Abstract
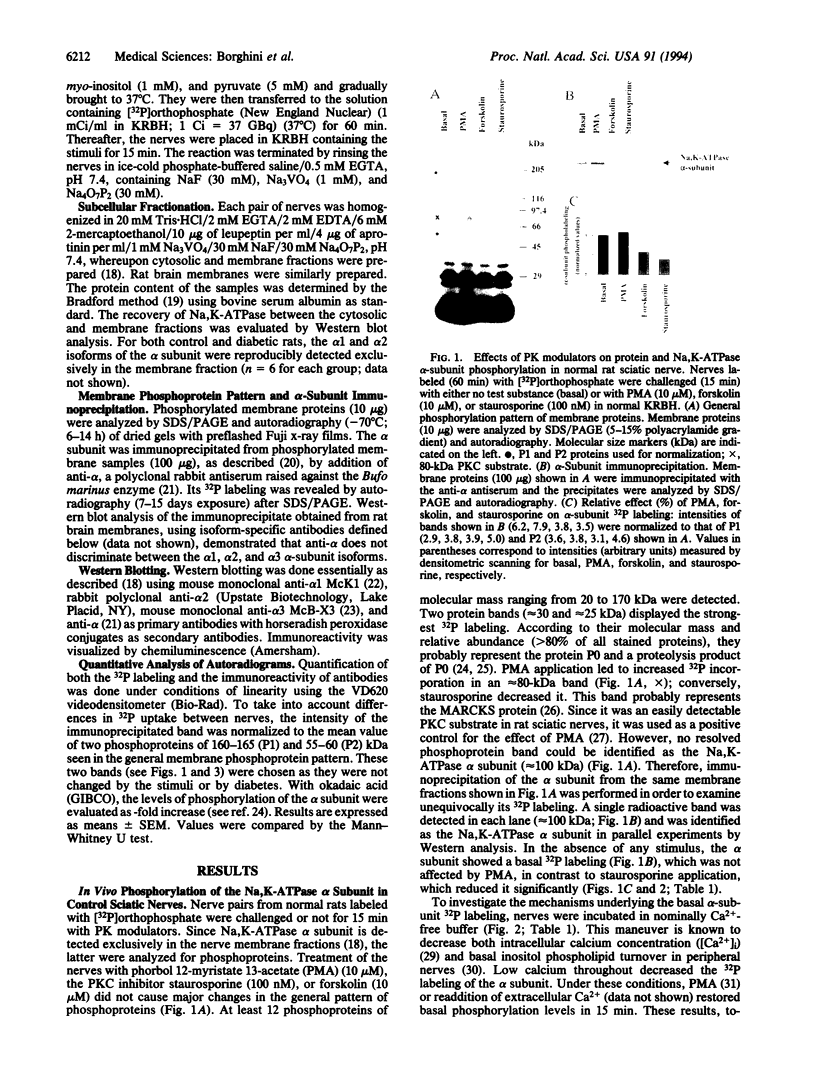
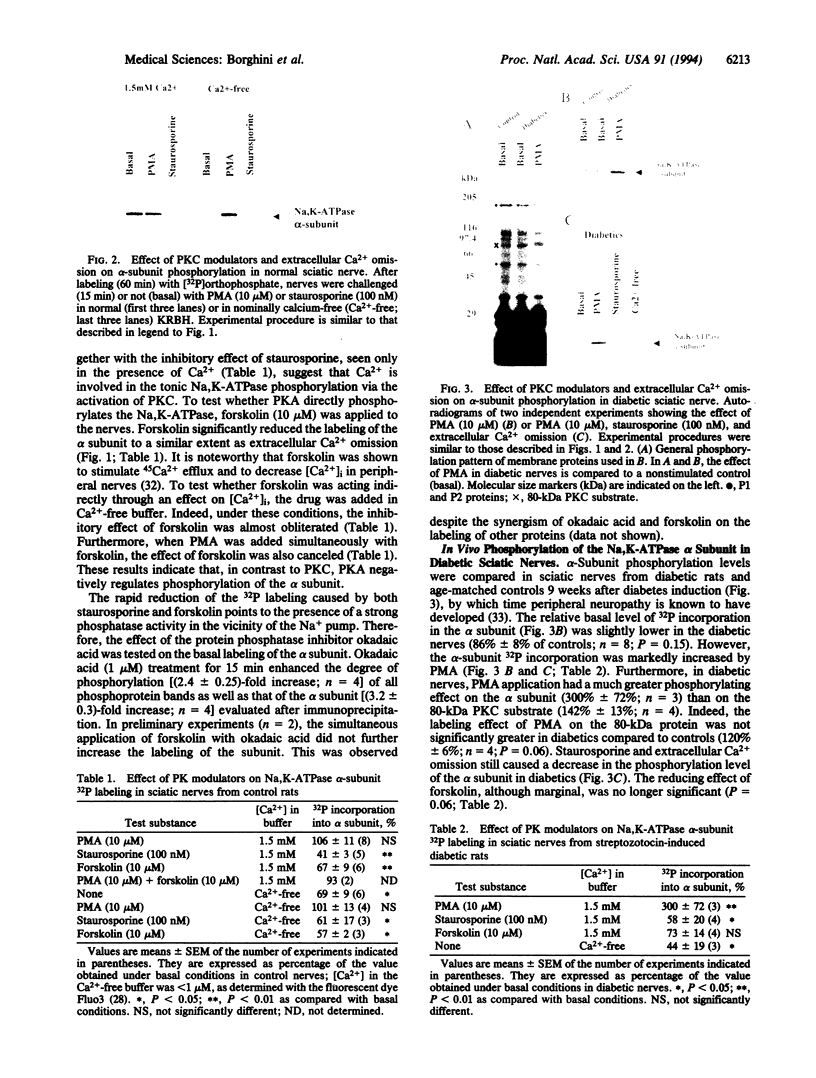
The phosphorylation state of the Na,K-ATPase alpha subunit has been examined in 32P-labeled sciatic nerves of control and streptozotocin-treated diabetic rats. Intact nerves were challenged with protein kinase (PK) modulators and alpha-subunit 32P labeling was analyzed after immunoprecipitation. In control nerves, the PKC activator phorbol 12-myristate 13-acetate (PMA) had little effect on alpha-subunit 32P labeling. In contrast, staurosporine, a PKC inhibitor, and extracellular calcium omission decreased it. In Ca(2+)-free conditions, PMA restored the labeling to basal levels. The cAMP-raising agent forskolin reduced the 32P labeling of the alpha subunit. The results suggest that nerve Na,K-ATPase is tonically phosphorylated by PKC in a Ca(2+)-dependent manner and that PKA modulates the phosphorylation process. In nerves of diabetic rats, PMA increased 32P labeling of the alpha subunit. In contrast to staurosporine or extracellular calcium omission, the decreased state of phosphorylation seen with forskolin was no longer significant in diabetic nerves. No change in the level of alpha-subunit isoforms (alpha 1 or alpha 2) was detected by Western blot analysis in such nerves. In conclusion, the altered effect of PK activators on Na,K-ATPase phosphorylation state is consistent with the view that a defect in PKC activation exists in diabetic nerves.
Full text
PDF
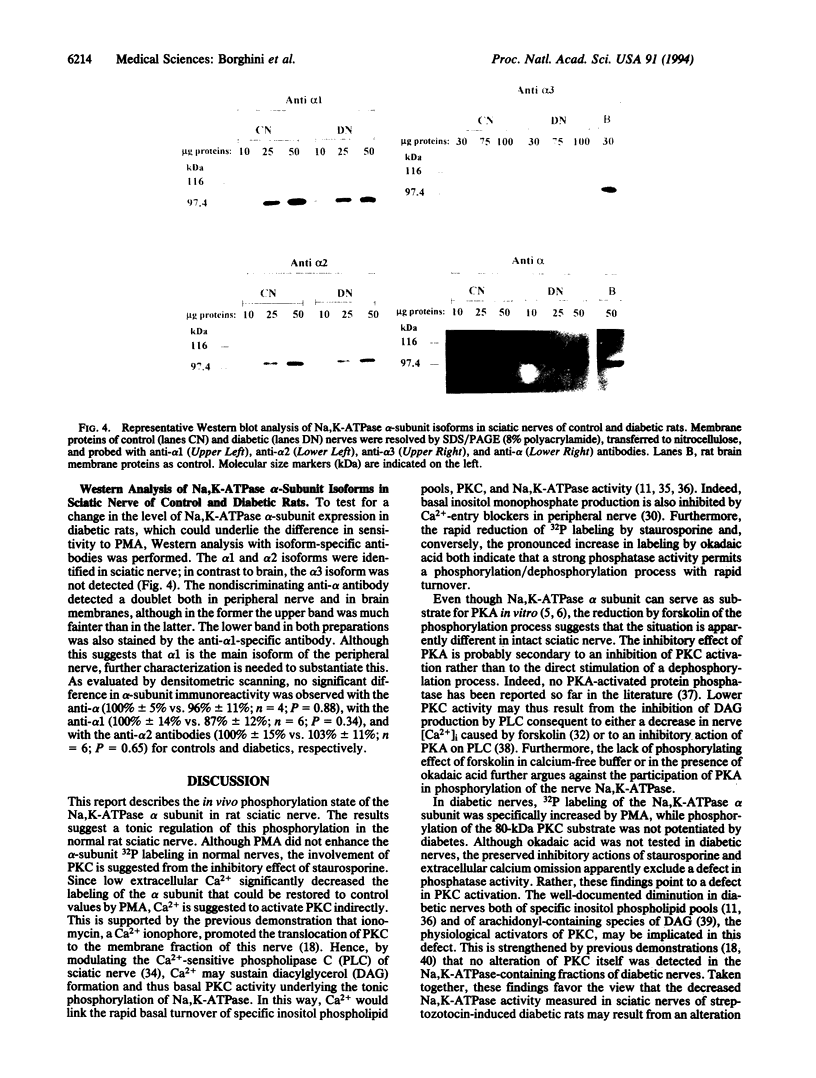

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992 Nov 27;71(5):713–716. doi: 10.1016/0092-8674(92)90546-o. [DOI] [PubMed] [Google Scholar]
- Bertorello A. M., Aperia A., Walaas S. I., Nairn A. C., Greengard P. Phosphorylation of the catalytic subunit of Na+,K(+)-ATPase inhibits the activity of the enzyme. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11359–11362. doi: 10.1073/pnas.88.24.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertorello A., Aperia A. Na+-K+-ATPase is an effector protein for protein kinase C in renal proximal tubule cells. Am J Physiol. 1989 Feb;256(2 Pt 2):F370–F373. doi: 10.1152/ajprenal.1989.256.2.F370. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Wen L., Glynn B. P., Witters L. A. Protein kinase C-stimulated phosphorylation in vitro of a Mr 80,000 protein phosphorylated in response to phorbol esters and growth factors in intact fibroblasts. Distinction from protein kinase C and prominence in brain. J Biol Chem. 1986 Jan 25;261(3):1459–1469. [PubMed] [Google Scholar]
