Abstract
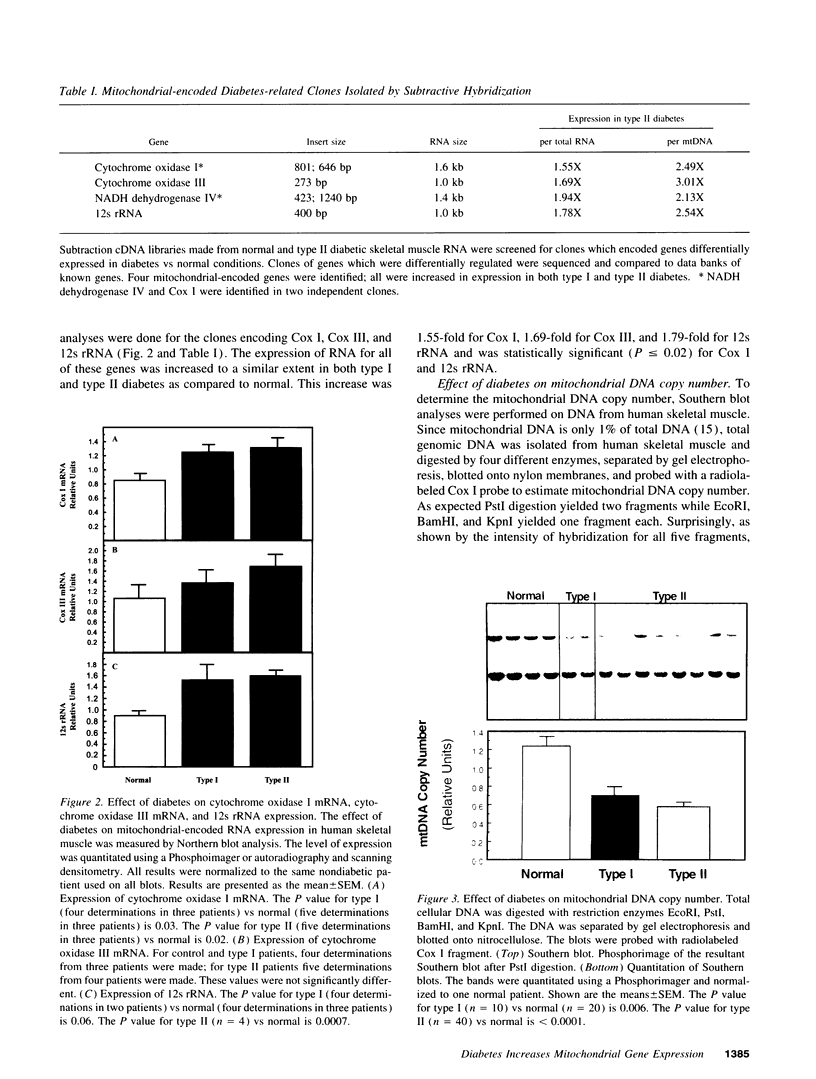
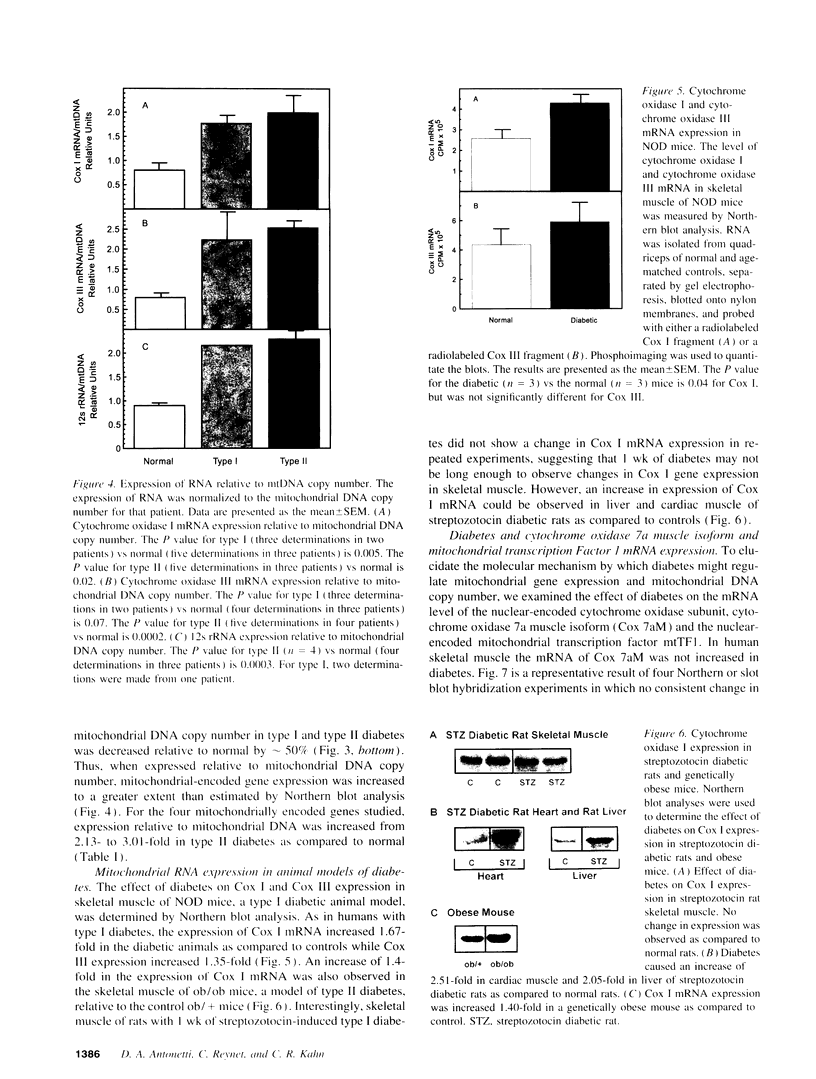
Screening subtraction libraries from normal and type II diabetic human skeletal muscle, we identified four different mitochondrially encoded genes which were increased in expression in diabetes. The genes were cytochrome oxidase I, cytochrome oxidase III, NADH dehydrogenase IV, and 12s rRNA, all of which are located on the heavy strand of the mitochondrial genome. There was a 1.5- to 2.2-fold increase in the expression of these mRNA molecules relative to total RNA in both type I and type II diabetes as assessed by Northern blot analyses. Since there was approximately 50% decrease in mitochondrial DNA copy number as estimated by Southern blot analyses, mitochondrial gene expression increased approximately 2.5-fold when expressed relative to mitochondrial DNA copy number. For cytochrome oxidase I similar changes in mitochondrial gene expression were observed in muscle of nonobese diabetic and ob/ob mice, models of type I and type II diabetes, respectively. By contrast there was no change or a slight decrease in expression of cytochrome oxidase 7a, a nuclear-encoded subunit of cytochrome oxidase, and the expression of mitochondrial transcription factor 1 in human skeletal muscle did not change with type I or type II diabetes. The increased mitochondrial gene expression may contribute to the increase in mitochondrial respiration observed in uncontrolled diabetes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaudo E., Hirano M., Seelan R. S., Milatovich A., Hsieh C. L., Fabrizi G. M., Grossman L. I., Francke U., Schon E. A. Tissue-specific expression and chromosome assignment of genes specifying two isoforms of subunit VIIa of human cytochrome c oxidase. Gene. 1992 Oct 1;119(2):299–305. doi: 10.1016/0378-1119(92)90287-y. [DOI] [PubMed] [Google Scholar]
- Attardi G., Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Ballinger S. W., Shoffner J. M., Hedaya E. V., Trounce I., Polak M. A., Koontz D. A., Wallace D. C. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet. 1992 Apr;1(1):11–15. doi: 10.1038/ng0492-11. [DOI] [PubMed] [Google Scholar]
- Beck-Nielsen H., Vaag A., Damsbo P., Handberg A., Nielsen O. H., Henriksen J. E., Thye-Rønn P. Insulin resistance in skeletal muscles in patients with NIDDM. Diabetes Care. 1992 Mar;15(3):418–429. doi: 10.2337/diacare.15.3.418. [DOI] [PubMed] [Google Scholar]
- Chau C. M., Evans M. J., Scarpulla R. C. Nuclear respiratory factor 1 activation sites in genes encoding the gamma-subunit of ATP synthase, eukaryotic initiation factor 2 alpha, and tyrosine aminotransferase. Specific interaction of purified NRF-1 with multiple target genes. J Biol Chem. 1992 Apr 5;267(10):6999–7006. [PubMed] [Google Scholar]
