Abstract
Purpose
Mutations in 60 known genes were previously identified by exome sequencing in 79 of 157 families with retinitis pigmentosa (RP). This study analyzed variants in 129 genes associated with other forms of hereditary retinal dystrophy in the same cohort.
Methods
Apart from the 73 genes previously analyzed, a further 129 genes responsible for other forms of hereditary retinal dystrophy were selected based on RetNet. Variants in the 129 genes determined by whole exome sequencing were selected and filtered by bioinformatics analysis. Candidate variants were confirmed by Sanger sequencing and validated by analysis of available family members and controls.
Results
A total of 90 candidate variants were present in the 129 genes. Sanger sequencing confirmed 83 of the 90 variants. Analysis of family members and controls excluded 76 of these 83 variants. The remaining seven variants were considered to be potential pathogenic mutations; these were c.899A>G, c.1814C>G, and c.2107C>T in BBS2; c.1073C>T and c.1669C>T in INPP5E; and c.3582C>G and c.5704–5C>G in CACNA1F. Six of these seven mutations were novel. The mutations were detected in five unrelated patients without a family history, including three patients with homozygous or compound heterozygous mutations in BBS2 and INPP5E, and two patients with hemizygous mutations in CACNA1F. None of the patients had mutations in the genes associated with autosome dominant retinal dystrophy.
Conclusions
Only a small portion of patients with RP, about 3% (5/157), had causative mutations in the 129 genes associated with other forms of hereditary retinal dystrophy.
Introduction
Retinitis pigmentosa (RP, OMIM 268000) is the most common and highly heterogeneous genetic group of hereditary retinal degeneration diseases, affecting one in about 3,500–5,000 individuals worldwide [1-3]. So far, mutations in over 60 genes have been reported to be responsible for about half of nonsyndromic RP [4-6]. Phenotypic and molecular genetic overlap has been observed in different forms of retinal degeneration [7,8]; for example, RP might be the main sign of syndromic RP or other related diseases [9-11]. Mutations in a few genes have been shown to cause different forms of retinal dystrophy [7], while a few genes originally held responsible for other forms of retinal dystrophy have been found to cause RP as well [12]. Systematic analysis of all genes responsible for other forms of retinal dystrophy in patients with RP is limited, especially in Chinese cohorts [13,14]. Therefore, systemic evaluation of the frequency of mutations in all genes responsible for other forms of retinal dystrophy, apart from known RP genes, would be valuable.
Our previous whole exome sequencing study detected potential pathogenic mutations in 73 known genes in 86 of 157 patients with RP [15-17]. Due to the highly heterogeneous and genetically and clinically complicated features of RP, mutations in the genes related to more severe or syndromic diseases might be ignored, and mutations in previously analyzed genes might be mistakenly used in molecular diagnosis and clinical evaluation. Therefore, it might be interested to know if mutations in genes associated with other forms of retinal dystrophy may also contribute to RP. In the present study, variants in 129 genes responsible for other forms of retinal dystrophy were analyzed based on the exome data set of the same cohort of 157 patients.
Methods
This study conformed to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of the Zhongshan Ophthalmic Center. Written informed consent was obtained from the participants or their guardians before the collection of their clinical data and preparation of genomic DNA from their peripheral venous leukocytes. The cohort of 157 probands with RP, including 34 autosomal dominant RP, 27 autosomal recessive RP, 10 X-linked RP, and 86 sporadic RP, were the same as described in our previous study [15]. All 157 probands were initially diagnosed as non-syndromic RP. The criteria for defining RP in the families was based on the oral descriptions of the features by the probands and their family members, such as poor vision and night blindness, or the clinical examination of the other RP patients; the clinical features of RP patients were similar to those of the probands and included attenuated retinal artery and pigment abnormality in the mid-peripheral retina. Whole exome sequencing was performed by the NimbleGen SeqCap EZ Exome (44M) array and Illumina Genome Analyzer II platform with a sequencing depth of 60-fold, as described previously [15,18,19].
