Abstract
To investigate the role of enhancer of zeste homolog (EZH) 1 and EZH2 in liver homeostasis, mice were generated that carried Ezh1−/− and EZH2fl/fl alleles and an Alb-Cre transgene. Only the combined loss of EZH1 and EZH2 in mouse hepatocytes caused a depletion of global trimethylation on Lys 27 of histone H3 (H3K27me3) marks and the specific loss of over ∼1900 genes at 3 mo of age. Ezh1−/−,Ezh2fl/flAlb-Cre mice exhibited progressive liver abnormalities manifested by the development of regenerative nodules and concomitant periportal fibrosis, inflammatory infiltration, and activation of A6-positive hepatic progenitor cells at 8 mo of age. In response to chronic treatment with carbon tetrachloride, all experimental mice, but none of the controls (n = 27 each), showed increased hepatic degeneration associated with liver dysfunction and reduced ability to proliferate. After two-thirds partial hepatectomy, mutant mice (n = 5) displayed increased liver injury and a blunted regenerative response. Genome-wide analyses at 3 mo of age identified 51 genes that had lost H3K27me3 marks, and their expression was significantly increased. These genes were involved in regulation of cell survival, fibrosis, and proliferation. H3K27me3 levels and liver physiology were unaffected in mice lacking either EZH1 globally or EZH2 specifically in hepatocytes. This work demonstrates a critical redundancy of EZH1 and EZH2 in maintaining hepatic homeostasis and regeneration.—Bae, W. K., Kang, K., Yu, J. H., Yoo, K. H., Factor, V. M., Kaji, K., Matter, M., Thorgeirsson, S., Hennighausen, L. The methyltransferases enhancer of zeste homolog (EZH) 1 and EZH2 control hepatocyte homeostasis and regeneration.
Keywords: H3-K27 trimethylation, liver regeneration, fibrosis
Polycomb group (PcG) proteins are essential epigenetic regulators in normal tissue homeostasis and are involved in transcriptional repression (1). Polycomb proteins form chromatin-remodeling complexes referred to as Polycomb-repressive complexes (PRCs) (2). PRC2 consists of 3 core subunits, including embryonic ectoderm development, suppressor of zeste 12 homolog, and enhancer of zeste homolog (EZH) 2, which catalyzes trimethylation on Lys 27 of histone H3 (H3K27me3) (3). Recent studies have shown that EZH1, a homolog of EZH2, also has histone H3K27 methyltransferase activity and binds to an overlapping subset of genes (4). EZH1 and EZH2 have different expression patterns. EZH2 is found in actively proliferating cells, whereas EZH1 expression is higher in nonproliferative adult tissues (4). EZH1 partially compensates for the loss of EZH2, as shown in cells lacking only Ezh2 (5).
The primary function of PRCs is the repression of gene expression through the introduction of H3K27me3 marks. However, little is known about the role of PRC2, and the associated degree of H3K27me3, in liver homeostasis and disease. Deregulation of PcG proteins has been observed in many solid tumors, and loss-of-function mutations in the Ezh2 gene have been linked to hematopoietic malignancies in mice and men (6). In contrast, elevated levels of EZH2 have been described in solid tumors and are associated with poor prognosis in hepatocellular carcinoma (HCC) (7). Although PRC2 was originally identified as a suppressor associated with H3K27me3 mark at target genes, recent evidence suggests that EZH2 can also control gene expression by serving as a transcription coactivator and by methylating cellular proteins (8, 9). The histone methyltransferase EZH1 also appears to strive beyond its enzymatic activity and binds to transcriptional start sites to promote RNA polymerase II elongation (10).
To directly address the role of PRC2 in adult liver homeostasis, as well as its impact on disease progression, Ezh1 and Ezh2 conditional knockout mouse models were generated. Our initial study demonstrated that the loss of EZH2 alone did not produce any apparent effect on liver phenotype, possibly due to compensation by EZH1. Therefore, we generated a double Ezh1 and Ezh2 knockout (E1/2KO) mouse model by breeding Ezh1 knockout (E1KO) mice (unpublished data; no specific phenotype) with Ezh2fl/flAlb-Cre [Ezh2 knockout (E2KO)] mice. Loss of EZH1 or EZH2 alone affected neither H3K27me3 marks nor liver biology. In contrast, the combined ablation of EZH1 and EZH2 in mouse hepatocytes disrupted H3K27me3 homeostasis, increased sensitivity to injury, and reduced the efficiency of liver regeneration in response to toxic stress or loss of tissue mass. These genetic experiments illustrate a critical role of EZH1 and EZH2 both for physiologic and compensatory liver regeneration.
MATERIALS AND METHODS
Generation of the E1/2KO mouse
E1KO mice were generated by the lab of Thomas Jenuwein (Research Institute of Molecular Pathology, Vienna, Austria) and Ezh2fl/fl mice by the lab of Alexander Tarakhovsky (Rockefeller University, New York, NY, USA) (11). Ezh2fl/fl mice were subsequently bred with Alb-Cre transgenic mice, and their offspring were bred with E1KO mice to generate double E1/2KO. This breeding scheme yielded Ezh1−/−, E2KO mice designated as E1/2KO and littermate control mice, Ezh2fl/fl [wild-type (WT)], E1KO, and E2KO.
Animal studies
Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care facility and cared for in accordance with the guidelines from the Animal Care and Use Committee at the National Institute of Diabetes and Digestive and Kidney Diseases. Only male mice were used in the experiments. To address the toxin-induced liver injury with carbon tetrachloride (CCl4), 3-mo-old male mice were divided into 4 experimental groups (n = 27 mice for each genotype): 1) WT, 2) E1KO, 3) E2KO, and 4) E1/2KO. Mice received intraperitoneal injections of CCl4 (0.2 ml/kg) in corn oil (Sigma-Aldrich, St. Louis, MO, USA) twice a week for 2 wk. Mice were killed 2 d after the first, second, and fourth injection (9 mice per group per time point). For the partial hepatectomy (PHx) experiment, mice were anesthetized with a mixture of isoflurane/oxygen, and right medial, left medial, and left lateral lobes were excised resulting in removal of two-thirds of the hepatic mass. Livers were harvested, weighed, and processed for subsequent analysis 48 h after surgery (5 mice per genotype). Liver regeneration was assessed by weight gain, analysis of proliferating cell nuclear antigen Ki67, and serum markers of liver integrity and function, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, and albumin. Blood samples were obtained from the heart under isoflurane anesthesia before killing. At least 3 animals were used for each time point.
