Abstract
Objective
Mechanical factors play a critical role in the physiology and pathology of articular cartilage, although the mechanisms of mechanical signal transduction are not fully understood. We examined the hypothesis that type VI collagen is necessary for mechanotransduction in articular cartilage, by determining the effects of type VI collagen knockout on the activation of the mechano-osmosensitive calcium-permeable channel, transient receptor potential vanilloid 4 (TRPV4), osmotically-induced chondrocyte swelling, and pericellular matrix (PCM) mechanical properties.
Methods
Confocal laser scanning microscopy was used to image TRPV4-mediated calcium signaling and osmotically-induced cell swelling in intact femora from 2 and 9 month old wild type (WT) and type VI collagen deficient (Col6a1−/−) mice. Immunofluorescence-guided atomic force microscopy was used to map PCM mechanical properties based on the presence of perlecan.
Results
Hypo-osmotic stress induced TRPV4-mediated calcium signaling was increased in Col6a1−/− mice relative to WT controls at 2 months. Col6a1−/− mice exhibited significantly increased osmotically-induced cell swelling and decreased PCM moduli relative to WT controls at both ages.
Conclusion
In contrast to our original hypothesis, type VI collagen was not required for TRPV4-mediated Ca2+ signaling; however, knockout of type VI collagen altered the mechanical properties of the PCM, which in turn increased the extent of cell swelling and osmotically-induced TRPV4 signaling in an age-dependent manner. These findings emphasize the role of the PCM as a transducer of mechanical and physicochemical signals, and suggest that alterations in PCM properties, as may occur with aging or osteoarthritis, can influence mechanotransduction via TRPV4 or other ion channels.
Keywords: mechanobiology, ion channel, mechanosensitivity, proteoglycan, AFM, chondron, osteoarthritis, arthritis, mechanical signal transduction, TRP channel, osmotic stress
INTRODUCTION
Osteoarthritis (OA) is a painful and debilitating joint disease that affects over 27 million adults nationwide and over 60% of the population over the age of 70, with tremendous impact on the world economy (1). Indeed, OA is likely a family of diseases with multifactorial etiopathogenesis that involve genetic, molecular, and environmental influences. In particular, biomechanical factors play a critical role in joint health, as well as in the initiation and progression of OA (2). Altered joint loading is well-recognized as a risk factor for OA and has been shown to lead to deleterious changes in the composition, structure, metabolism, and mechanical properties of articular cartilage (3). For example, joint instability due to ligamentous or meniscal injury often results in progressive joint degeneration with a clinical risk of OA approaching 50% within 5-10 years (4). Joint immobilization, on the other hand, causes atrophic changes in cartilage composition and properties (5), which can be recovered with remobilization of the joint (6). Interestingly, however, joint immobilization at the time of a joint destabilizing injury is protective of the development of OA in mice due to reduced mechanical activation of metalloproteases (7). These findings indicate that there is an important balance of chondrocyte anabolic and catabolic activities that are mediated by the cartilage mechanical environment, and factors that alter chondrocyte mechanotransduction may contribute to the pathogenesis of OA (8).
Within the cartilage extracellular matrix (ECM), chondrocytes are surrounded by a unique matrix region termed the pericellular matrix (PCM) (9). Because the PCM surrounds each cell, any biochemical or biophysical signal the chondrocyte perceives is likely to be influenced, and potentially regulated, by the properties of the PCM (10). In addition to the constituents of the ECM, the PCM is rich in proteoglycans and is the exclusive location of type VI collagen in adult cartilage (9). Type VI collagen anchors the chondrocyte to the PCM through β-integrin receptors as well as through the transmembrane proteoglycan NG2 (11, 12). In mice, knockout of type VI collagen decreases the mechanical properties of the PCM and leads to accelerated development of OA in the hip (13) but not the knee (14). Collagen VI-deficient mice also show dysfunctional tendon fibrillogenesis, which may be associated with site-specific joint laxity (15). Interestingly, mice lacking type VI collagen also exhibit a myopathic disorder that is characterized by mitochondrial dysfunction and Ca2+ dysregulation (16), which affects muscle cell metabolism and mechanotransduction (17). It is possible, therefore, that type VI collagen may also regulate Ca2+ signaling in cell types from other tissues, such as cartilage.
Deformation of cartilage during normal daily activities exposes the chondrocytes to varying stresses, strains, hydrostatic pressure, interstitial fluid flow, electrokinetic effects and, interestingly, osmotic pressure (18). Compression of the cartilage matrix causes water to be exuded from the tissue, while the negatively charged proteoglycans are retained and attract positive counter-ions. This phenomenon results in fluctuations in the interstitial osmolarity of ECM (19) and the PCM (20). Chondrocytes respond to changes in osmolarity with transient increases in intracellular Ca2+ (21) that ultimately regulate the cells’ metabolic function and matrix synthesis (22). While the mechanisms regulating this response are complex, recent studies suggest that this mechano-osmotic transduction occurs via the ion channel TRPV4 (transient receptor potential vanilloid 4), a non-selective, Ca2+-preferring channel that is both osmotically (23) and mechanically activated (24). TRPV4 is highly expressed in chondrocytes and appears to be the primary regulator of Ca2+ signaling in response to osmotic change in chondrocytes (23). TRPV4 mediated Ca2+ influx can also activate Sox9 expression, a transcription factor that functions as a master regulator of chondrogenesis (25). Additionally, TRPV4 expression during development corresponds with the aggrecan and collagen II gene expression profiles (26), which are both components of the cartilage matrix. Importantly, TRPV4 appears to play a critical role in the transduction of mechanical loading into an intracellular signal that regulates chondrocyte matrix production (27), and genetic knockout of TRPV4 leads to a loss of osmotic Ca2+ signaling and subsequently, age-dependent OA (28), whereas mutations in TRPV4 can lead to skeletal dysplasias or arthropathies, depending on the site of the mutation within the gene (29).