A total of 221 genes were listed in RetNet, accessed on January 27, 2014. Of these, 60 of the 62 genes with known association with RP had been analyzed in our previous study; the remaining two were not analyzed because EMC1 was not captured and FSCN2 (MIM: 607643) was confirmed not to be an RP gene [20]. Of the remaining 159 genes, the following 30 were not included in this study: 1) nine genes associated with age-related macular degeneration, since no evidence shows that these genes were associated with monogenetic retinal diseases; 2) seven genes encoded by mitochondrial DNA that were not captured by the NimbleGen SeqCap EZ Exome (44M) array; and 3) CHM (MIM: 300390), CYP4V2 (MIM: 608614), and 12 genes associated with Leber congenital amaurosis (LCA) that were independently analyzed in our previous studies [16,17,21]. Ultimately, 129 genes were included in this study. All genes screened in the earlier studies and in the current study are listed in Appendix 1.
Variants in the 129 genes were selected based on the data set from whole exome sequencing. Candidate variants were filtered out with the following multiple-step bioinformatics analysis: 1) the SNPs and short indels in the exome region were filtered against data from dbSNP132, the 1000 Genome Project, HapMap, the YH project, and the Exome Variant Server, removing minor allele frequency (MAF) values that were greater than 0.01, the frequency based on the incidence of RP (1/4000), and the fraction of heterozygotes in Hardy–Weinberg equilibrium; 2) excluded non-coding variants without altering splicing sites predicted by BDGP; 3) excluded the synonymous variants without altering splicing sites in the genes; 4) excluded missense variants predicted to be benign by both Polyphen-2 and SIFT in autosomal dominant and X-linked genes; and 5) compared with the whole exome sequencing data of 633 individuals in ethnic-matched regions without retinal degeneration.
Sanger sequencing was used to confirm the candidate variants with primer pairs targeting the fragments encompassing the candidate variants. Primers (Appendix 2) were designed using Primer3 [22]. The procedures used for amplification, sequencing, and analysis of the target fragments were as previously described [18,19]. Segregation analysis of potential pathogenic variants were performed in available family members. Candidate causative mutations were validated in 192 normal Chinese individuals.
Results
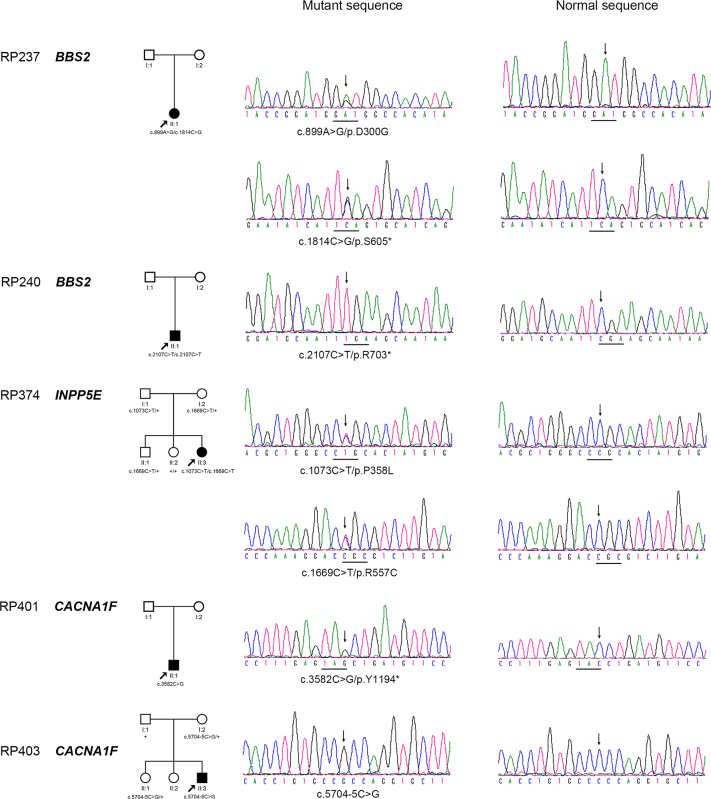
Multiple-step bioinformatics analysis was used to select 90 candidate variants from the 129 genes from the whole exome sequencing of the 157 patients. Of the 90 variants, 83 (92%) were confirmed by Sanger sequencing, while the other 7 (8%) with the low sequencing depth were false-positives. Of the 83, 76 (Appendix 3) were considered as unlikely causes of RP for the following reasons: 1) mutations that are more likely to be pathogenic have already been detected in one of the 72 genes related to RP and LCA or CHM in our previous studies [15-17]; 2) the genotype did not match the patterns of inheritance; 3) the variants did not segregate with the disease; 4) the variants were found in controls or in the 1000 Genome/Exome Variant Server database with a frequency higher than the prevalence rate of RP as causative mutations; 5) the variants were unlikely or less likely to be pathogenic mutations based on previous reports; 6) the phenotype did not match the genotype; and 7) the other allele for compound heterozygous variants if one of them was confirmed to be a false-positive. The remaining seven variants involved three genes: BBS2 (MIM: 606151), INPP5E (MIM: 613037), and CACNA1F (MIM: 300110); these were considered to be potentially causative in five patients (Figure 1 and Table 1), including three patients with homozygous (1) or compound heterozygous (2) mutations in genes associated with an autosomal recessive trait, and two patients with hemizygous mutation in genes associated with an X-linked trait. Limited cosegregation analysis and family structure did not reject the association of the mutations with RP (Figure 1). The carriers with the heterozygous mutant alleles were evaluated as asymptomatic for the disease through follow-up examinations and revisits by phone calls. All 7 mutations were absent in the 192 normal individuals (Table 1).
