Abstract
We asked whether chronic tolerance and the hyperthermic sign-reversal induced by repeated 60% N2O exposures could be extinguished using a cue-exposure paradigm. Rats received 18 N2O administrations in a total calorimetry system that simultaneously measures core temperature (Tc), metabolic heat production (HP), and body heat loss (HL). Each exposure entailed a 2-h baseline period followed by a 1.5-h N2O exposure. The 18 drug exposures induced a robust intra-administration hyperthermia in which the initial hypothermic effect of N2O inverted to a significant hyperthermic sign-reversal during N2O inhalation due primarily to an acquired robust increase in HP. The rats were then randomized to one of 3 extinction procedures (n = 8/procedure) over a 20-d interval: 1) a N2O-abstinent home-cage group (HC) that received only the usual animal care; 2) a cue-exposure group (CEXP) in which the animals were placed in the calorimeter 8 times but received no N2O; and 3) a drug-onset-cue group (DOC) in which animals received a brief N2O exposure in the calorimeter that mimicked the first 3 min of an actual 60% N2O trial. Following the extinction sessions, all rats received a 60% N2O test trial and Tc, HP and HL were assessed. The hyperthermic sign-reversal remained fully intact during the test trial, with no significant differences observed among groups in any post-baseline change in any thermal outcome. These data suggest that cue exposure may not be an efficacious strategy to reduce sign-reversals that develop with chronic drug use.
Keywords: allostasis, drug addiction, extinction, homeostasis
Abbreviations
- CEXP
cue exposure
- CR
conditioned response
- CS
conditioned stimulus
- DHL
dry heat loss
- DOC
drug onset cue
- EHL
evaporative heat loss
- HC
home cage
- HL
heat loss
- HP
heat production
- N2O
nitrous oxide
- Tc
core temperature
Introduction
Drug use fosters a web of biopsychosocial phenomena that can promote a transition to drug addiction in some individuals and under some conditions.1 Among the processes thought to contribute to this progression is associative learning, whereby drug-associated cues acquire the power to elicit motivationally salient symptoms such as drug craving and withdrawal-like effects that encourage further drug taking.2,3 Cues associated with repeated drug administration have been found to acquire the ability to elicit responses that counter the effects of the drugs, and when these responses are elicited in the presence of the drug, they consequently lessen the drug's impact and thereby contribute to drug tolerance.2,4,5 These cues can include obvious stimuli such as the specific drug environment or drug-taking paraphernalia, or else arbitrary stimuli such as novel odors. Repeated presentation of drug-relevant conditioned stimuli (CS), in the absence of the drug itself, can extinguish the ability of the CS to elicit compensatory conditioned responses (CR), thereby attenuating the ability of the relevant cues to elicit responses that contribute to tolerance and withdrawal effects.2 For example, if rats tolerant to the hypothermic effects of ethanol are given repeated presentations of ethanol-paired stimuli, but now in the absence of ethanol, the learned compensatory responses mediating tolerance are extinguished and the hypothermic effect of ethanol is reinstated.6-9 This extinction strategy, often called cue-exposure therapy, remains a focus of interest among researchers and clinicians seeking effective treatments for drug addiction.10,11
The evidence for the efficacy of cue-exposure therapy has been inconsistent, and one possibility for this is that principles of learning may not always have been appropriately applied.12 A vital consideration in this regard involves the identity of the critical drug-associated CSs that elicit CRs. Most approaches to extinction-based therapy have focused on traditional exteroceptive cues (e.g., environmental cues such as drug paraphernalia), whereas evidence suggests that interoceptive cues exerted by the drug itself during a drug administration can play dominant roles as CSs, especially in situations where the onset of the drug effect is sufficiently gradual so that synchronous build-up of interoceptive cues provides a reliable and salient predictor of the upcoming larger drug effect.4,13-17. Failures of cue-exposure therapy may consequently reflect failures to target and extinguish the most salient interoceptive cues in the form of the effect of the drug itself, which reliably signals an impending greater drug effect. In the context of drug taking, these kinds of interoceptive CSs are called drug-onset cues (DOCs).14
Our laboratory has developed a rat model for investigating drug tolerance, using nitrous oxide (N2O) and assessing thermal parameters.18-20 This model permits us to deliver N2O, a pharmacologically active gas that promotes significant hypothermia upon initial administration, while synchronously measuring core temperature (Tc), heat production (HP) and heat loss (HL). Using this approach, we have demonstrated that serial administration of N2O to rats causes tolerance to develop in the form of a lessened hypothermia over trials resulting primarily from the growth of a HP response during repeated N2O administrations.19,21 Further, if N2O administrations are continued after full tolerance has developed, rats eventually exhibit a significant hyperthermic sign-reversal during steady-state N2O inhalation.19,21-23 As is true for the tolerance itself, this thermal sign-reversal is caused by a brisk and robust increase in the generation of metabolic HP upon commencement of N2O administration, and to a lesser extent by adaptations that slightly impede HL.21 This hyperthermic sign-reversal during N2O administration has been suggested to have motivational consequences because rats will oppose their own dysregulated hyperthermia by moving to a cooler ambient temperature if one is available.23 This finding is compatible with an allostatic interpretation of addiction, which proposes that withdrawal-like sign-reversal states may motivate behaviors that oppose the sign-reversal state.24 For example, making a behavioral response that increases the amount of drug in the body (i.e., drug taking) could cause a greater pharmacological effect that opposes, and thereby reduces, the aversive sign-reversal.
