Abstract
The genioglossus (GG) is an extrinsic muscle of the human tongue that plays a critical role in preserving airway patency. In the last quarter century, >50 studies have reported on respiratory-related GG electromyographic (EMG) activity in human subjects. Remarkably, of the studies performed, none have duplicated subject body position, electrode recording locations, and/or breathing task(s), making interpretation and integration of the results across studies extremely challenging. In addition, more recent research assessing lingual anatomy and muscle contractile properties has identified regional differences in muscle fiber type and myosin heavy chain expression, giving rise to the possibility that the anterior and posterior regions of the muscle fulfill distinct functions. Here, we assessed EMG activity in anterior and posterior regions of the GG, across upright and supine, in rest breathing and in volitionally modulated breathing tasks. We tested the hypotheses that GG EMG is greater in the posterior region and in supine, except when breathing is subject to volitional modulation. Our results show differences in the magnitude of EMG (%regional maximum) between anterior and posterior muscle regions (7.95 ± 0.57 vs. 11.10 ± 0.99, respectively; P < 0.001), and between upright and supine (8.63 ± 0.73 vs. 10.42 ± 0.90, respectively; P = 0.008). Although the nature of a task affects the magnitude of EMG (P < 0.001), the effect is similar for anterior and posterior muscle regions and across upright and supine (P > 0.2).
Keywords: electromyography, respiration, electrode
the genioglossus (GG) muscle of the human tongue is involved in functions critical to survival, including swallowing, speech, and breathing. In view of the muscle's role as an airway dilator, the preponderance of research has focused on electromyographic (EMG) activity during sleep and wakefulness when subjects are in the supine or side-lying position (Eastwood et al. 2003; Malhotra et al. 2004; Fogel et al. 2005; Bailey et al. 2007; Eckert et al. 2009; Wilkinson et al. 2010; Jordan et al. 2010; Richardson and Bailey 2010; Saboisky et al. 2010; Laine and Bailey 2011; Trinder et al. 2013). Numerous other studies have incorporated manipulations that impact upon respiratory-related GG activity, including head/body position (i.e., head up vs. head back; upright vs. supine) (Douglas et al. 1993; Wasicko et al. 1993; Ono et al. 1996; Otsuka et al. 2000; Tsuiki et al. 2000; Williams et al. 2000; Pae et al. 2002 2004; Takahashi et al. 2002; Walsh et al. 2008), assessment of EMG activity in multiple muscle regions (Eastwood et al. 2003; Wilkinson et al. 2008 2010; Nicholas et al. 2010; Richardson and Bailey 2010; McSharry et al. 2013; Trinder et al. 2013), and in different tasks (i.e., rest breathing, voluntary hyperventilation, maximal inspiratory effort, or exercise) (Mezzanotte et al. 1992; Williams et al. 2000; Eastwood et al. 2003; Walls et al. 2013). In addition, studies of human tongue muscle tissue highlight regional differences in GG muscle fiber type, myosin heavy chain composition, cross-sectional area, innervation, and motor end-plate banding (Sanguineti and Laboissi 1997; Saigusa et al. 2001; Zur et al. 2004; Buchaillard et al. 2009; Mu and Sanders 2010; Daugherty et al. 2012; Sanders et al. 2013), which suggest different regions of the muscle may fulfill different functions (Saigusa et al. 2001; Daugherty et al. 2012).
In this case, we sought to integrate across the breadth of approaches reported in the literature and recorded multiunit EMG activity in rest breathing, deep breathing, voluntary hyperventilation, and Mueller maneuvers. Given the suggestion that different regions of the muscle are driven preferentially by certain inputs (Eastwood et al. 2003), or that different regions of the muscle may have different mechanical effectiveness in dilation of the airway (Bilston and Gandevia 2014), we recorded activity in the most anterior and most posterior regions of the muscle. In view of gravitational effects on the upper airway (Pae et al. 1994) and recently reported evidence that the posterior region of the GG muscle exhibits greater respiratory-related activation (Cheng et al. 2008), we predicted that EMG activity would be greatest in the posterior region and in supine. Second, given indications that (motor) cortical input may be a more potent driver of the anterior tongue (Laine and Bailey 2011) and anatomical evidence that the anterior GG may be more important for volitional activities (Saigusa et al. 2001), we predicted that anterior EMG would exceed posterior EMG activity in the context of volitionally modulated breathing.
METHODS
We recruited 14 healthy young adults (11 women and 3 men, age 20.6 ± 2.1 yr; height 167.7 ± 9.0 cm; weight 62.0 ± 11.2 kg; body mass index 21.9 ± 2.8). Subjects were nonsmokers, without history of sleep disorders, respiratory, neuromuscular, or cardiovascular disease, and a forced expiratory volume (1.0 s) to forced vital capacity ratio >80% predicted value based on height, weight, age, and sex (Miller et al. 2005). Experimental procedures were approved by The University of Arizona Human Subjects Protection Program, and subjects gave their written informed consent before participation.
General procedures.
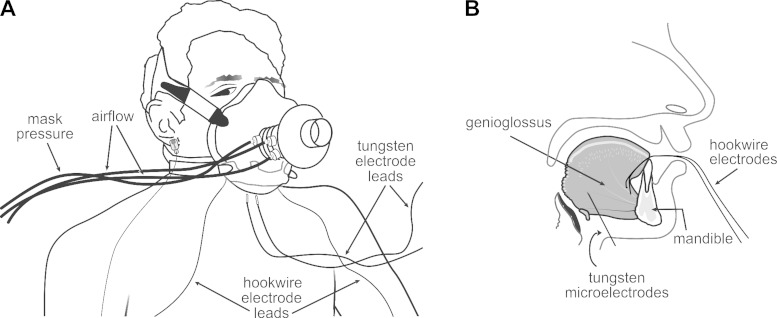
Subjects were fitted with an oronasal facemask that allowed for oral and nasal breathing (series 8900; Hans Rudolph). The mask was held in place with a head cap (series 7450; Hans Rudolph) and self-sealed to the face. The seal was verified by a vacuum leak test. Inspiratory and expiratory airflows were measured using a pneumotachometer attached in series to the mask (Fig. 1) and connected to an amplifier (model 1110; Hans Rudolph) that transmitted mask pressure and airflow signals to a data acquisition system (Cambridge Electronic Design, 1401 and Spike 2 software).
Fig. 1.
A: schematic of experimental setup. B: lateral view of the tongue and mandible showing approximate locations of hook-wire and tungsten electrodes in the anterior and posterior genioglossus (GG) muscle regions.
Electromyography.
