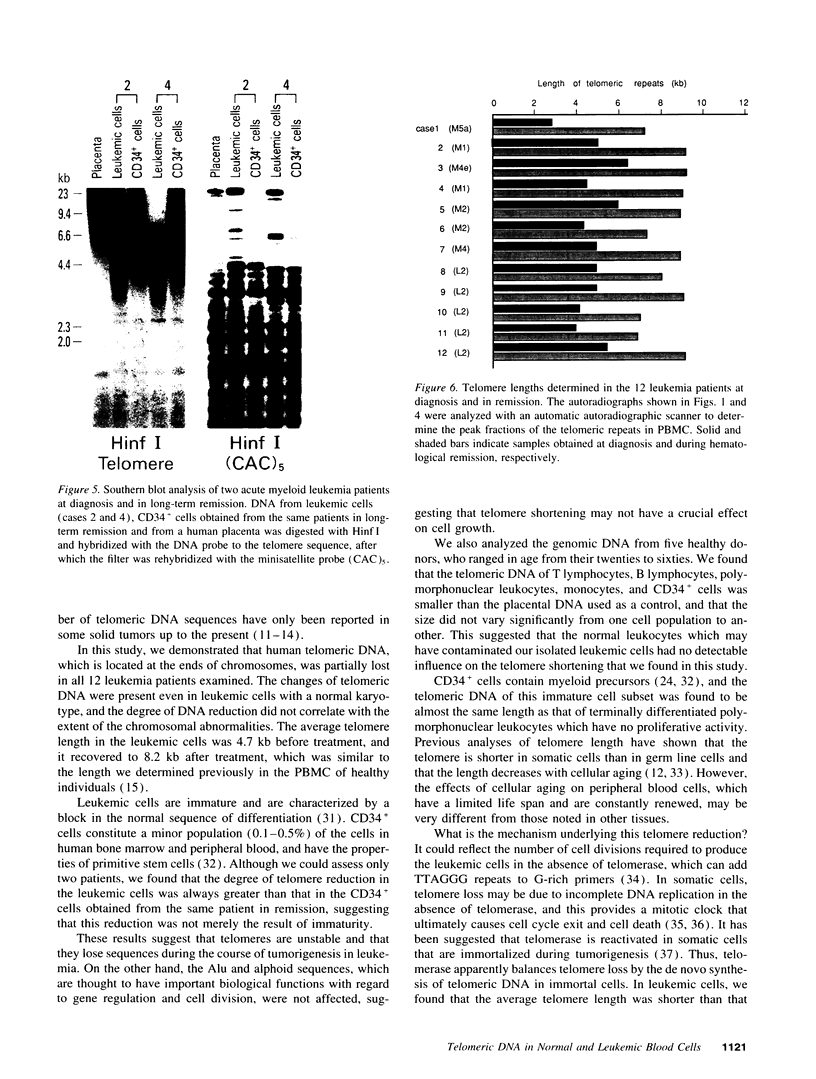
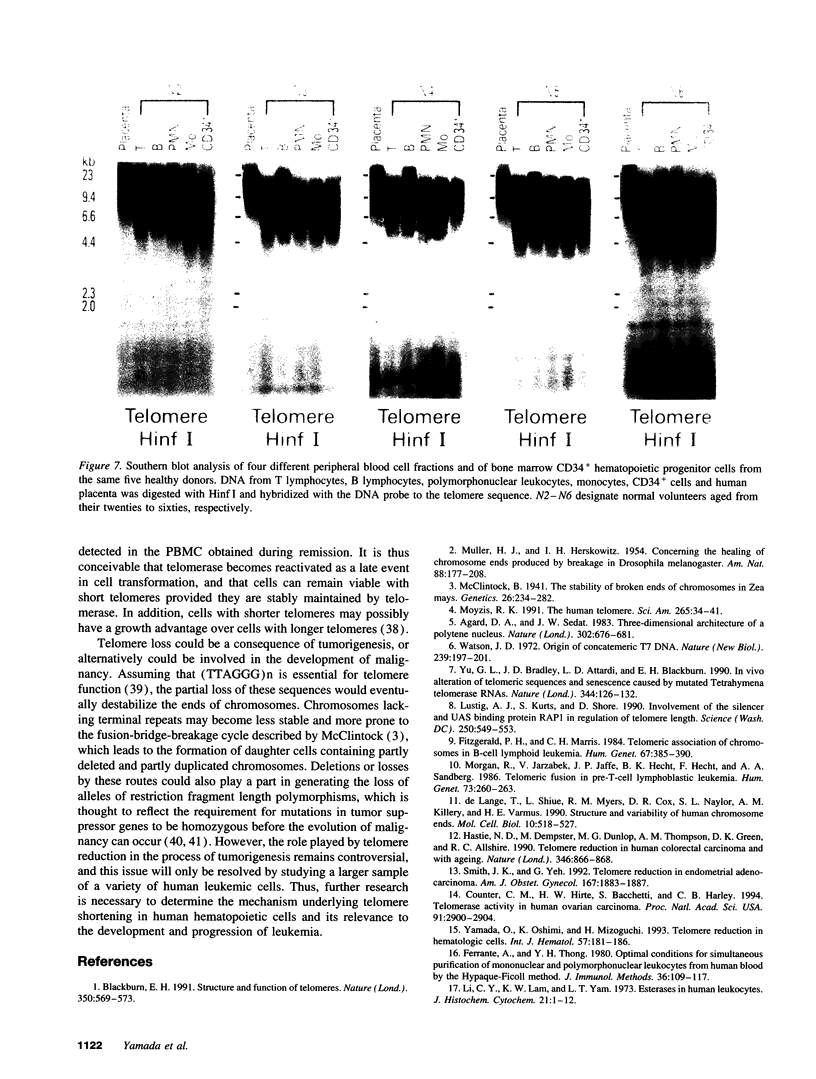
We studied telomeric DNA in leukemic cells as well as in normal T cells, B cells, monocytes, polymorphonuclear leukocytes, and bone marrow hematopoietic progenitor cells. No marked differences were observed in the sizes of the telomeric repeats in the various populations of normal blood cells obtained from donors in their twenties to sixties, and the telomere length ranged between 8.5 and 9.0 kb. The leukemic cells of 12 patients with acute leukemia (seven with myeloid and five with lymphoid leukemia) showed a variable reduction in the length of telomeric DNA, ranging from 2.7 to 6.4 kb. The average telomere length was 4.8 and 4.7 kb in myeloid and lymphoid leukemia, respectively, while the telomere length in peripheral blood mononuclear cells obtained from the same patients during complete remission was 8.5 and 7.9 kb, respectively. When the same Southern blots were hybridized with Alu or alphoid sequences, no marked changes in the sizes of the repetitive DNA sequences were observed, indicating that the DNA abnormality in the leukemic cells was specific to the telomere region. Investigation of telomeric DNA changes may be helpful in determining the biological properties of leukemic cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agard D. A., Sedat J. W. Three-dimensional architecture of a polytene nucleus. Nature. 1983 Apr 21;302(5910):676–681. doi: 10.1038/302676a0. [DOI] [PubMed] [Google Scholar]
- Andrews R. G., Bryant E. M., Bartelmez S. H., Muirhead D. Y., Knitter G. H., Bensinger W., Strong D. M., Bernstein I. D. CD34+ marrow cells, devoid of T and B lymphocytes, reconstitute stable lymphopoiesis and myelopoiesis in lethally irradiated allogeneic baboons. Blood. 1992 Oct 1;80(7):1693–1701. [PubMed] [Google Scholar]
- Andrews R. G., Singer J. W., Bernstein I. D. Monoclonal antibody 12-8 recognizes a 115-kd molecule present on both unipotent and multipotent hematopoietic colony-forming cells and their precursors. Blood. 1986 Mar;67(3):842–845. [PubMed] [Google Scholar]
- Blackburn E. H. Structure and function of telomeres. Nature. 1991 Apr 18;350(6319):569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counter C. M., Hirte H. W., Bacchetti S., Harley C. B. Telomerase activity in human ovarian carcinoma. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):2900–2904. doi: 10.1073/pnas.91.8.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald G. W., Dahl R. J., Spurbeck J. L., Carney J. A., Gordon H. Chromosomally abnormal clones and nonrandom telomeric translocations in cardiac myxomas. Mayo Clin Proc. 1987 Jul;62(7):558–567. doi: 10.1016/s0025-6196(12)62293-9. [DOI] [PubMed] [Google Scholar]