- Clayton D. A. Nuclear gadgets in mitochondrial DNA replication and transcription. Trends Biochem Sci. 1991 Mar;16(3):107–111. doi: 10.1016/0968-0004(91)90043-u. [DOI] [PubMed] [Google Scholar]
- Clayton D. A. Replication and transcription of vertebrate mitochondrial DNA. Annu Rev Cell Biol. 1991;7:453–478. doi: 10.1146/annurev.cb.07.110191.002321. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Scarpulla R. C. NRF-1: a trans-activator of nuclear-encoded respiratory genes in animal cells. Genes Dev. 1990 Jun;4(6):1023–1034. doi: 10.1101/gad.4.6.1023. [DOI] [PubMed] [Google Scholar]
- Gerbitz K. D. Does the mitochondrial DNA play a role in the pathogenesis of diabetes? Diabetologia. 1992 Dec;35(12):1181–1186. doi: 10.1007/BF00401375. [DOI] [PubMed] [Google Scholar]
- Gross M. D., Harris S., Beyer R. E. The effect of streptozotocin-induced diabetes on oxidative phosphorylation and related reactions in skeletal muscle mitochondria. Horm Metab Res. 1972 Jan;4(1):1–7. doi: 10.1055/s-0028-1094106. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S. Lilly Lecture 1979: role of insulin in the regulation of protein synthesis. Diabetes. 1980 Jun;29(6):487–496. doi: 10.2337/diab.29.6.487. [DOI] [PubMed] [Google Scholar]
- Koga Y., Davidson M., Schon E. A., King M. P. Fine mapping of mitochondrial RNAs derived from the mtDNA region containing a point mutation associated with MELAS. Nucleic Acids Res. 1993 Feb 11;21(3):657–662. doi: 10.1093/nar/21.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Mackerer C. R., Paquet R. J., Mehlman M. A., Tobin R. B. Oxidation and phosphorylation in live mitochondria from alloxan and steptozotocin diabetic rats. Proc Soc Exp Biol Med. 1971 Jul;137(3):992–995. doi: 10.3181/00379727-137-35712. [DOI] [PubMed] [Google Scholar]
- Meisler M. H., Howard G. Effects of insulin on gene transcription. Annu Rev Physiol. 1989;51:701–714. doi: 10.1146/annurev.ph.51.030189.003413. [DOI] [PubMed] [Google Scholar]
- Minchenko A. G. Ekspressiia mitokhondrial'nogo genoma v pecheni pri diabete. Probl Endokrinol (Mosk) 1981 Jan-Feb;27(1):62–66. [PubMed] [Google Scholar]
- Montoya J., Gaines G. L., Attardi G. The pattern of transcription of the human mitochondrial rRNA genes reveals two overlapping transcription units. Cell. 1983 Aug;34(1):151–159. doi: 10.1016/0092-8674(83)90145-9. [DOI] [PubMed] [Google Scholar]
- Mutvei A., Nelson B. D. The response of individual polypeptides of the mammalian respiratory chain to thyroid hormone. Arch Biochem Biophys. 1989 Jan;268(1):215–220. doi: 10.1016/0003-9861(89)90582-1. [DOI] [PubMed] [Google Scholar]
