Abstract
Chronic intermittent ethanol consumption is associated with neurodegeneration and cognitive deficits in preclinical laboratory animals and in the clinical population. While previous work suggests a role for neuroadaptations in the N-methyl-D-aspartate (NMDA) receptor in the development of ethanol dependence and manifestation of withdrawal, the relative roles of ethanol exposure and ethanol withdrawal in producing these effects have not been fully characterized. To examine underlying cytotoxic mechanisms associated with CIE exposure, organotypic hippocampal slices were exposed to 1–3 cycles of ethanol (50 mM) in cell culture medium for 5 days, followed by 24-hours of ethanol withdrawal in which a portion of slices were exposed to competitive NMDA receptor antagonist (2R)-amino-5-phosphonovaleric acid (APV; 40 µM). Cytotoxicity was assessed using immunohistochemical labeling of neuron specific nuclear protein (NeuN; Fox-3), a marker of mature neurons, and thionine (2%) staining of Nissl bodies. Multiple cycles of CIE produced neurotoxicity, as reflected in persisting losses of neuron NeuN immunoreactivity and thionine staining in each of the primary cell layers of the hippocampal formation. Hippocampi aged in vitro were significantly more sensitive to the toxic effects of multiple CIEs than were non-aged hippocampi. This effect was not demonstrated in slices exposed to continuous ethanol, in the absence of withdrawal, or to a single exposure/withdrawal regimen. Exposure to APV significantly attenuated the cytotoxicity observed in the primary cell layers of the hippocampus. The present findings suggest that ethanol withdrawal is required to produce NMDA receptor-dependent hippocampal cytotoxicity, particularly in the aging hippocampus in vitro.
Keywords: Hippocampus, CIE, NMDA Receptor, APV, NeuN, Thionine
Introduction
Prolonged alcohol dependence is known to produce neurodegeneration and cognitive decline that may be specifically associated with a common drinking pattern characterized by periods of heavy consumption followed by periods of abstinence (Mello & Mendelson 1972; for review, see Duka et al., 2004). This intermittent pattern of intake is known to progressively increase the incidence of seizures during periods of withdrawal from alcohol (Ballenger & Post, 1978; Shaw et al., 1998; Wojnar et al., 1999). Retrospective analyses of patient records have established a significant relationship between multiple prior withdrawals and seizures during acute withdrawal (Lechtenberg & Worner 1991; Booth& Blow, 1993; Worner, 1996; see Duka et al., 2004 for a review). Brain volume abnormalities and cognitive decline are also thought to be expedited in dependent individuals who have experienced multiple seizures or detoxifications. For example, Sullivan et al. (1996) reported that temporal lobe white matter volume was inversely associated with prior alcohol withdrawal seizures. Duka et al. (2003) reported deficits in inhibitory control of prepotent motor responses in alcohol-dependent individuals that were statistically associated with a history of a greater number of prior detoxifications. Loeber et al. (2010) similarly reported that patients with a history of multiple prior detoxifications showed delays in their cognitive recovery at 3 months post detoxification, when compared with those with fewer prior detoxifications.
Use of preclinical models of chronic intermittent ethanol (CIE) in rodents suggests that CIE exposure increases the rate, intensity, and duration of subsequent seizures (Stevens et al. 2001; Veatch & Becker, 2002), decreases the development of subsequent long-term potentiation (Stephens et al., 2005), and produces neurodegeneration of the hippocampal formation (Corso et al., 1998; Collins et al., 1998; Zhao et al., 2013). For example, mice exposed to 3 cycles of vaporized ethanol for 16 hours a day followed by 8 hours of ethanol withdrawal demonstrated significant increases in handling-induced convulsions and electroencephalogram (EEG) activity (Veatch & Becker, 2005). As another example, Zhao et al. (2013) reported that a CIE model employing gavage ethanol administration produced neurodegeneration of the medial temporal lobe and working memory deficits in rats. In vitro, cultured cortical neurons were exposed to a CIE treatment regimen of 75 mM ethanol for 14 hours followed by 10 hours of withdrawal from ethanol, and repeated a total of 5 times and terminated by either a 2 or 5 day period of withdrawal (Qiang et al., 2007). Western blot and immunoblot analyses revealed that CIE produced selective increases in GluN1 and GluN2B subunit expression on the surface membrane. While collectively, these studies demonstrate that neuroadaptive changes in excitatory neurotransmission are produced by CIE, the functional role of the NMDA receptor in mediating these neurodegenerative effects has yet to be established. The purpose of the present studies was, therefore, to examine the distinct roles of ethanol exposure and ethanol withdrawal, as well assess the influence of the NMDA receptor in promotion of hippocampal neurodegeneration produced by CIE in rat hippocampal explants.
Materials and Methods
Preparation of Organotypic Hippocampal Slice Cultures
Whole brains from eight-day-old Sprague-Dawley rats (Harlan Laboratories; Indianapolis, IN) were aseptically removed and transferred to culture dishes containing frozen dissecting medium composed of Minimum Essential Medium (MEM; Invitrogen, Carlsbad, CA), 25 mM HEPES (Sigma, St. Louis, MO), and 50 µM streptomycin/penicillin (Invitrogen). Bilateral hippocampi were then removed and transferred to plates containing culture medium composed of dissecting medium, distilled water, 36 mM glucose (Fisher, Pittsburg, PA), 25% Hanks’ Balanced Salt Solution (HBSS; Invitrogen), 25% (v/v) heat-inactivated horse serum (HIHS; Sigma), and 0.05% streptomycin/penicillin (Invitrogen). Excess hippocampal tissue was removed using a stereoscopic microscope and unilateral hippocampi were sectioned at 200 µM using a McIlwain Tissue Chopper (Mickle Laboratory Engineering Co. Ltd., Gomshall, UK). Three to 4 intact hippocampal slices were plated onto Millicell-CM 0.4 µM biopore membrane inserts containing 1 mL of pre-incubated culture medium, with each plate containing 18 to 24 intact hippocampal slices. The tissue was maintained in an incubator at 37°C with a gas composition of 5% CO2/95% air for 5 days before any experiments were conducted. Care of all animals was carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996), as well as the University of Kentucky’s Institutional Animal Care and Use Committee. All experiments included in the present report were replicated using at least two different rat litters.