We recorded multiunit EMG activity in the GG using two electrode types (see below). In the anterior region, EMG activities were recorded via bipolar intramuscular hook-wire electrodes (50 μm; California Finewire, Grover Beach, CA) inserted via the mouth, as described previously (Sauerland and Harper 1976; Williams et al. 2000; Pittman and Bailey 2009; Richardson and Bailey 2010). Each hook wire was bared of ∼2–3 mm insulation at the tip, threaded through a 30-gauge needle (0.3 × 13 mm; Becton-Dickinson, Franklin Lakes, NJ), and inserted bilaterally, immediately posterior to the lingual sulcus at points equidistant from the lingual frenulum to a depth ∼12 mm from the mucosal surface (Sauerland and Harper 1976; Eastwood et al. 2003). The needle subsequently was removed, leaving the hook wire in the muscle belly. Hook wires were taped to the chin and anchored by the breathing mask. This recording area corresponds well with the region identified previously as GG-A comprising anterior and superior muscle fibers before entry into the tongue (Daugherty et al. 2012).
Multiunit EMG activity in the posterior GG was recorded via bipolar intramuscular tungsten microelectrodes (1–5 μm tip diameter, 250 μm shaft diameter; Frederick Haer, Bowdoin, ME). Electrodes were inserted bilaterally into the skin underlying the jaw, ∼2.0 cm posterior to the mandible, ∼0.5–1.0 cm from the midline, and 1.0–2.0 cm from the other electrode. Based on our calculations and depending on subject size, electrodes were inserted ∼15–18 mm posterior to the mandible at an angle ∼120–130 degrees to the horizontal when seated upright. This recording area is in the most posterior region of the muscle and corresponds well with the area GG-P identified previously as comprising inferior oblique and horizontal muscle fibers (Daugherty et al. 2012). For these recordings, the distance to the GG in each subject was determined via ultrasonography (Aloka Pro Sound 3500, Tokyo, Japan) (Eastwood et al. 2003), and this information was used to place a mark on the electrode to indicate the distance to the middle of the muscle. This mark served as an indicator of the target depth and was used to ensure that the electrode placement was preserved throughout. Subjects were grounded with a gold cup electrode ear clip (Grass Technologies, Warwick, RI).
Tungsten microelectrodes were manufactured without insulation on the last 5.0 mm (Frederick Haer). Similarly, we prepared hook-wire electrodes baring ∼2–3 mm insulation at the terminal tip to match the impedance of the tungsten electrodes. Both electrode types subsequently were assessed and found to have equivalent negligible impedances at 1,000 Hz (Electrode Impedance Tester; Bak Electronics, Sanford, FL) and thus equivalent recording surface areas. EMG signals were sampled at 5 kHz and preamplified (3×), amplified (1,000×), and band-pass filtered from 30 to 3,000 Hz using CED 1902 amplifiers and head stages (Cambridge Electronic Design, Cambridge, UK). The signals were digitized and stored using a Cambridge Electronic Design 1401 interface and Spike2 software (Cambridge Electronic Design).
Experimental protocol.
Subjects were assigned randomly to begin the experiment in supine or seated upright. Head placement was kept in the Frankfort plane throughout the experiment (Johnson 1950).
GG EMG activity, inspiratory and expiratory airflow, and mask pressure were subsequently recorded for 2 min in rest breathing via the mouth and rest breathing via the nose. Subjects next performed deep breathing, maximal voluntary hyperventilation, and Mueller maneuvers. For deep breathing, subjects were instructed to inspire to approximately two times their normal breath (Fox et al. 1986). For maximal voluntary hyperventilation, subjects were instructed to breathe as fast and as deeply as they could. In view of the high airflows associated with these tasks and to minimize airway resistance, subjects were directed to breathe through the mouth (Fregosi and Lansing 1995; Williams et al. 2000). For Mueller maneuvers, subjects made an inspiratory effort at end-expiration against the occluded intake port on the pneumotachometer. For purposes of EMG normalization, subjects were asked to perform sharp sniffs, unimpeded tongue protrusions out of the mouth, and swallows at end-expiration to determine maximal GG activation. The maneuver in which the maximum EMG amplitude was observed served as the maneuver against which EMG in all other maneuvers or tasks were normalized. Note that subjects rested for 1–2 min between maneuvers/tasks to ensure that EMG and breathing frequency returned to baseline before proceeding. The entire protocol, including maximum maneuvers, was repeated for the seated upright or supine body position, whichever body position had yet to be completed.
Data analysis.
All data were analyzed offline using Spike2 software and customized scripts. EMG signals were rectified and integrated at a time constant of 100 ms. Breathing frequency (breaths/min) was determined for each task. Measures of inspiratory flow were integrated to obtain inspiratory tidal volume (VT) and ventilation. Mean inspiratory flow (VT/TI, ml/s) was calculated as an accepted index of ventilatory drive (Milic-Emili and Grunstein 1976; Boggs and Tenney 1984) in each breathing task. Average multiunit EMG activity was determined for the phasic (i.e., inspiratory) and tonic (i.e., expiratory) portions of each breath cycle. Because breathing frequencies varied between tasks, EMG measures at each electrode location were based on average EMG amplitude for 10 consecutive breaths (Mateika et al. 1999; Eastwood et al. 2003; Saboisky et al. 2006 2007; Bailey et al. 2007; Richardson and Bailey 2010). Because subtle differences in electrode impedance may alter the signal in terms of raw voltage, EMG values were normalized with regard to the regional maximal EMG activity (%maximum) and averaged across breaths. The maximum EMG for both the anterior (range: 0.5–1.6 mV) and posterior (range: 0.2–1.3 mV) muscle regions occurred during unimpeded tongue protrusion in all subjects (Pittman and Bailey 2009).
EMG averages were not normally distributed and were converted into logarithms for statistical purposes (Douglas et al. 1993). Statistical evaluation was by general linear model ANOVA (2 × 2 × 2 × 5), testing for significant differences in EMG, airflow (VT/TI), and breathing frequency as a function of muscle region (anterior vs. posterior), body position (upright vs. supine), and task (rest breathing via nose, rest breathing via mouth, deep breathing, voluntary hyperventilation, Mueller maneuver). Significance was set at P < 0.05. Post hoc comparisons were performed using paired t-tests, with significance adjusted according to the Bonferroni correction.