- Erickson D. Model mice. Transgenic animals and Alzheimer's research. Sci Am. 1991 Sep;265(3):34–34. [PubMed] [Google Scholar]
- Ferrante A., Thong Y. H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J Immunol Methods. 1980;36(2):109–117. doi: 10.1016/0022-1759(80)90036-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. H., Morris C. M. Telomeric association of chromosomes in B-cell lymphoid leukemia. Hum Genet. 1984;67(4):385–390. doi: 10.1007/BF00291396. [DOI] [PubMed] [Google Scholar]
- Harley C. B. Telomere loss: mitotic clock or genetic time bomb? Mutat Res. 1991 Mar-Nov;256(2-6):271–282. doi: 10.1016/0921-8734(91)90018-7. [DOI] [PubMed] [Google Scholar]
- Harrington L. A., Greider C. W. Telomerase primer specificity and chromosome healing. Nature. 1991 Oct 3;353(6343):451–454. doi: 10.1038/353451a0. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Houck C. M., Rinehart F. P., Schmid C. W. A ubiquitous family of repeated DNA sequences in the human genome. J Mol Biol. 1979 Aug 15;132(3):289–306. doi: 10.1016/0022-2836(79)90261-4. [DOI] [PubMed] [Google Scholar]
- Kiyama R., Matsui H., Oishi M. A repetitive DNA family (Sau3A family) in human chromosomes: extrachromosomal DNA and DNA polymorphism. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4665–4669. doi: 10.1073/pnas.83.13.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs G., Müller-Brechlin R., Szücs S. Telomeric association in two human renal tumors. Cancer Genet Cytogenet. 1987 Oct;28(2):363–366. doi: 10.1016/0165-4608(87)90225-1. [DOI] [PubMed] [Google Scholar]
- Kurtzberg J., Denning S. M., Nycum L. M., Singer K. H., Haynes B. F. Immature human thymocytes can be driven to differentiate into nonlymphoid lineages by cytokines from thymic epithelial cells. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7575–7579. doi: 10.1073/pnas.86.19.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson D. D., Spangler E. A., Blackburn E. H. Dynamics of telomere length variation in Tetrahymena thermophila. Cell. 1987 Jul 31;50(3):477–483. doi: 10.1016/0092-8674(87)90501-0. [DOI] [PubMed] [Google Scholar]
- Levy M. Z., Allsopp R. C., Futcher A. B., Greider C. W., Harley C. B. Telomere end-replication problem and cell aging. J Mol Biol. 1992 Jun 20;225(4):951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- Lithotripsy. Health and Public Policy Committee, American College of Physicians. Ann Intern Med. 1985 Oct;103(4):626–629. [PubMed] [Google Scholar]