Immunofluorescence and special stains
Livers were fixed in 10% neutral buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E) for routine histopathologic examination, and processed for Masson’s trichrome and immunofluorescence staining. Primary antibodies used were anti-Ezh2 (5246S; Cell Signaling Technology, Beverly, MA, USA); anti-H3K27me3 (07-449; Millipore, Temecula, CA, USA); anti-CTGF (connective tissue growth factor) (ab59681; Abcam, Cambridge, MA, USA); anti-Ki67 (ab16667; Abcam); anti–β-catenin (BD Transduction Laboratories, San Jose, CA, USA); and A6 (12).
RNA-sequencing analysis
RNA-sequencing (RNA-seq) analysis was performed using each 3 WT, E1KO, E2KO, and E1/2KO mice at 3 and 8 mo of age, and 3-mo-old mice exposed to CCl4 (2 d after the first and fourth injection). Poly(A) RNA was purified twice from 1 mg total RNA, and cDNA was synthesized using SuperScript II (Invitrogen, Life Technologies, Carlsbad, CA, USA) and TruSeq RNA Sample Preparation Kit (Illumina Incorporated, San Diego, CA, USA) and sequenced using HiSeq 2000 (Illumina). The single-end reads of biologic replicates obtained from each sample were aligned to the mouse reference genome (mm9 assembly) using the TopHat program (13). Transcript abundance was estimated by means of fragment per kilobase of exon per million fragments mapped (FPKM) by Cufflinks (14). Differentially expressed genes were identified with Cufflinks with an adjusted P value of 0.05 (14). The RNA-seq data set has been deposited in the gene expression omnibus (GEO) database under accession number GSE53627.
Chromatin immunoprecipitation and parallel sequencing
Histone H3K4me3 and H3K27me3 chromatin immunoprecipitation with parallel sequencing (ChIP sequencing) were initially performed and processed by Active Motif, Incorporated (Carlsbad, CA, USA), on liver tissues of WT, E1KO, E2KO, and E1/2KO mice. Antibodies against histone H3K4me3 (17-614; Millipore) and H3K27me3 (07-449; Millipore) were used for ChIP. H3K4me3 ChIP-seq was checked again by using hepatocytes. Primary hepatocytes were isolated from WT and E1/2KO mice using a 2-step collagenase digestion method, as previously reported (15). The yield and viability of the isolated hepatocytes were 1.0–1.5 × 107 cells per animal and >90%, respectively. Primary hepatocytes were cross-linked with 1% formaldehyde for 10 min. Nuclei were extracted in Farnham lysis buffer [5 mM PIPES (pH 8.0), 85 mM KCl, 0.5% NP-40, and protease inhibitor]. Chromatin was fragmented to 200–300 bp by sonication using a MISONIX Sonicator 3000 (QSonica; Newtown, CT, USA). The ChIP DNA fragments were blunt ended and ligated to the Illumina Indexed DNA adaptors using NEBNext ChIP-seq Library Prep Master Mix Set for Illumina (New England BioLabs, Ipswich, MA, USA), and sequenced using the Illumina HiSeq 2000. The single-end reads were aligned to the mouse reference genome (mm9 assembly). Spatial clustering for Identification of ChIP-Enriched Regions (SICER) identified H3K27me3-enriched regions with a false discovery rate (FDR) cutoff of 0.01 (16). Hypergeometric Optimization of Motif EnRichment (HOMER) pinpointed H3K4me3 enriched regions with an FDR cutoff of 0.001 (17). Total number of reads in each sample was normalized to 10 million by HOMER. All snapshots were obtained from an integrative genomics viewer (18). The ChIP-seq set has been deposited in the GEO database under accession number GSE53627.
Statistical analysis
All biochemical data were expressed as the mean ± sd. The Student’s t test and ANOVA test were used to determine significance. The survival study was determined using the Kaplan-Meier survival curve. For survival studies, statistical significance (P < 0.05) was analyzed using the log-rank test. The Mann-Whitney U test was used for expression data (P < 0.01). Cuffdiff (14) was used to detect significantly up-regulated genes in RNA-seq data (FDR-adjusted P value <0.05).
RESULTS
Combined loss of EZH1 and EZH2 results in liver damage and activation of hepatic progenitor cells
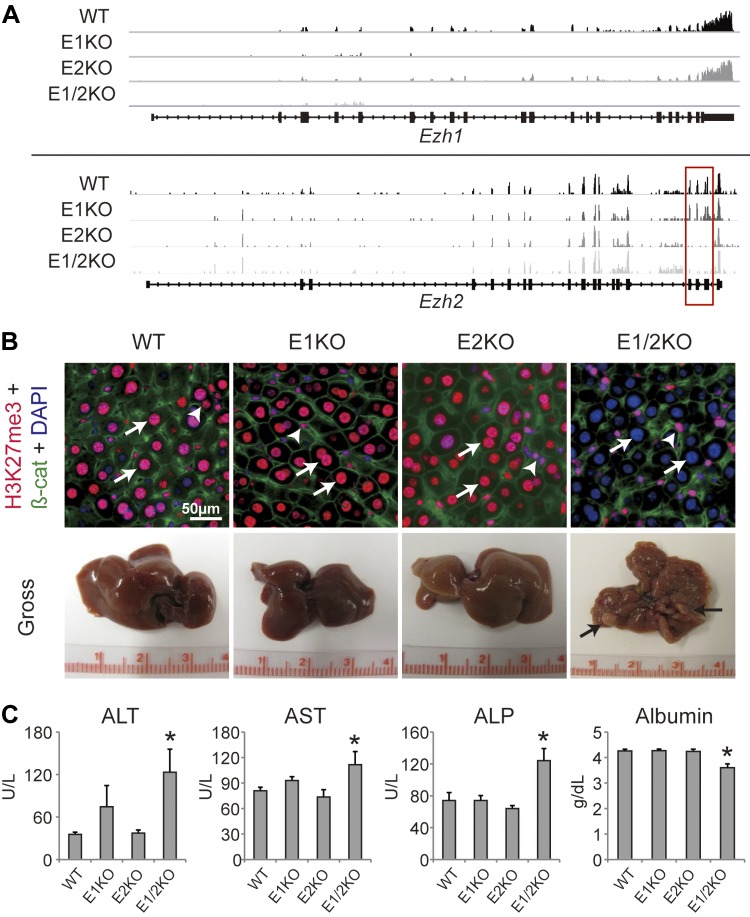
To elucidate the role of Ezh1 and Ezh2 in postnatal liver biology, Ezh1−/− mice (E1KO) and mice harboring Ezh2 floxed alleles were used (5, 11). The Alb-Cre transgene (19) was introduced into Ezh2fl/fl mice to generate liver-specific conditional Ezh2 mice (E2KO) that were subsequently crossed with E1KO mice to generate double-knockout mice referred to as E1/2KO. RNA-seq from liver tissue confirmed the absence of Ezh1 mRNA and transcripts corresponding to the deleted exons (exons encoding the SET domain of Ezh2) of Ezh2 (Fig. 1A) (n = 3 mice per each genotype per time point). H3K27me3 immunofluorescence staining was performed to assess the extent to which EZH1 and EZH2 control the presence of H3K27me3 marks in hepatocytes (n = 9 mice for each genotype). The staining patterns for H3K27me3 in E1KO and E2KO livers were indistinguishable from that found in WT controls (96 and 92% of positive hepatocytes vs. 89% in WT controls), supporting the notion that these 2 methyltransferases have overlapping redundant functions (Fig. 1B, top panel). However, the loss of both Ezh1 and Ezh2 caused a strong down-regulation of H3K27me3 expression in hepatocytes (Fig. 1B, top panel). Quantitative estimates of H3K27me3 staining revealed just 4% of positive hepatocytes in E1/2KO livers, whereas the H3K27me3 expression in nonparenchymal cells was not affected (78, 81, 78, and 92% in WT, E1KO, E2KO, and E1/2KO livers, respectively).