CIE Treatment Regimen
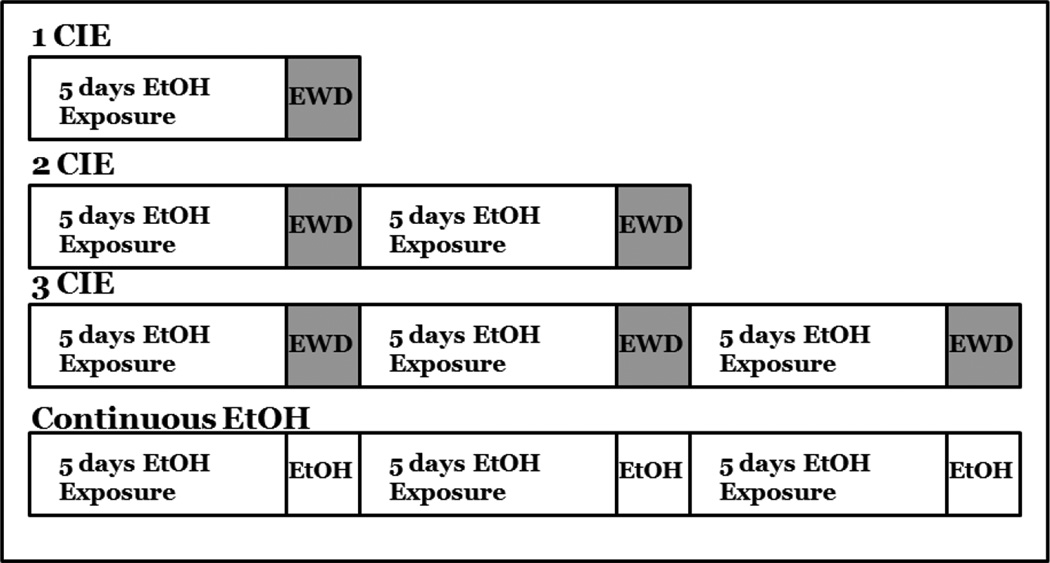
The current studies examined the number of cycles of CIE required to produce cytotoxicity in hippocampal slice cultures. At 5 days in vitro (DIV), slices were assigned to one of the following treatment conditions: 1) 1 cycle of CIE, in which slices were exposed to medium or medium containing 50 mM ethanol for 5 DIV, followed by a 24-hour withdrawal period; 2) 2 cycles of CIE, in which slices were exposed to medium or medium containing 50 mM ethanol for 5 DIV, followed by a 24-hour withdrawal period, and repeated two times; 3) 3 cycles of CIE, in which slices were exposed to medium with or without 50 mM ethanol for 5 DIV, followed by a 24-hour withdrawal period, repeated a total of three times; 4) continuous ethanol exposure, in which slices were maintained in medium with or without50 mM ethanol for 18 consecutive days, in the absence of any withdrawal, and fixed (Figure 1). An additional study assessed the effects of 3 cycles of CIE and protracted withdrawal for 7 days on neuron specific nuclear protein (NeuN) immunofluorescence. Slices were exposed to medium with or without 50 mM ethanol for 5 DIV, followed by a 24-hour withdrawal period, repeated a total of three times, followed by 7 days of withdrawal. This concentration of ethanol (50 mM) was selected in order to reflect binge alcohol use (Eckardt et al., 1998; Jones and Sternebring, 1992). For example, prior studies conducted in our laboratory demonstrate a decline in the actual ethanol concentration over the 5-day exposure (i.e., from 198.39 mg/dl to 123.93 mg/dl) (Butler et al, 2008). During each 5-day exposure period, ethanol and controltreated slices were maintained inside of Ziploc bags filled with 5%CO2/95% air and water bath solutions containing either distilled water (50 mL) for control plates or distilled water (50 mL) containing 50 mM ethanol for ethanol-treated plates so as to minimize evaporation of ethanol described in prior reports (Prendergast et al., 2004). Slices were fixed by placing 1 mL of 10% formalin solution on the top and bottom of each well for 30 minutes. Slices were then washed twice with phosphate buffered saline (PBS) and stored at 4°C until immunohistochemistry was initiated.
Figure 1.
Representative timelines of chronic, intermittent ethanol treatment of hippocampal slice cultures.
Effects of Aging in Vitro on CIE-induced Cytotoxicity
Following the initial studies described above, a separate set of experiments were conducted in which tissue was maintained in culture for the same length of time, and fixed at the same time point, as 3 cycles of CIE. These studies were designed to account for potential effects of aging on CIE-induced loss of NeuN immunoreactivity and thionine staining. In these studies, hippocampal slices were prepared, and maintained, as described above. At 5 DIV, slices were randomly assigned to one of the following treatment conditions: 1) 1 cycle of CIE, in which slices were maintained in ethanol-free medium for 16 days (with a medium change every 5 days prior to ethanol treatment) and then exposed to medium or medium containing 50 mM ethanol for 5 DIV, followed by a 24-hour withdrawal period; or 2) 2 cycles of CIE, in which slices were maintained in ethanol-free medium for 11 days (with medium changes every 5 days prior to ethanol treatment) and then exposed to medium or medium containing 50 mM ethanol for 5 DIV, followed by a 24-hour withdrawal period, and repeated two times.
Effects of Competitive NMDA Receptor Antagonist-APV on Cytotoxicity
To determine if cytotoxicity observed in tissue exposed to 3 CIE was NMDA receptor-dependent, the competitive NMDA receptor antagonist (2R)-amino-5-phosphonovaleric acid (APV; 40 µM; Sigma) was administered during periods of withdrawal. In these studies, slices were exposed to 50 mM ethanol for 5 days, followed by a 24-hour withdrawal period in which slices were exposed to ethanol-naïve medium either with or without APV. This treatment regimen was repeated during each of the 3 ethanol withdrawal periods. The concentration of APV (40 µM) was selected based on a previous report demonstrating that lower concentrations (i.e., 20 µM) of APV significantly attenuated the excitotoxic effects of 24-hour NMDA exposure in the pyramidal cell layer (Butler et al., 2010).
Immunoreactivity of neuron specific nuclear protein (NeuN)
Immunohistochemistry was performed on slice cultures with labeling of NeuN to compare the density of mature neurons in the pyramidal cell layers of the cornu ammonis (CA1 and CA3), and granule cell layer of the dentate gyrus. NeuN is expressed in nearly all post-mitotic and differentiating neurons and is detected by the monoclonal anti-NeuN antibody (Mullen et al., 1992). NeuN has been recently identified as Fox-3, a member of the Fox-1 gene family, which is a splicing regulator of pre-mRNA (Kim et al., 2009). Following fixation, inserts were transferred to a plate containing 1 mL of permeabilization (wash) buffer (200 mL PBS [Invitrogen], 200 µL Triton X-100 [Sigma], 0.010 mg Bovine Serum [Sigma]), 1 ml of buffer was added to the top of each well and allowed to incubate at room temperature for 45 minutes to permeate cell membranes. Inserts were transferred to a plate containing 1 mL of PBS and 1mL of the primary monoclonal antibody mouse anti-NeuN (1:200; Sigma) was placed on the top of each well (after Butler et al., 2010) and stored at 4°C for 24-hours. Slices were washed twice with PBS and transferred to a plate containing 1 mL of PBS and 1 mL of goat anti-mouse secondary antibody fluorescein isothiocyanate (FITC) for NeuN-labeled cultures (1:200; Sigma) was placed on top of the well and stored at 4°C for 24 hours. Slices were washed twice with PBS and imaged immediately with a 5× objective lens. Imaging of cultures is described in detail below. A range of antibody concentrations was examined prior to the conduct of studies included in the present report. The concentrations of NeuN and FITC antibodies chosen were shown to have the least amount of background signal while maintaining a strong, specific signal within the regions of interest (Butler et al., 2010).
Hippocampal slices were imaged with SPOT software 4.0.2 (advanced version) for Windows (W. Nuhsbahm Inc.; McHenry, IL, USA) through a 5x objective with a Leica DMIRB microscope (W. Nuhsbahm Inc.; McHenry, IL, USA) connected to a computer and captured with a SPOT 7.2 color mosaic camera (W. Nuhsburg). FITC fluorescence was detected with a band-pass filter at 495 nm (520 nm emission). Densitometry using Image J software (National Institutes of Health, Bethesda, MD) was conducted to measure the intensity of FITC fluorescence. A background measurement was recorded for each slice and then subtracted from the measurement of each region of the hippocampus (the granule cell layer of the dentate gyrus and the pyramidal cell layers of the CA3 and CA1 regions). In the quantitative analyses of NeuN, raw values of NeuN immunoreactivity were converted to percent control values, and combined. N = 38–40 for each hippocampal cell layer.
Thionine Staining
Following immunohistochemistry, some of the slices were also exposed to 1 mL of 0.2% thionine stock on the top of each with 1 mL of PBS on the bottom of each well for 5 minutes. The inserts were then transferred to a plate containing 1 mL of PBS and 1 mL of 70% ethanol was placed on the top of the well for a 2-minute dehydration period. Slices were then washed twice with PBS for 5 minutes each and subsequently imaged. Hippocampal slices stained with thionine were observed with SPOT software 4.0.2 (advanced version) for Windows (W. Nuhsbahm Inc.; McHenry, IL, USA) through a 5× objective with a Leica DMIRB microscope (w. Nuhsbahm Inc.; McHenry, IL, USA) connected to a computer and captured with a SPOT 7.2 color mosaic camera (W. Nuhsburg). Densitometry using Image J software (National Institutes of Health, Bethesda, MD) was conducted to measure the brightfield intensity. Raw brightfield values were converted to percent control values within each region of the hippocampus to control for differences among replications. Thionine is a monochromatic dye which binds to Nissl substance[s] located on cytoplasmic RNA (Kadar et al., 2009) and DNA content of all cell nuclei (Scott and Willett, 1966). N = 17–35 per treatment condition for each hippocampal region due to tissue loss during thionine staining.