The primary goal of the present study was to determine whether the responses mediating the hyperthermic sign-reversal that eventually develops with repeated N2O administrations might be acquired through an associative mechanism and therefore be eliminated using extinction procedures. If extinction could inhibit the elicitation of HP responses during N2O administration, the hyperthermic sign-reversal of Tc should diminish, and the degree of tolerance development should be reduced as well. The present study was designed to induce thermal tolerance, including a hyperthermic sign-reversal of Tc during repeated N2O administrations in rats. After the sign-reversal was well-established, rats were randomly assigned to one of 3 groups: 1) a N2O-abstinent home-cage control group, 2) a cue-exposure group that received extinction trials to the environmental cues associated with the N2O delivery chamber, or 3) a drug-onset cue group that received extinction trials that provided both the environmental cues plus a brief interoceptive drug-onset cue of N2O. Following the extinction trials, a final N2O administration determined whether the responses mediating tolerance or the hyperthermic sign-reversal of Tc were diminished by the extinction intervention.
Materials and Methods
Subjects
Male Long-Evans rats (Charles River, N = 24, 25–28 d of age upon arrival) were maintained on a 12-h:12-h light/dark cycle (lights on at 0700 h) at an ambient temperature of 22 ± 1°C. Rats were group-housed in polycarbonate tubs with free access to tap water and pelleted chow (5053 PicoLab Rodent Diet 20, Animal Specialties and Provisions, Quakertown, PA). All animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Experimental design and procedures
Figure 1 presents an overview of the experimental design. Rats were placed individually in a calorimeter for 18 exposure sessions to 60% N2O so as to induce thermal tolerance followed by a hyperthermic sign-reversal as observed in previous research.19,21 Exposures were conducted on Monday, Wednesday and Friday for 6 consecutive weeks. Each exposure entailed a 2-h baseline pre-exposure period followed by a 1.5-h 60% N2O administration. Exposures began at either 0900 h (morning) or 1300 h (afternoon) and each rat was consistently tested at the same time of day. The first exposure session began >7 d after surgery and recovery [body mass was 157.2 ± 25.0 g (mean ± SD)]. After the 18 trials, the 24 rats were randomized (counterbalancing for morning and afternoon exposure sessions) into 3 extinction groups: 1) a home-cage group (HC), 2) a cue-exposure group (CEXP), and 3) a drug-onset cue group (DOC). The 3 extinction procedures were conducted over a 20-d interval. During this time, the CEXP and DOC groups were provided with 8 calorimetry sessions that occurred on the same days of the week and with the same starting times and durations as in the induction period. Except for a brief 3-min delivery of N2O in the DOC group (described below), the control gas was delivered for the entire 3.5-h extinction session. HC rats were maintained in their home cages during the entirety of the extinction period.
Figure 1.
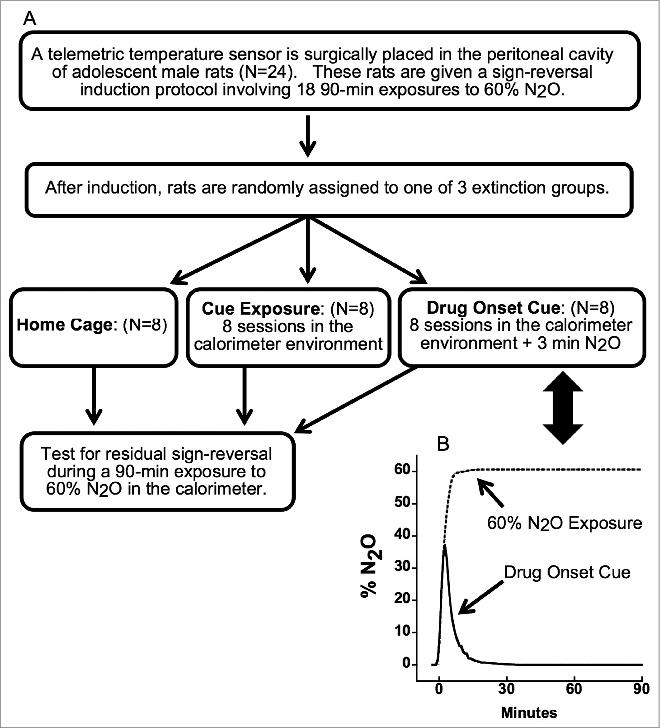
(A) Study design. (B) Temporal profiles of N2O concentration during the drug-onset cue (solid line) in comparison to a standard 60% N2O administration (dashed line).