Figure 1.
Pedigrees of the five families with mutations and sequence chromatography. The family members and their corresponding mutations are shown just above the pedigrees (+: wild-type allele). Sequence changes that were detected in the patients are shown (left column) and compared with corresponding normal sequences (right column).
Table 1. The seven potential pathogenic variants from genes associated with other forms of retinal dystrophy in the five of 157 patients with retinitis pigmentosa.
| Gene | Inheri-tance | Patient ID | Chromo-some | Start position | Nucleotide change | Amino acid change | State |
Computational prediction |
Allele frequency in |
Reported | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P/SS | SIFT | Phast Cons | GERP | 1000 G # | Patients | NC | |||||||||
|
BBS2 |
AR |
RP237 |
chr16 |
56536626 |
c.899A>G |
p.D300G |
Het |
PoD |
D |
0.992 |
5.920 |
1/2184 |
1/314 |
0/384 |
Novel |
| |
|
|
chr16 |
56530975 |
c.1814C>G |
p.S605* |
Het |
- |
- |
1.000 |
5.120 |
1/2184 |
1/314 |
0/384 |
Novel |
| |
|
RP240 |
chr16 |
56518732 |
c.2107C>T |
p.R703* |
Homo |
- |
- |
0.992 |
5.300 |
NA |
1/314 |
0/384 |
Deveault et al. 2011 |
|
INPP5E |
AR |
RP374 |
chr9 |
139327693 |
c.1073C>T |
p.P358L |
Het |
PrD |
D |
0.955 |
4.870 |
NA |
1/314 |
0/384 |
Novel |
| |
|
|
chr9 |
139324862 |
c.1669C>T |
p.R557C |
Het |
PrD |
D |
0.999 |
5.010 |
NA |
1/314 |
0/384 |
Novel |
|
CACNA1F |
XL |
RP401 |
chrX |
49071594 |
c.3582C>G |
p.Y1194* |
Hemi |
- |
- |
0.999 |
1.720 |
NA |
1/205† |
0/274‡ |
Novel |
| RP403 | chrX | 49061832 | c.5704-5C>G | Splicing defect | Hemi | SSA | - | 0.480 | 3.150 | NA | 1/205† | 0/274‡ | Novel | ||
Note: #, These variants were absent from the Exome Variant Server database and were evaluated in 2184 alleles of autosome from 1000 Genome database. †, In the 157 patients, 109 are male and 48 are female, and there are 205 alleles for genes on the X chromosome. ‡, In the 327 controls, 175 are male and 152 are female, and there are 479 alleles for genes on the X chromosome. The following abbreviations are used: AR, autosomal recessive; AD, autosomal dominate; XL, X-linked; P/SS, Polyphen-2/Splice Site Prediction; 1000 G, 1000 Genome; NC, normal control; Het, heterozygous; Hom, homozygous; Hemi, hemizygous; PrD, probably damaging; PoD, possibly damaging; D, damaging; SSA, splicing site abolished;, not applicable.