RESULTS
Means and SEs for ventilation parameters in upright and supine and for each task are reported in Table 1. There were no differences in inspiratory time (P = 0.952), expiratory time (P = 0.798), breathing frequency (P = 0.847), tidal volume (P = 0.514), inspiratory flow rate (P = 0.806), or minute ventilation (P = 0.741) between upright and supine within a task. VT/TI and breathing frequency were comparable for rest breathing via the nose (185.12 ± 16.20 ml/s; 13.95 ± 0.84 breaths/min) and rest breathing via the mouth (222.51 ± 18.92 ml/s; 14.12 ± 0.86 breaths/min). Given the absence of any difference between the two conditions (P > 0.3), only data for breathing via the mouth are presented in Figs. 2–5. As expected, inspiratory flow rates and breathing frequency at rest differed from deep breathing (328.80 ± 29.43 ml/s; 10.09 ± 0.55 breaths/min) (P < 0.005) and voluntary hyperventilation (789.66 ± 89.54 ml/s; 40.24 ± 2.55 breaths/min) (P < 0.001). Because a Mueller maneuver entails an inspiratory effort against an occlusion, no airflow or volume change was anticipated nor detected in this condition.
Table 1.
TI, TE, fR, VT, VT/TI, and VE in each task and as a function of body position
| Task: | Nasal Breathing |
Oral Breathing |
Deep Breathing |
Hyperventilation |
||||
|---|---|---|---|---|---|---|---|---|
| Body position: | Upright | Supine | Upright | Supine | Upright | Supine | Upright | Supine |
| TI , s | 1.62 ± 0.16 | 1.73 ± 0.20 | 1.60 ± 0.19 | 1.71 ± 0.17 | 2.37 ± 0.43 | 2.06 ± 0.30 | 0.62 ± 0.05 | 0.62 ± 0.07 |
| TE, s | 2.82 ± 0.24 | 2.64 ± 0.22 | 2.75 ± 0.25 | 2.66 ± 0.27 | 3.79 ± 0.36 | 3.81 ± 0.38 | 0.89 ± 0.07 | 0.86 ± 0.08 |
| fR, breaths/min | 13.82 ± 1.12 | 14.08 ± 1.30 | 14.04 ± 1.19 | 14.19 ± 1.30 | 9.96 ± 0.75 | 10.22 ± 0.82 | 39.54 ± 3.33 | 40.94 ± 3.97 |
| VT, ml | 306.01 ± 39.21 | 312.62 ± 38.36 | 345.29 ± 47.65 | 391.83 ± 47.83 | 726.25 ± 91.13 | 723.28 ± 118.69 | 486.68 ± 72.15 | 492.45 ± 69.51 |
| VT/TI, ml/s | 189.19 ± 24.41 | 181.0 ± 22.16 | 215.27 ± 27.63 | 229.76 ± 26.73 | 306.21 ± 34.22 | 351.3 ± 48.46 | 782.6 ± 122.93 | 796.6 ± 134.85 |
| VE, l·breaths−1·min−1 | 4.23 ± 0.55 | 4.40 ± 0.57 | 4.85 ± 0.60 | 5.56 ± 0.59 | 7.23 ± 0.94 | 7.39 ± 0.97 | 19.24 ± 2.89 | 20.16 ± 3.38 |
Values are means ± SE for inspiratory time (TI), expiratory time (TE), breathing frequency (fR), tidal volume (VT), inspiratory flow (VT/TI), and minute ventilation (VE) in each task and as a function of body position (i.e., upright and supine).
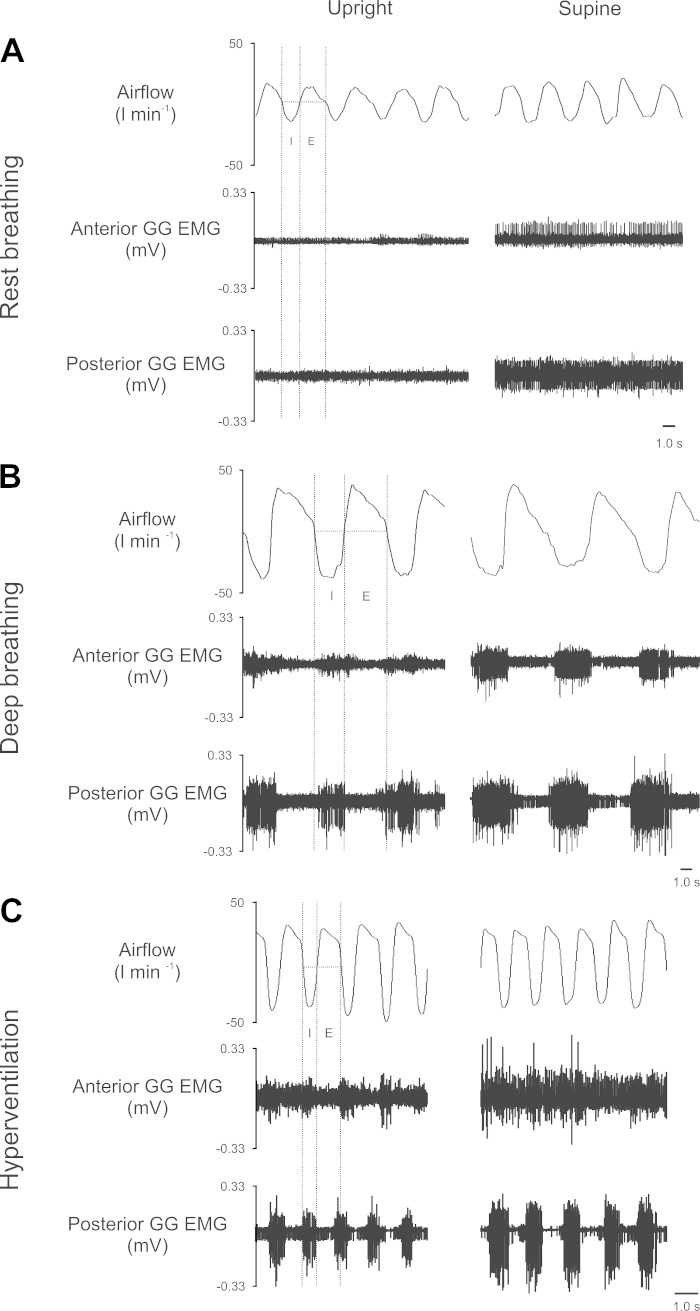
Fig. 2.
Representative recordings obtained from one subject during rest breathing via the mouth (A), deep breathing (B), and voluntary hyperventilation (C). Airflow (l/min) where inspiration is negative (trace on top), anterior multiunit muscle GG electromyographic (EMG) (trace in middle), and posterior multiunit muscle GG EMG (trace on bottom). Broken lines demonstrate inspiratory (I) and expiratory (E) breath phase information to illustrate which portions of the electromyogram were used during analysis. [Note for this individual, average EMG (mV) recorded in the maximum maneuver was 1.3 mV in the anterior region and 1.1 mV in the posterior region].