Total calorimetry and N2O administration systems
Total calorimetry simultaneously measures the rates of total HL and metabolic HP, the 2 underlying determinants of Tc that reflect the influence of control mechanisms involved in regulating Tc. This system is described in detail elsewhere19-22 (see Part I of the online supplement). In brief, 6 total-calorimetry systems equipped for telemetric Tc assessment served as gas-tight exposure chambers for N2O. All gas mixtures were delivered to each chamber at a constant flow rate of 1.5 L/min. In the 60% N2O gas condition, the drug was administered such that the target concentration of 60% N2O was achieved rapidly. This goal was accomplished by a 2-stage administration in which 72% N2O, 21% O2, and 7% N2 was delivered for the first 6.5 minutes. Subsequently, the gas blend was switched to 60% N2O, 21% O2, and 19% N2 for the remainder of the delivery of the 60% N2O gas condition. The drug concentration in the chamber did not exceed 60% N2O using this procedure. The control gas condition consisted of a blend of 0% N2O, 21% Oxygen (O2), and 79% nitrogen (N2). A drug-onset-cue was made by delivering the first 3 minutes of the 60% N2O gas condition followed by delivery of the control gas condition. This procedure for the drug-onset cue produced a maximum 39% N2O concentration in the chamber, whereupon the incurrent gas was switched to control gas such that the percent N2O in the chamber decayed exponentially to 0% over ∼15 min (graphically depicted in Fig. 1).21 The DOC and CEXP protocols were identical except for the initial period of N2O inhalation in the DOC group.
Telemetric measurement of Tc, data acquisition and instrument control
Telemetric measurement of Tc was accomplished using a comm-ercial system from Data Sciences International (Saint Paul, MN) that consists of a Data-Exchange Matrix, Physio-Tel Receiver (Model RPC-1), Dataquest ART 4.2 software, and an implan-table battery-powered temperature sensor (model TA-F40) imp-lanted in the rat's peritoneal cavity. The antenna system used in each direct calorimeter consists of 2 radio ferrite coils oriented perpendicularly to each other and epoxied underneath a Plexiglas platform that holds them 2 mm above the floor of the direct calorimeter. The antennae wires exit the calorimeter through a gas-tight port and are connected to the RPC-1 receiver base. All other instrument control and data acquisition were performed using custom programs written in LabVIEW 6.8 (National Instruments, Austin, Texas).
Surgical placement of the telemetric temperature sensor
A telemetric temperature sensor was implanted surgically into each rat's peritoneal cavity using isoflurane anesthesia (3–5% for induction and 1–3% for maintenance) while the rat was on a 39 °C heating pad. Meloxicam (an NSAID) was provided in the drinking water (0.02 mg/ml H2O) from 1 d before to 2 d after surgery.
Data reduction for total calorimetry and Tc
Dependent variables were Tc, HP, DHL (dry HL) and EHL (evaporative HL). Tc was recorded at 15-s intervals while HP and HL data were recorded at 10-s intervals. Average Tc, HP and HL were calculated for each 6-min bin. Data were also analyzed in terms of change (Δ) from baseline values that were defined as the means in the final 12 min of the baseline period. Gas concentration data were recorded from each calorimeter at 1-min intervals.
Statistical analyses
Time zero (t0) was defined as the end of the 2-h baseline period corresponding to commencement of N2O administration (or continued control gas depending on the protocol). The first 2 6-min bins of HP and HL data after t0 were omitted from analysis due to the potential for artifact in this interval.20 For analyses focused on early changes, an early analysis period was defined as the 3rd and 4th 6-min bins following t0.
Correlated within-subjects longitudinal data were analyzed using the linear mixed-model program in SPSS Statistics 20 (IBM, Somers, NY). Session and condition were treated as fixed effects. Either unstructured or compound symmetry covariance structures were employed for statistical analyses, based on examination of regression diagnostics and information criteria.25 Data are summarized as means ±95% confidence limits (CL), i.e., the values that define the 95% confidence intervals (CI) to convey the magnitude and uncertainty range of each outcome. Baseline values were defined as means over the 12-min prior to N2O onset. The magnitude of hypothermia during the induction phase was defined as mean Tc during N2O minus baseline Tc. For the test session, the null hypothesis for baseline-adjusted comparisons was that μ CEXP = μ DOC = μ HC where μ denotes the mean value. For comparisons between conditions, difference scores and associated 95% CIs were adjusted for baseline values. 95% CIs for difference scores that do not include the null hypothesis value are significant at P < 0.05, and the distance of the null hypothesis value from a 95% confidence bound provides a measure of effect size.
We did not adjust for multiple comparisons due to the conundrums and misplaced emphasis that accompany this class of procedures when implemented in the context of basic preclinical research26-28 (see Part II of the online supplement for a more detailed explanation). Readers are urged to judge our results on the basis of the 95% confidence intervals and their coherence across sessions.
Data are presented as means ±95% CLs unless otherwise specified.
Results
Body mass and baseline core temperature during the induction phase. Body mass increased markedly during the induction phase but did not differ among groups (F2,21 = 0.04; P = 0.96). At the first exposure, animals weighed 157.2 ± 5.1 g whereas by the 18th they weighed 424.3 ± 6.8 g. Baseline core temperature decreased slightly but significantly over the induction phase (t = −7.44; P < 0.0001; coefficient estimate = −0.017 ± 0.002°C per exposure) but did not differ among groups (F2,21 = 1.18; P = 0.33). On the first exposure, baseline core temperature was 37.3 ± 0.06°C; on the 18th it was 37.0 ± 0.04°C. Thus, consistent with our use of random assignment, groups were well matched across exposures in terms of body mass and baseline core temperature, 2 variables with potentially important effects on changes of core temperature during N2O administration.