Figure 1.
Combined deletion of Ezh1 and Ezh2 resulted in the loss of H3K27me3 marks in hepatocytes. A) Representative photographs depict the mRNA expression of Ezh1 and Ezh2 by RNA-seq in WT, E1KO, E2KO, and E1/2KO livers at 3 mo of age. E1/2KO livers showed the lack of Ezh1 and Ezh2 expression. Exons encoding the SET domain of Ezh2 (red rectangle). B) Top panels are representative images of H3K27me3 immunofluorescence at 3 mo of age. H3K27me3 marks are absent in E1/2KO hepatocytes (arrows), but present in nonparenchymal cells (arrowheads). Bottom panels show gross liver morphology in mice of indicated genotypes at 8 mo of age. Only E1/2KO livers showed a nodular appearance (arrows). β-cat, β-catenin. C) Serum levels of ALT, AST, ALP, and albumin at 8 mo of age. Data are the mean ± sd (n = 5). *P < 0.05 as compared to the age-matched WT, E1KO, and E2KO.
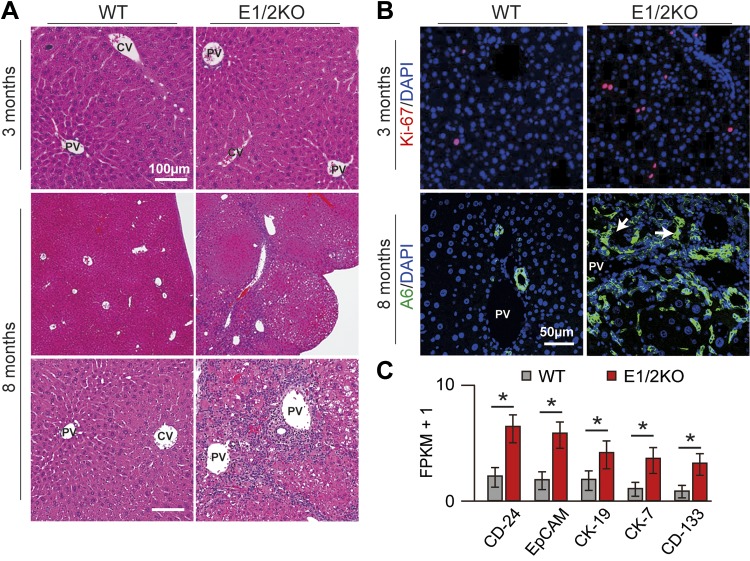
Consistent with this, mice lacking either Ezh1 globally or Ezh2 specifically in hepatocytes did not display any abnormalities in gross liver appearance and histology, at least up to 8 mo of age (Fig. 1B, bottom panel) (n = 18 mice per each genotype per time point). In striking contrast, 16 out of 18 E1/2KO livers had acquired a nodular surface by 8 mo of age (Fig.1B, bottom panel). In addition, E1/2KO mice displayed a broad impairment of liver function as evidenced by reduced serum albumin levels and a significant increase in serum levels of ALT and AST, sensitive indicators of hepatocyte integrity. ALP, a marker of biliary obstruction, was also increased in the combined absence of EZH1 and EZH2 as compared to WT or any single knockout mice (Fig. 1C). Histologically, E1/2KO livers did not show any obvious structural abnormalities at 3 mo of age (Fig. 2A, top panel). However, there was a modest increase in proliferation as judged by Ki67 staining (2.1-fold; P < 0.003) in E1/2KO livers as compared to age-matched WT controls, a reflection of ongoing tissue damage (Fig. 2B, top panel). By 8 mo, E1/2KO livers had developed numerous regenerative nodules surrounded by extensive portal and periportal inflammation (Fig. 2A, middle and bottom panels, and Supplemental Fig. 1). The nodular regeneration was paralleled by a prominent accumulation of ductular structures that expressed the progenitor/biliary cell marker A6 (Fig. 2B, bottom panel). This was corroborated by results of RNA-seq analysis, which revealed a strong up-regulation of several hepatic progenitor cell (HPC)–related genes in 8-mo-old livers, including keratin (Krt) 7 (CK-7), Krt19 (CK-19), epithelial cell adhesion molecule (EpCAM), prominin 1 [(Prom1) CD-133], and cd24a antigen [(Cd24a) CD-24] in E1/2KO livers (Fig. 2C). This was corroborated by these data that genetic deletion of both Ezh1 and Ezh2 caused liver damage and activation of HPCs.
Figure 2.
Histologic abnormalities in E1/2KO livers. A) Liver morphology at 3 and 8 mo of age. Paraffin-embedded sections were stained with H&E. Top panels are E1/2KO livers showing normal histology at 3 mo. Middle and bottom panels are E1/2KO livers showing a nodular appearance and inflammatory infiltration in portal and periportal areas at 8 mo. PV, portal vein; CV, central vein. B) Top panels show representative immunofluorescence staining for cell proliferation marker Ki67 (red) at 3 mo. Nuclei were counterstained with DAPI. Bottom panels show immunofluorescence staining with A6, a biliary/progenitor cell marker at 8 mo. Representative confocal images show the presence of numerous A6-positive ductular cells (arrows) only in E1/2KO livers. Nuclei were counterstained with DAPI. C) Increased expression of progenitor cell-related genes in E1/2KO livers at 8 mo determined by RNA-seq. Cuffdiff (14) was used to detect significantly up-regulated genes with an FDR-adjusted P value of 0.05 (asterisk).