Statistical Analyses
Each experiment included in the present report was conducted a minimum of two times using different rat litters composed of 10–12 rat pups per litter. Data from each replication were converted into percent control values and then combined to control for potential litter effects. Previous studies from our laboratory have demonstrated that the CA1 region may be particularly sensitive to excitotoxic insult (Butler, et al., 2010). Thus, for the initial CIE immunohistochemical studies, a separate two-way ANOVA was conducted (treatment × sex) for each region of the hippocampus: pyramidal cells of the CA1 and CA3, and granule cells of the dentate gyrus. As no main effect of sex was observed, all data were collapsed into a one-way ANOVA (treatment). Statistical significance was determined at p<0.05. Post-hoc analyses were conducted using Fisher’s Least Significant Difference (LSD). For graphical representation of thionine data, mean data from each were condition were converted using the formula [(x-100)-100*(−1)] so as to express data on the same scale used for the immunohistochemical data.
Results
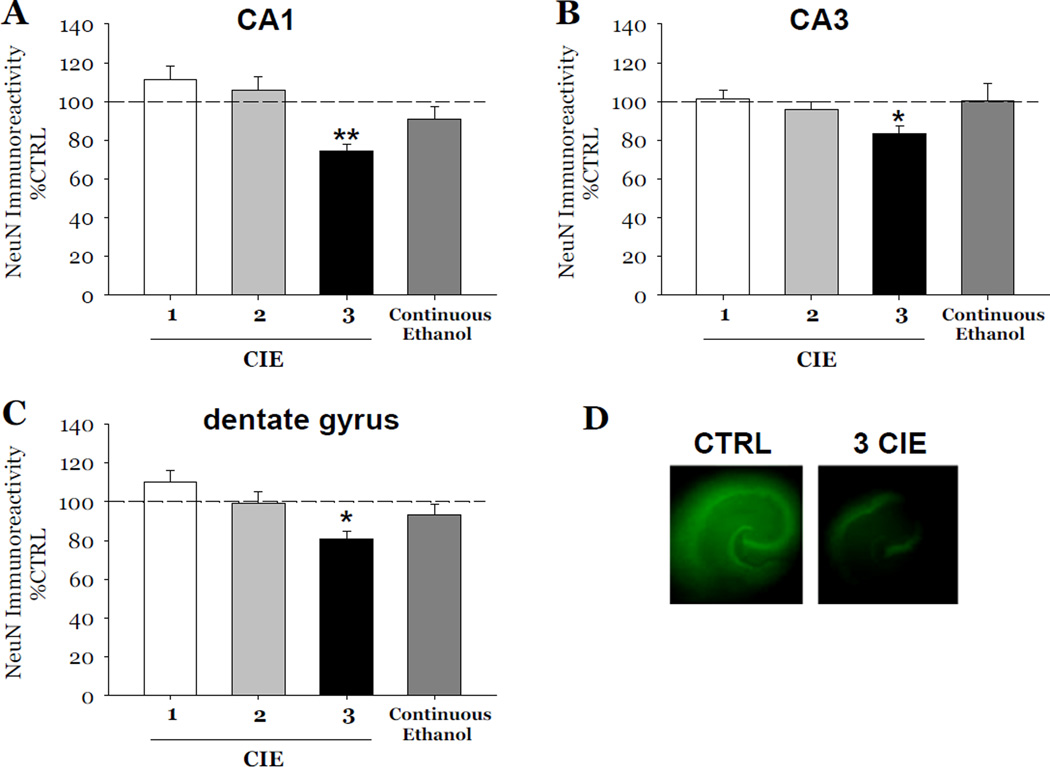
Initial studies included in this report examined the number of cycles of CIE required to produce cytotoxicity (reflected by reductions of NeuN and thionine staining) in hippocampal slice cultures exposed ethanol (50 mM), followed by 1 day of withdrawal, and repeated either a total of 1, 2, or 3 times (i.e., 1, 2, or 3 CIE). Exposure to 3 cycles of CIE produced a 26% loss of NeuN immunoreactivity in the CA1 (F[1,39]= 23.16, p<0.001; Figure 2A), a 17% loss of NeuN immunoreactivity in the CA3 (F[1,39]= 6.46, p<0.05; Figure 2B), and 20% loss of NeuN immunoreactivity as compared to control values in dentate gyrus (F[1,39]= 9.67, p<0.05; Figure 2C). Notably, these decreases in NeuN immunoflourescence did not recover at 7 days post-withdrawal (data not shown).
Figure 2.
Effects of exposure to 50 mM ethanol for 5 DIV, followed by one 24-hour ethanol withdrawal period, and repeated for 1, 2, and 3 cycles of CIE, or continuous ethanol exposure (50 mM), in the absence of withdrawal, on the immunoreactivity of neuron specific nuclear protein (NeuN) observed in organotypic slice cultures. Within the pyramidal cell layers of the CA1 (A) and CA3 (B), and granule cell layer of the dentate gyrus (C), exposure to 1 or 2 cycles of CIE, exposure to continuous ethanol did not result in significant decreases of NeuN immunoreactivity as compared to control values (Figure 2). By contrast, 3 cycles of CIE in slices resulted in significant decreases of NeuN immunoreactivity as compared to control values in these hippocampal subregions (Figure 2). NeuN immunoflourescence was significantly decreased in hippocampi exposed to 3 cycles of CIE compared to hippocampi exposed to control medium (D). p < 0.05 vs control; ** p < 0.001 vs control.
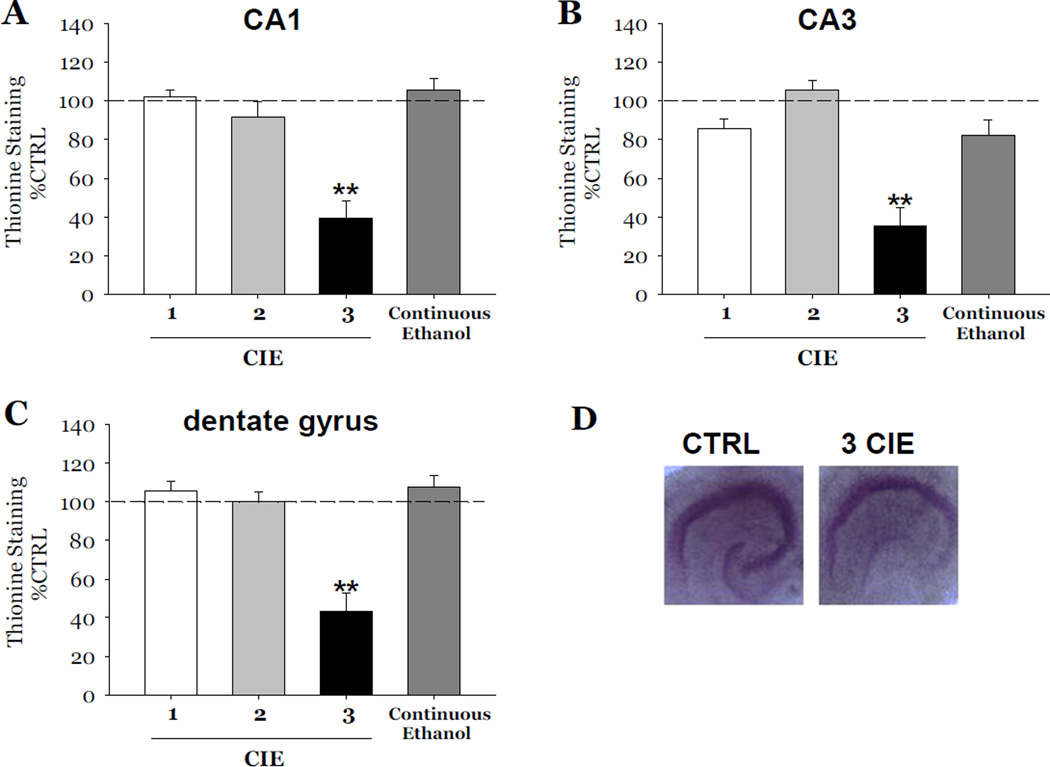
Thionine staining was also consistently decreased as compared to control values by 61% in the CA1 (F[1,34]= 35.31, p<0.001, Figure 3A), 65% in the CA3 (F[1,34]= 28.89, p<0.001, Figure 3B), and 57% in the dentate gyrus (F[1,34]= 25.97, p<0.001, Figure 3C). Exposure to either 1 or 2 cycles of CIE did not result in decreases of either marker of neuronal viability in any subregion of the hippocampal formation (Figures 2 and 3). Notably, exposure to 50 mM ethanol for 18 consecutive DIV, in the absence of any withdrawal, did not result in significant decreases of either marker in any hippocampal subregion (Figures 2 and 3).