Development of thermal tolerance with repeated N2O administration
Consistent with our previous work,19,21-23,29 repeated 90-min 60% N2O administrations engendered the development of thermal tolerance, defined as the absence of N2O-induced hypothermia, and a subsequent sign-reversal, defined as the occurrence of hyperthermia during drug administration (Fig. 2 A,B). The thermal sign-reversal occurred reliably during exposures 6–18, as indicated by the 95% CIs in Figure 2A. The magnitude of hyperthermia during exposures 7–18 did not differ significantly among the 3 groups (adjusted for exposure number and baseline core temperature: F2,28.5 = 0.71; P = 0.50).
Figure 2.
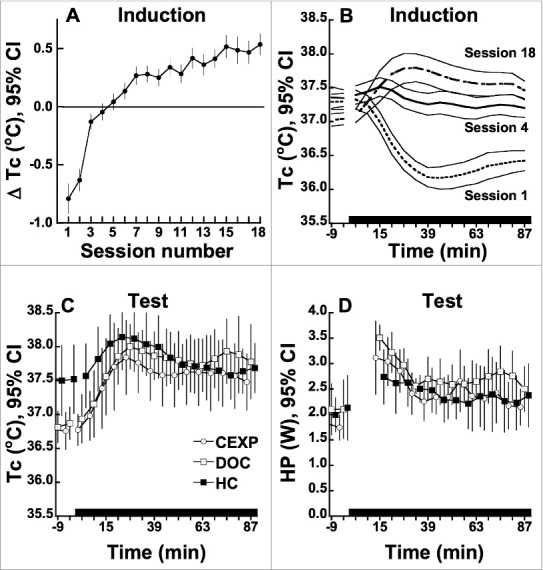
(A) Change in Tc from baseline during N2O inhalation in the induction phase in rats (n = 24). Change values are adjusted for pre-N2O baseline Tc. 95% confidence intervals that exclude zero denote means that are significantly different from baseline at P < 0.05. (B) Temporal profiles of Tc during N2O inhalation in the induction phase. (C) Temporal profiles of Tc during N2O inhalation in the post-extinction test session. Note the elevation in baseline Tc in the HC extinction group. (D) Temporal profiles of HP during N2O inhalation in the post-extinction test session (n = 8 per group). Extinction group key: CEXP: placement in calorimeter during extinction trials; DOC: initial brief N2O administration and placement in calorimeter during extinction trials; HC = remained in home cage during extinction period.
Effect of drug-onset cue on thermal outcomes during extinction trials
Comparisons between the DOC and CEXP groups indicated that the DOC had thermal consequences in the early period of measurement (min 12–24 after the t0 presentation of the DOC; see Table 1). Specifically, transient physiological effects were elicited by the drug-onset cue's abbreviated N2O administration (achieving a peak value of 39% N2O and a mean concentration of 9% N2O during the first 20 min after time zero). These DOC-associated changes did not continue after this early period (12–24 min) and there was no evidence that DOC-associated early thermal changes increased or decreased across extinction trials (0.21 ≤ P ≤ 0.97 for within subjects analysis of exposure number for the baseline-adjusted thermal outcomes).
Table 1.
Effect of DOC on thermal outcomes measured in the 3rd and 4th 6-min bins following t0
| Outcome (units) | DOC minus CEXP group difference in change from baseline ± SEa | P-value |
|---|---|---|
| ΔTc (°C) |
0.20 ± 0.1025 |
0.07 |
| ΔHP (W) |
0.40 ± 0.180 |
0.047 |
| ΔDHL (W) |
0.16 ± 0.032 |
<0.0005 |
| ΔEHL (W) | 0.03 ± 0.024 | 0.25 |
aBased on repeated measures regression analysis encompassing the 8 extinction trials. Values are adjusted for baseline thermal values.
Effect of extinction procedures on thermal outcomes during the test trial
Figure 2 C and D depicts Tc and HP profiles for each extinction group during the post-extinction-period test session. Unexpectedly, the home-cage group had reliably higher baseline Tc than either the CEXP or DOC groups (by 0.74 ± 0.21°C and 0.67 ± 0.21°C, respectively; P < 0.005). Because this result contrasts with the absence of group differences in baseline Tc during the induction of the thermal sign-reversal, it suggests that the rats that remained in their home cages during the extinction period generated a greater stress response when returned to the calorimeter for testing than did the 2 groups that had experienced the calorimeter environment 8 times each over the 20-d extinction period.
Notably, N2O exposure in the test session evoked hyperthermic changes in Tc (Fig. 2C) that were undiminished in comparison with those observed in the latter sessions of the induction protocol (F1,18.7 = 3.14, P = 0.09; test session minus session 18 difference = 0.16 ± 0.193°C).