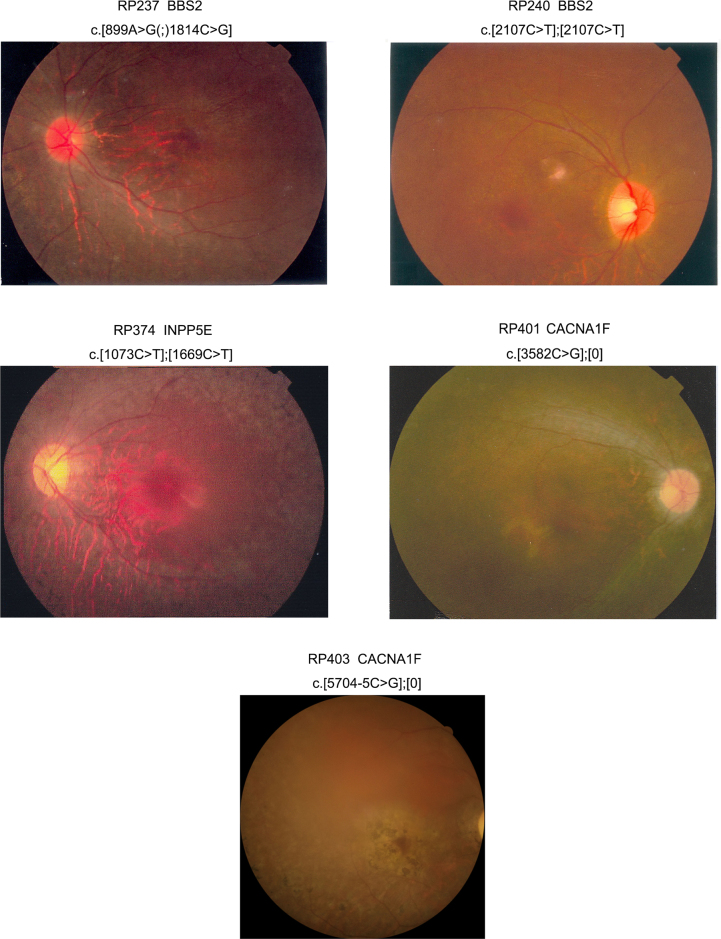
Clinical data of the five patients with RP are summarized in Table 2. Ocular signs and symptoms in all five patients met the diagnosis of RP (Figure 2). Two patients (RP237 and RP240) had BBS2 mutations; one had RP alone with a missense mutation and a nonsense mutation, while the other one had BBS syndrome (misdiagnosed as RP) and a homozygous nonsense mutation. The patient (RP374) with INPP5E mutations had typical RP but without noticeable extraocular or systemic anomalies (unfortunately, a brain CT scan was not available). The two patients (RP401 and RP403) with CACNA1F truncation mutations showed generalized retinal degeneration involving both rods and cones.
Table 2. Clinical features of the five patients with mutations identified in this study.
| Patient ID | Gene | Variations | Inheri- tance | Gender |
Age at |
First symptom |
Visual acuity |
Fundus examination |
Electroretinography responses |
Other systemic symptom | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| exam | onset | OD/OS | rod | cone | ||||||||
| RP237 |
BBS2 |
c.[899A>G(;)
1814C>G] |
Sporadic |
F |
14 |
EC |
PV; NB |
0.1/0.1 |
ARA; PBSL |
Extinguished |
Extinguished |
None |
| RP240 |
BBS2 |
c.[2107C>T];
[2107C>T] |
Sporadic |
M |
13 |
7 |
PV; NB |
0.1/0.2 |
ARA; SP |
Extinguished |
Extinguished |
Polydactyly; Obesity; Diabetes mellitus |
| RP374 |
INPP5E |
c.[1073C>T];
[1669C>T] |
Sporadic |
F |
20 |
EC |
PV; NB |
0.2/0.2 |
ARA; TLP; PBSL |
Extinguished |
Extinguished |
None |
| RP401 |
CACNA1F |
c.[3582C>G];[
0] |
Sporadic |
M |
9 |
NA |
PV |
0.2/0.08 |
PD |
Extinguished |
Extinguished |
None |
| RP403 | CACNA1F | c.[5704–5C>G];[0] | Sporadic | M | 32 | 22 | PV | 0.2/0.3 | ARA; PBSL | Severe reduced | Severe reduced | None |
Note: M, male; F, female; EC, early childhood; OD, right eye; OS, left eye; PV, poor vision; NB, night blindness; ARA, attenuated retinal arteries; PD, pigment deposit; PBSL, pigment bone spicule-like; TLR, tapetal-like retinal degeneration.
Figure 2.
Fundus photographs of five patients with the mutations identified in this study. The corresponding patient identification numbers and gene mutations are listed above each photo. Further clinical information about these patients is listed in Table 2.