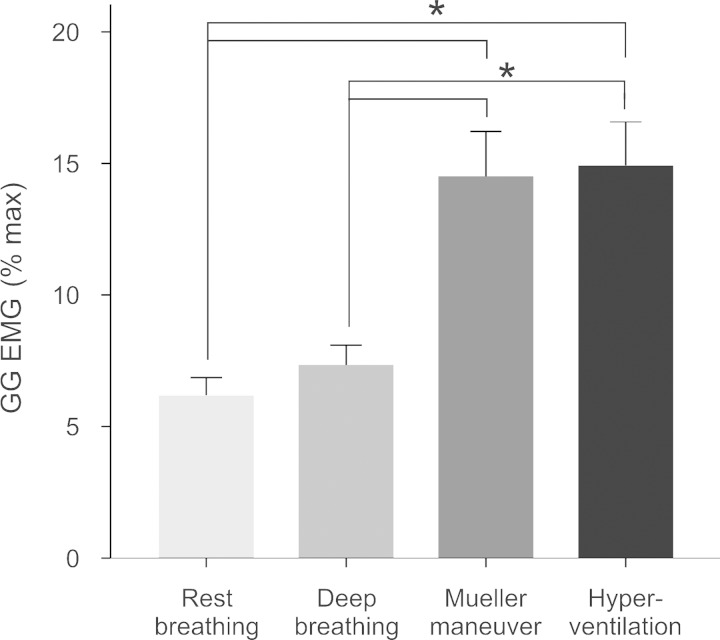
Fig. 5.
Effect of breathing task on average (±SE) multiunit muscle EMG (%maximum), collapsed across muscle region and body position. *P < 0.005 between factors; brackets indicate significantly different pairwise comparisons between breathing tasks.
The three panels in Fig. 2 contain representative recordings obtained from one subject during rest breathing (Fig. 2A), volitionally modulated deep breathing (Fig. 2B), and voluntary hyperventilation (Fig. 2C) upright (Fig. 2, left) and supine (Fig. 2, right). The example recordings provided here are qualitatively similar to the patterns of activity in the subject pool more broadly and show multiunit EMG activities in the anterior and posterior locations during rest breathing. As shown for rest breathing, the muscle is tonically active in the anterior and posterior regions across upright and supine, although additional unit(s) are recruited in supine and thus the magnitude of the activity appears somewhat greater in that position. A majority (10/14) of our young adult subjects exhibited tonic GG activation during rest breathing. In contrast, deep breathing and voluntary hyperventilation were characterized by phasic activity, with the greatest phasic activation evident in the posterior region (Fig. 2, B and C).
Task averages for both the inspiratory and expiratory components of the GG electromyogram are shown in Fig. 3. Consistent with the representative recording shown in Fig. 2, average data showed inspiratory and expiratory components of the EMG of comparable magnitude in rest breathing (P = 0.7); however, in deep breathing, Mueller maneuver, and hyperventilation, evident differences in the magnitude of the inspiratory and expiratory components of the EMG emerged (P < 0.05). Thus, there is a shift from predominantly tonic to predominantly phasic activation in both the anterior and posterior muscle regions as subjects progressed from rest breathing to hyperventilation. As shown, the inspiratory component of the multiunit EMG increases in the context of deep breathing, Mueller Maneuver, and voluntary hyperventilation (P < 0.05). Increases in the expiratory component attained significance only in voluntary hyperventilation. Accordingly, average data for the inspiratory component of the breath cycle are presented Figs. 4 and 5.
Fig. 3.
Average (±SE) multiunit muscle EMG (%maximum), comparing phasic vs. tonic activation in each breathing task. Phasic activation represents EMG activity during the inspiratory phase of the breath cycle, whereas tonic activation represents EMG activity during the expiratory phase of the breath cycle. In the case of Mueller maneuver, tonic activity was determined from the exhale immediately following the inspiratory maneuver. *P < 0.05, phasic vs. tonic; #P < 0.05 relative to rest breathing.
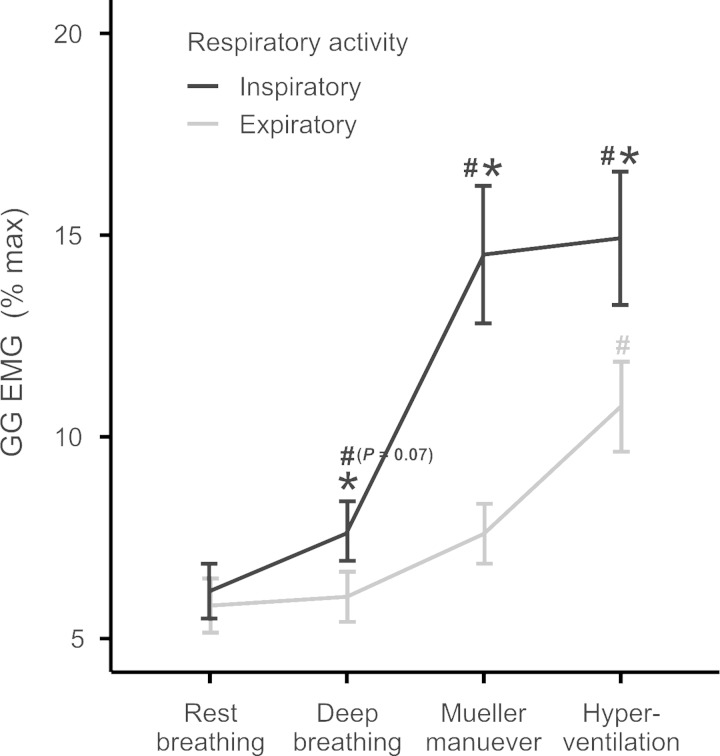
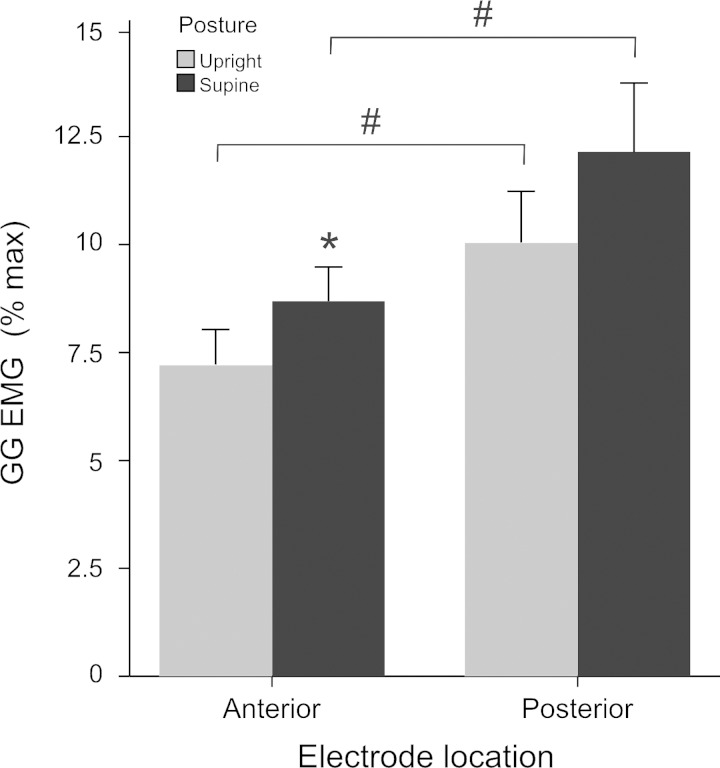
Fig. 4.
