Abstract
Aims/hypothesis
As insulin entry into muscle interstitium is rate-limiting for its overall peripheral action, defining the route and regulation of its entry is critical. Caveolin-1 is required for caveola formation in vascular endothelial cells (ECs) and for EC insulin uptake. Whether this requirement reflects simply the need for caveola availability or involves a more active role for caveolae/caveolin-1 is not known. Here, we examined the role of insulin-stimulated tyrosine 14 (Tyr14)-caveolin-1 phosphorylation in mediating EC insulin uptake and the role of cellular Src-kinase (cSrc), TNF-α/IL-6 and high fat diet (HFD) in regulating this process.
Methods
Freshly-isolated ECs from normal or HFD-fed rats and/or cultured ECs were treated with FITC-labelled or regular insulin with or without a Src or phosphotidylinositol-3-kinase inhibitor, TNF-α or IL-6, or transfecting FLAG-tagged wild-type (WT) or mutant (Y14F) caveolin-1. Tyr14-caveolin-1/Tyr416 cSrc phosphorylation and FITC-insulin uptake were quantified by immunostaining and/or Western blots.
Results
Insulin stimulated Tyr14-caveolin-1 phosphorylation during EC insulin uptake. Inhibiting cSrc, but not phosphotidylinositol-3-kinase, reduced insulin-stimulated caveolin-1 phosphorylation. Furthermore, inhibiting cSrc reduced FITC-insulin uptake by ~50%. Overexpression of caveolin-1Y14F inhibited, while overexpression of WT caveolin-1 increased, FITC-insulin uptake. Exposure of ECs to TNF-α or IL-6, or to 1-week HFD feeding eliminated insulin-stimulated caveolin-1 phosphorylation and inhibited FITC-insulin uptake to a similar extent.
Conclusions/interpretation
Insulin stimulation of its own uptake requires caveolin-1 phosphorylation and Src-kinase activity. HFD in vivo and proinflammatory cytokines in vitro both inhibit this process.
Keywords: Caveolin-1, Endothelial cells, IL-6, Insulin uptake, TNF-α, Tyrosine phosphorylation
Introduction
Work from several laboratories has shown that the rate of insulin delivery to the interstitial fluid compartment of skeletal muscle is rate-limiting for muscle insulin action in vivo [1]. The rate of insulin delivery is slowed in insulin resistant, obese subjects, suggesting this process may contribute to peripheral insulin resistance [2,3].
Caveolae, the flask-shaped plasmalemmal invaginations, are abundant in microvascular endothelial cells (ECs) [4] constituting >95% of the cell vesicles [5]. In cultured cells, caveolae actively engage in the endocytosis of a variety of macromolecules [6] and this is also the case for vascular endothelium in vivo, thus caveolae can mediate the transcellular transport of plasma proteins through the endothelial barrier [7]. Caveolin-1 is the main structural unit and biological marker of EC caveolae, and is essential for their formation [8]. Additionally, caveolae are enriched with a variety of receptors and signalling molecules at the plasma membrane that facilitate cellular signal transduction [9]. For example, binding of albumin to its receptor gp60 induces gp60 clustering in caveolae and the tyrosine (Tyr) phosphorylation of both gp60 and caveolin-1, accompanied by cellular Src-kinase (cSrc) activation and increased albumin transendothelial transport (TET) [10]. Interestingly, in vascular ECs, Tyr14-caveolin-1 phosphorylation has been related to caveola budding and fusion, suggesting a role in macromolecule transcytosis [11].
We and others have previously reported that the insulin’s TET is mediated by insulin receptors (IRs) [12,13] and requires caveolin-1 [14] and intact post-receptor insulin signalling [15,16], consistent with a caveola-mediated transcytosis process. However, whether or how insulin action on caveolin-1 mediates insulin uptake is not clear.
We have previously shown that TNF-α interferes with EC insulin signalling and induces insulin resistance both in vivo [17] and in vitro [18]. We have also reported that exposure of ECs to TNF-α or IL-6 (20 ng/ml each) for 24 h inhibited the expression of caveolin-1 and blunted insulin’s entry into ECs without affecting cell viability [14]. Treatment with either TNF-α or IL-6 inhibits insulin-stimulated caveolin-1 phosphorylation at Tyr14 in both 3T3L1 adipocytes and fibroblasts [19]. In rodents and humans, short-term high fat diet (HFD) feeding (~1 week) can induce metabolic insulin resistance [20,21]. Whether or not physiological insulin concentrations regulate (and by what pathway) EC Tyr14-caveolin-1 phosphorylation, and whether or not this is required for insulin uptake and inhibited by insulin resistance is not known.
Therefore, we examined the effect of insulin on Tyr14-caveolin-1 phosphorylation in ECs and whether or not this was required for insulin uptake (the first step of its transendothelial transport). We also examined the signalling pathways mediating insulin-stimulated Tyr14-caveolin-1 phosphorylation and the effects of insulin resistance induced either in vitro by exposing ECs to TNF-α or IL-6 or in vivo by 1 week of HFD feeding.