Discussion
Seven putative pathogenic mutations in 3 of the 129 genes known to associate with other forms of retinal dystrophy have been detected in 5 of 157 (3%, Figure 3) patients with RP based on whole exome sequencing. These mutations are predicted to be causative based on bioinformatics analysis and comparison with previously reported mutations in BBS2, INPP5E, and CACNA1F. Ocular changes in the five patients with mutations are consistent with RP (Figure 2 and Table 2). These findings suggest that a small portion of RP may be caused by mutations in genes responsible for other forms of retinal dystrophy.
Figure 3.
Proportions of mutations in 129 genes associated with other forms of retinal dystrophy in 157 unrelated patients with retinitis pigmentosa.
Systematic analysis of genes responsible for other forms of retinal dystrophy in patients with RP has been limited. Previously, genotyping microarrays were used to analyze all known mutations in several genes associated with retinal dystrophy in patients with retinal dystrophy [23-25]. However, only a small subset of known mutations are covered by this technology, and the total diagnostic rate is less than 15% [26,27]. Systematic analysis of most of these genes in patients with RP or hereditary retinal dystrophies has been conducted using targeted-capture sequencing. The frequencies of detected mutations in these genes are from 4% to 11% [13,28,29]. Analysis of individual genes also revealed that mutations in a few genes known to associate with other forms of retinal dystrophy also cause a fraction of RP [12,30,31]. This suggests that the frequency of mutations in genes associated with other forms of retinal dystrophy in our cohort of RP is close to that reported previously.
Consistencies and inconsistencies were both evident in the associations between the phenotypes and the identified mutations in the five patients. The two patients with BBS2 mutations included one with nonsyndromic RP that has missense and nonsense mutations, while the other with Bardet-Biedl syndrome had a homozygous nonsense mutation. A missense and a nonsense mutation in BBS2 have previously been reported to be responsible for Bardet-Biedl syndrome [32,33], and two missense mutations in BBS2 have been described recently in patients with nonsyndromic RP [34]. Comparisons of these findings suggest that the phenotypes might be affected by the type of mutation. Further study is needed to clarify this interesting issue.
The patient (RP374) with INPP5E mutations had no other noticeable signs of systemic anomalies, although mutations in INPP5E have been identified in patients with MORM syndrome [35] and Joubert syndrome [36]. Unexpectedly, two patients (RP401 and RP403) with RP had hemizygous truncation mutations in CACNA1F; these kinds of mutations have been identified in patients with congenital stationary night blindness [37,38], cone-rod dystrophy [39], and Aland Island eye disease [40], but not in patients with RP. The association of CACNA1F mutations with RP in these two patients is uncertain, although the phenotype in the patients is consistent with RP, and mutations in CACNA1F are predicted to be null.
Overall, mutations in all known genes—including these 129 genes associated with other forms of retinal dystrophy, 72 genes related to RP and LCA, and CHM—could be identified in less than 58% of 157 patients with RP. In each of the disease categories, mutations were detected in 71% (24/34) autosomal dominant RP, 48% (13/27) autosomal recessive RP, 80% (8/10) X-linked RP, and 53% (46/86) sporadic RP. Over 42% of the patients without identified mutations might carry other pathogenic variants in the noncoding regions of the corresponding genes, or may have gross deletion involving whole exons that might be missed by whole exome sequencing and Sanger sequencing, or may be caused by mutations in novel genes yet to be identified. The information about these patients without identified mutations is a valuable resource for seeking out new genes that are responsible for RP.
Acknowledgments
The authors are grateful to all of the patients and controls for their participation in this study. This study was supported by the National Natural Science Foundation of China (U1201221), Guangdong Department of Science & Technology Translational Medicine Center grant 2011A080300002, and the Fundamental Research Funds of the State Key Laboratory of Ophthalmology.
Appendix 1. Genomic information of all the genes responsible for other forms of retinal dystrophy in the earlier studies and the current study.
To access these data, click or select the words "Appendix 1".
Appendix 2. Primers used in Sanger sequencing.
To access these data, click or select the words "Appendix 2".
Appendix 3. Other variants detected in 129 genes responsible for other forms of retinal dystrophy from the 157 unrelated patients with retinitis pigmentosa.
To access these data, click or select the words "Appendix 3".