- Borghini I., Ania-Lahuerta A., Regazzi R., Ferrari G., Gjinovci A., Wollheim C. B., Pralong W. F. Alpha, beta I, beta II, delta, and epsilon protein kinase C isoforms and compound activity in the sciatic nerve of normal and diabetic rats. J Neurochem. 1994 Feb;62(2):686–696. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Campbell M. D., Subramaniam S., Kotlikoff M. I., Williamson J. R., Fluharty S. J. Cyclic AMP inhibits inositol polyphosphate production and calcium mobilization in neuroblastoma X glioma NG108-15 cells. Mol Pharmacol. 1990 Aug;38(2):282–288. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chibalin A. V., Vasilets L. A., Hennekes H., Pralong D., Geering K. Phosphorylation of Na,K-ATPase alpha-subunits in microsomes and in homogenates of Xenopus oocytes resulting from the stimulation of protein kinase A and protein kinase C. J Biol Chem. 1992 Nov 5;267(31):22378–22384. [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- Das P. K., Bray G. M., Aguayo A. J., Rasminsky M. Diminished ouabain-sensitive, sodium-potassium ATPase activity in sciatic nerves of rats with streptozotocin-induced diabetes. Exp Neurol. 1976 Oct;53(1):285–288. doi: 10.1016/0014-4886(76)90299-5. [DOI] [PubMed] [Google Scholar]
- Felsenfeld D. P., Sweadner K. J. Fine specificity mapping and topography of an isozyme-specific epitope of the Na,K-ATPase catalytic subunit. J Biol Chem. 1988 Aug 5;263(22):10932–10942. [PubMed] [Google Scholar]
- Geering K., Girardet M., Bron C., Kraehenbühl J. P., Rossier B. C. Hormonal regulation of (Na+,K+)-ATPase biosynthesis in the toad bladder. Effect of aldosterone and 3,5,3'-triiodo-L-thyronine. J Biol Chem. 1982 Sep 10;257(17):10338–10343. [PubMed] [Google Scholar]
- Girardet M., Geering K., Frantes J. M., Geser D., Rossier B. C., Kraehenbuhl J. P., Bron C. Immunochemical evidence for a transmembrane orientation of both the (Na+, K+)-ATPase subunits. Biochemistry. 1981 Nov 10;20(23):6684–6691. doi: 10.1021/bi00526a025. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Impaired rat sciatic nerve sodium-potassium adenosine triphosphatase in acute streptozocin diabetes and its correction by dietary myo-inositol supplementation. J Clin Invest. 1983 Sep;72(3):1058–1063. doi: 10.1172/JCI111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A. Protein kinase C agonists acutely normalize decreased ouabain-inhibitable respiration in diabetic rabbit nerve. Implications for (Na,K)-ATPase regulation and diabetic complications. Diabetes. 1986 Feb;35(2):242–245. doi: 10.2337/diab.35.2.242. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Sima A. A., Stevens M. J., Feldman E. L., Lattimer S. A. Complications: neuropathy, pathogenetic considerations. Diabetes Care. 1992 Dec;15(12):1902–1925. doi: 10.2337/diacare.15.12.1902. [DOI] [PubMed] [Google Scholar]
- Hermenegildo C., Felipo V., Miñana M. D., Grisolía S. Inhibition of protein kinase C restores Na+,K(+)-ATPase activity in sciatic nerve of diabetic mice. J Neurochem. 1992 Apr;58(4):1246–1249. doi: 10.1111/j.1471-4159.1992.tb11335.x. [DOI] [PubMed] [Google Scholar]
- Hermenegildo C., Felipo V., Miñana M. D., Romero F. J., Grisolía S. Sustained recovery of Na(+)-K(+)-ATPase activity in sciatic nerve of diabetic mice by administration of H7 or calphostin C, inhibitors of PKC. Diabetes. 1993 Feb;42(2):257–262. doi: 10.2337/diab.42.2.257. [DOI] [PubMed] [Google Scholar]
- Jørgensen P. L. Mechanism of the Na+, K+ pump. Protein structure and conformations of the pure (Na+ +K+)-ATPase. Biochim Biophys Acta. 1982 Aug 11;694(1):27–68. doi: 10.1016/0304-4157(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Kim J., Kyriazi H., Greene D. A. Normalization of Na(+)-K(+)-ATPase activity in isolated membrane fraction from sciatic nerves of streptozocin-induced diabetic rats by dietary myo-inositol supplementation in vivo or protein kinase C agonists in vitro. Diabetes. 1991 May;40(5):558–567. doi: 10.2337/diab.40.5.558. [DOI] [PubMed] [Google Scholar]
- Kim J., Rushovich E. H., Thomas T. P., Ueda T., Agranoff B. W., Greene D. A. Diminished specific activity of cytosolic protein kinase C in sciatic nerve of streptozocin-induced diabetic rats and its correction by dietary myo-inositol. Diabetes. 1991 Nov;40(11):1545–1554. doi: 10.2337/diab.40.11.1545. [DOI] [PubMed] [Google Scholar]