Taken together, these previous studies suggest that alterations in the mechanotransduction process, either due to changes in the mechanical or physicochemical properties of the PCM or dysfunctional mechanosensation at the cellular level, may lead to a loss of chondrocyte homeostasis and potentially OA. Furthermore, growing evidence suggests that cellular and cytoskeletal interactions with the surrounding matrix may in fact regulate the activity of sensory ion channels such as TRPV4 (30). In the present study, we hypothesized that type VI collagen was necessary for TRPV4 channel activation, and that mice lacking type VI collagen would exhibit a loss of TRPV4-mediated Ca2+ signaling in an age-dependent manner. Using fluorescence confocal laser scanning microscopy, we measured the activation of TRPV4 in response to a hypo-osmotic stimulus in chondrocytes in situ within wild type (WT) and collagen VI deficient (Col6a1−/−) mouse cartilage. To further determine the mechanisms involved in the observed response, we examined the change in chondrocyte volume in response to osmotic swelling, and applied immunofluorescence-guided atomic force microscopy (AFM) to spatially map the properties of the PCM in Col6a1−/− mice.
MATERIALS & METHODS
All procedures were performed in accordance with an Institutional Animal Care and Use Committee–approved protocol. Male and female CD1 mice (Charles River Laboratories) were obtained at 6 weeks and grown to 2 and 9 months as wild type (WT) controls. Type VI collagen deficient mice were on a CD1 genetic background with the Col6a1 gene disrupted, which eliminates the α1(VI) chain and results in a lack of triple helical collagen VI (13). WT and Col6a1−/− mice (13) were sacrificed at 2 months or 9 months of age. Hind limbs were disarticulated from the body, and the femora were isolated and cleaned of muscle, ligament, and tendon under a dissection microscope. During dissection, the femora were frequently sprayed with PBS so that the cartilage remained moist. Care was taken throughout dissection to avoid direct contact with articular cartilage. In separate experiments, nearly 100% viability of the chondrocytes was confirmed after dissection of test limbs using a fluorescence viability assay (Invitrogen). After harvest, femora were submerged in medium (phenol red-free Dulbecco’s modified Eagle’s medium, 15mM HEPES, 2mM L-Glutamine, 1mM Sodium Pyruvate, pH 7.4) at 37°C and 5% CO2 for use within 12 hours of sacrifice.
Fluorescence imaging of Ca2+ in intact femora
Fluorescence imaging. All femora were imaged on the day of isolation. Prior to imaging, the chondrocytes in the intact femoral articular cartilage were loaded for 40 minutes at 37°C with two visible light fluorescent Ca2+ indicators, Fura-Red AM (30μM) and Fluo-4 AM (16 μM) (Invitrogen-Molecular Probes). Femora were held on a custom-built apparatus inside heated perfusion chamber (Zeiss) that was mounted on an inverted confocal laser scanning microscope (LSM 510; Zeiss). Femora were submerged in 2.5 ml of isotonic (300mOsm) medium with the articular cartilage resting on a cover slip over the microscope objective (Figure 1A). The temperature of the medium within chamber was maintained at 37°C throughout experiment using a feedback controlled heated stage.
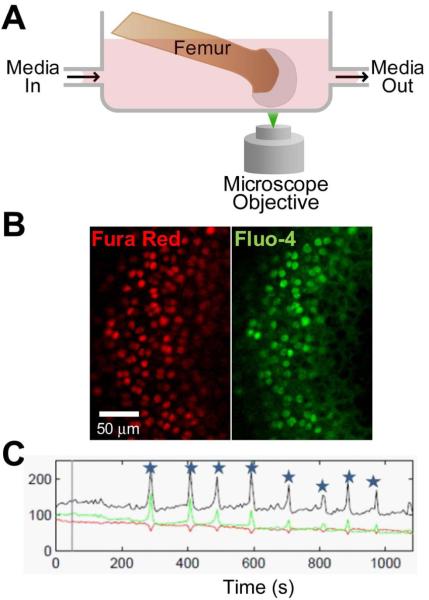
Figure 1. Schematic of in situ Ca2+ imaging configuration.
A. Experimental system for fluorescence imaging of Ca2+ in chondrocytes within intact murine femoral condyle on a confocal laser scanning microscope. Media was exchanged with a peristaltic pump while fluorescence ratio imaging was performed. B. Representative frame of fluorescence imaging with Fura Red (red) and Fluo-4 (green). C. Representative fluorescence recording of single signaling cell over time. Red and green lines show Fura Red and Fluo-4 intensity (respectively), and black shows ratio of green to red. Stars mark significant increases in intracellular Ca2+ concentration.
Transient changes in intracellular Ca2+ concentration ([Ca2+]i) were measured using an adaptation (28) of a previously described ratiometric imaging technique (31). The sample was excited using an argon ion laser (488 nm), and fluorescence emission was recorded using a 20x objective lens at 505-550 (Fluo-4) and at greater than 650 (Fura-Red) (Figure 1B). Nine scans were performed with the femora submerged in 300 mOsm media or 300 mOsm + 100 μM GSK205 before media was withdrawn and replaced by a vehicle control (1:100 DMSO), hypotonic (200 mOsm), isotonic (300 mOsm) or hypotonic/isotonic + 100 μM GSK205 medium. In the experiments where GSK205 was used to inhibit TRPV4, a 40 minute pre-incubation of 100μM GSK205 was also performed. Sequential images were recorded at a scan rate of 0.28Hz for 12 minutes to measure relative [Ca2+]i. Medium osmolarity was adjusted before experiment by adding distilled water and verified using a freezing point osmometer (Osmette A; Precision Systems).