Figure 3.
Effects of exposure to 50 mM ethanol for 5 DIV, followed by one 24-hour period of ethanol withdrawal, and repeated for 1, 2, and 3 cycles of CIE, or exposure to continuous ethanol (50 mM) on thionine staining observed in organotypic slice cultures. Within the pyramidal cell layers of the CA1 (A) and CA3 (B), and granule cell layer of the dentate gyrus (C), exposure to 1 or 2 cycles of CIE, or continuous ethanol exposure did not result in significant decreases of thionine staining as compared to control values; whereas exposure 3 cycles of CIE in slices resulted in significant decreases of thionine staining as compared to control values in these hippocampal subregions (Figure 3). ** p < 0.001 vs control. Thionine staining of Nissl bodies was significantly decreased in hippocampi exposed to 3 cycles of CIE compared to hippocampi exposed to control medium (D).
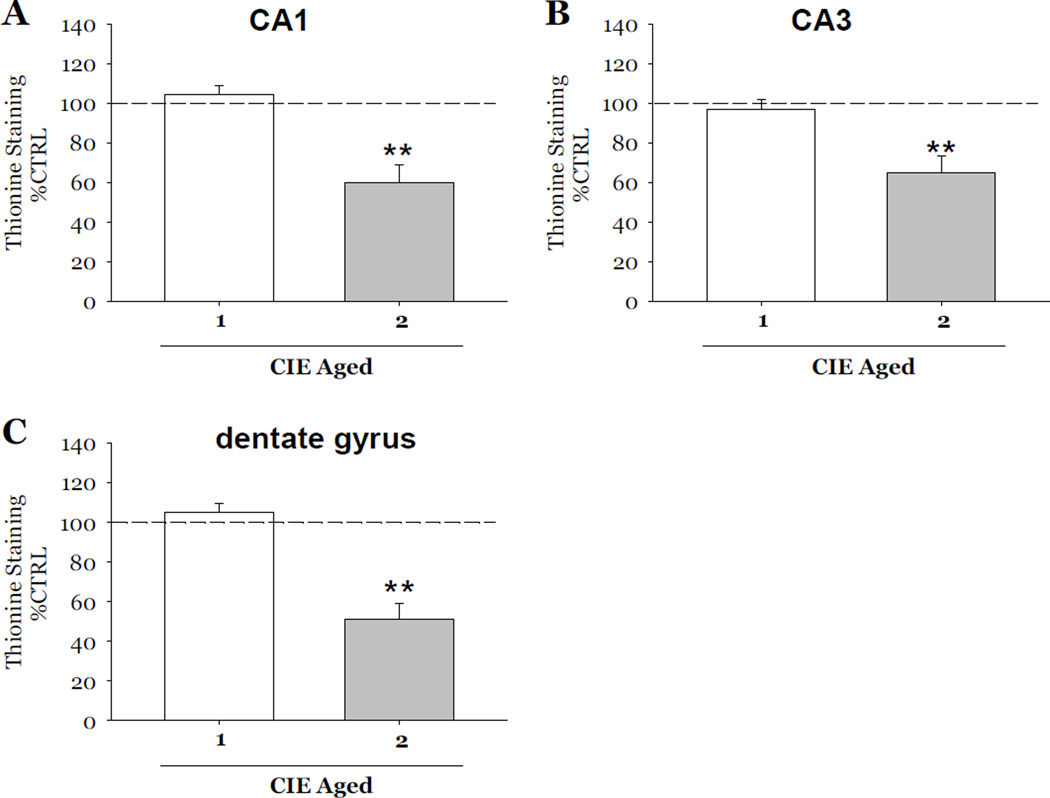
Effects of Aging in Vitro on CIE-induced Cytotoxicity
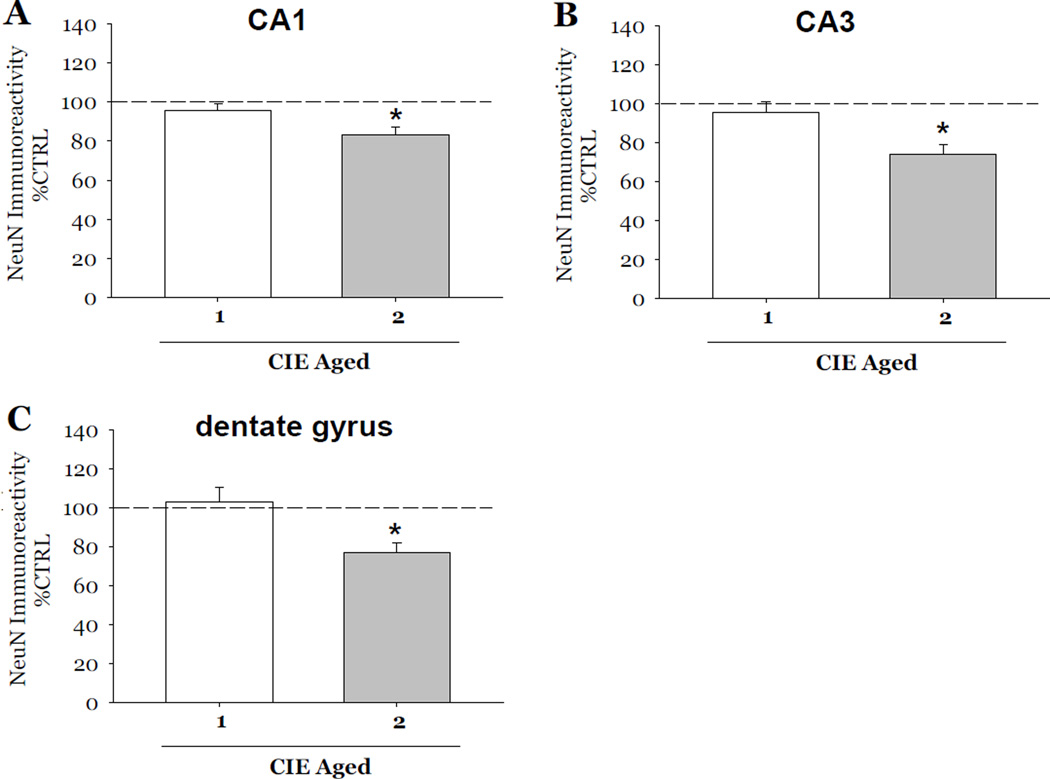
Following the initial studies described above, a separate set of experiments were conducted in order to account for potential effects of aging on CIE-induced loss of NeuN immunoreactivity and thionine staining. Therefore, in these studies, all tissue was maintained in vitro for a total of 23 days. Exposure to 2 cycles of CIE produced a 17% loss of NeuN immunoreactivity as compared to control values in the CA1 (F[1,38]= 7.94, p<0.05; Figure 4A), a 26% loss in the CA3 (F[1,38]= 8.15, p<0.05; Figure 4B) and a 23% loss in the dentate gyrus (F[1,38]= 9.67, p<0.05; Figure 4C). Thionine staining was also significantly decreased as compared to control values by 40% in the CA1 (F[1,34]= 13.73, p<0.001; Figure 5A), 35% in the CA3 (F[1,34]= 12.01, p<0.001; Figure 5B), and 48% in the dentate gyrus (F[1,34]= 25.22, p<0.001; Figure 5C). Worthwhile to note is that exposure to 1 cycle of CIE in these aged slice cultures did not result in decreases of either marker of neuronal viability in any subregion of the hippocampal formation (Figures 4 and 5).
Figure 4.