- Kowluru R., Bitensky M. W., Kowluru A., Dembo M., Keaton P. A., Buican T. Reversible sodium pump defect and swelling in the diabetic rat erythrocyte: effects on filterability and implications for microangiopathy. Proc Natl Acad Sci U S A. 1989 May;86(9):3327–3331. doi: 10.1073/pnas.86.9.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattimer S. A., Sima A. A., Greene D. A. In vitro correction of impaired Na+-K+-ATPase in diabetic nerve by protein kinase C agonists. Am J Physiol. 1989 Feb;256(2 Pt 1):E264–E269. doi: 10.1152/ajpendo.1989.256.2.E264. [DOI] [PubMed] [Google Scholar]
- Lingrel J. B., Orlowski J., Shull M. M., Price E. M. Molecular genetics of Na,K-ATPase. Prog Nucleic Acid Res Mol Biol. 1990;38:37–89. doi: 10.1016/s0079-6603(08)60708-4. [DOI] [PubMed] [Google Scholar]
- Lowery J. M., Berti-Mattera L. N., Zhu X., Peterson R. G., Eichberg J. Relationship of ATP turnover, polyphosphoinositide metabolism, and protein phosphorylation in sciatic nerve and derived peripheral myelin subfractions from normal and streptozotocin diabetic rats. J Neurochem. 1989 Mar;52(3):921–932. doi: 10.1111/j.1471-4159.1989.tb02543.x. [DOI] [PubMed] [Google Scholar]
- Lowndes J. M., Hokin-Neaverson M., Bertics P. J. Kinetics of phosphorylation of Na+/K(+)-ATPase by protein kinase C. Biochim Biophys Acta. 1990 Apr 9;1052(1):143–151. doi: 10.1016/0167-4889(90)90069-p. [DOI] [PubMed] [Google Scholar]
- Middleton J. P., Khan W. A., Collinsworth G., Hannun Y. A., Medford R. M. Heterogeneity of protein kinase C-mediated rapid regulation of Na/K-ATPase in kidney epithelial cells. J Biol Chem. 1993 Jul 25;268(21):15958–15964. [PubMed] [Google Scholar]
- Minta A., Kao J. P., Tsien R. Y. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989 May 15;264(14):8171–8178. [PubMed] [Google Scholar]
- Natarajan V., Schmid H. H. Inositol phospholipid hydrolysis by rat sciatic nerve phospholipase C. J Neurochem. 1987 Dec;49(6):1878–1887. doi: 10.1111/j.1471-4159.1987.tb02450.x. [DOI] [PubMed] [Google Scholar]
- Roomi M. W., Ishaque A., Khan N. R., Eylar E. H. The PO protein. The major glycoprotein of peripheral nerve myelin. Biochim Biophys Acta. 1978 Sep 26;536(1):112–121. doi: 10.1016/0005-2795(78)90057-0. [DOI] [PubMed] [Google Scholar]
- Schrama L. H., Berti-Mattera L. N., Eichberg J. Altered protein phosphorylation in sciatic nerve from rats with streptozocin-induced diabetes. Diabetes. 1987 Nov;36(11):1254–1260. doi: 10.2337/diab.36.11.1254. [DOI] [PubMed] [Google Scholar]