Image Analysis. The ratiometric fluorescence was normalized to the average value over the first 9 scans for each individual cell. A positive [Ca2+]i response (a ‘signal’) was defined as an increase in normalized ratiometric fluorescence greater than 4 standard deviations over background noise, with both fluorescence indicators responding (Figure 1C). Background noise was calculated based on 60 non-signaling cells with no stimuli. Data was analyzed using a custom MATLAB program (MathWorks). Each experimental group included 50-134 individual cells from at least 3 different animals. The percentage of cells responding with signal was calculated and chi-squared test were used to determine significant differences between groups.
Cellular swelling of chondrocytes in intact femora
Fluorescence imaging. Femora were isolated as described above. Prior to imaging, the chondrocytes in intact femoral articular cartilage were loaded for 30 minutes at room temperature with Calcein-AM (10μm) (Invitrogen). Femora were held and imaged on an inverted confocal laser scanning microscope as described above. The temperature of the medium within chamber was maintained at 37°C throughout the experiment. Volume changes of chondrocytes in intact femora were measured before and after exposing the specimens to isotonic (300 mOsm) or hypotonic (200 mOsm) medium. Two sets of image stacks comprising typically 35×1μm Z increments using a 40x objective lens at a scan rate of 0.06 Hz, once before and once after media exchange. Samples were left for 5 minutes after media exchange before second set was taken to allow for diffusion of the media through the intact cartilage.
Image Analysis. Confocal laser scanning microscopy using this Z-stack method provides three-dimensional information, which can be reconstructed to provide quantitative volume measurements of imaged chondrocytes (32, 33). Three-dimensional reconstruction of the image stacks was performed using MATLAB to determine cell volume before and after the medium exchange. For defining the cell boundary, a 40% baseline threshold segmentation method was used to take into account variations in fluorescence intensity of individual chondrocytes (32). Both genotypes and age groups exhibited similar low levels of volume change in response to isotonic media exchange, which was attributed to a small amount of photobleaching. As such, percent volume change was calculated by normalization to isotonic exchange. Each treatment group included at least 16 cells from 2-3 different animals. A two-way ANOVA was used to examine the effects of genotype and age on cell swelling.
Atomic force microscopy (AFM) for measuring PCM mechanical properties
Specimen preparation. Femora from 2 or 9 month old WT or Col6a1−/− mice were embedded in water-soluble embedding medium and condyles were sectioned in 5 μm-thick slices using a cryostat microtome. Only a thin cartilage layer was included with no underlying subchondral bone. Samples were collected on glass slides and rinsed with PBS to remove the embedding medium.
Elastic moduli were quantified using an AFM system integrated with an optical microscope to allow for phase contrast imaging of cartilage samples as described previously (34). A 5 μm diameter glass sphere was attached to a 4.5 N/m AFM cantilever for microscale mechanical measurements. Indentations were applied with a force trigger of 300 nN and an indentation velocity of 15μm/s, based on previous work in this lab showing little or no rate-dependence of moduli collected at velocities ranging from 5 μm/s to 25 μm/s. Previous studies have used type VI collagen to define the edges of the PCM (34), but because the present study used Col6a1−/− mice, perlecan, which is also specific to the PCM (35), was used to define the PCM. Thus, perlecan was labeled using immunohistochemical techniques as described previously (35). Labeling of type VI collagen on adjacent sections was performed to confirm presence in WT, and absence in Col6a1−/− samples. Scan regions (10×10 μm) were selected based on presence (PCM) or absence (ECM) of perlecan and AFM force maps were taken in a 20×20 grid (PCM) or 4×4 grid (ECM).
Data Analysis. Raw data for cantilever deflection and z-piezo movement were analyzed using a custom MATLAB script. A modified Hertz contact mechanics model was used to calculate tissue modulus (36). The Poisson's ratio was assumed to be 0.04 for all tissue regions (37). Probe–surface contact was identified using a contact point extrapolation method described previously (38). The mean moduli of perlecan-labeled (PCM) or perlecan free (ECM) regions were calculated. Groups contained measurements from 24-43 separate sites across at least 3 different animals. The effects of genotype and age on PCM and ECM moduli were determined using a 2-way ANOVA on log-transformed data (p < 0.05).
RESULTS
Immunohistochemistry
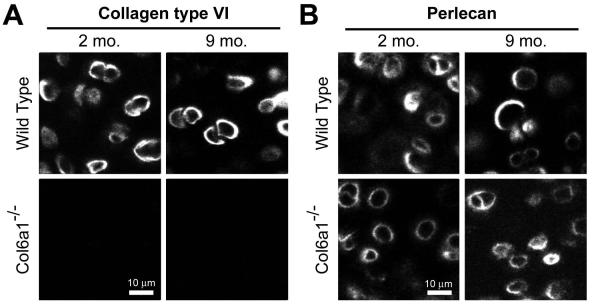
Articular cartilage of WT mice showed extensive pericellular labeling for type VI collagen, whereas type VI collagen was absent in Col6a1−/− mice (Figure 2a). Both wild-type and Col6a1−/− mice stained positively for perlecan in a region immediately around the cell of approximately 1-2 μm, consistent with the demarcation of the PCM (34) (Figure 2b). Neither type VI collagen nor perlecan labeling were found in the ECM.