Masson’s trichrome staining confirmed the presence of dense collagen deposition in periportal areas in double-knockout livers (Fig. 3A, top panel). This was paralleled by a stronger activation of hepatic stellate cells, a key contributor to fibrosis (Fig. 3A, middle panel), and immunofluorescence staining for the CTGF, which was observed only in E1/2KO livers (Fig. 3A, bottom panel). Further reinforcing the presence of advanced fibrosis, expression of genes associated with fibrosis was elevated in E1/2KO tissue by 8 mo of age. Among these were collagen type I α1 (Col1a1), collagen type I α2 (Col1a2), collagen type IV α2 (Col4a2), Ctgf, TGF-β induced (Tgfbi), and annexin A2 (Anxa2) (FDR-adjusted P value <0.05) (Fig. 3B). In the absence of both Ezh1 and Ezh2, the expression of Cidea, a gene linked to liver metabolism and hepatic steatosis, was also elevated (FDR-adjusted P value <0.05) (Supplemental Table 1) (18). In addition, the combined loss of Ezh1 and Ezh2 in mouse livers caused activation of genes that are normally quiescent, including members of the Hox and Pax gene families (Supplemental Table 2). These lineage-specific genes are particularly sensitive to the status of H3K27me3 at 3 mo of age, and inappropriate activation in the absence of EZH2 has been observed in other systems (17).
Figure 3.
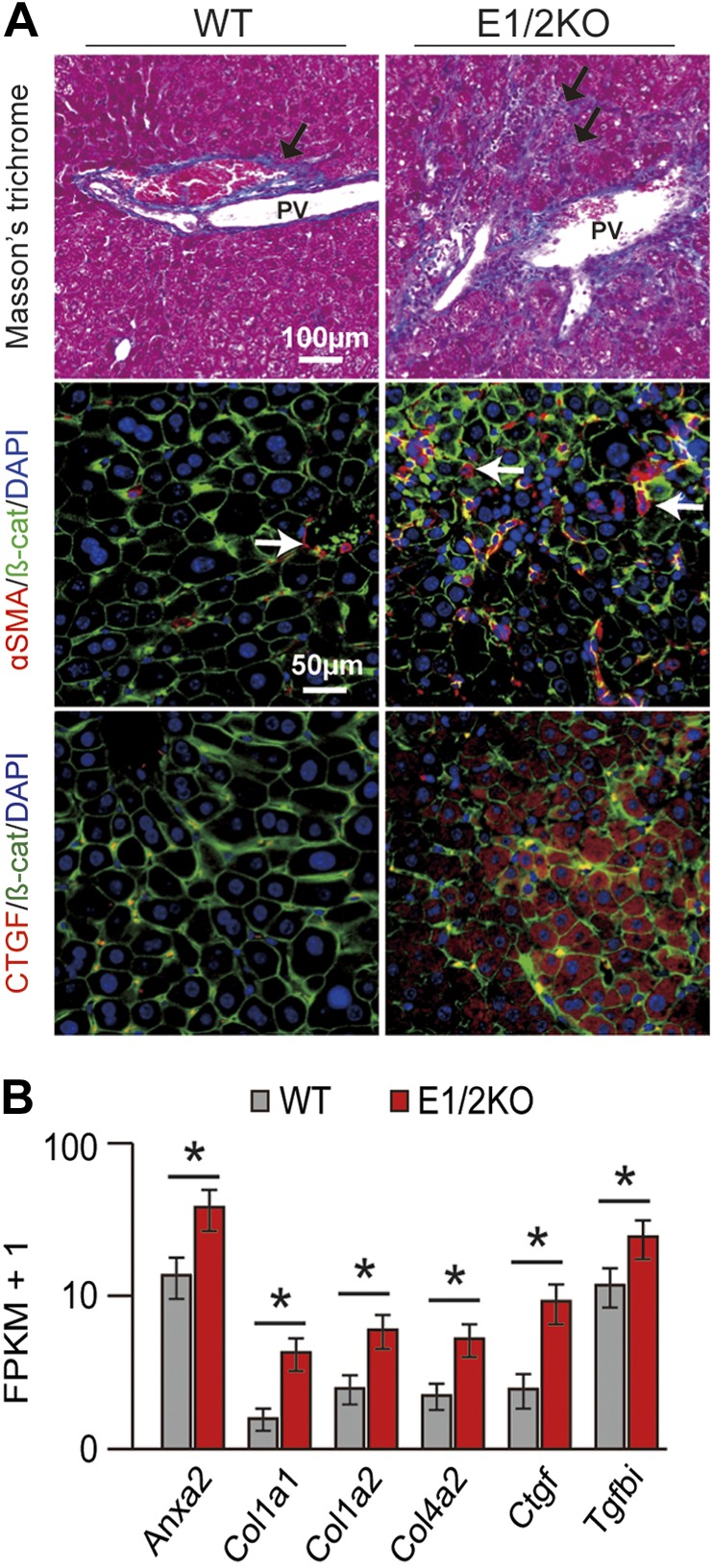
E1/2KO mice developed liver fibrosis by 8 mo of age. A) Top panels show Masson’s trichrome staining on paraffin-embedded liver sections revealing increased density of collagen fibers (arrows) in portal and periportal areas in E1/2KO livers. PV, portal vein. Middle and bottom panels show representative double-immunofluorescence staining for stellate cell marker, αSMA (red) and fibrogenic growth factor, CTGF (red) with β-catenin (β-cat; green). Nuclei were counterstained with DAPI. B) Up-regulation of fibrosis-related genes in E1/2KO livers as determined by RNA-seq. Cuffdiff (14) was used to detect significantly up-regulated genes with an FDR-adjusted P value of 0.05 (asterisk).