Effects of exposure to 50 mM ethanol for 5 DIV, followed by one 24-hour period of ethanol withdrawal, and repeated for 1 or 2 CIE in aged slice cultures on NeuN immunoreactivity observed in organotypic slice cultures. Within the pyramidal cell layers of the CA1 and CA3, and granule cell layer of the dentate gyrus, exposure to 1 cycle of CIE in aged slice cultures did not result in significant decreases NeuN immunoreactivity (Figure 4) as compared to control values; whereas exposure 2 cycles of CIE in aged slices resulted in significant decreases of NeuN immunoreactivity compared to control values in these hippocampal subregions (Figure 4). *p <0.05 vs control.
Figure 5.
Effects of exposure to 50 mM ethanol for 5 DIV, followed by one 24-hour period of ethanol withdrawal, and repeated for 1 or 2 CIE in aged slice cultures on thionine staining observed in organotypic slice cultures. Within the pyramidal cell layers of the CA1 and CA3, and granule cell layer of the dentate gyrus, exposure to 1 cycle of CIE in aged slice cultures did not result in significant decreases in thionine staining of Nissl bodies (Figure 5) as compared to control values; whereas exposure 2 cycles of CIE in aged slices resulted in significant decreases of thionine compared to control values in these hippocampal subregions (Figure 5). **p <0.001 vs control.
Effects of NMDA Receptor Antagonist-APV on CIE-induced Cytotoxicity
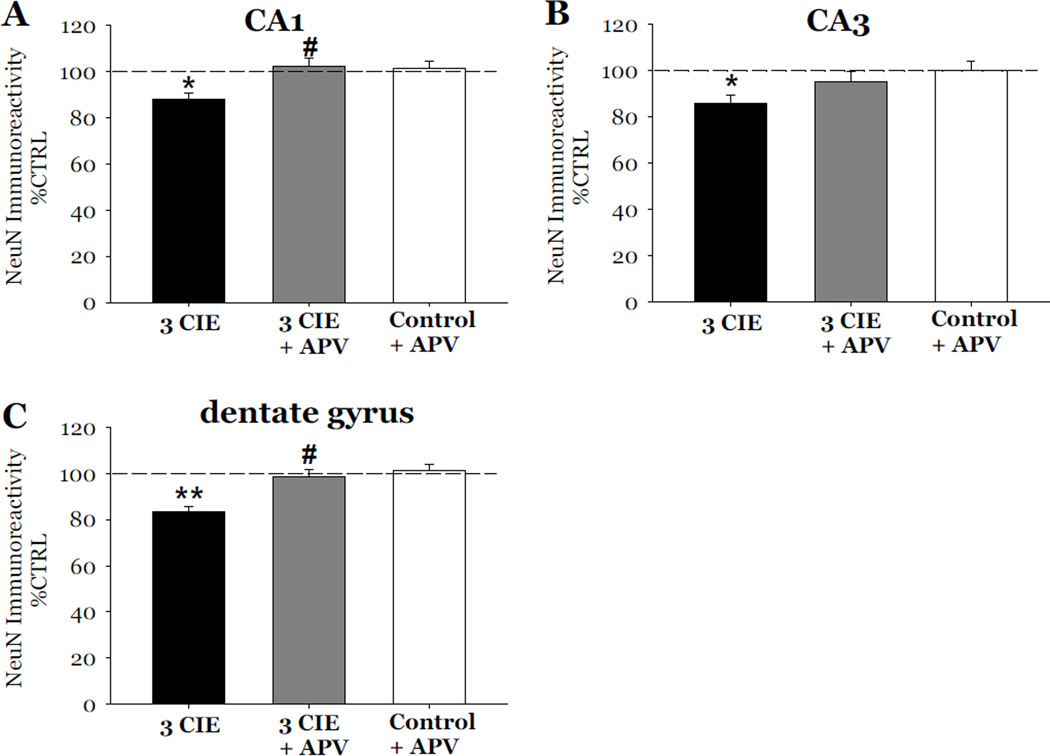
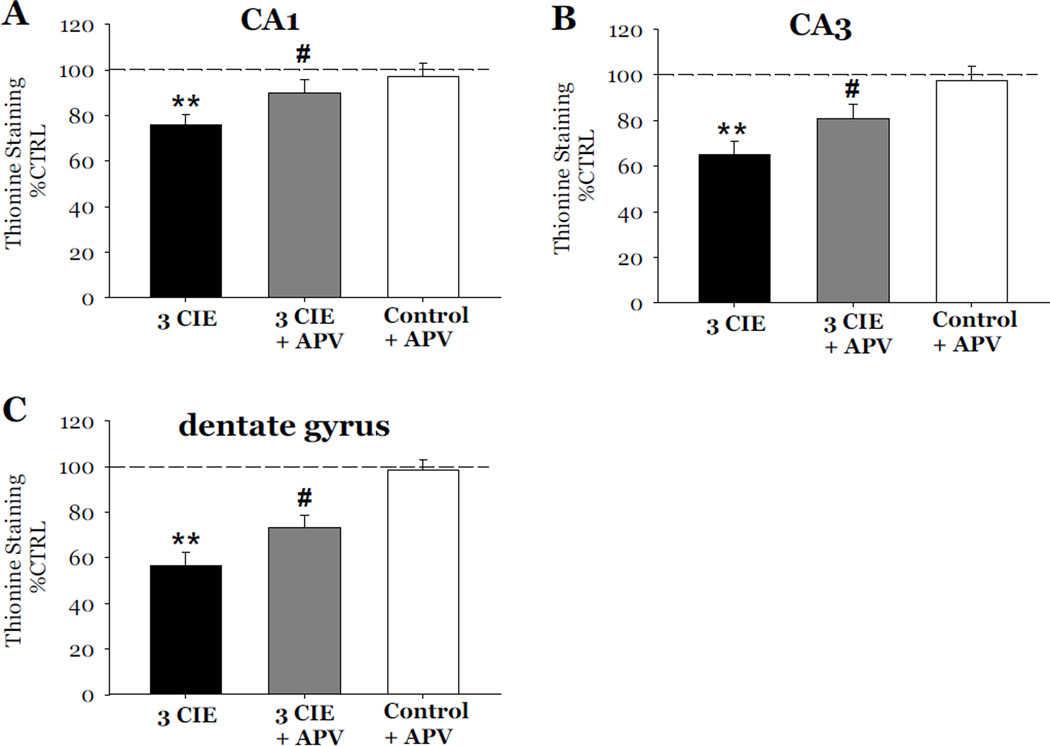
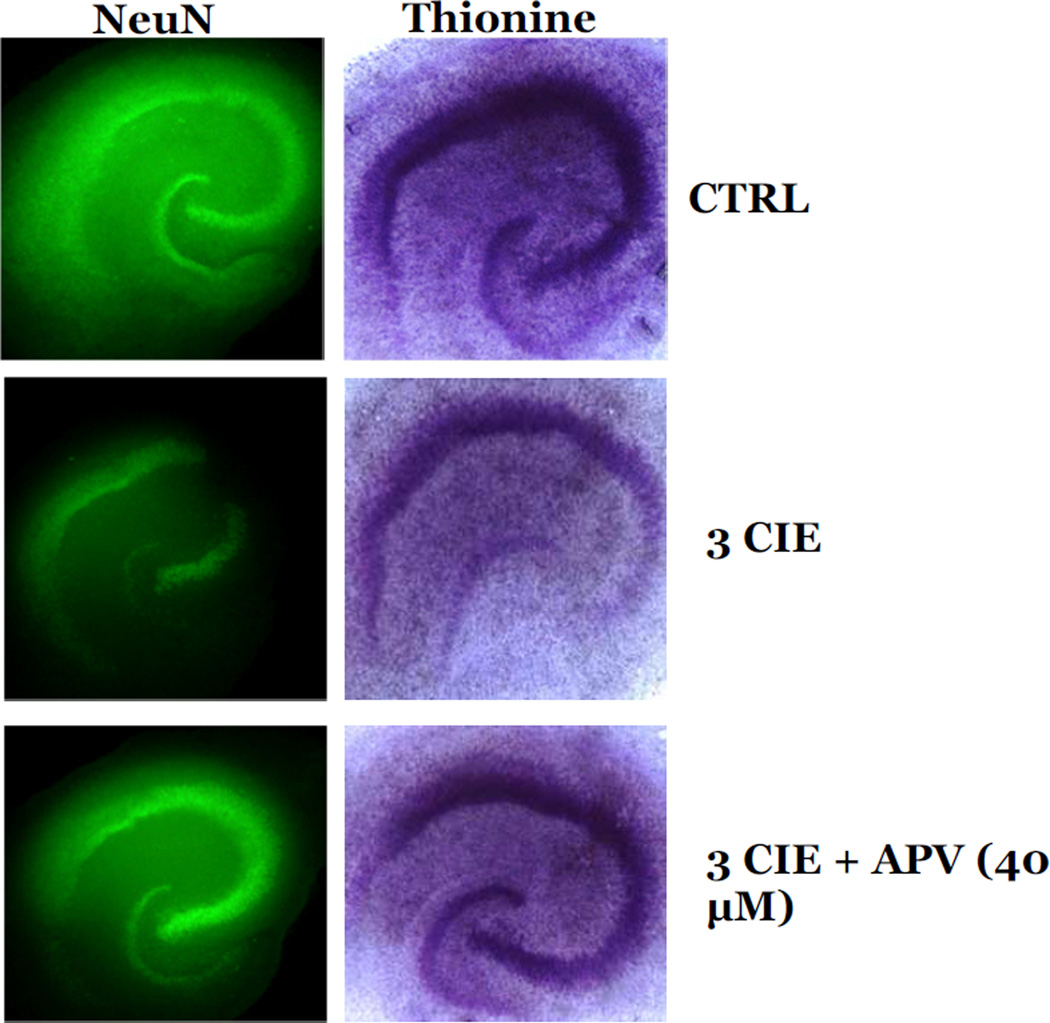
The role of the NMDA receptor on CIE-induced cytotoxicity was examined following 3 cycles of CIE. In these studies, slices exposed to 3 cycles of CIE were also exposed to 40 µM APV during periods of withdrawal from CIE. Exposure to 3 cycles of CIE produced a 12% loss of NeuN immunoreactivity as compared to control values in the CA1 (F[3,37]= 4.73, p<0.05; Figure 6A), a 15% loss in the CA3 (F[3,37]= 7.01, p<0.05; Figure 6B), and a 17% loss in the dentate gyrus (F[3,37]= 9.72, p<0.001; Figure 6C). Exposure to 40 µM APV reversed the decreases of NeuN immunoreactivity in the CA1 and dentate gyrus (p<0.05; Figure 6A and 6C), but only significantly attenuated these effects in the CA3 (p<0.05; Figure 6B). Thionine staining of slices yielded similar results as exposure to APV significantly attenuated the decreases of thionine staining in the CA1 (F[3,34]= 5.89; p<0.05; Figure 7A), CA3 (F[3,34]= 8.43, p<0.05; Figure 7B), and dentate gyrus (F[3,34]= 16.331, p<0.05; Figure 7C). Exposure to APV (40 µM) alone, in ethanol-naïve slices, did not significantly alter NeuN immunoreactivity nor thionine staining as compared to control values (Figures 6 and 7). Representative images of hippocampi exposed to ethanol-naïve medium and 3 cycles of CIE with or without the addition of APV are shown in Figure 8.
Figure 6.
Effects of exposure to 50 mM ethanol for 5 DIV, followed by exposure to APV (40µM) during the 24-hour period of ethanol withdrawal, and repeated for 3 CIE on the immunoreactivity of neuron specific nuclear protein (NeuN) in organotypic slice cultures. Exposure to 50 mM ethanol for 5 DIV, followed by a 24-hour period of ethanol withdrawal, and repeated for 3 CIE resulted in consistent and significant decreases of NeuN immunoreactivity as compared to control values in the pyramidal cell layers of the CA1 (A) and CA3 (B), and granule cell layer of the dentate gyrus (C). Exposure to APV (40 µM) during periods of withdrawal prevented the decreases of NeuN immunoreactivity in the CA1, CA3, and dentate gyrus; whereas exposure to APV in ethanol naïve slices did not significantly alter levels of NeuN immunoreactivity (Figure 6). *p <0.05 vs control; **p <0.001 vs control; #p <0.05 vs 3 CIE.
Figure 7.