Figure 2. Immunohistochemistry for type VI collagen and perlecan in mouse cartilage.
A. Immunohistochemistry shows the localized pericellular presence of type VI collagen in WT but not Col6a1−/− mice at both 2 and 9 month ages. B. Immunolabeling shows clear presence of perlecan in the pericellular region of WT as well as Col6a1−/− mice at both 2 and 9 month time points.
Ca2+ signaling in intact femoral cartilage
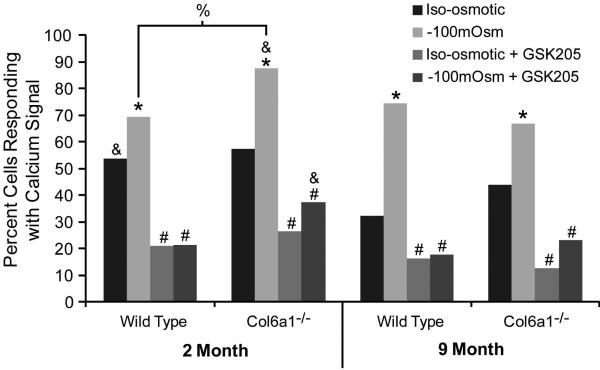
In mice of all genotypes and ages, exposure to hypotonic media (-100 mOsm) induced significant Ca2+ signaling in chondrocytes that was blocked with TRPV4 specific antagonist GSK205, indicating that the signaling was mediated by TRPV4 (Figure 3). Both 2 month old Col6a1−/− and WT mice had similar levels of Ca2+ signaling at baseline (with iso-osmotic media exchange), however the percent of cells signaling when under hypotonic stress was significantly greater in Col6a1−/− mice than in their WT counterparts (Figure 3). Nine month old Col6a1−/− mice trended towards increased signaling at baseline, however they responded to the same level of signaling as the WT mice with hypotonic stress (Figure 3). This finding indicates that lack of collagen VI does alter mechano/osmo-responsiveness in chondrocytes.
Figure 3. Osmotically-induced Ca2+ signaling in chondrocytes in situ.
The percentage of cells responding with a Ca2+ signal was significantly higher in response to −100mOsm change in osmolarity than to iso-osmotic control, and this hypo-osmotic signaling was significantly greater in Col6a1−/− mice than in WT for 2 month old mice. All signaling was significantly inhibited with the TRPV4 antagonist GSK205, indicating that the response was mediated by TRPV4. *: Significantly greater than iso-osmotic control, #: Significantly less than −100mOsm treatment, %: Significant difference between WT and Col6a1−/−, & Significantly greater than 9 month group. All analyses performed with a chi-squared test (p<0.05).
The increase in Ca2+ signaling relative to isotonic exchange was also different in Col6a1−/− mice as compared to controls. In WT mice, the relative increase in signaling with hypotonic stress was much greater for 9 month old mice compared to 2 month old mice (2 months: Hypo:Iso 69%:54% =29% increase, 9 months: Hypo:Iso75%:32% =132% increase). In Col6a1−/− mice, however, both 2 month old and 9 month old groups exhibited the same relative increase in signaling in response to hypotonic stress (2 months: Hypo:Iso 88%:57% =53% increase, 9 months: Hypo:Iso 67%:44% =52% increase).
Cellular Swelling of chondrocytes in intact femora
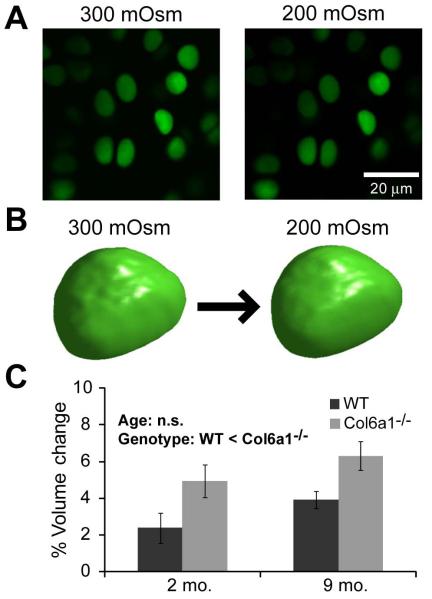
The baseline volume of the chondrocytes from 2 month old mice was not significantly different between WT and Col6a1−/− mice (2 months WT: 289±16μm3, Col6a1−/−: 266±14μm3) ; however, at 9 months the WT chondrocytes were significantly smaller than the Col6a1−/− (9 months WT: 237±7μm3, Col6a1−/−: 262±8μm3) and significantly smaller than their 2 month counterparts (ANOVA on log transformed data, age*genotype p=0.01). All chondrocytes swelled in response to hypotonic (-100 mOsm) media exchange (Figure 4). Chondrocytes from Col6a1−/− mice, however, swelled significantly more than their WT counterparts. There was no effect of age on chondrocyte swelling (Figure 4).
Figure 4. In situ measurements of chondrocyte volume and osmotically-induced swelling.
A. Representative images of one z-stack slice for volume reconstructions obtained with Calcein-AM intracellular label before (300mOsm) and after hypotonic fluid exchange (200mOsm). B. Rendering of 3-D reconstruction of a representative cell before (left) and after (right) hypotonic fluid exchange. C. Percent increase in chondrocyte volume in intact femoral condyle in response to hypotonic media change. WT chondrocytes showed a significantly smaller volume increase with hypo-osmotic stress than Col6a1−/− chondrocytes, regardless of age (2-way ANOVA, genotype p<0.0085, age p=0.12, interaction p=0.93). Bars show mean±SEM.