CCl4 triggered hepatocyte death, decreased liver regeneration, and caused activation of HPCs in E1/2KO livers
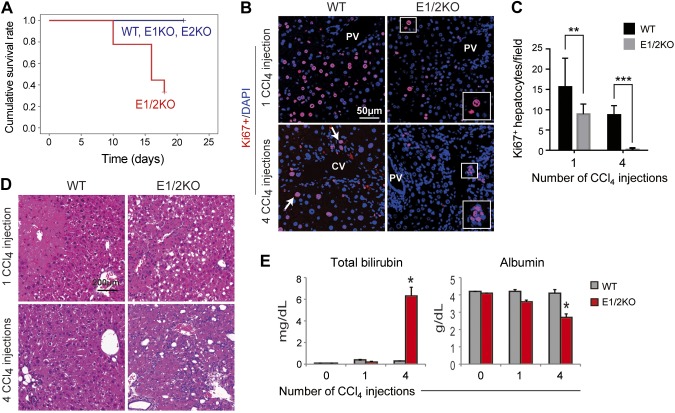
To assess the role of EZH1 and EZH2 under a stress condition, we subjected 3-mo-old EZH1, EZH2, and E1/2KO mice and WT controls to chronic (twice a week) treatment with CCl4 and compared the extent of liver injury (n = 9 mice per each genotype per time point). Remarkably, all (9 out of 9) E1/2KO mice died within 3 wk of treatment after receiving 4 injections of CCl4 (2 ml/kg, 2 times per week) (Fig. 4A). No deaths were observed in the similarly treated WT, E1KO, and E2KO littermates.
Figure 4.
The loss of EZH1 and EZH2 impaired functional performance, reduced survival, and decreased hepatocyte proliferation in E1/2KO mice during chronic liver injury induced by CCl4 injections twice a week. A) Kaplan-Meier survival curves after fourth injection of CCl4 to 3-mo-old mice show reduced survival in E1/2KO mice (n = 9 for each genotype). B) Representative immunofluorescence staining for cell proliferation marker Ki67 (red) of hepatocytes (arrows). Nuclei were counterstained with DAPI. Insets show higher magnifications of the boxed areas. Note that in E1/2KO livers, only nonparenchymal cells proliferate after the fourth CCl4 injection. PV, portal vein. C) Quantification of Ki67-positive hepatocytes. The number of Ki67-positive cells was determined in 5 independent confocal images taken at ×200 magnification and expressed as numbers per field. Data are the mean ± sd (n = 3 mice for each genotype). **P < 0.01; ***P < 0.0001. D) H&E staining of WT and E1/2KO livers. E) Serum levels of total bilirubin and albumin 2 d after the first and fourth injection of CCl4 to 3-mo-old mice. Data are the mean ± sd (n = 5 mice per each genotype per time point).
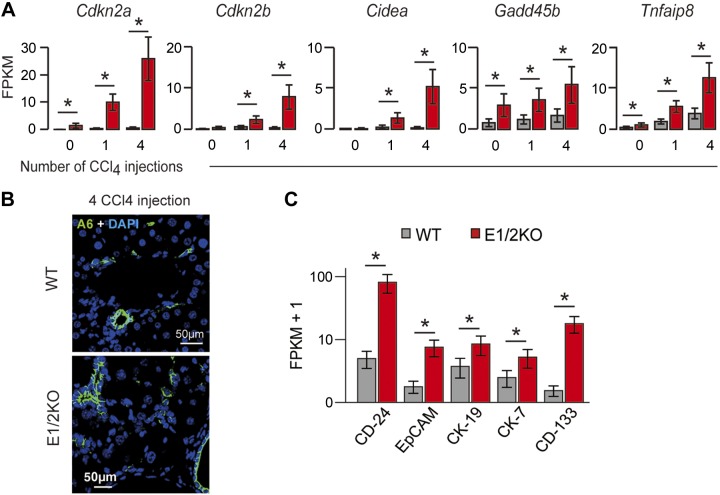
An analysis of compensatory regeneration using the proliferative marker Ki67 corroborated these findings. The quantitative estimates of Ki67 staining revealed that hepatocytes lacking both Ezh1 and Ezh2 were unable to elicit an adequate proliferative response to CCl4-mediated tissue injury (Fig. 4B). The rate of hepatocyte proliferation in E1/2KO livers was significantly reduced already after a single injection of CCl4 and severely blunted after 4 injections (Fig. 4C). In addition, mutant livers showed a greater cellularity in the periportal areas suggestive of increased inflammatory reaction, and more severe hydropic degeneration developed after a single injection of CCl4 (Fig. 4D). E1/2KO mice also displayed a sharp elevation in serum bilirubin, and a decrease in albumin content as compared to the CCl4-treated WT controls as a reflection of the reduced functional performance of mutant hepatocytes (Fig. 4E). Notably, these observations were supported by RNA-seq experiments used to identify gene expression changes after 1 and 4 injections of CCl4 (n = 3 mice per group per time point). Based on the online tool “Database for Annotation, Visualization and Integrated Discovery” version 6.7, the activated genes were involved in cell death and cell cycle regulation. The expression of cyclin-dependent kinase inhibitor 2A (Cdkn2a), cyclin-dependent kinase inhibitor 2B (Cdkn2b), cell death-inducing DNA fragmentation factor α subunit-like effector A (Cidea), TNF-α-induced protein 8 (Tnfaip8), and growth arrest and DNA-damage-inducible 45 β (Gadd45b) was significantly elevated in E1/2KO livers (Fig. 5A).
Figure 5.
Liver damage and activation of HPCs during CCl4-induced hepatotoxicity in E1/2KO mice. A) Up-regulation of cell cycle inhibitors and cell death-related genes in E1/2KO livers as determined by RNA-seq after CCl4 injection. Cuffdiff (14) was used to detect significantly up-regulated genes with an FDR-adjusted P value of 0.05 (asterisk). B) Immunofluorescence staining for A6, a biliary/progenitor cell marker. Representative confocal images show the accumulation of A6-positive cells in portal and periportal areas of E1/2KO livers. Nuclei were counterstained with DAPI. C) Up-regulation of HPC-related genes in E1/2KO livers after the fourth injection of CCl4 as determined by RNA-seq. Cuffdiff (14) was used to detect significantly up-regulated genes with an FDR-adjusted P value of 0.05 (asterisk).
In response to CCl4 treatment, E1/2KO livers also displayed an increase of A6-positive bile ductules and small A6-positive progenitor-like cells located in the periportal areas, which is consistent with the notion that HPCs are activated when mature hepatocytes are damaged and unable to proliferate (20). Histologically, A6-positive cells increased in number and expanded into parenchyma after 4 CCl4 injections, whereas A6-positive immunofluorescence was limited to the periportal regions in WT livers (Fig. 5B). RNA-seq analyses also revealed that the expression levels of several HPC-related genes, including CK-19 (Krt19), Epcam, CD-133 (Prom1), CD-24 (Cd24a), and CK-7 (Krt7), were significantly increased in E1/2KO livers following 4 injections of CCl4 in E1/2KO (Fig. 5C).