Effects of exposure to 50 mM ethanol for 5 DIV, followed by exposure to APV (40µM) during the 24-hour period of ethanol withdrawal, and repeated for 3 CIE on thionine staining in organotypic slice cultures. Exposure to 50 mM ethanol for 5 DIV, followed by a 24-hour period of ethanol withdrawal, and repeated for 3 CIE resulted in consistent and significant decreases of thionine staining as compared to control values in the pyramidal cell layers of the CA1 (A) and CA3 (B), and granule cell layer of the dentate gyrus (C). Exposure to APV (40 µM) during periods of withdrawal attenuated the decreases of thionine staining in the CA1, CA3, and dentate gyrus; whereas exposure to APV in ethanol naïve slices did not significantly alter levels of thionine (Figure 7). **p <0.001 vs control; #p <0.05 vs 3 CIE.
Figure 8.
Representative images of NeuN immunoreactivity and thionine staining in hippocampal slices exposed to 3 CIE, 3 CIE and APV (40 µM) during withdrawal, or ethanol- naïve control media.
Discussion
The present studies found that multiple cycles of CIE are required to produce cytotoxicity in hippocampal slice cultures, as reflected by significant decreases of NeuN immunoreactivity and thionine staining. Exposure to 50 mM ethanol for 5 DIV, followed by a single withdrawal period, did not result in significant decreases of NeuN immunoreactivity or thionine staining in any hippocampal subregion. These data are consistent with previous studies conducted in our laboratory in which exposure to ethanol (50 mM), followed by a single period of withdrawal, did not produce excitotoxicity in vitro (Butler et al., 2009; Self et al., 2005). However, prior work has shown that chronic exposure to this concentration of ethanol produces a heightened sensitivity of hippocampal glutamatergic receptors systems to agonists (Self et al., 2004). In contrast, exposure to 2 and 3 cycles of CIE in aged hippocampal slices produced consistent and significant decreases of NeuN immunoreactivity and thionine staining in the pyramidal cell layers of the CA1 and CA3, as well as the granule cell layer of the dentate gyrus. These data are consistent with findings in which exposure to CIE produced deficits, such as neurotoxicity in cortical neurons (Nagy & Laszlo, 2002), increased seizure susceptibility (Kokka et al., 1993) and EEG activity (Veatch et al., 1996), as well as hippocampal neurodegeneration in vivo (Collins et al., 1998; Zhao et al., 2013). The present findings expand on this literature by characterizing the distinct roles of ethanol exposure and withdrawal on the hippocampal injury produced by the CIE regimen. For example, exposure to 50 mM ethanol for 18 consecutive days (in the absence of any withdrawal) did not produce significant loss of NeuN immunoreactivity or thionine staining in any hippocampal subregion in the present studies. Further, NeuN immunoreactivity did not recover following 7 days of protracted withdrawal. These findings demonstrate the critical importance of the intermittent withdrawal in producing the long-lasting cytotoxicity observed.
Important to note is that the hippocampal explants were harvested from 8 day old animals, and maintained in vitro for up to 28 consecutive days. Thus, the present studies may represent the developing brain. However, prior studies conducted by Martens and Wree (2001) suggest that there are marked similarities between hippocampal cultures maintained in vitro cultures and age-matched in situ hippocampi, such as glutamate receptor binding distribution. Others suggest that that these cultures maintain mature features similar to the adult live animal, such as glial distribution and synaptic plasticity (Baher et al., 1995). The present studies may, therefore, model aspects of both the developing and mature brain.