Average (±SE) multiunit inspiratory GG EMG. GG EMG (%maximum) in anterior and posterior muscle regions, in the upright and supine positions. *P < 0.05, upright vs. supine body position; #P < 0.05, anterior vs. posterior muscle region.
We report main effects for muscle region, posterior greater than anterior (P < 0.001) and body position, supine greater than upright (P = 0.008). There was a significant interaction between muscle region and body position (P < 0.05) (Fig. 4), such that EMG activity in the anterior region alone was greater in supine than in upright. Note that activity in the posterior GG consistently exceeded the anterior EMG independent of body position. There were no systematic differences in regional muscle activities as a function of task. Thus, although voluntary hyperventilation and Mueller maneuver were associated with greater EMG activation relative to rest breathing and deep breathing (P < 0.001) (Fig. 5), these effects were not specific to muscle region or body position (P > 0.2). Interestingly, there is a progressive increase in the magnitude of the EMG such that in rest breathing activity approaches ∼5% maximum, deep breathing ∼7.5% maximum, and voluntary hyperventilation and Mueller maneuvers approach ∼15% maximum.
DISCUSSION
In summary, body position and breathing task are key determinants of the magnitude of GG EMG activity in both the anterior and posterior regions of the GG. These healthy young adults exhibited tonic activation in the anterior and posterior regions of the muscle across upright and supine that shifted to phasic activity in volitionally modulated deep breathing and hyperventilation. The increase in the magnitude of tonic and phasic components of the EMG from rest breathing to volitionally modulated breathing is consistent with the recruitment of increasing numbers of GG motor units (Richardson and Bailey 2010; Bailey 2011; Walls et al. 2013). We found no evidence of differential activation of the anterior vs. posterior GG, rather the magnitude of EMG activities in each region was similarly impacted by the requirements of the particular task.
Critique of method.
We studied 14 healthy young adults reportedly free of sleep-disordered breathing. Although we did not perform overnight sleep studies, subjects were interviewed and health history was provided by self-report, which in low-risk subjects is known to be effective in ruling out individuals with sleep apnea (Mezzanotte et al. 1992; Young et al. 1993). Second, the population comprised a majority of women, whereas previous studies recruited exclusively male subjects (Tangel et al. 1992; Wasicko et al. 1993; Mezzanotte et al. 1996; Tsuiki et al. 2000; Saboisky et al. 2007) or a majority of men (Sauerland and Harper 1976; Leiter and Andrew 1990; Douglas et al. 1993; Williams et al. 2000; Jordan et al. 2010; Saboisky et al. 2010; Wilkinson et al. 2010). We do not consider that the proportion of women in the study lessens the credibility of the findings.
In light of studies showing heterogeneity in GG contractile properties (Sanguineti and Laboissi 1997; Saigusa et al. 2001; Zur et al. 2004; Buchaillard et al. 2009; Mu and Sanders 2010; Sanders et al. 2013), we recorded from the most anterior and posterior regions of the muscle taking care to avoid the midregion of the muscle that is considered an area of transition (Saigusa et al. 2001; Sanders et al. 2013). To access the anterior muscle, we used traditional hook-wire electrodes inserted per orally into the floor of the mouth, ∼5.0 mm from the internal aspect of the mandible close to the muscle's origin on the mandible (Sauerland and Mitchell 1975; Sauerland and Harper 1976). This approach is well documented, well tolerated by subjects, and yielded excellent recordings in these 14 subjects.
To access the posterior region of the GG we used tungsten microelectrodes inserted via the skin under the chin, using ultrasound to establish distance to the GG muscle in each subject. In our experience, tungsten electrodes cause less discomfort upon percutaneous insertion than hook-wire electrodes (which require a hypodermic needle) and permit the monitoring of electrode position and depth throughout the experiment. Tungsten electrodes also yield remarkably stable recordings across diverse behaviors and protocols, as has been demonstrated previously (Pittman and Bailey 2009; Richardson and Bailey 2010; Laine and Bailey 2011; Walls et al. 2013). Finally, the signal detection properties of the two types of electrodes are virtually identical and depend primarily on the surface area of exposed, i.e., uninsulated, electrode. In both cases, the electrode head was without insulation and confirmed by the equivalent negligible impedances measured at ∼1,000 Hz for both electrode types. Although electrodes were of equivalent impedance and EMG activity was normalized to the regional maximum, we acknowledge that the surface areas of the two electrodes differed, and as a result electrode pick up also differed slightly. In this case, the hook-wire electrode had the smaller pick up, which may have favored detection of single motor unit activity in the anterior muscle region.
Ventilatory-related findings.
The averages reported here for inspiratory flow rates during breathing at rest are comparable with previous reports (Williams et al. 2000; Parreira et al. 2010), and the average VT/TI attained in voluntary hyperventilation tasks approximates the values reported previously by Eastwood et al. (2003) for hyperventilation in the supine position and during moderate cycling exercise upright and supine (Williams et al. 2000; Walls et al. 2013). Previous studies show voluntary hyperventilation lasting ∼20–30 s exerts a negligible effect on EMG amplitude (Shea et al. 2000). Nevertheless, any resultant hypocapnia may have reduced the magnitude of overall EMG activity.
EMG findings.
GG EMG amplitude during rest breathing in supine was greater than in upright but was significant only in the anterior muscle region. Although average EMG in the posterior region showed a trend toward increased activation in supine (see Fig. 4), the increase failed to attain significance. The overall greater magnitude of EMG activity in the posterior tongue may have obscured the more subtle effects of posture on EMG activity. Our findings in supine are consistent with previously published work by Douglas et al. (1993) and Pae et al. (2002) but differ from other studies that documented transient increases in EMG activity followed by a decrease in activity (Wasicko et al. 1993), and no effect of body position (Williams et al. 2000). Divergent outcomes are to be expected if the conditions and/or tasks performed by the subjects also differ. For example, Wasicko et al. (1993) manipulated vestibular input and/or blood pressure secondary to passive tilt, whereas Williams et al. (2000) assessed GG EMG upright and supine within the context of cycling exercise. Thus, the stimulus may exert a unique and/or combinatorial effect on the GG electromyogram that renders meaningful comparison difficult.