References
- 1.Chizzolini M, Galan A, Milan E, Sebastiani A, Costagliola C, Parmeggiani F. Good epidemiologic practice in retinitis pigmentosa: from phenotyping to biobanking. Curr Genomics. 2011;12:260–6. doi: 10.2174/138920211795860071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boughman JA, Conneally PM, Nance WE. Population genetic studies of retinitis pigmentosa. Am J Hum Genet. 1980;32:223–35. [PMC free article] [PubMed] [Google Scholar]
- 3.Hu DN. Prevalence and mode of inheritance of major genetic eye diseases in China. J Med Genet. 1987;24:584–8. doi: 10.1136/jmg.24.10.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 5.Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol. 2007;125:151–8. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrari S, Di Iorio E, Barbaro V, Ponzin D, Sorrentino FS, Parmeggiani F. Retinitis pigmentosa: genes and disease mechanisms. Curr Genomics. 2011;12:238–49. doi: 10.2174/138920211795860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.den Hollander AI, Black A, Bennett J, Cremers FP. Lighting a candle in the dark: advances in genetics and gene therapy of recessive retinal dystrophies. J Clin Invest. 2010;120:3042–53. doi: 10.1172/JCI42258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger W, Kloeckener-Gruissem B, Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases. Prog Retin Eye Res. 2010;29:335–75. doi: 10.1016/j.preteyeres.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Boughman JA, Vernon M, Shaver KA. Usher syndrome: definition and estimate of prevalence from two high-risk populations. J Chronic Dis. 1983;36:595–603. doi: 10.1016/0021-9681(83)90147-9. [DOI] [PubMed] [Google Scholar]
- 10.Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet. 1999;36:437–46. [PMC free article] [PubMed] [Google Scholar]
- 11.Ayala-Ramirez R, Graue-Wiechers F, Robredo V, Amato-Almanza M, Horta-Diez I, Zenteno JC. A new autosomal recessive syndrome consisting of posterior microphthalmos, retinitis pigmentosa, foveoschisis, and optic disc drusen is caused by a MFRP gene mutation. Mol Vis. 2006;12:1483–9. [PubMed] [Google Scholar]
- 12.Fukui T, Yamamoto S, Nakano K, Tsujikawa M, Morimura H, Nishida K, Ohguro N, Fujikado T, Irifune M, Kuniyoshi K, Okada AA, Hirakata A, Miyake Y, Tano Y. ABCA4 gene mutations in Japanese patients with Stargardt disease and retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002;43:2819–24. [PubMed] [Google Scholar]
- 13.Fu Q, Wang F, Wang H, Xu F, Zaneveld JE, Ren H, Keser V, Lopez I, Tuan HF, Salvo JS, Wang X, Zhao L, Wang K, Li Y, Koenekoop RK, Chen R, Sui R. Next-generation sequencing-based molecular diagnosis of a Chinese patient cohort with autosomal recessive retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2013;54:4158–66. doi: 10.1167/iovs.13-11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Wang H, Sun V, Tuan HF, Keser V, Wang K, Ren H, Lopez I, Zaneveld JE, Siddiqui S, Bowles S, Khan A, Salvo J, Jacobson SG, Iannaccone A, Wang F, Birch D, Heckenlively JR, Fishman GA, Traboulsi EI, Li Y, Wheaton D, Koenekoop RK, Chen R. Comprehensive molecular diagnosis of 179 Leber congenital amaurosis and juvenile retinitis pigmentosa patients by targeted next generation sequencing. J Med Genet. 2013;50:674–88. doi: 10.1136/jmedgenet-2013-101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Guan L, Shen T, Zhang J, Xiao X, Jiang H, Li S, Yang J, Jia X, Yin Y, Guo X, Wang J, Zhang Q. Mutations of 60 known causative genes in 157 families with retinitis pigmentosa based on exome sequencing. Hum Genet. 2014;133:1255–71. doi: 10.1007/s00439-014-1460-2. [DOI] [PubMed] [Google Scholar]