In general, multiple cycles of CIE produced a more modest reduction of NeuN immunoreactivity as compared to the robust reductions of thionine staining. The reasons for these effects are unknown but probably reflect inherent differences regarding the neuronal selectivity of these markers (Kadar et al., 2009; Mullen et al., 1992; Scott & Willett, 1996; Wolf et al., 1996). The robust decreases in thionine observed in the present studies could reflect a loss of neuronal and non-neuronal cells. For example, our previous work has shown that a single exposure to withdrawal produced significant and transient decreases of glial fibrillary acidic protein immunoreactivity in hippocampal slice cultures (Wilkins et al., 2006). Further, astrocytes express each of the known GluN subunits and are susceptible to prototypical NMDA receptor-mediated excitotoxicity (Lee et al. 2010). Thus, we suggest that both neurons and astrocytes demonstrate sensitivity to excitotoxicity during periods of withdrawal from CIE. It is interesting that decreases of both NeuN immunoreactivity and thionine staining produced by multiple cycles of CIE were ubiquitous in nature as they were observed in each examined subregion of the hippocampus (i.e., CA1, CA3, and dentate gyrus). Prior work employing this model with a single ethanol withdrawal has demonstrated a selective vulnerability of the CA1 pyramidal cell layer to the excitotoxic effects of withdrawal from a much higher concentration of ethanol (Prendergast et al. 2004). One possible interpretation of the present findings is the CIE regimen employed in these studies produced a greater extent of withdrawal excitotoxicity than did previous regimens using a single withdrawal given that multiple exposure and withdrawal cycles were employed in the current studies, producing a robust spreading of excitotoxicity from the pyramidal cell layer of the CA1 to other, proximal hippocampal cell layers. For example, Guiterrez and Heinemann (1999) found that de novo sprouting of mossy fiber tracts from granule cells of the dentate gyrus to the CA1 in organotypic explants promoted epileptiform-like activity in the dentate gyrus. Thus, this de novo plasticity of the model may be expected to contribute to this form of spreading excitotoxicity.
The present studies also examined the effects of in vitro aging on the loss of NeuN and thionine following exposure to multiple cycles of CIE. An effect of in vitro aging was observed in studies that employed a regimen of 2 exposure and withdrawal cycles. For example, exposure to 2 cycles of CIE initiated on DIV 11 (and maintained in vitro for 23 total DIV) resulted in significant decreases of both NeuN immunoreactivity and thionine staining in each examined hippocampal subregion. This effect was not demonstrated in slices exposed to the 2 cycles of CIE treatment regimen initiated on DIV 5 (and maintained in vitro for 16 total DIV). These findings are consistent with the notion that aged neurons are particularly vulnerable to excitotoxicity, likely thorough alterations in expression and/or trafficking of glutamate receptor subunits (Brewer et al., 2007; Choi et al., 1987). In sum, the collective loss of NeuN and thionine suggest a decrease in the density of mature neurons following multiple cycles of CIE in the hippocampus.
The Role of NMDA Receptor Activation during Withdrawal from CIE
NMDA receptors are heteromeric ionotrophic glutamatergic receptors, comprised of an obligatory NR1 and combination of NR2 (A–D) subunits (Dingledine et al., 1999; for a review, see Paoletti & Neyton, 2007). During withdrawal from ethanol, overactivation of these receptors leads to excessive calcium (Ca2+) influx and subsequent cell death (i.e., excitotoxicity; Choi, 1992). Following CIE exposure, neuroadaptations in NMDA receptors can further potentiate Ca2+-mediated excitotoxicity. In one study, for example, exposure to CIE produced increases in NMDA receptor-mediated excitatory post-synaptic potentials (EPSPs) in the pyramidal cell layer of the CA1 of the rat hippocampus (Nelson et al, 2005). As another example, administration of MK-801, a non-competitive NMDA receptor antagonist (0.1–0.3 g/kg), during periods of withdrawal attenuated CNS hyperexcitability during withdrawal from ethanol (Veatch & Becker, 2005).
In the current studies, exposure to the competitive NMDA receptor antagonist APV completely prevented the loss of mature neurons in the pyramidal CA1 and Ca3 cell layers of the hippocampal formation, as well as in the granule cell layer of the dentate gyrus. These data suggest that the loss of mature neurons following multiple cycles of CIE is produced by overactivation of NMDA receptors during periods of withdrawal. This interpretation is supported by the work of Ticku and colleagues who demonstrated upregulation of GluN1 and GluN2B subunits in cortical neurons exposed to ethanol via a CIE regimen in vitro (Qiang et al. 2007). Further, while the mechanistic underpinnings associated with producing these neuroadaptive changes in the NMDA receptor are not yet entirely understood, ethanol exposure is known to indirectly enhance NMDA receptor function through protein kinase A-dependent phosphorylation of these receptors prior to trafficking from extrasynaptic to synaptic sites (Carpenter-Hyland et al., 2004). Therefore, it is likely that cytoxicity produced by CIE could be, in part, mediated by protein kinase A. In sum, the present findings indicate that withdrawal is required for NMDA receptor-dependent cytotoxicity in the rat hippocampus. These findings suggest that increased function of the NMDA receptor is likely associated with both the behavioral and neurodegenerative effects observed following multiple bouts of heavy ethanol consumption (Duka et al., 2003; Duka et al., 2004; Sullivan et al., 1996) observed in the clinical population.
Highlights.
CIE exposure produces persisting NMDA-receptor dependent cytotoxicity in the hippocampus
Withdrawal is required to produce cytotoxicity during CIE
Aged slices are more susceptible to cytotoxic effects of CIE
Acknowledgements
This research was supported by Grant AA013388 from the National Institute on Alcohol and Alcoholism (NIAAA) awarded to MAP and National Institute of Drug Abuse (NIDA) DA035200. This funding agency had no role in study design, data collection or analysis or preparation and submission of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest relevant to this research.
References
- Bahr BA. Long-term hippocampal slices: A model system for investigating synaptic mechanisms and pathologic processes. Journal of Neuroscience Research. 1995;42(3):294–305. doi: 10.1002/jnr.490420303. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. Br J Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Booth BM, Blow FC. The kindling hypothesis: further evidence from a U.S. national study of alcoholic men. Alcohol Alcohol. 1993;28(5):593–598. [PubMed] [Google Scholar]
- Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, Garcia-Ramos G, Porter NM. Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res. 2007;1151:20–31. doi: 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Butler TR, Self RL, Smith KJ, Sharrett-Field LJ, Berry JN, Littleton JM, Prendergast MA. Selective vulnerability of hippocampal cornu ammonis 1 pyramidal cells to excitotoxic insult is associated with the expression of polyamine-sensitive N-methyl-D-asparate-type glutamate receptors. Neuroscience. 2010;165(2):525–534. doi: 10.1016/j.neuroscience.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Smith KJ, Berry JN, Sharrett-Field LJ, Prendergast MA. Sex differences in caffeine neurotoxicity following chronic ethanol exposure and withdrawal. Alcohol Alcohol. 2009;44(6):567–574. doi: 10.1093/alcalc/agp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TR, Smith KJ, Self RL, Braden BB, Prendergast MA. Sex differences in the neurotoxic effects of adenosine A1 receptor antagonism during ethanol withdrawal: reversal with an A1 receptor agonist or an NMDA receptor antagonist. Alcohol Clin Exp Res. 2008;32(7):1260–1270. doi: 10.1111/j.1530-0277.2008.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24(36):7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23(9):1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. Journal of Neurobiology. 1992;23(9):1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Collins MA, Zou JY, Neafsey EJ. Brain damage due to episodic alcohol exposure in vivo and in vitro: furosemide neuroprotection implicates edemabased mechanism. FASEB J. 1998;12(2):221–230. doi: 10.1096/fasebj.12.2.221. [DOI] [PubMed] [Google Scholar]