The baseline-adjusted early and overall increases in HP, DHL or EHL during N2O exposure did not differ reliably among groups in the test session (F2,20 ≤ 2.34; P ≥ 0.12).
Discussion
The primary aim of this study was to test the hypothesis that a robust hyperthermic sign-reversal state acquired by repeated inhalation of 60% N2O might be attenuated by extinction trials involving unpaired associations with cues that were previously associated with drug inhalation. This concept is important because some models of drug addiction suggest that a sign-reversal state may contribute to the escalation of drug consumption.23,24 Thus, a procedure that would diminish or eliminate a sign-reversal could have potential therapeutic significance. In addition to evaluating extinction trials using environmental cues, the present study also evaluated the effectiveness of extinction trials that use an interoceptive drug-onset cue (DOC).15,16 This approach was founded on an extension of the traditional extinction model that holds that interoceptive cues caused by the sensory consequences of the drug itself can be critical CSs in eliciting CRs to drug administrations.4,13–17 According to this view, to successfully extinguish the effect of a DOC to elicit CRs in conditioned individuals, the extinction protocol must disassociate the initial sensory sequellae of the drug onset with the subsequent impending drug effects. It is thought that the extinction process does not erase or destroy the original learning, but rather depends on learning a new altered contingency.30-32
Our data, however, revealed that a robust acquired intra-N2O-administration hyperthermic state (i.e., the sign-reversal hyperthermia) remained fully intact in a N2O test session following a 20-d interval in which rats received extinction trials. The sign-reversal remained fully intact during the test session and, not surprisingly, the major driver of the hyperthermic state was a prompt and substantial increase in metabolic HP with the onset of N2O.
The inefficacy of the extinction procedures in our work must be interpreted with caution, given both the details of our experimental model and study design and a host of broader theoretical questions related to the mixed record of cue exposure therapy.12 An obvious question is whether we measured outcomes that are subject to conditioning. Thermal outcomes have been exploited extensively in addiction research33,34 and have been found to be conditionable in studies involving ethanol,6-9,35 morphine36 and non-drug hypothermic stressors.37,38 Our experimental model makes use of Tc, a centrally controlled variable in homeothermic animals, which is maintained within a narrow range in rats. Tc was measured non-invasively and with excellent sensitivity and precision via telemetry throughout the calorimetry sessions, and sufficient time (2 h) was allowed prior to drug onset to allow for abatement of handling-induced hyperthermia. Therefore, Tc is a highly appropriate outcome for evaluating extinction of the hyperthermic sign-reversal and chronic tolerance development to N2O-induced hypothermia.
An important advantage of our system is that it measures the underlying determinants of Tc, specifically HL and HP, via simultaneous direct gradient-layer calorimetry (HL) and indirect calorimetry (HP).20 These outcomes permit valuable insights into the behavior of the effector responses that underlie perturbations and adaptations in Tc during drug administration. We have previously documented that the initial hypothermia evoked by N2O primarily reflects a marked increase in HL, whereas the intra-administration hyperthermic state that develops with repeated N2O treatments primarily reflects an acquired increase in HP that occurs briskly upon N2O onset.18-20 It must also be emphasized that HP and HL can exhibit substantial reciprocal changes during N2O administration such that Tc does not change, i.e., N2O's effect to promote temperature loss via increased HL was fully obviated by a countervailing increase in HP.18 Thus, our model enables identification of agonistic pharmacological effects (HL) and antagonistic effector responses (HP) that would be masked or inferred by a model that measures only Tc. Because of this, the failure of the employed extinction procedures to attenuate changes of HP or HL demonstrates that the persistent change in the regulated variable (Tc) reflects a persistent adaptation in the behavior of its underlying determinants during N2O administration. Interestingly,39 Metzger et al. were unable to extinguish an acquired thermal tolerance to a cold exposure challenge despite evidence that associative processes played a role in tolerance development using the same method.37,38
Of fundamental importance to interpreting our results is the issue of whether the rats are able to sense the interoceptive DOC employed in our study. Our DOC was an initial 3-min rise in the N2O concentration with a time-concentration profile that was identical to the first 3 min of a standard 60% N2O administration. Presumably any change in sensory information or effector activity that occurred on the induction trials would also have occurred with the 3-min N2O administration. In the present study, the brief N2O DOC did in fact exert measurable physiological effects. A previous study employed a shorter duration N2O DOC that also caused significant changes in early ΔHP and early ΔDHL.21
In a previous study, we were unable to demonstrate classical conditioning of N2O-associated thermal responses using exteroceptive cues as well as interoceptive drug-onset-cues.21 As a possible explanation, we previously suggested21 that N2O's effect as an NMDA receptor antagonist40 might impair the development of conditioned thermal responses owing to the important role of NMDA-glutamatergic signaling in memory acquisition.41 In light of the present failure to attenuate thermal responses using a N2O DOC in a cue-exposure paradigm, we now suggest that studies are warranted to determine whether co-administration of N2O with another drug that is known to permit conditioning (e.g., ethanol) would prevent conditioning to that drug.