The results obtained here are consistent with prior results also demonstrating predominantly tonic activation during rest breathing (Mezzanotte et al. 1992; Mateika et al. 1999; Laine et al. 2012; Walls et al. 2013) and underscore the role of GG as a pharyngeal airway dilator active in both the inspiratory and expiratory phases of the respiratory cycle in the healthy adult (Bailey 2011). Phasic activation has been observed previously in deep breathing associated with moderate exercise (Williams et al. 2000; Walls et al. 2013). The apparent absence of phasic activation in hyperventilation (Fig. 3) is an intriguing finding and resembles increased tonic GG activation seen under conditions of inspiratory loading (Tangel et al. 1992). Tonic activity may be a more energetically efficient means of ensuring airway patency, especially at very high breathing rates.
Importantly, this study is distinct from the earlier study by Eastwood and colleagues (2003). Eastwood et al. recorded GG EMG in rest breathing, in a 10-breath transition from rest breathing to hyperventilation, in response to negative pressure application, and during inspiration against an occluded airway. Moreover, all tasks were performed with subjects in the supine position. This study documents EMG activity in rest breathing via nose, rest breathing via the mouth, deep breathing, voluntary hyperventilation, and Mueller maneuvers, performed both upright and supine. The recording locations accessed in this study are somewhat more anterior and further posterior than those reported by Eastwood et al. (2003).
Volitionally modulated breathing vs. rest breathing.
Relative to rest breathing, the various volitionally modulated breathing tasks were associated with significantly greater magnitude EMG overall as well as differences in the phasic and tonic EMG components. These effects held true across recording location and body positions. As noted above, the progression from rest breathing to deep breathing, hyperventilation, and Mueller maneuver was characterized by successive increases in the magnitude of the GG electromyogram (%maximum) in each subject (see Fig. 5). This increase in the magnitude of the EMG activity may be related to the source(s) of neural drives converging onto hypoglossal motoneurons. For example, rest breathing presumably is driven primarily by the respiratory central pattern generator and by mechanoreceptor and chemoreceptor inputs. For volitionally modulated breathing, however, additional inputs arise in motor cortex but may also include inputs arising in pontine, parabrachial nuclei that control respiratory phase switching and permit adjustment of the respiratory cycle to fit the particular behavioral requirements (Sawczuk and Mosier 2001; Martelli et al. 2013; Dutschmann and Dick 2014). Although reflex activation secondary to negative pressure pulse application was not attempted in this protocol, voluntary hyperventilation and Mueller maneuvers, large negative (inspiratory) pressures, also may have contributed to GG activation via stimulation of upper airway receptors, resulting in reflex augmentation of the EMG activity in a manner distinct from cortical drive (Horner et al. 1991; Eastwood et al. 2003).
Finally, studies in other respiratory motoneuron pools, including intercostal and abdominal muscles, have documented greater EMG activation in the context of volitionally modulated breathing (McKenzie et al. 1988; Gandevia et al. 1990); however, none assessed the magnitude of EMG activity across the number of tasks attempted here. The present data obtained across a range of behaviors may be of use in assaying regional muscle activation and in gauging the proportion of the motoneuron pool that can be recruited into activity in healthy adults.
In summary, we selected for use the traditional per oral hook-wire electrodes and lesser-known tungsten microelectrodes inserted percutaneously. Importantly, both electrodes were constructed to equivalent impedance specifications and yielded excellent recordings from their respective muscle locations. We conducted a comprehensive assessment of upper airway EMG in healthy adults across a range of breathing behaviors, in two body positions and two recording locations. Respiratory behaviors were selected for inclusion as tasks that reasonably might be encountered in the course of daily life and, in the case of the Mueller maneuvers, to approximate the response of the muscle to airway obstruction (Hanly et al. 1989; Andreas et al. 1992; Morgan et al. 1993; Somers et al. 1993; Koshino et al. 2010; Camen et al. 2013). Given evidence that individuals with sleep apnea exhibit augmented GG activity in wakefulness (Mezzanotte et al. 1992; Saboisky et al. 2007 and 2012), findings obtained from healthy individuals will serve as a valuable dataset against which to compare anterior and posterior GG activity in this population.
GRANTS
This work was supported by a National Institute on Deafness and Other Communication Disorders Grant DC-009587 and start-up monies from The University of Arizona awarded to E. F. Bailey and by The Finley and Florence Brown Predoctoral Fellowship (Sarver Heart Center, University of Arizona) awarded to J. R. Vranish.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.R.V. and E.F.B. conception and design of research; J.R.V. performed experiments; J.R.V. analyzed data; J.R.V. and E.F.B. interpreted results of experiments; J.R.V. prepared figures; J.R.V. and E.F.B. drafted manuscript; J.R.V. and E.F.B. edited and revised manuscript; J.R.V. and E.F.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Mark Borgstrom and Santiago Barreda for assistance with statistical analyses; Philip Putnam, Ian Kidder, and Jordan Mudery for assistance with experiments; and Drs. Ralph Fregosi and Andrew Fuglevand for helpful comments regarding the manuscript.