- Loken M. R., Shah V. O., Dattilio K. L., Civin C. I. Flow cytometric analysis of human bone marrow. II. Normal B lymphocyte development. Blood. 1987 Nov;70(5):1316–1324. [PubMed] [Google Scholar]
- Lustig A. J., Kurtz S., Shore D. Involvement of the silencer and UAS binding protein RAP1 in regulation of telomere length. Science. 1990 Oct 26;250(4980):549–553. doi: 10.1126/science.2237406. [DOI] [PubMed] [Google Scholar]
- Mandahl N., Heim S., Kristoffersson U., Mitelman F., Röser B., Rydholm A., Willén H. Telomeric association in a malignant fibrous histiocytoma. Hum Genet. 1985;71(4):321–324. doi: 10.1007/BF00388457. [DOI] [PubMed] [Google Scholar]
- McClintock B. The Stability of Broken Ends of Chromosomes in Zea Mays. Genetics. 1941 Mar;26(2):234–282. doi: 10.1093/genetics/26.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan R., Jarzabek V., Jaffe J. P., Hecht B. K., Hecht F., Sandberg A. A. Telomeric fusion in pre-T-cell acute lymphoblastic leukemia. Hum Genet. 1986 Jul;73(3):260–263. doi: 10.1007/BF00401240. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols J., Nimer S. D. Transcription factors, translocations, and leukemia. Blood. 1992 Dec 15;80(12):2953–2963. [PubMed] [Google Scholar]
- Oshimi K., Oshimi Y., Yamada O., Wada M., Hara T., Mizoguchi H. Cytotoxic T lymphocyte triggering via CD16 is regulated by CD3 and CD8 antigens. Studies with T cell receptor (TCR)-alpha beta+/CD3+16+ and TCR-gamma delta+/CD3+16+ granular lymphocytes. J Immunol. 1990 May 1;144(9):3312–3317. [PubMed] [Google Scholar]
- Ryan D. H., Chapple C. W., Kossover S. A., Sandberg A. A., Cohen H. J. Phenotypic similarities and differences between CALLA-positive acute lymphoblastic leukemia cells and normal marrow CALLA-positive B cell precursors. Blood. 1987 Sep;70(3):814–821. [PubMed] [Google Scholar]
- Smith J. K., Yeh G. Telomere reduction in endometrial adenocarcinoma. Am J Obstet Gynecol. 1992 Dec;167(6):1883–1887. doi: 10.1016/0002-9378(92)91791-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Yamada O., Oshimi K., Mizoguchi H. Telomere reduction in hematologic cells. Int J Hematol. 1993 Apr;57(2):181–186. [PubMed] [Google Scholar]
- Yu G. L., Bradley J. D., Attardi L. D., Blackburn E. H. In vivo alteration of telomere sequences and senescence caused by mutated Tetrahymena telomerase RNAs. Nature. 1990 Mar 8;344(6262):126–132. doi: 10.1038/344126a0. [DOI] [PubMed] [Google Scholar]
- de Lange T., Shiue L., Myers R. M., Cox D. R., Naylor S. L., Killery A. M., Varmus H. E. Structure and variability of human chromosome ends. Mol Cell Biol. 1990 Feb;10(2):518–527. doi: 10.1128/mcb.10.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]