- 16.Shen T, Guan L, Li S, Zhang J, Xiao X, Jiang H, Yang J, Guo X, Wang J, Zhang Q. Mutation analysis of Leber congenital amaurosis associated genes in patients with retinitis pigmentosa. Mol Med Rep. 2015;11:1827–32. doi: 10.3892/mmr.2014.2894. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Guan L, Fang S, Jiang H, Xiao X, Yang J, Wang P, Yin Y, Guo X, Wang J, Zhang J, Zhang Q. Exome sequencing reveals CHM mutations in six families with atypical choroideremia initially diagnosed as retinitis pigmentosa. Int J Mol Med. 2014;34:573–7. doi: 10.3892/ijmm.2014.1797. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Zhang Q, Shen T, Xiao X, Li S, Guan L, Zhang J, Zhu Z, Yin Y, Wang P, Guo X, Wang J, Zhang Q. Comprehensive mutation analysis by whole-exome sequencing in 41 Chinese families with Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2013;54:4351–7. doi: 10.1167/iovs.13-11606. [DOI] [PubMed] [Google Scholar]
- 19.Huang L, Zhang Q, Li S, Guan L, Xiao X, Zhang J, Jia X, Sun W, Zhu Z, Gao Y, Yin Y, Wang P, Guo X, Wang J, Zhang Q. Exome sequencing of 47 chinese families with cone-rod dystrophy: mutations in 25 known causative genes. PLoS ONE. 2013;8:e65546. doi: 10.1371/journal.pone.0065546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin ZB, Mandai M, Homma K, Ishigami C, Hirami Y, Nao IN, Takahashi M. Allelic copy number variation in FSCN2 detected using allele-specific genotyping and multiplex real-time PCRs. Invest Ophthalmol Vis Sci. 2008;49:3799–805. doi: 10.1167/iovs.07-1656. [DOI] [PubMed] [Google Scholar]
- 21.Xiao X, Mai G, Li S, Guo X, Zhang Q. Identification of CYP4V2 mutation in 21 families and overview of mutation spectrum in Bietti crystalline corneoretinal dystrophy. Biochem Biophys Res Commun. 2011;409:181–6. doi: 10.1016/j.bbrc.2011.04.112. [DOI] [PubMed] [Google Scholar]
- 22.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 23.Mandal MN, Heckenlively JR, Burch T, Chen L, Vasireddy V, Koenekoop RK, Sieving PA, Ayyagari R. Sequencing arrays for screening multiple genes associated with early-onset human retinal degenerations on a high-throughput platform. Invest Ophthalmol Vis Sci. 2005;46:3355–62. doi: 10.1167/iovs.05-0007. [DOI] [PubMed] [Google Scholar]
- 24.Henderson RH, Waseem N, Searle R, van der Spuy J, Russell-Eggitt I, Bhattacharya SS, Thompson DA, Holder GE, Cheetham ME, Webster AR, Moore AT. An assessment of the apex microarray technology in genotyping patients with Leber congenital amaurosis and early-onset severe retinal dystrophy. Invest Ophthalmol Vis Sci. 2007;48:5684–9. doi: 10.1167/iovs.07-0207. [DOI] [PubMed] [Google Scholar]
- 25.Vallespin E, Cantalapiedra D, Riveiro-Alvarez R, Wilke R, Aguirre-Lamban J, Avila-Fernandez A, Lopez-Martinez MA, Gimenez A, Trujillo-Tiebas MJ, Ramos C, Ayuso C. Mutation screening of 299 Spanish families with retinal dystrophies by Leber congenital amaurosis genotyping microarray. Invest Ophthalmol Vis Sci. 2007;48:5653–61. doi: 10.1167/iovs.07-0007. [DOI] [PubMed] [Google Scholar]
- 26.Ávila-Fernandez A, Cantalapiedra D, Aller E, Vallespin E, Aguirre-Lamban J, Blanco-Kelly F, Corton M, Riveiro-Alvarez R, Allikmets R, Trujillo-Tiebas MJ, Millan JM, Cremers FP, Ayuso C. Mutation analysis of 272 Spanish families affected by autosomal recessive retinitis pigmentosa using a genotyping microarray. Mol Vis. 2010;16:2550–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Blanco-Kelly F, Garcia-Hoyos M, Corton M, Avila-Fernandez A, Riveiro-Alvarez R, Gimenez A, Hernan I, Carballo M, Ayuso C. Genotyping microarray: mutation screening in Spanish families with autosomal dominant retinitis pigmentosa. Mol Vis. 2012;18:1478–83. [PMC free article] [PubMed] [Google Scholar]