- Corso TD, Mostafa HM, Collins MA, Neafsey EJ. Brain neuronal degeneration caused by episodic alcohol intoxication in rats: effects of nimodipine, 6,7-dinitro-quinoxaline-2,3-dione, and MK-801. Alcohol Clin Exp Res. 1998;22(1):217–224. [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51(1):7–61. [PubMed] [Google Scholar]
- Duka T, Gentry J, Malcolm R, Ripley TL, Borlikova G, Stephens DN, Crews FT. Consequences of multiple withdrawals from alcohol. Alcohol Clin Exp Res. 2004;28(2):233–246. doi: 10.1097/01.alc.0000113780.41701.81. [DOI] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res. 2003;27(10):1563–1572. doi: 10.1097/01.ALC.0000090142.11260.D7. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, Tabakoff B. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22(5):998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Heinemann U. Synaptic reorganization in explanted cultures of rat hippocampus. Brain Res. 1999;815(2):304–316. doi: 10.1016/s0006-8993(98)01101-9. [DOI] [PubMed] [Google Scholar]
- Jones AW, Sternebring B. Kinetics of ethanol and methanol in alcoholics during detoxification. Alcohol Alcohol. 1992;27(6):641–647. [PubMed] [Google Scholar]
- Kadar A, Wittmann G, Liposits Z, Fekete C. Improved method for combination of immunocytochemistry and Nissl staining. J Neurosci Methods. 2009;184(1):115–118. doi: 10.1016/j.jneumeth.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem. 2009;284(45):31052–31061. doi: 10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokka N, Sapp DW, Taylor AM, Olsen RW. The kindling model of alcohol dependence: similar persistent reduction in seizure threshold to pentylenetetrazol in animals receiving chronic ethanol or chronic pentylenetetrazol. Alcohol Clin Exp Res. 1993;17(3):525–531. doi: 10.1111/j.1530-0277.1993.tb00793.x. [DOI] [PubMed] [Google Scholar]
- Lechtenberg R, Worner TM. Relative kindling effect of detoxification and non-detoxification admissions in alcoholics. Alcohol Alcohol. 1991;26(2):221–225. doi: 10.1093/oxfordjournals.alcalc.a045104. [DOI] [PubMed] [Google Scholar]
- Lee MC, Ting KK, Adams S, Brew BJ, Chung R, Guillemin GJ. Characterisation of the expression of NMDA receptors in human astrocytes. PLoS One. 2010;5(11):e14123. doi: 10.1371/journal.pone.0014123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel Marquez H, Nakovics H, Heinz A, Mann K, Flor H. Effects of repeated withdrawal from alcohol on recovery of cognitive impairment under abstinence and rate of relapse. Alcohol Alcohol. 2010;45(6):541–547. doi: 10.1093/alcalc/agq065. [DOI] [PubMed] [Google Scholar]
- Martens U, Wree A. Distribution of [3H]MK-801, [3H]AMPA and [3H]Kainate binding sites in rat hippocampal long-term slice cultures isolated from external afferents. Anat Embryol (Berl) 2001;203(6):491–500. doi: 10.1007/s004290100174. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. Drinking patterns during work-contingent and noncontingent alcohol acquisition. Psychosom Med. 1972;34(2):139–164. doi: 10.1097/00006842-197203000-00007. [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116(1):201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nagy J, Laszlo L. Increased sensitivity to NMDA is involved in alcohol-withdrawal induced cytotoxicity observed in primary cultures of cortical neurones chronically pre-treated with ethanol. Neurochem Int. 2002;40(7):585–591. doi: 10.1016/s0197-0186(01)00131-0. [DOI] [PubMed] [Google Scholar]
- Nelson TE, Ur CL, Gruol DL. Chronic intermittent ethanol exposure enhances NMDA-receptor-mediated synaptic responses and NMDA receptor expression in hippocampal CA1 region. Brain Res. 2005;1048(1–2):69–79. doi: 10.1016/j.brainres.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7(1):39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mullholland PJ, Blanchard JA, 2nd, Gibson DA, Holley RC, Littleton JM. Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of N-methyl-Daspartate receptors. Neuroscience. 2004;124(4):869–877. doi: 10.1016/j.neuroscience.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Qiang M, Denny AD, Ticku MK. Chronic intermittent ethanol treatment selectively alters N-methyl-D-aspartate receptor subunit surface expression in cultured cortical neurons. Mol Pharmacol. 2007;72(1):95–102. doi: 10.1124/mol.106.033043. [DOI] [PubMed] [Google Scholar]
- Scott JE, Willett, Irene H. Binding of Cationic Dyes to Nucleic Acids and Other Biological Polyanions. Nature. 1966;209(5027):985–987. doi: 10.1038/209985a0. [DOI] [PubMed] [Google Scholar]
- Self RL, Mulholland PJ, Harris BR, Nath A, Prendergast MA. Cytotoxic effects of exposure to the human immunodeficiency virus type 1 protein Tat in the hippocampus are enhanced by prior ethanol treatment. Alcohol Clin Exp Res. 2004;28(12):1916–1924. doi: 10.1097/01.alc.0000148108.93782.05. [DOI] [PubMed] [Google Scholar]
- Self RL, Smith KJ, Mulholland PJ, Prendergast MA. Ethanol exposure and withdrawal sensitizes the rat hippocampal CA1 pyramidal cell region to beta-amyloid (25–35)-induced cytotoxicity: NMDA receptor involvement. Alcohol Clin Exp Res. 2005;29(11):2063–2069. doi: 10.1097/01.alc.0000187591.82039.b2. [DOI] [PubMed] [Google Scholar]
- Shaw GK, Waller S, Latham CJ, Dunn G, Thomson AD. The detoxification experience of alcoholic in-patients and predictors of outcome. Alcohol Alcohol. 1998;33(3):291–303. doi: 10.1093/oxfordjournals.alcalc.a008393. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Brown G, Duka T, Ripley TL. Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. Eur J Neurosci. 2001;14(12):2023–2031. doi: 10.1046/j.0953-816x.2001.01824.x. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Ripley TL, Borlikova G, Schubert M, Albrecht D, Hogarth L, Duka T. Repeated ethanol exposure and withdrawal impairs human fear conditioning and depresses long-term potentiation in rat amygdala and hippocampus. Biol Psychiatry. 2005;58(5):392–400. doi: 10.1016/j.biopsych.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Relationship between alcohol withdrawal seizures and temporal lobe white matter volume deficits. Alcohol Clin Exp Res. 1996;20(2):348–354. doi: 10.1111/j.1530-0277.1996.tb01651.x. [DOI] [PubMed] [Google Scholar]
- Wojnar M, Bizon Z, Wasilewski D. Assessment of the role of kindling in the pathogenesis of alcohol withdrawal seizures and delirium tremens. Alcohol Clin Exp Res. 1999;23(2):204–208. [PubMed] [Google Scholar]
- Worner TM. Relative kindling effect of readmissions in alcoholics. Alcohol Alcohol. 1996;31(4):375–380. doi: 10.1093/oxfordjournals.alcalc.a008164. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Becker HC. Electrographic and behavioral indices of ethanol withdrawal sensitization. Brain Res. 2002;946(2):272–282. doi: 10.1016/s0006-8993(02)02895-0. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Becker HC. Lorazepam and MK-801 effects on behavioral and electrographic indices of alcohol withdrawal sensitization. Brain Res. 2005;1065(1–2):92–106. doi: 10.1016/j.brainres.2005.10.047. [DOI] [PubMed] [Google Scholar]
- Veatch LM, Gonzalez LP. Repeated ethanol withdrawal produces site-dependent increases in EEG spiking. Alcohol Clin Exp Res. 1996;20(2):262–267. doi: 10.1111/j.1530-0277.1996.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Wilkins LH, Jr, Prendergast MA, Blanchard J, Holley RC, Chambers ER, Littleton JM. Potential value of changes in cell markers in organotypic hippocampal cultures associated with chronic EtOH exposure and withdrawal: comparison with NMDA-induced changes. Alcohol Clin Exp Res. 2006;30(10):1768–1780. doi: 10.1111/j.1530-0277.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Wolf HK, Buslei R, Schmidt-Kastner R, Schmidt-Kastner PK, Pietsch T, Wiestler OD, Blumcke I. NeuN: a useful neuronal marker for diagnostic histopathology. J Histochem Cytochem. 1996;44(10):1167–1171. doi: 10.1177/44.10.8813082. [DOI] [PubMed] [Google Scholar]
- Zhao YN, Wang F, Fan YX, Ping GF, Yang JY, Wu CF. Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behav Brain Res. 2013;236(1):270–282. doi: 10.1016/j.bbr.2012.08.052. [DOI] [PubMed] [Google Scholar]