- Sharma A. K., Thomas P. K. Peripheral nerve structure and function in experimental diabetes. J Neurol Sci. 1974 Sep;23(1):1–15. doi: 10.1016/0022-510x(74)90136-1. [DOI] [PubMed] [Google Scholar]
- Shindo H., Tawata M., Onaya T. Cyclic adenosine 3',5'-monophosphate enhances sodium, potassium-adenosine triphosphatase activity in the sciatic nerve of streptozotocin-induced diabetic rats. Endocrinology. 1993 Feb;132(2):510–516. doi: 10.1210/endo.132.2.7678791. [DOI] [PubMed] [Google Scholar]
- Sierro C. D., Vitus J., Dunant Y. Effects of muscarinic agonists and depolarizing agents on inositol monophosphate accumulation in the rabbit vagus nerve. J Neurochem. 1992 Aug;59(2):456–466. doi: 10.1111/j.1471-4159.1992.tb09392.x. [DOI] [PubMed] [Google Scholar]
- Simmons D. A., Winegrad A. I., Martin D. B. Significance of tissue myo-inositol concentrations in metabolic regulation in nerve. Science. 1982 Aug 27;217(4562):848–851. doi: 10.1126/science.6285474. [DOI] [PubMed] [Google Scholar]
- Sweadner K. J. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta. 1989 May 9;988(2):185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- Urayama O., Shutt H., Sweadner K. J. Identification of three isozyme proteins of the catalytic subunit of the Na,K-ATPase in rat brain. J Biol Chem. 1989 May 15;264(14):8271–8280. [PubMed] [Google Scholar]
- Wang J. K., Walaas S. I., Greengard P. Protein phosphorylation in nerve terminals: comparison of calcium/calmodulin-dependent and calcium/diacylglycerol-dependent systems. J Neurosci. 1988 Jan;8(1):281–288. doi: 10.1523/JNEUROSCI.08-01-00281.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Eichberg J. 1,2-diacylglycerol content and its arachidonyl-containing molecular species are reduced in sciatic nerve from streptozotocin-induced diabetic rats. J Neurochem. 1990 Sep;55(3):1087–1090. doi: 10.1111/j.1471-4159.1990.tb04604.x. [DOI] [PubMed] [Google Scholar]
- Zhu X., Eichberg J. A myo-inositol pool utilized for phosphatidylinositol synthesis is depleted in sciatic nerve from rats with streptozotocin-induced diabetes. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9818–9822. doi: 10.1073/pnas.87.24.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]