Thus, there is an apparent conundrum with regard to using extinction procedures in an attempt to eliminate a sign-reversal that has been suggested to encourage further drug taking. It may be that associative mechanisms are not the underlying cause of the sign-reversed hyperthermia, such that extinction attempts are futile. This would be consistent with the NMDA-antagonistic pharmacologic action of N2O to prevent conditioning from occurring. However, that would imply that a different mechanism, one that is not reliant upon conditioning, accounts for the sign-reversal, and tolerance as well. In either instance, the promise for using extinction procedures to successfully reduce sign-reversals as a therapeutic strategy is not supported by the current data from this pre-clinical model.
Our study employed 8 extinction trials distributed across a 20-d interval. It is possible that a greater number of extinction trials is required to bring about a reduction in the sign-reversal hyperthermic state that was established during the 18 induction administrations. A major review on cue-exposure therapy noted that the number of cue-extinction trials necessary to achieve extinction had a range of 2-35 sessions.12 In addition, in our study, each extinction session involved only one DOC exposure, whereas some studies have used multiple cue exposures per trial (reviewed in12). Some evidence indicates that the number of cue extinction trials needs to equal or exceed the number of induction trials,42) whereas our study involved a ratio of 18 induction : 8 cue extinction trials. Accordingly, future cue exposure studies involving our paradigm should involve more extinction trials and perhaps more cues per trial.
There are important implications of these findings for theories of physiological regulation. A homeostatic interpretation of N2O-elicited hypothermia and its subsequent tolerance would suggest that the initial hypothermia that begins within minutes of the first N2O session acts like a US (unconditioned stimulus) that elicits compensatory responses to counter the hypothermia. As discussed above, we have found that the initial N2O-induced hypothermia is due to a concomitant N2O-induced increase of HL. The compensatory increase of HP is initiated later, beginning from 30 min to an hour or 2, with considerable variability among animals.20,21 Thus, while the increased HL (the unconditioned drug effect) persists during the N2O administration, the subsequent increase of HP lags and ultimately raises Tc, accounting for acute tolerance.20 This is perfectly consistent with the principles of homeostasis. A perturbation (decreased Tc) elicits a UR (unconditioned response) that ultimately restores Tc to normal. Over trials, the compensatory response increases in magnitude and occurs earlier within the N2O trial, eventually completely mitigating the unconditioned hypothermia (i.e., chronic tolerance develops).19,21
However, while these phenomena are easily explained and in fact predicted by a homeostatic adaptation model, the changes that occur with further N2O trials are not. The finding that rats develop a sign-reversal hyperthermia when administered N2O would not be predicted by theories of homeostasis and in fact is counter to its premises.22,23 It should be noted that sign-reversals that occur after the prior development of tolerance has been achieved have been described for other drugs, e.g., opioid-induced hyperalgesia.43,44
In part because of sign-reversals and other phenomena inconsistent with homeostatic principles, many have advocated that a different model of physiological regulation be adopted, and the current front-runner is allostasis. Allostasis was in fact formulated specifically to account for instances of regulation that do not meet homeostatic criteria.24 The important point is that an allostatic interpretation can accommodate the present hyperthermic sign-reversal and the concurrent activation of effector responses that have opposing actions on the same variable, Tc, as has been found.22,23 The point is that whereas homeostasis can explain the development of tolerance, it falls short and thereby invokes an allostatic model of regulation to explain the persistent hyperthermic sign-reversal observed in this study. The failure of the sign-reversal hyperthermia, and indeed of the tolerance itself, to be extinguished using the present paradigm does not speak to either allostatic or homeostatic interpretations as both models acknowledge the importance of associative mechanisms to regulation. However, contemporary views of allostasis emphasize the important role of central stress effects and peripheral stress hormones as possible mediators of allostatic effects. Stress may play a role in mediating the sign-reversal hyperthermia observed in this study.
In conclusion, the hyperthermic sign-reversal remained fully intact during the test trial, which suggests that cue exposure may not be an efficacious strategy to reduce sign-reversals that develop with chronic drug use.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors gratefully acknowledge the contributions of Christopher W Prall and Hoang Yen Ho for their technical contributions to this study. The Helen Riaboff Whiteley Center, located at the University of Washington's Friday Harbor Laboratories, provided an ideal environment for working on this manuscript.