- Nelson B. D., Mutvei A., Joste V. Regulation of biosynthesis of the rat liver inner mitochondrial membrane by thyroid hormone. Arch Biochem Biophys. 1984 Jan;228(1):41–48. doi: 10.1016/0003-9861(84)90044-4. [DOI] [PubMed] [Google Scholar]
- Nelson B. D. Thyroid hormone regulation of mitochondrial function. Comments on the mechanism of signal transduction. Biochim Biophys Acta. 1990 Jul 25;1018(2-3):275–277. doi: 10.1016/0005-2728(90)90266-7. [DOI] [PubMed] [Google Scholar]
- O'Brien R. M., Granner D. K. Regulation of gene expression by insulin. Biochem J. 1991 Sep 15;278(Pt 3):609–619. doi: 10.1042/bj2780609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi M. A., Clayton D. A. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science. 1991 May 17;252(5008):965–969. doi: 10.1126/science.2035027. [DOI] [PubMed] [Google Scholar]
- Reardon W., Ross R. J., Sweeney M. G., Luxon L. M., Pembrey M. E., Harding A. E., Trembath R. C. Diabetes mellitus associated with a pathogenic point mutation in mitochondrial DNA. Lancet. 1992 Dec 5;340(8832):1376–1379. doi: 10.1016/0140-6736(92)92560-3. [DOI] [PubMed] [Google Scholar]
- Reynet C., Kahn C. R. Rad: a member of the Ras family overexpressed in muscle of type II diabetic humans. Science. 1993 Nov 26;262(5138):1441–1444. doi: 10.1126/science.8248782. [DOI] [PubMed] [Google Scholar]
- Rötig A., Bessis J. L., Romero N., Cormier V., Saudubray J. M., Narcy P., Lenoir G., Rustin P., Munnich A. Maternally inherited duplication of the mitochondrial genome in a syndrome of proximal tubulopathy, diabetes mellitus, and cerebellar ataxia. Am J Hum Genet. 1992 Feb;50(2):364–370. [PMC free article] [PubMed] [Google Scholar]
- Savabi F., Kirsch A. Alteration of the phosphocreatine energy shuttle components in diabetic rat heart. J Mol Cell Cardiol. 1991 Nov;23(11):1323–1333. doi: 10.1016/0022-2828(91)90089-5. [DOI] [PubMed] [Google Scholar]
- Van Itallie C. M. Thyroid hormone and dexamethasone increase the levels of a messenger ribonucleic acid for a mitochondrially encoded subunit but not for a nuclear-encoded subunit of cytochrome c oxidase. Endocrinology. 1990 Jul;127(1):55–62. doi: 10.1210/endo-127-1-55. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Williams R. S. Mitochondrial gene expression in mammalian striated muscle. Evidence that variation in gene dosage is the major regulatory event. J Biol Chem. 1986 Sep 15;261(26):12390–12394. [PubMed] [Google Scholar]
- Williams R. S., Salmons S., Newsholme E. A., Kaufman R. E., Mellor J. Regulation of nuclear and mitochondrial gene expression by contractile activity in skeletal muscle. J Biol Chem. 1986 Jan 5;261(1):376–380. [PubMed] [Google Scholar]