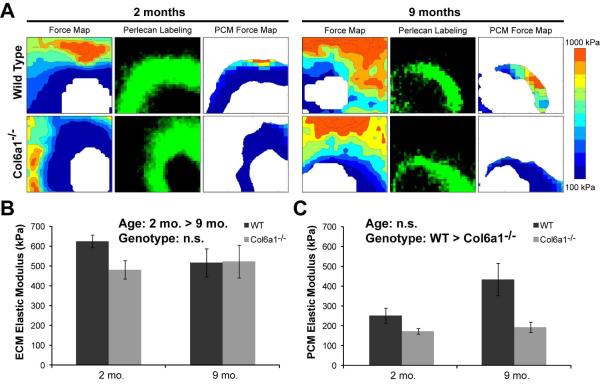
Mechanical properties of PCM
In WT and Col6a1−/− mice, AFM-generated stiffness maps revealed clear regions of lower compressive modulus around the cells that matched perlecan labeling of the PCM (Figure 5a). The ECM elastic modulus decreased significantly with age and there was a trend towards a lower ECM modulus of Col6a1−/− mice (Figure 5b). The PCM of Col6a1−/− mice was significantly less stiff than that of the WT mice, indicating loss of mechanical properties of the PCM with knockout of collagen VI (Figure 5c). There was no significant effect of aging on the PCM modulus.
Figure 5. Immunofluorescence-guided AFM for mapping ECM and PCM moduli.
A. Sample AFM force maps show matrix stiffness around cells in cartilage from 2 month and 9 month old mice. Perlecan labeling was used to define the boundaries of the PCM around each cell. B. ECM elastic moduli were greater in 2 month mice than 9 month mice. There was a trend towards WT having a greater ECM modulus than Col6a1−/− (2-way ANOVA on log transformed data, age p=0.016, genotype p=0.087, interaction p=0.23). C. PCM moduli of Col6a1−/− mice were significantly lower than those of WT mice regardless of age (2-way ANOVA on log transformed data, genotype p=0.0013, age p=0.67, interaction p=0.21). Bars show mean±SEM.
DISCUSSION
Our findings suggest that chondrocyte mechano-osmotic signaling through TRPV4 is mediated by the mechanical properties of the PCM in an age-related manner, based on the presence of type VI collagen. In contrast to our original hypothesis that type VI collagen is necessary for TRPV4 activity, both WT and Col6a1−/− mice showed spontaneous and osmotically-induced TRPV4-mediated Ca2+ signaling. At 2 months of age, mice lacking collagen VI exhibited an increased TRPV4-mediated response to hypo-osmotic stress as compared to WT controls. The increased signaling in Col6a1−/− mice corresponded with increased cell swelling in response to hypo-osmotic stress, which was associated with a loss of mechanical properties in the Col6a1−/− PCM as compared to WT controls. Taken together, these findings show that type VI collagen regulates the mechanical properties of the PCM, cell swelling, and therefore osmotically-induced TRPV4 signaling in an age-dependent manner. Additionally, these findings further support the role of the PCM as a transducer of mechanical and physicochemical signals in cartilage, and suggest that alterations in PCM properties can influence the mechanotransduction processes necessary for maintaining cartilage homeostasis.
TRPV4 is a polymodally-activated channel that can respond to a variety of physical signals including changes in osmolarity, deformation, or temperature, and may serve as an “integrator” of various signals at the cell surface. In this regard, previous studies have provided evidence to suggest that TRPV4 activation may occur through interactions between cell surface receptors and the surrounding matrix (39). For example, the presence of a PCM has been shown to enhance the chondrogenic biosynthetic response to mechanical load (40), and recent studies have shown that this effect is mediated by TRPV4 (27) as well as collagen VI (41). Type VI collagen anchors the chondrocyte to the PCM through various integrin receptors (9) as well as through the transmembrane proteoglycan NG2 (11, 12). TRPV4 expressed on endothelial cells can be rapidly activated by pulling on magnetic beads attached to β-integrins (24), and co-immunoprecipitates with α2 integrin (42). Furthermore, intracellular Ca2+ signaling in response to hypo-osmotic stimuli or the TRV4 agonist GSK1016790A is lost in mice lacking the collagen binding receptor α1 integrin subunit (30). While this data together suggests a potential linkage between TRPV4, α2β1 integrins and collagen VI in chondrocytes, our findings suggest that collagen VI is not required for TRPV4-mediated signaling in these cells, but rather that changes in chondrocytes’ volume secondary to the hypotonic stimulus are responsible for activating the channel. This finding is also consistent with studies that show that hypo-osmotic stress or specific agonists can activate TRPV4 in isolated chondrocytes that do not have a PCM (23) and furthermore, TRPV4 signaling is more closely correlated to the change in cell volume rather than the change in osmolarity (43). Nonetheless, it is unlikely that TRPV4 is directly gated by mechanical stretch of the chondrocytic cell membrane during cell swelling, as the channel does not respond to membrane distortion when isolated (44) and neither negative patch-clamp pressure nor micropipette aspiration activate chondrocyte Ca2+ signaling at physiologic levels of membrane stretch (45). One potential explanation for this lack of responsiveness to stretch is the fact that chondrocytes possess large amounts of excess membrane area, such that under physiologic conditions the membrane is more likely to unfold than to stretch (46).