Failure of efficient hepatocyte regeneration after PHx
To support the results obtained after CCl4 injection, two-thirds PHx were performed on WT and E1/2KO mice at 3 mo of age (n = 5 mice for each genotype). Consistently, E1/2KO mice showed a sharp increase in serum ALT, AST, and total bilirubin and a significant decrease in serum albumin 48 h after PHx (Fig. 6A). Furthermore, E1/2KO livers showed a significant decline in the number of Ki67-positive cells as compared to WT (Fig. 6B) as well as a reduced recovery of liver mass calculated as a percentage of body mass (Fig. 6C). Histologic examination also revealed less frequent mitosis and pronounced lipid accumulation in E1/2KO livers (Fig. 6D). The results of CCl4 and PH experiments demonstrate that deletion of Ezh1 and Ezh2 in hepatocytes predisposes liver to damage and impairs its regenerative potential.
Figure 6.
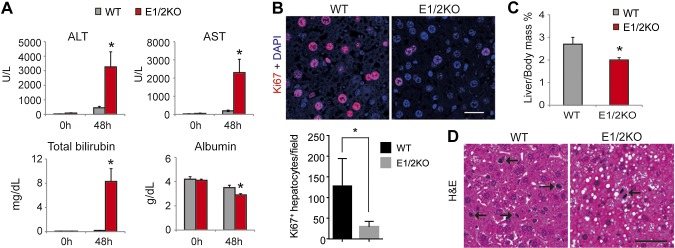
The genetic loss of EZH1 and EZH2 increases hepatocyte damage and reduces hepatocyte proliferation during compensatory liver regeneration induced by two-thirds PHx. A) Serum levels of ALT, AST, and total bilirubin are increased and levels of albumin decreased in E1/2KO mice at 48 h after PHx. B) Top panels show representative immunofluorescence staining for cell proliferation marker Ki67 (red). Nuclei were counterstained with DAPI. Bottom panels show quantification of Ki67-positive hepatocytes. The number of Ki67-positive cells was determined in 5 independent confocal images taken at ×200 magnification and expressed as numbers per field. Data are the mean ± sd (n = 5 mice for each genotype). *P < 0.05 as compared to the age-matched WT. C) Reduced recovery of liver mass in E1/2KO mice calculated as percentage of body mass. D) Representative H&E staining. Arrows indicate dividing hepatocytes. Scale bars in (B) and (D) are 50 and 100 μm, respectively.
Gene-specific reduction of H3K27me3 is associated with increased transcription
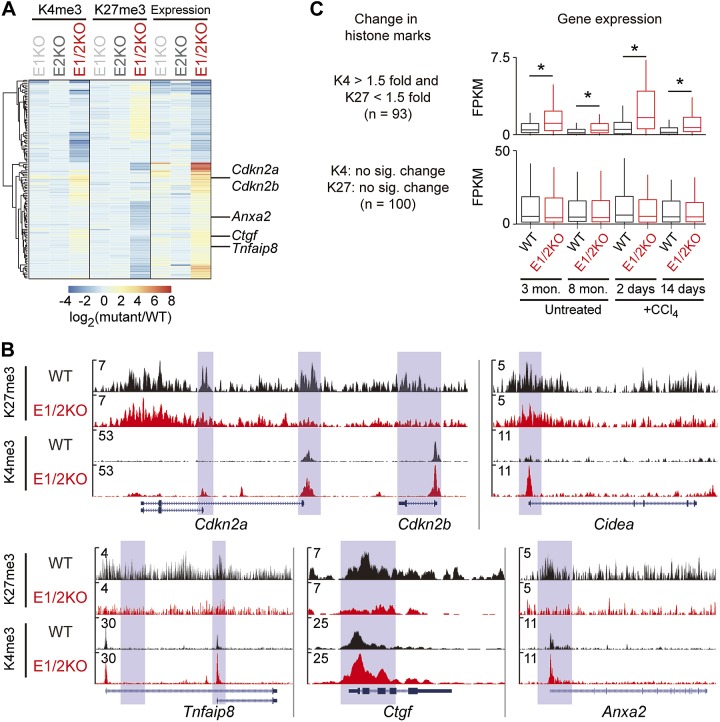
ChIP-seq was used to establish global and gene-specific H3K27me3 marks in liver tissue from 3-mo-old WT, E1KO, E2KO, and E1/2KO mice (Fig. 7A). Consistent with the results of H3K27me3 immunofluorescence staining (Fig. 1B), H3K27me3 marks were reduced only in E1/2KO samples (Fig. 7A and Supplemental Table 3). A total of 1956 genes showed at least a 1.5-fold reduction of H3K27me3 in their promoter regions, and 51 genes showed a clear correlation between H3K27me3 marks and gene expression (Supplemental Table 3). In particular, H3K27me3 marks were decreased in genes linked to fibrosis (Ctgf, Tgfbi, and Anxa2) and the locus encoding the cell cycle regulatory genes Cdkn2a and Cdkn2b (Fig. 7B). Correspondingly, H3K4me3 marks in the same genes were elevated (Fig. 7B), with the increased expression observed already at 3 mo of age (Fig. 7A). Of note, there were no significant changes of H3K4me3 marks between whole-liver tissues and primary hepatocytes in WT and E1/2KO mice (Supplemental Fig. 2).
Figure 7.
Genes displaying altered histone marks due to the combined loss of EZH1 and EZH2 are highly susceptible to epigenetic dysregulation. A) Heatmap shows changes in H3K4me3, H3K27me3, and gene expression levels in tissues with different genotypes (3-mo-old mice). Genes showing a 2-fold change of H3K4me3 or 1.5-fold change of H3K27me3 are shown (n = 124). B) Enrichment of H3K27me3 and H3K4me3 on 5 loci is shown through the UCSC Genome Browser. The light-blue-shaded boxes represent the promoter of genes. C) Expression levels of deregulated histone and nonaltered gene sets in the context of aging (3- and 8-mo-old mice) and toxic (CCl4) administration were determined by RNA-seq. no sig. change, no significant change. *P < 0.01, Mann-Whitney U test.
Based on these results, it can be hypothesized that altered histone modifications might change the susceptibility of specific genes to environmental stimuli, such as age and exposure to toxins. To test this hypothesis, expression levels of genes displaying reduced H3K27me3 were compared to those without significant changes in histone modifications (Fig. 7C). RNA-seq analyses were performed using 3- and 8-mo-old mice and mice treated with 1 and 4 injections of CCl4. Neither age nor CCl4 exposure affected the expression of genes with an unaltered H3K27me3 pattern, whereas those displaying altered histone modifications were significantly up-regulated in response to aging and CCl4 treatment. This result suggests that the status of H3K27me3 and H3K4me3 is a critical molecular determinant for genes responding to environmental stimuli and that EZH1 and EZH2 are essential for this process.