Funding
This investigation was supported by the National Institutes of Health (NIDA grant DA023484). Supplemental data for this article can be accessed on the publisher's website
References
- 1. Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology (Berl) 2013; 229:387-413; PMID:23963530; http://dx.doi.org/ 10.1007/s00213-013-3224-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel S, Baptista MA, Kim JA, McDonald RV, Weise-Kelly L. Pavlovian psychopharmacology: the associative basis of tolerance. Exp Clin Psychopharmacol 2000; 8:276-93; PMID:10975617; http://dx.doi.org/ 10.1037/1064-1297.8.3.276 [DOI] [PubMed] [Google Scholar]
- 3. Siegel S, Ramos BM. Applying laboratory research: drug anticipation and the treatment of drug addiction. Exp Clin Psychopharmacol 2002; 10:162-83; PMID: 12233979; http://dx.doi.org/ 10.1037/1064-1297.10.3.162 [DOI] [PubMed] [Google Scholar]
- 4. Dworkin BR. Learning and physiological regulation. Chicago: University of Chicago Press; 1993 [Google Scholar]
- 5. Ramsay DS, Woods SC. Biological consequences of drug administration: implications for acute and chronic tolerance. Psychol Rev 1997; 104:170-93; PMID:9009884; http://dx.doi.org/ 10.1037/0033-295X.104.1.170 [DOI] [PubMed] [Google Scholar]
- 6. Crowell CR, Hinson RE, Siegel S. The role of conditional drug responses in tolerance to the hypothermic effects of ethanol. Psychopharmacology (Berl) 1981; 73:51-4; PMID:6785789; http://dx.doi.org/ 10.1007/BF00431101 [DOI] [PubMed] [Google Scholar]
- 7. Lê AD, Poulos CX, Cappell H. Conditioned tolerance to the hypothermic effect of ethyl alcohol. Science 1979; 206:1109-10; http://dx.doi.org/ 10.1126/science.493999 [DOI] [PubMed] [Google Scholar]
- 8. Mansfield JG, Cunningham CL. Conditioning and extinction of tolerance to the hypothermic effect of ethanol in rats. J Comp Physiol Psychol 1980; 94:962-9; PMID:7430477; http://dx.doi.org/ 10.1037/h0077824 [DOI] [PubMed] [Google Scholar]
- 9. Ramos BM, Siegel S, Bueno JL. Occasion setting and drug tolerance. Integr Physiol Behav Sci 2002; 37:165-77; PMID:12435209; http://dx.doi.org/ 10.1007/BF02734179 [DOI] [PubMed] [Google Scholar]
- 10. Myers KM, Carlezon WA. Extinction of drug- and withdrawal-paired cues in animal models: Relevance to the treatment of addiction. Neurosci Biobehav Rev 2010; 35:285-302; PMID:20109490; http://dx.doi.org/ 10.1016/j.neubiorev.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science 2012; 336:241-5; PMID: 22499948; http://dx.doi.org/ 10.1126/science.1215070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction 2002; 97:155-67; PMID: 11860387; http://dx.doi.org/ 10.1046/j.1360-0443.2002.00014.x [DOI] [PubMed] [Google Scholar]
- 13. Greeley J, Lê DA, Poulos CX, Cappell H. Alcohol is an effective cue in the conditional control of tolerance to alcohol. Psychopharmacology (Berl) 1984; 83:159-62; PMID:6431466; http://dx.doi.org/ 10.1007/BF00429726 [DOI] [PubMed] [Google Scholar]
- 14. Kim JA, Siegel S, Patenall VR. Drug-onset cues as signals: intraadministration associations and tolerance. J Exp Psychol Anim Behav Process 1999; 25:491-504; PMID:10531660; http://dx.doi.org/ 10.1037/0097-7403.25.4.491 [DOI] [PubMed] [Google Scholar]
- 15. McDonald RV, Siegel S. Intra-administration associations and withdrawal symptoms: morphine-elicited morphine withdrawal. Exp Clin Psychopharmacol 2004a; 12:3-11; http://dx.doi.org/ 10.1037/1064-1297.12.1.3 [DOI] [PubMed] [Google Scholar]
- 16. McDonald RV, Siegel S. The potential role of drug onset cues in drug dependence and withdrawal: Reply to Bardon (2004), Bossert and Shaham (2004), Bouton (2004), and Stewart (2004). Exp Clin Psychopharmacol 2004b; 12:23-6 [DOI] [PubMed] [Google Scholar]
- 17. Sokolowska M, Siegel S, Kim JA. Intraadministration associations: conditional hyperalgesia elicited by morphine onset cues. J Exp Psychol Anim Behav Process 2002; 28:309-20; PMID:12136706; http://dx.doi.org/ 10.1037/0097-7403.28.3.309 [DOI] [PubMed] [Google Scholar]
- 18. Kaiyala KJ, Butt S, Ramsay DS. Direct evidence for systems-level modulation of initial drug (in)sensitivity in rats. Psychopharmacology (Berl) 2007a; 191:243-51; http://dx.doi.org/ 10.1007/s00213-006-0657-z [DOI] [PubMed] [Google Scholar]
- 19. Kaiyala KJ, Butt S, Ramsay DS. Systems-level adaptations explain chronic tolerance development to nitrous oxide hypothermia in young and mature rats. Psychopharmacology (Berl) 2007b; 191:233-42; http://dx.doi.org/ 10.1007/s00213-006-0655-1 [DOI] [PubMed] [Google Scholar]
- 20. Kaiyala KJ, Ramsay DS. Assessment of heat production, heat loss, and core temperature during nitrous oxide exposure: a new paradigm for studying drug effects and opponent responses. Am J Physiol Regul Integr Comp Physiol 2005; 288:R692-701; PMID: 15563578; http://dx.doi.org/ 10.1152/ajpregu.00412.2004 [DOI] [PubMed] [Google Scholar]