Under physiologic conditions, chondrocytes undergo dynamic changes in osmolarity due to loading-induced consolidation and recovery of the cartilage ECM. In this study, we used osmotic stress as a physiologically-relevant means of activating TRPV4. The downstream effect of chondrocyte swelling are multifocal and include increases in intracellular Ca2+, cytoskeletal rearrangement (21), gene activation (47) and activation of regulatory volume decrease (48). As the chondrocyte is fully surrounded by the PCM, this region plays a critical role in the transduction of ECM compression into local physicochemical (i.e., osmotic) changes [reviewed in (10)]. Furthermore, the PCM can regulate cellular swelling by physically preventing the enlargement of the cell. Several computational and experimental studies have shown that alterations in composition and mechanical properties of the PCM can influence the swelling behavior of chondrocytes (49, 50). Our data showed that mice lacking type VI collagen exhibited significantly greater chondrocyte swelling in response to osmotic stress. Under these test conditions, the chondrocyte is expected to behave as an “ideal” osmometer such that the swelling pressure will be directly related to the applied osmotic changes (46). Thus, the differences in chondrocyte swelling in situ are likely due to differences in the mechanical properties of the PCM, rather than any active chondrocyte responses or differences in cellular biophysical properties.
Thus, to directly test the hypothesis that collagen VI regulates PCM mechanical properties, immunofluorescence-guided AFM was used to spatially map the in situ modulus of the PCM. Col6a1−/− mice showed significantly lower PCM moduli as compared to WT controls, consistent with a previous study that used micropipette aspiration to determine PCM properties in extracted chondrons (13). Based on previous theoretical models of cell-PCM-ECM interactions, such changes in PCM properties would be expected to significantly influence the transmission and transduction of both mechanical and osmotic stresses from the ECM to the PCM (10). Similarly, the PCM has been shown to soften and expand with the development of osteoarthritis (37 , 51). As such, it is likely that the mechanical signals transmitted to the chondrocyte are altered in OA cartilage, and may contribute to the loss of homeostasis in matrix production (10).
In summary, our findings indicate that type VI collagen can play an important role in regulating chondrocyte mechanotransduction via TRPV4. However, our findings do not support a direct role of collagen VI in the function of the channel, as chondrocytes from Col6a1−/− mice were functionally responsive to osmotic stimuli via TRPV4 activation. Rather, our findings suggest that type VI collagen plays a critical role in the mechanical properties of the PCM, and therefore regulates the transduction and transmission of mechanical and osmotic stresses from the ECM to the PCM, and thus to the chondrocyte (10). Future studies may wish to examine the effects of direct mechanical loading in this context (52), as well as the potential roles of other pericellular components, such as type IX collagen (53) or other mechanosensitive channels (54). These findings also emphasize the importance of in situ measurements of cell behavior and matrix properties to allow for a more physiologic context for studies of mechanotransduction (28, 35). Finally, these findings indicate that changes in matrix properties, such as those occurring with OA, could thus affect mechanotransduction in chondrocytes and matrix metabolism. In this regard, modulation of mechanotransduction pathways, possibly by altering TRPV4 function, could provide a basis for therapeutic interventions for OA. A more thorough understanding of the mechanotransduction cascade in chondrocytes and stem/progenitor cells will also have important implications for enhancing chondrogenesis in engineered cartilage replacement (27, 40 , 41).
ACKNOWLEDGMENTS
The authors thank Stephen Johnson for assistance with animal handling. This work was supported in part by the Arthritis Foundation and National Institutes of Health grants AR48182, AR48852, AG15768, AR50245, AG46927.
Financial Support: This work was supported by the Arthritis Foundation and National Institutes of Health grants AR48182, AR48852, AG15768, AR50245, AG46927
REFERENCES
- 1.Turkiewicz A, Petersson IF, Bjork J, Hawker G, Dahlberg LE, Lohmander LS, et al. Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarth Cartilage. 2014;22(11):1826–32. doi: 10.1016/j.joca.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25(6):815–23. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helminen H, Jurvelin J, Kiviranta I, Paukkonen K, Saamanen A, Tammi M. Joint loading effects on articular cartilage: A historical review. In: Helminen HJ, Kiviranta I, Tammi M, Saamanen AM, K P, Jurvelin J, editors. Joint Loading: Biology and Health of Articular Structures. Wright and Sons; Bristol: 1987. pp. 1–46. [Google Scholar]
- 4.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–69. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 5.Leroux MA, Cheung HS, Bau JL, Wang JY, Howell DS, Setton LA. Altered mechanics and histomorphometry of canine tibial cartilage following joint immobilization. Osteoarth Cartilage. 2001;9(7):633–40. doi: 10.1053/joca.2001.0432. [DOI] [PubMed] [Google Scholar]
- 6.Palmoski M, Perricone E, Brandt KD. Development and reversal of a proteoglycan aggregation defect in normal canine knee cartilage after immobilization. Arthritis Rheum. 1979;22(5):508–17. doi: 10.1002/art.1780220511. [DOI] [PubMed] [Google Scholar]