REFERENCES
- Andreas S, Hajak G, von Breska B, Ruther E, Kreuzer H. Changes in heart rate during obstructive sleep apnoea. Eur Respir J 5: 853–857, 1992. [PubMed] [Google Scholar]
- Bailey EF, Fridel KW, Rice AD. Sleep/wake firing patterns of human genioglossus motor units. J Neurophysiol 97: 3284–3291, 2007. [DOI] [PubMed] [Google Scholar]
- Bailey EF. Activities of human genioglossus motor units. Respir Physiol Neurobiol 179: 14–22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilston LE, Gandevia SC. Biomechanical properties of the human upper airway and their effect on its behavior during breathing and in obstructive sleep apnea. J Appl Physiol 116: 314–324, 2014. [DOI] [PubMed] [Google Scholar]
- Boggs D, Tenney S. Scaling respiratory pattern and respiratory “drive.” Respir Physiol 58: 245–251, 1984. [DOI] [PubMed] [Google Scholar]
- Buchaillard S, Perrier P, Payan Y. A biomechanical model of cardinal vowel production: muscle activations and the impact of gravity on tongue positioning. J Acoust Soc Am 126: 2033–2051, 2009. [DOI] [PubMed] [Google Scholar]
- Camen G, Clarenbach C, Stowhas A, Rossi V, Sieve N, Stradling J, Kohler M. The effects of simulated obstructive apnea and hypopnea on arrhthmic potential in healthy subjects. Eur J Appl Physiol 113: 489–496, 2013. [DOI] [PubMed] [Google Scholar]
- Cheng S, Butler JE, Gandevia SC, Bilston LE. Movement of the tongue during normal breathing in awake healthy humans. J Physiol 586: 4283–4294, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty M, Luo Q, Sokoloff A. Myosin heavy chain composition of the human genioglossus muscle. J Speech Lang Hear Res 55: 609–625, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas NJ, Jan a M, Yildirim N, Warren PM, Drummond GB. Effect of posture and breathing route on genioglossal electromyogram activity in normal subjects and in patients with the sleep apnea/hypopnea syndrome. Am Rev Respir Dis 148: 1341–1345, 1993. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Dick T. Pontine mechanisms of respiratory control. Compr Physiol 4: 2443–2469, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood PR, Allison GT, Shepherd KL, Szollosi I, Hillman DR. Heterogeneous activity of the human genioglossus muscle assessed by multiple bipolar fine-wire electrodes. J Appl Physiol 94: 1849–1858, 2003. [DOI] [PubMed] [Google Scholar]
- Eckert DJ, Malhotra A, Lo YL, White DP, Jordan AS. The Influence of Obstructive Sleep Apnea and Gender on Genioglossus Activity During Rapid Eye Movement Sleep. Chest 135: 1–14, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel RB, Trinder J, White DP, Malhotra A, Raneri J, Schory K, Kleverlaan D, Pierce RJ. The effect of sleep onset on upper airway muscle activity in patients with sleep apnoea versus controls. J Physiol 564: 549–562, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Kreisman H, Colacone A, Kreismanj H. Respiratory volume perception through the nose and mouth determined noninvasively. J Appl Physiol 61: 436–439, 1986. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Lansing RW. Neural drive to nasal dilator muscles: influence of exercise intensity and oronasal flow partitioning. J Appl Physiol 79: 1330–1337, 1995. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Mckenzie DK, Plassman BL. Activation of human respiratory muscles during different voluntary manoeuvres. J Physiol 428: 387–403, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanly PJ, George CF, Millar TW, Kryger MH. Heart rate response to breath-hold, valsalva and Mueller maneuvers in obstructive sleep apnea. Chest 95: 735–739, 1989. [DOI] [PubMed] [Google Scholar]
- Horner BYRL, Murphy JAIK, Guz A. Evidence for Reflex Upper Airway Dilator Muscle Activation by Sudden Negative Airway Pressure in Man. J Physiol 436: 15–29, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EL. The Frankfort-Mandibular plane angle and the facial pattern. Am J Orthod 36: 516–533, 1950. [DOI] [PubMed] [Google Scholar]
- Jordan AS, White DP, Owens RL, Eckert DJ, Rahangdale S, Yim-Yeh S, Malhotra A. The effect of increased genioglossus activity and end-expiratory lung volume on pharyngeal collapse. J Appl Physiol 109: 469–475, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino Y, Villarraga HR, Orban M, Bruce CJ, Pressman GS, Leinveber P, Saleh HK, Konecny T, Kara T, Somers VK, Lopez-Jimenez F. Changes in left and right ventricular mechanics during the Mueller maneuver in healthy adults: a possible mechanism for abnormal cardiac function in patients with obstructive sleep apnea. Circ Cardiovasc Imaging 3: 282–289, 2010. [DOI] [PubMed] [Google Scholar]
- Laine CM, Bailey EF. Common synaptic input to the human hypoglossal motor nucleus. J Neurophysiol 105: 380–387, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine CM, Nickerson a L, Bailey EF. Cortical entrainment of human hypoglossal motor unit activities. J Neurophysiol 107: 493–499, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiter JC, Andrew J. Selective reflex activation in humans of the genioglossus. J Appl Physiol 68: 2581–2587, 1990. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Trinder J, Fogel R, Stanchina M, Patel SR, Schory K, Kleverlaan D, White DP. Postural effects on pharyngeal protective reflex mechanisms. Sleep 27: 1105–1112, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli D, Stanić D, Dutschmann M. The emerging role of the parabrachial complex in the generation of wakefulness drive and its implication for respiratory control. Respir Physiol Neurobiol 188: 318–323, 2013. [DOI] [PubMed] [Google Scholar]
- Mateika JH, Millrood DL, Kim J, Rodriguez HP, Samara GJ. Response of human tongue protrudor and retractors to hypoxia and hypercapnia. Am J Respir Crit Care Med 160: 1976–1982, 1999. [DOI] [PubMed] [Google Scholar]
- McKenzie DK, Plassman BL, Gandevia SC. Maximal activation of the human diaphragm but not inspiratory intercostal muscles during static inspiratory efforts. Neurosci Lett 89: 63–68, 1988. [DOI] [PubMed] [Google Scholar]
- McSharry DG, Saboisky JP, Deyoung P, Matteis P, Jordan AS, Trinder J, Smales E, Hess L, Guo M, Malhotra A. A mechanism for upper airway stability during slow wave sleep. Sleep 36: 555–563, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 89: 1571–1579, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med 153: 1880–7, 1996. [DOI] [PubMed] [Google Scholar]
- Milic-Emili J, Grunstein M. Drive and timing components of ventilation. Chest 70: 131–133, 1976. [DOI] [PubMed] [Google Scholar]
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J 26: 319–338, 2005. [DOI] [PubMed] [Google Scholar]
- Morgan BJ, Denahan T, Ebert TJ. Neurocirculatory consequences of negative intrathoracic pressure vs. asphyxia during voluntary apnea. J Appl Physiol 74: 2969–2975, 1993. [DOI] [PubMed] [Google Scholar]