DISCUSSION
The requirement of PRC2 in liver homeostasis and regeneration was investigated in double-knockout mice generated by crossing Ezh1-null and Ezh2 conditional-null mice. Only the combined loss of EZH1 and EZH2 in mouse hepatocytes caused a global depletion of H3K27me3 marks in genes associated with hepatocyte homeostasis and regeneration. E1/2KO mice developed progressive liver abnormalities during postnatal ontogenesis such as fibrosis, regenerative nodules, and activation of HPCs. E1/2KO livers also displayed increased hepatic degeneration and reduced ability to proliferate in response to toxic treatment with CCl4 or standard PH.
EZH1 partially complements for EZH2 loss in embryonic and skin stem cell (5, 21). Our studies show that a similar mechanism may likely play a role in mouse hepatocytes. Because H3K27me3 is quantitatively abolished only upon the combined loss of both EZH1 and EZH2, it is clear that these 2 proteins possess compensatory methyltransferase activities in hepatocytes. However, up-regulation of Ezh2 has been reported in HCC, and down-regulation of Ezh2 reduced H3K27me3 in HCC cells (7). In vitro knockdown of Ezh2 in human HCC cell lines reduced H3K27me3, but as shown here, in vivo E2KO in hepatocyte in genetically engineered mice did not show significant reduction of H3K27me3 expression in hepatocytes. The possibility that EZH2 contributes differently to the establishment of H3K27me3 marks in tissue culture cells in vitro and in liver tissue in vivo needs to be studied separately. The loss of Ezh1 and Ezh2 in mouse skin results in a change in fate determination in epidermal progenitor cells by repressing the transcription factor Sox2 (22). However, Sox2 expression was not increased in E1/2KO livers even though this gene was targeted by H3K27me3. E1/2KO livers displayed the expansion of A6-positive cells and increased expression of HPC-related genes. Compared to previous reports (23–25), the E1/2KO model showed a late-onset expansion of HPCs (around 8 mo of age). Several HPC-related genes were up-regulated in E1/2KO livers after developing severe liver injury, and these were not related to the H3K27me3 marks’ changes. These findings suggest that the activation of HPCs in E1/2KO livers may be caused by liver damage mediated by the loss of Ezh1 and Ezh2 function, rather than directly controlled by H3K27me3.
Among the most striking pathology of E1/2KO mice was the progressive loss of regenerative capacity of mutant hepatocytes and the reduced life span as compared to WT littermates. Most of the male E1/2KO mice had died by 12.6 mo, and they also showed high lethality after CCl4 exposure. The likely explanation of the reduced survival may be the inability to maintain an adequate level of hepatocyte proliferation. Of note, the consistent activation of HPCs found in aging and CCl4-treated double-knockout livers was not sufficient to support the postnatal and compensatory liver regeneration, suggesting that the loss of both EZH1 and EZH2 may have direct or indirect effects on HPC proliferation and/or differentiation.
The loss of regenerative potential of E1/2KO livers was associated with the decrease of H3K27me3 marks and increase of H3K4me3 marks in Cdkn2a and Cdkn2b genes. Cdkn2a and Cdkn2b genes encode p16 and p15 inhibitors of the G1/S phase of the cell cycle (26), and derepression of Cdkn2a has been suggested to induce cell death through downstream targets of p53, including p21 and Wig1 (27). In E1/2KO hepatocytes, expression of Cdkn2a and Cdkn2b genes was up-regulated. CCl4 experiments and PHx also revealed the decrease of regenerative function in E1/2KO mice. Taken together, these findings demonstrated that the loss of Ezh1 and Ezh2 was sufficient for the transcriptional activation of Cdkn2a and Cdkn2b genes. Notably, these PRC2 targets are also up-regulated in Ezh1/2-null hair follicles (5). However, the loss of Bmi-1 in neural stem cells did not alter H3K27me3 but caused activation of Cdkn4a genes (28). It remains to be determined whether EZH1 and EZH2 deregulation results in aberrant expression of these PRC2 target genes in other tissues.
In conclusion, this study demonstrates essential roles of the histone methyltransferases EZH1 and EZH2 in liver homeostasis and regeneration. We demonstrate for the first time that the combined loss of EZH1 and EZH2 in mouse hepatocytes and the concomitant disruption of H3K27me3 homeostasis promote liver fibrosis, decrease regenerative potential, and reduce overall survival both under physiologic conditions and stress.
Supplementary Material
Acknowledgments
The authors thank Harold Smith [U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases’ (NIDDK) genomics core] for conducting next-generation sequencing. Alexander Tarakhovsky (Rockefeller University, New York, NY, USA) provided Ezh2 floxed mice. Ezh1 mutant mice were generated at the Research Institute of Molecular Pathology (Vienna, Austria) by Donal O’Carroll (laboratory of Thomas Jenuwein) with the help of Maria Sibilia (Laboratory of Erwin Wagner). The research was supported by the Intramural Research Program of the NIH NIDDK. The authors declare no conflicts of interest.