- 7.Burleigh A, Chanalaris A, Gardiner MD, Driscoll C, Boruc O, Saklatvala J, et al. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 2012;64(7):2278–88. doi: 10.1002/art.34420. [DOI] [PubMed] [Google Scholar]
- 8.Vincent TL. Targeting mechanotransduction pathways in osteoarthritis: a focus on the pericellular matrix. Curr Opin Pharmacol. 2013;13(3):449–54. doi: 10.1016/j.coph.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Poole CA, Ayad S, Schofield JR. Chondrons from articular cartilage: I. Immunolocalization of type VI collagen in the pericellular capsule of isolated canine tibial chondrons. J Cell Sci. 1988;90(4):635. doi: 10.1242/jcs.90.4.635. [DOI] [PubMed] [Google Scholar]
- 10.Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014;39C:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcelino J, McDevitt CA. Attachment of articular cartilage chondrocytes to the tissue form of type VI collagen. Biochim Biophys Acta. 1995;1249(2):180–8. doi: 10.1016/0167-4838(95)00026-q. [DOI] [PubMed] [Google Scholar]
- 12.Doane KJ, Howell SJ, Birk DE. Identification and functional characterization of two type VI collagen receptors, alpha 3 beta 1 integrin and NG2, during avian corneal stromal development. Invest Opth Vis Sci. 1998;39(2):263–75. [PubMed] [Google Scholar]
- 13.Alexopoulos LG, Youn I, Bonaldo P, Guilak F. Developmental and osteoarthritic changes in Col6a1-knockout mice: Biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis Rheum. 2009;60(3):771–9. doi: 10.1002/art.24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen SE, Coles JM, Zelenski NA, Furman BD, Leddy HA, Zauscher S, et al. Altered trabecular bone structure and delayed cartilage degeneration in the knees of collagen VI null mice. PLoS One. 2012;7(3):e33397. doi: 10.1371/journal.pone.0033397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izu Y, Ansorge HL, Zhang G, Soslowsky LJ, Bonaldo P, Chu ML, et al. Dysfunctional tendon collagen fibrillogenesis in collagen VI null mice. Matrix Biol. 2011;30(1):53–61. doi: 10.1016/j.matbio.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin WA, Bergamin N, Sabatelli P, Reggiani C, Megighian A, Merlini L, et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003;35(4):367–71. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- 17.De Palma S, Leone R, Grumati P, Vasso M, Polishchuk R, Capitanio D, et al. Changes in muscle cell metabolism and mechanotransduction are associated with myopathic phenotype in a mouse model of collagen VI deficiency. PLoS One. 2013;8(2):e56716. doi: 10.1371/journal.pone.0056716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mow VC, Bachrach NM, Setton LA, Guilak F, Mow VC, Tran-Son-Tay R. Stress, Strain, Pressure and Flow Fields in Articular Cartilage and Chondrocytes. In: Guilak F, Hochmuth RM, editors. Cell Mechanics and Cellular Engineering. Springer-Verlag New York, LLC; New York: 1994. pp. 345–79. [Google Scholar]
- 19.Maroudas A. Freeman MAR, editor. Physicochemical properties of articular cartilage. Adult Articular Cartilage. Bath: Pitman Medical. 1979:215–90. [Google Scholar]
- 20.Haider MA, Schugart RC, Setton LA, Guilak F. A mechano-chemical model for the passive swelling response of an isolated chondron under osmotic loading. Biomech Model Mechanobiol. 2006;5(2-3):160–71. doi: 10.1007/s10237-006-0026-1. [DOI] [PubMed] [Google Scholar]
- 21.Erickson G, Northrup D, Guilak F. Hypo-osmotic stress induces calcium-dependent actin reorganization in articular chondrocytes. Osteoarth Cartilage. 2003;11(3):187–97. doi: 10.1053/s1063-4584(02)00347-3. [DOI] [PubMed] [Google Scholar]
- 22.Chao PG, West AC, Hung CT. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol Cell Physiol. 2006;291(4):C718–C25. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]
- 23.Phan MN, Leddy HA, Votta BJ, Kumar S, Levy DS, Lipshutz DB, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60(10):3028–37. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matthews B, Thodeti C, Tytell J, Mammoto A, Overby D, Ingber D. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integrative Biology. 2010:435–42. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muramatsu S, Wakabayashi M, Ohno T, Amano K, Ooishi R, Sugahara T, et al. Functional gene screening system identified TRPV4 as a regulator of chondrogenic differentiation. J Biol Chem. 2007;282(44):32158–67. doi: 10.1074/jbc.M706158200. [DOI] [PubMed] [Google Scholar]
- 26.Cameron TL, Belluoccio D, Farlie PG, Brachvogel B, Bateman JF. Global comparative transcriptome analysis of cartilage formation in vivo. BMC Dev Biol. 2009;9:20. doi: 10.1186/1471-213X-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Conor CJ, Leddy HA, Benefield HC, Liedtke WB, Guilak F. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci U S A. 2014;111(4):1316–21. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark AL, Votta BJ, Kumar S, Liedtke W, Guilak F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: Age-and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010;62(10):2973–83. doi: 10.1002/art.27624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leddy HA, McNulty AL, Lee SH, Rothfusz NE, Gloss B, Kirby ML, et al. Follistatin in chondrocytes: the link between TRPV4 channelopathies and skeletal malformations. FASEB J. 2014 doi: 10.1096/fj.13-245936. doi: 10.1096/fj.13-245936 fj.13-245936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jablonski CL, Ferguson S, Pozzi A, Clark AL. Integrin alpha1beta1 participates in chondrocyte transduction of osmotic stress. Biochem Biophys Res Commun. 2014;445(1):184–90. doi: 10.1016/j.bbrc.2014.01.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipp P, Niggli E. Ratiometric confocal Ca(2+)-measurements with visible wavelength indicators in isolated cardiac myocytes. Cell Calcium. 1993;14(5):359–72. doi: 10.1016/0143-4160(93)90040-d. [DOI] [PubMed] [Google Scholar]