- 21. Kaiyala KJ, Chan B, Ramsay DS. Robust thermoregulatory overcompensation, rather than tolerance, develops with serial administrations of 70% nitrous oxide to rats. J Therm Biol 2012; 37:30-40; PMID:22247586; http://dx.doi.org/ 10.1016/j.jtherbio.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramsay DS, Woods SC, Kaiyala KJ. Repeated nitrous oxide exposure in rats causes a thermoregulatory sign-reversal with concurrent activation of opposing thermoregulatory effectors. Temperature; 2014; 1:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ramsay DS, Woods SC, Kaiyala KJ. Drug-induced regulatory overcompensation has motivational consequences: implications for homeostatic and allostatic models of drug addiction. Temperature; 2014; 1:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramsay DS, Woods SC. Clarifying the roles of homeostasis and allostasis in physiological regulation. Psychol Rev 2014; 121:225-47; PMID:24730599; http://dx.doi.org/ 10.1037/a0035942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, New Jersey: John Wiley & Sons, Inc; 2004. [Google Scholar]
- 26. Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Med Res Methodol 2002; 2:8; PMID:12069695; http://dx.doi.org/ 10.1186/1471-2288-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Perneger TV. What's wrong with Bonferroni adjustments. BMJ 1998; 316:1236-8; PMID:9553006; http://dx.doi.org/ 10.1136/bmj.316.7139.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1:43-6; PMID:2081237; http://dx.doi.org/ 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 29.Ramsay DS, Kaiyala KJ, Leroux BG, Woods SC. Individual differences in initial sensitivity and acute tolerance predict patterns of chronic drug tolerance to nitrous-oxide-induced hypothermia in rats. Psychopharmacology (Berl); 2005; 181:48-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bouton ME. Context and behavioral processes in extinction. Learn Mem 2004; 11:485-94; PMID:15466298; http://dx.doi.org/ 10.1101/lm.78804 [DOI] [PubMed] [Google Scholar]
- 31. Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry 2006; 60:352-60; PMID:16616731; http://dx.doi.org/ 10.1016/j.biopsych.2005.12.015 [DOI] [PubMed] [Google Scholar]
- 32. Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 2008; 33:56-72; PMID:17882236; http://dx.doi.org/ 10.1038/sj.npp.1301555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kalant H, LeBlanc AE, Gibbins RJ. Tolerance to, and dependence on, some non-opiate psychotropic drugs. Pharmacol Rev 1971; 23:135-91; PMID:4398655 [PubMed] [Google Scholar]
- 34. Lovinger DM, Crabbe JC. Laboratory models of alcoholism: treatment target identification and insight into mechanisms. Nat Neurosci 2005; 8:1471-80; PMID:16251990; http://dx.doi.org/ 10.1038/nn1581 [DOI] [PubMed] [Google Scholar]
- 35. Cunningham CL, Crabbe JC, Rigter H. Pavlovian conditioning of drug-induced changes in body temperature. Pharmacol Ther 1983; 23:365-91; PMID:6371843; http://dx.doi.org/ 10.1016/0163-7258(83)90019-0 [DOI] [PubMed] [Google Scholar]
- 36. Siegel S. Tolerance to the hyperthermic effect of morphine in the rat is a learned response. J Comp Physiol Psychol 1978; 92:1137-49; PMID:755060; http://dx.doi.org/ 10.1037/h0077525 [DOI] [PubMed] [Google Scholar]
- 37. Kissinger SC, Riccio DC. Stimulus Conditions Influencing the Development of Tolerance to Repeated Cold-Exposure in Rats. Anim Learn Behav 1995; 23:9-16; http://dx.doi.org/ 10.3758/BF03198010 [DOI] [Google Scholar]
- 38. Riccio DC, MacArdy EA, Kissinger SC. Associative processes in adaptation to repeated cold exposure in rats. Behav Neurosci 1991; 105:599-602; PMID:1930727; http://dx.doi.org/ 10.1037/0735-7044.105.4.599 [DOI] [PubMed] [Google Scholar]
- 39. Metzger MM, Harrod SB, Kissinger SC, Riccio DC. Is acquired tolerance to hypothermia susceptible to extinction? Psychol Rec 1998; 48:33-44. [Google Scholar]
- 40. Jevtovic-Todorovic V, Todorovic S, Mennerick S, Powell S, Dikranian K, Beenshoff N, Zorumski CF, Olney JW. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med 1998; 4:460-63; PMID:9546794; http://dx.doi.org/ 10.1038/nm0498-460 [DOI] [PubMed] [Google Scholar]
- 41. Li F, Tsien JZ. Memory and the NMDA receptors. N Engl J Med 2009; 361:302-3; PMID:19605837; http://dx.doi.org/ 10.1056/NEJMcibr0902052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chelonis JJ, Calton JL, Hart JA, Schachtman TR. Attenuation of the renewal effect by extinction in multiple contexts. Learn Motiv 1999; 30:1-14; http://dx.doi.org/ 10.1006/lmot.1998.1022 [DOI] [Google Scholar]
- 43. Ossipov MH, Lai J, King T, Vanderah TW, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers 2005; 80:319-24; PMID:15795927; http://dx.doi.org/ 10.1002/bip.20254 [DOI] [PubMed] [Google Scholar]
- 44.Vanderah TW, Suenaga NM, Ossipov MH, Malan TP Jr, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci 2001; 21:279-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