Glossary
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- Anxa2
annexin A2
- AST
aspartate aminotransferase
- CCl4
carbon tetrachloride
- CD-24
Cd24a cd24a antigen
- Cdkn2a
cyclin-dependent kinase inhibitor 2A
- Cdkn2b
cyclin-dependent kinase inhibitor 2B
- ChIP
chromatin immunoprecipitation
- ChIP-seq
chromatin immunoprecipitation with parallel sequencing
- Cidea
cell death-inducing DNA fragmentation factor α subunit-like effector A
- Col1a1
collagen type I α1
- Col1a2
collagen type I α2
- Col4a2
collagen type IV α2
- CTGF
connective tissue growth factor
- E1/2KO
Ezh1 and Ezh2 knockout
- E1KO
Ezh1 knockout
- E2KO
Ezh2 knockout
- EpCAM
epithelial cell adhesion molecule
- EZH
enhancer of zeste homolog
- FDR
false discovery rate
- FPKM
fragment per kilobase of exon per million fragments mapped
- Gadd45b
growth arrest and DNA-damage-inducible 45 β
- GEO
gene expression omnibus
- H3K27me3
trimethylation on Lys 27 of histone H3
- HCC
hepatocellular carcinoma
- H&E
hematoxylin and eosin
- HOMER
Hypergeometric Optimization of Motif EnRichment
- HPC
hepatic progenitor cell
- Krt
keratin
- PcG
Polycomb group
- PHx
partial hepatectomy
- PRC
Polycomb-repressive complex
- Prom1
prominin 1
- RNA-seq
RNA sequencing
- Tgfbi
TGF-β induced
- Tnfaip8
TNF-α–induced protein 8
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Sauvageau M., Sauvageau G. (2010) Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell 7, 299–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ringrose L., Paro R. (2004) Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu. Rev. Genet. 38, 413–443 [DOI] [PubMed] [Google Scholar]
- 3.Margueron R., Reinberg D. (2011) The Polycomb complex PRC2 and its mark in life. Nature 469, 343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margueron R., Li G., Sarma K., Blais A., Zavadil J., Woodcock C. L., Dynlacht B. D., Reinberg D. (2008) Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol. Cell 32, 503–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezhkova E., Lien W. H., Stokes N., Pasolli H. A., Silva J. M., Fuchs E. (2011) EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 25, 485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ntziachristos P., Tsirigos A., Van Vlierberghe P., Nedjic J., Trimarchi T., Flaherty M. S., Ferres-Marco D., da Ros V., Tang Z., Siegle J., Asp P., Hadler M., Rigo I., De Keersmaecker K., Patel J., Huynh T., Utro F., Poglio S., Samon J. B., Paietta E., Racevskis J., Rowe J. M., Rabadan R., Levine R. L., Brown S., Pflumio F., Dominguez M., Ferrando A., Aifantis I. (2012) Genetic inactivation of the polycomb repressive complex 2 in T cell acute lymphoblastic leukemia. Nat. Med. 18, 298–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Au S. L., Wong C. C., Lee J. M., Fan D. N., Tsang F. H., Ng I. O., Wong C. M. (2012) Enhancer of zeste homolog 2 epigenetically silences multiple tumor suppressor microRNAs to promote liver cancer metastasis. Hepatology 56, 622–631 [DOI] [PubMed] [Google Scholar]
- 8.Xu K., Wu Z. J., Groner A. C., He H. H., Cai C., Lis R. T., Wu X., Stack E. C., Loda M., Liu T., Xu H., Cato L., Thornton J. E., Gregory R. I., Morrissey C., Vessella R. L., Montironi R., Magi-Galluzzi C., Kantoff P. W., Balk S. P., Liu X. S., Brown M. (2012) EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science 338, 1465–1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J. M., Lee J. S., Kim H., Kim K., Park H., Kim J. Y., Lee S. H., Kim I. S., Kim J., Lee M., Chung C. H., Seo S. B., Yoon J. B., Ko E., Noh D. Y., Kim K. I., Kim K. K., Baek S. H. (2012) EZH2 generates a methyl degron that is recognized by the DCAF1/DDB1/CUL4 E3 ubiquitin ligase complex. Mol. Cell 48, 572–586 [DOI] [PubMed] [Google Scholar]
- 10.Mousavi K., Zare H., Wang A. H., Sartorelli V. (2012) Polycomb protein Ezh1 promotes RNA polymerase II elongation. Mol. Cell 45, 255–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su I. H., Basavaraj A., Krutchinsky A. N., Hobert O., Ullrich A., Chait B. T., Tarakhovsky A. (2003) Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat. Immunol. 4, 124–131 [DOI] [PubMed] [Google Scholar]
- 12.Factor V. M., Radaeva S. A., Thorgeirsson S. S. (1994) Origin and fate of oval cells in dipin-induced hepatocarcinogenesis in the mouse. Am. J. Pathol. 145, 409–422 [PMC free article] [PubMed] [Google Scholar]
- 13.Langmead B., Trapnell C., Pop M., Salzberg S. L. (2009) Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trapnell C., Roberts A., Goff L., Pertea G., Kim D., Kelley D. R., Pimentel H., Salzberg S. L., Rinn J. L., Pachter L. (2012) Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi N., Ito M., Nakamura J., Cai J., Gao C., Hammel J. M., Fox I. J. (2000) Hepatocyte transplantation in rats with decompensated cirrhosis. Hepatology 31, 851–857 [DOI] [PubMed] [Google Scholar]
- 16.Zang C., Schones D. E., Zeng C., Cui K., Zhao K., Peng W. (2009) A clustering approach for identification of enriched domains from histone modification ChIP-Seq data. Bioinformatics 25, 1952–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinz S., Benner C., Spann N., Bertolino E., Lin Y. C., Laslo P., Cheng J. X., Murre C., Singh H., Glass C. K. (2010) Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorvaldsdóttir H., Robinson J. T., Mesirov J. P. (2013) Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yakar S., Liu J. L., Stannard B., Butler A., Accili D., Sauer B., LeRoith D. (1999) Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc. Natl. Acad. Sci. USA 96, 7324–7329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams M. J., Clouston A. D., Forbes S. J. (2014) Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology 146, 349–356 [DOI] [PubMed] [Google Scholar]
- 21.Shen X., Liu Y., Hsu Y. J., Fujiwara Y., Kim J., Mao X., Yuan G. C., Orkin S. H. (2008) EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol. Cell 32, 491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardot E. S., Valdes V. J., Zhang J., Perdigoto C. N., Nicolis S., Hearn S. A., Silva J. M., Ezhkova E. (2013) Polycomb subunits Ezh1 and Ezh2 regulate the Merkel cell differentiation program in skin stem cells. EMBO J. 32, 1990–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakubowski A., Ambrose C., Parr M., Lincecum J. M., Wang M. Z., Zheng T. S., Browning B., Michaelson J. S., Baetscher M., Wang B., Bissell D. M., Burkly L. C. (2005) TWEAK induces liver progenitor cell proliferation. J. Clin. Invest. 115, 2330–2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benhamouche S., Curto M., Saotome I., Gladden A. B., Liu C. H., Giovannini M., McClatchey A. I. (2010) Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 24, 1718–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin T., Ibrahim W., Peng C. Y., Finegold M. J., Tsai R. Y. (2013) A novel role of nucleostemin in maintaining the genome integrity of dividing hepatocytes during mouse liver development and regeneration. Hepatology 58, 2176–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherr C. J., Roberts J. M. (2004) Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 18, 2699–2711 [DOI] [PubMed] [Google Scholar]
- 27.Park I. K., Qian D., Kiel M., Becker M. W., Pihalja M., Weissman I. L., Morrison S. J., Clarke M. F. (2003) Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature 423, 302–305 [DOI] [PubMed] [Google Scholar]
- 28.Molofsky A. V., Pardal R., Iwashita T., Park I. K., Clarke M. F., Morrison S. J. (2003) Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425, 962–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.