- 32.Bush PG, Hall AC. The volume and morphology of chondrocytes within non-degenerate and degenerate human articular cartilage. Osteoarth Cartilage. 2003;11(4):242–51. doi: 10.1016/s1063-4584(02)00369-2. [DOI] [PubMed] [Google Scholar]
- 33.Guilak F. Volume and surface area measurement of viable chondrocytes in situ using geometric modelling of serial confocal sections. J Microsc. 1994;173(Pt 3):245–56. doi: 10.1111/j.1365-2818.1994.tb03447.x. [DOI] [PubMed] [Google Scholar]
- 34.Wilusz RE, DeFrate LE, Guilak F. Immunofluorescence-guided atomic force microscopy to measure the micromechanical properties of the pericellular matrix of porcine articular cartilage. J R Soc Lond Interface. 2012;9(76):2997–3007. doi: 10.1098/rsif.2012.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilusz RE, Defrate LE, Guilak F. A biomechanical role for perlecan in the pericellular matrix of articular cartilage. Matrix Biol. 2012;31(6):320–7. doi: 10.1016/j.matbio.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darling EM, Zauscher S, Block JA, Guilak F. A thin-layer model for viscoelastic, stress-relaxation testing of cells using atomic force microscopy: do cell properties reflect metastatic potential? Biophys J. 2007;92(5):1784–91. doi: 10.1529/biophysj.106.083097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alexopoulos LG, Haider MA, Vail TP, Guilak F. Alterations in the mechanical properties of the human chondrocyte pericellular matrix with osteoarthritis. J Biomech Eng. 2003;125(3):323. doi: 10.1115/1.1579047. [DOI] [PubMed] [Google Scholar]
- 38.Guo S, Akhremitchev BB. Packing density and structural heterogeneity of insulin amyloid fibrils measured by AFM nanoindentation. Biomacromolecules. 2006;7(5):1630–6. doi: 10.1021/bm0600724. [DOI] [PubMed] [Google Scholar]
- 39.Mobasheri A, Carter SD, Martin-Vasallo P, Shakibaei M. Integrins and stretch activated ion channels; putative components of functional cell surface mechanoreceptors in articular chondrocytes. Cell Biol Int. 2002;26(1):1–18. doi: 10.1006/cbir.2001.0826. [DOI] [PubMed] [Google Scholar]
- 40.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122(3):252–60. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 41.Twomey JD, Thakore PI, Hartman DA, Myers EG, Hsieh AH. Roles of type VI collagen and decorin in human mesenchymal stem cell biophysics during chondrogenic differentiation. Eur Cell Mater. 2014;27:237–50. doi: 10.22203/ecm.v027a17. discussion 49-50. [DOI] [PubMed] [Google Scholar]
- 42.Alessandri-Haber N, Dina O, Joseph E, Reichling D, Levine J. Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neuroscience. 2008;28(5):1046–57. doi: 10.1523/JNEUROSCI.4497-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leddy HA, Liedtke W, Guilak F. Effect of initial osmolarity on hypo-osmotically induced calcium signaling in chondrocytes. Trans Orthop Res Soc. 2010;35:0876. [Google Scholar]
- 44.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2(10):695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 45.Erickson GR, Minkhorst R, Guilak F. The Calcium transients elicited in chondrocytes as a result of exposure to osmotic pressure change are not due to simple membrane deformation. Trans Orthop Res Soc. 2001;26:555. [Google Scholar]
- 46.Guilak F, Erickson GR, Ting-Beall HP. The effects of osmotic stress on the viscoelastic and physical properties of articular chondrocytes. Biophys J. 2002;82(2):720–7. doi: 10.1016/S0006-3495(02)75434-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hung CT, LeRoux MA, Palmer GD, Chao PH, Lo S, Valhmu WB. Disparate aggrecan gene expression in chondrocytes subjected to hypotonic and hypertonic loading in 2D and 3D culture. Biorheology. 2003;40(1-3):61–72. [PubMed] [Google Scholar]
- 48.Bush PG, Hall AC. Regulatory volume decrease (RVD) by isolated and in situ bovine articular chondrocytes. J Cell Physiol. 2001;187(3):304–14. doi: 10.1002/jcp.1077. [DOI] [PubMed] [Google Scholar]
- 49.Tanska P, Turunen SM, Han SK, Julkunen P, Herzog W, Korhonen RK. Superficial collagen fibril modulus and pericellular fixed charge density modulate chondrocyte volumetric behaviour in early osteoarthritis. Comput Math Methods Med. 2013;2013:164146. doi: 10.1155/2013/164146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bush PG, Hall AC. Passive osmotic properties of in situ human articular chondrocytes within non-degenerate and degenerate cartilage. Journal of cellular physiology. 2005;204(1):309–19. doi: 10.1002/jcp.20294. [DOI] [PubMed] [Google Scholar]
- 51.Lee GM, Paul TA, Slabaugh M, Kelley SS. The incidence of enlarged chondrons in normal and osteoarthritic human cartilage and their relative matrix density. Osteoarth Cartilage. 2000;8(1):44–52. doi: 10.1053/joca.1999.0269. [DOI] [PubMed] [Google Scholar]
- 52.Han SK, Wouters W, Clark A, Herzog W. Mechanically induced calcium signaling in chondrocytes in situ. J Orthop Res. 2012;30(3):475–81. doi: 10.1002/jor.21536. [DOI] [PubMed] [Google Scholar]
- 53.Hu K, Xu L, Cao L, Flahiff CM, Brussiau J, Ho K, et al. Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis Rheum. 2006;54(9):2891–900. doi: 10.1002/art.22040. [DOI] [PubMed] [Google Scholar]
- 54.Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci U S A. 2014;111(47):E5114–22. doi: 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]