- Mu L, Sanders I. Human tongue neuroanatomy: nerve supply and motor endplates. Clin Anat 23: 777–791, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas CL, Bei B, Worsnop C, Malhotra A, Jordan AS, Saboisky JP, Chan JKM, Duckworth E, White DP, Trinder J. Motor unit recruitment in human genioglossus muscle in response to hypercapnia. Sleep 33: 1529–1538, 2010. [PMC free article] [PubMed] [Google Scholar]
- Ono T, Lowe Ferguson K, Pae EK, Fleetham J. The effect of the tongue retaining device on awake genioglossus muscle activity in patients with obstructive sleep apnea. Am J Orthod Dentofac Orthop 110: 28–35, 1996. [DOI] [PubMed] [Google Scholar]
- Otsuka R, Ono T, Ishiwata Y, Kuroda T. Respiratory-related genioglossus electromyographic activity in response to head rotation and changes in body position. Angle Orthod 70: 63–69, 2000. [DOI] [PubMed] [Google Scholar]
- Pae E, Lowe A, Sasaki K, Price C, Tsuchiya M, Fleetham J. A cephalometric and electromyographic study of upper airway structures in the upright and supine positions. Am J Orthod Dentofac Orthop 106: 52–59, 1994. [DOI] [PubMed] [Google Scholar]
- Pae EK, Blasius JJ, Nanda R. Sex differences in genioglossus muscle response to changes in pharyngeal resistance. Am J Orthod Dentofac Orthop 122: 500–505, 2002. [DOI] [PubMed] [Google Scholar]
- Pae EK, Blasius JJ, Nanda R. Heterogeneity in vertical positioning of the hyoid bone in relation to genioglossal activity in men. Angle Orthod 74: 343–348, 2004. [DOI] [PubMed] [Google Scholar]
- Parreira VF, Bueno CJ, França DC, Vieira DSR, Pereira DR, Britto RR. Breathing pattern and thoracoabdominal motion in healthy individuals: influence of age and sex. Brazilian J Phys Ther 14: 411–416, 2010. [PubMed] [Google Scholar]
- Pittman LJ, Bailey EF. Genioglossus and intrinsic electromyographic activities in impeded and unimpeded protrusion tasks. J Neurophysiol 101: 276–282, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PA, Bailey EF. Tonically discharging genioglossus motor units show no evidence of rate coding with hypercapnia. J Neurophysiol 103: 1315–1321, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, Fogel RB, Taylor JL, Trinder JA, White DP, Gandevia SC. Tonic and phasic respiratory drives to human genioglossus motoneurons during breathing. J Neurophysiol 95: 2213–2221, 2006. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Butler JE, McKenzie DK, Gorman RB, Trinder a J, White DP, Gandevia SC. Neural drive to human genioglossus in obstructive sleep apnoea. J Physiol 585: 135–146, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Jordan AS, Eckert DJ, White DP, Trinder a J, Nicholas CL, Gautam S, Malhotra A. Recruitment and rate-coding strategies of the human genioglossus muscle. J Appl Physiol 109: 1939–1949, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Stashuk DW, Hamilton-Wright A, Carusona AL, Campana LM, Trinder J, Eckert DJ, Jordan AS, McSharry DG, White DP, Nandedkar S, David WS, Malhotra A. Neurogenic changes in the upper airway of patients with obstructive sleep apnea. Am J Respir Crit Care Med 185: 322–329, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigusa H, Niimi S, Yamashita K, Gotoh T, Kumada M. Morphological and histochemical studies of the genioglossus muscle. Ann Otol Rhinol Laryngol 110: 779–784, 2001. [DOI] [PubMed] [Google Scholar]
- Sanders I, Mu L, Amirali A, Su H, Sobotka S. The human tongue slows down to speak: muscle fibers of the human tongue. Anat Rec (Hoboken) 296: 1615–1627, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguineti V, Laboissi R. A control model of human tongue movements in speech. Biol Cybern 22: 11–22, 1997. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. Human tongue during sleep: electromyographic activity of the genioglossus muscle. Exp Neurol 51: 160–170, 1976. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Mitchell SP. Electromyographic activity of intrinsic and extrinsic muscles of the human tongue. Tex Rep Biol Med 33: 444–455, 1975. [PubMed] [Google Scholar]
- Sawczuk A, Mosier KM. Neural control of tongue movement with respect to respiration and swallowing. Crit Rev Oral Biol Med 12: 18–37, 2001. [DOI] [PubMed] [Google Scholar]
- Shea SA, Akahoshi T, Edwards JK, White DP. Influence of chemoreceptor stimuli on genioglossal response to negative pressure in humans. Am J Respir Crit Care Med 162: 559–565, 2000. [DOI] [PubMed] [Google Scholar]
- Somers V, Dyken M, Skinner J. Autonomic and hemodynamic responses and interactions during the Mueller maneuver in humans. J Aut Nerv Syst 44: 253–259, 1993. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Ono T, Ishiwata Y, Kuroda T. Breathing modes, body positions, and suprahyoid muscle activity. J Orthod 29: 307–313, 2002. [DOI] [PubMed] [Google Scholar]
- Tangel DJ, Mezzanotte WS, Sandberg EJ, White DP. Influences of NREM sleep on the activity of tonic vs. inspiratory phasic muscles in normal men. J Appl Physiol 73: 1055–1062, 1992. [DOI] [PubMed] [Google Scholar]
- Trinder J, Woods M, Nicholas CL, Chan JKM, Jordan AS, Semmler JG. Motor unit activity in upper airway muscles genioglossus and tensor palatini. Respir Physiol Neurobiol 188: 362–369, 2013. [DOI] [PubMed] [Google Scholar]
- Tsuiki S, Ono T, Ishiwata Y, Kuroda T. Functional divergence of human genioglossus motor units with respiratory-related activity. Eur Respir J 15: 906–910, 2000. [DOI] [PubMed] [Google Scholar]
- Walls CE, Laine CM, Kidder IJ, Bailey EF. Human hypoglossal motor unit activities in exercise. J Physiol 591: 3579–3590, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JH, Maddison KJ, Platt PR, Hillman DR, Eastwood PR. Influence of head extension, flexion, and rotation on collapsibility of the passive upper airway. Sleep 31: 1440–1447, 2008. [PMC free article] [PubMed] [Google Scholar]
- Wasicko MJ, Knuth SL, Leiter JC. Response of genioglossus EMG activity to passive tilt in men. J Appl Physiol 74: 73–81, 1993. [DOI] [PubMed] [Google Scholar]
- Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during sleep onset. Sleep 31: 525–533, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson V, Malhotra A, Nicholas CL, Worsnop C, Jordan AS, Butler JE, Saboisky JP, Gandevia SC, White DP, Trinder J. Discharge patterns of human genioglossus motor units during arousal from sleep. Sleep 33: 379–387, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JS, Janssen PL, Fuller DD, Fregosi RF. Influence of posture and breathing route on neural drive to upper airway dilator muscles during exercise. J Appl Physiol 89: 590–598, 2000. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230–1235, 1993. [DOI] [PubMed] [Google Scholar]
- Zur KB, Mu L, Sanders I. Distribution pattern of the human lingual nerve. Clin Anat 17: 88–92, 2004. [DOI] [PubMed] [Google Scholar]