- 28.Wang F, Wang H, Tuan HF, Nguyen DH, Sun V, Keser V, Bowne SJ, Sullivan LS, Luo H, Zhao L, Wang X, Zaneveld JE, Salvo JS, Siddiqui S, Mao L, Wheaton DK, Birch DG, Branham KE, Heckenlively JR, Wen C, Flagg K, Ferreyra H, Pei J, Khan A, Ren H, Wang K, Lopez I, Qamar R, Zenteno JC, Ayala-Ramirez R, Buentello-Volante B, Fu Q, Simpson DA, Li Y, Sui R, Silvestri G, Daiger SP, Koenekoop RK, Zhang K, Chen R. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum Genet. 2014;133:331–45. doi: 10.1007/s00439-013-1381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glöckle N, Kohl S, Mohr J, Scheurenbrand T, Sprecher A, Weisschuh N, Bernd A, Rudolph G, Schubach M, Poloschek C, Zrenner E, Biskup S, Berger W, Wissinger B, Neidhardt J. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies. Eur J Hum Genet. 2014;22:99–104. doi: 10.1038/ejhg.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or leber congenital amaurosis. Proc Natl Acad Sci USA. 1998;95:3088–93. doi: 10.1073/pnas.95.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweeney MO, McGee TL, Berson EL, Dryja TP. Low prevalence of lecithin retinol acyltransferase mutations in patients with Leber congenital amaurosis and autosomal recessive retinitis pigmentosa. Mol Vis. 2007;13:588–93. [PMC free article] [PubMed] [Google Scholar]
- 32.Deveault C, Billingsley G, Duncan JL, Bin J, Theal R, Vincent A, Fieggen KJ, Gerth C, Noordeh N, Traboulsi EI, Fishman GA, Chitayat D, Knueppel T, Millan JM, Munier FL, Kennedy D, Jacobson SG, Innes AM, Mitchell GA, Boycott K, Heon E. BBS genotype-phenotype assessment of a multiethnic patient cohort calls for a revision of the disease definition. Hum Mutat. 2011;32:610–9. doi: 10.1002/humu.21480. [DOI] [PubMed] [Google Scholar]
- 33.Janssen S, Ramaswami G, Davis EE, Hurd T, Airik R, Kasanuki JM, Van Der Kraak L, Allen SJ, Beales PL, Katsanis N, Otto EA, Hildebrandt F. Mutation analysis in Bardet-Biedl syndrome by DNA pooling and massively parallel resequencing in 105 individuals. Hum Genet. 2011;129:79–90. doi: 10.1007/s00439-010-0902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shevach E, Ali M, Mizrahi-Meissonnier L, McKibbin M, El-Asrag M, Watson CM, Inglehearn CF, Ben-Yosef T, Blumenfeld A, Jalas C, Banin E, Sharon D. Association Between Missense Mutations in the BBS2 Gene and Nonsyndromic Retinitis Pigmentosa. JAMA Ophthalmol. 2015;133:312–8. doi: 10.1001/jamaophthalmol.2014.5251. [DOI] [PubMed] [Google Scholar]
- 35.Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compere P, Schiffmann SN, Gergely F, Riley JH, Perez-Morga D, Woods CG, Schurmans S. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet. 2009;41:1027–31. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- 36.Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Sztriha L, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, Kayserili H, Swistun D, Scott LC, Bertini E, Boltshauser E, Fazzi E, Travaglini L, Field SJ, Gayral S, Jacoby M, Schurmans S, Dallapiccola B, Majerus PW, Valente EM, Gleeson JG. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet. 2009;41:1032–6. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strom TM, Nyakatura G, Apfelstedt-Sylla E, Hellebrand H, Lorenz B, Weber BH, Wutz K, Gutwillinger N, Ruther K, Drescher B, Sauer C, Zrenner E, Meitinger T, Rosenthal A, Meindl A. An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:260–3. doi: 10.1038/940. [DOI] [PubMed] [Google Scholar]
- 38.Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel alpha1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:264–7. doi: 10.1038/947. [DOI] [PubMed] [Google Scholar]
- 39.Jalkanen R, Demirci FY, Tyynismaa H, Bech-Hansen T, Meindl A, Peippo M, Mantyjarvi M, Gorin MB, Alitalo T. A new genetic locus for X linked progressive cone-rod dystrophy. J Med Genet. 2003;40:418–23. doi: 10.1136/jmg.40.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jalkanen R, Bech-Hansen NT, Tobias R, Sankila EM, Mantyjarvi M, Forsius H, de la Chapelle A, Alitalo T. A novel CACNA1F gene mutation causes Aland Island eye disease. Invest Ophthalmol Vis Sci. 2007;48:2498–502. doi: 10.1167/iovs.06-1103. [DOI] [PubMed] [Google Scholar]