Methods
An expanded Methods section containing details for all methods used can be found in electronic supplementary material (ESM) Methods.
Animals
Adult male Sprague–Dawley rats (Charles River Laboratories, Wilmington, MA, USA) were either on HFD (60% fat) (Research Diets, New Brunswick, NJ, USA) or control diet (chow) for 1 week. The aortic ECs were freshly harvested and treated ex vivo [16]. The study procedure was approved by the Animal Care and Use Committee of the University of Virginia.
Serum analyses
Measurement of serum glucose, insulin and triacylglycerol was carried out as detailed in the ESM Methods.
Cell culture
Small interfering RNA design and transfection
Small interfering RNA (SiRNA) knockdown of IR was carried out in ECs as described previously [16,22,23].
DNA constructs and transfection
Plasmids encoding FLAG-tagged wild-type (WT) caveolin-1 and caveolin-1 mutation of tyrosine 14 to phenylalanine (Y14F) were obtained from M. A. Schwartz (University of Virginia) and transfected in ECs as described previously [14,24].
Western blotting
Western blotting was performed as described previously [14,23].
Immunocytochemistry
The double-staining protocols have been described previously [13,15].
Imaging
Immunocytochemical staining was examined using a confocal microscope as described previously [16].
Statistical analysis
Data are presented as mean ± SEM. Comparisons among different groups were made using one-way ANOVA with post-hoc testing performed by the method of Student–Newman–Keuls. Statistical significance was defined as p≤0.05.
Results
Insulin stimulates caveolin-1 Tyr14 phosphorylation
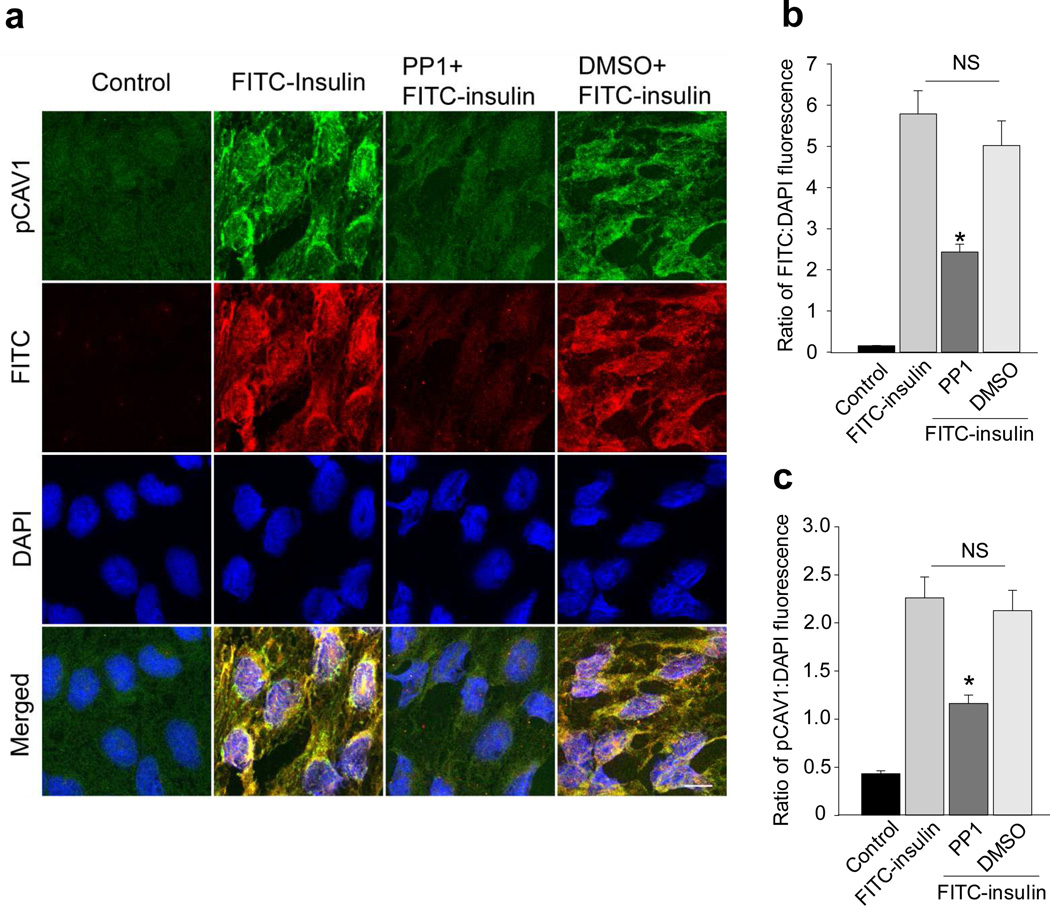
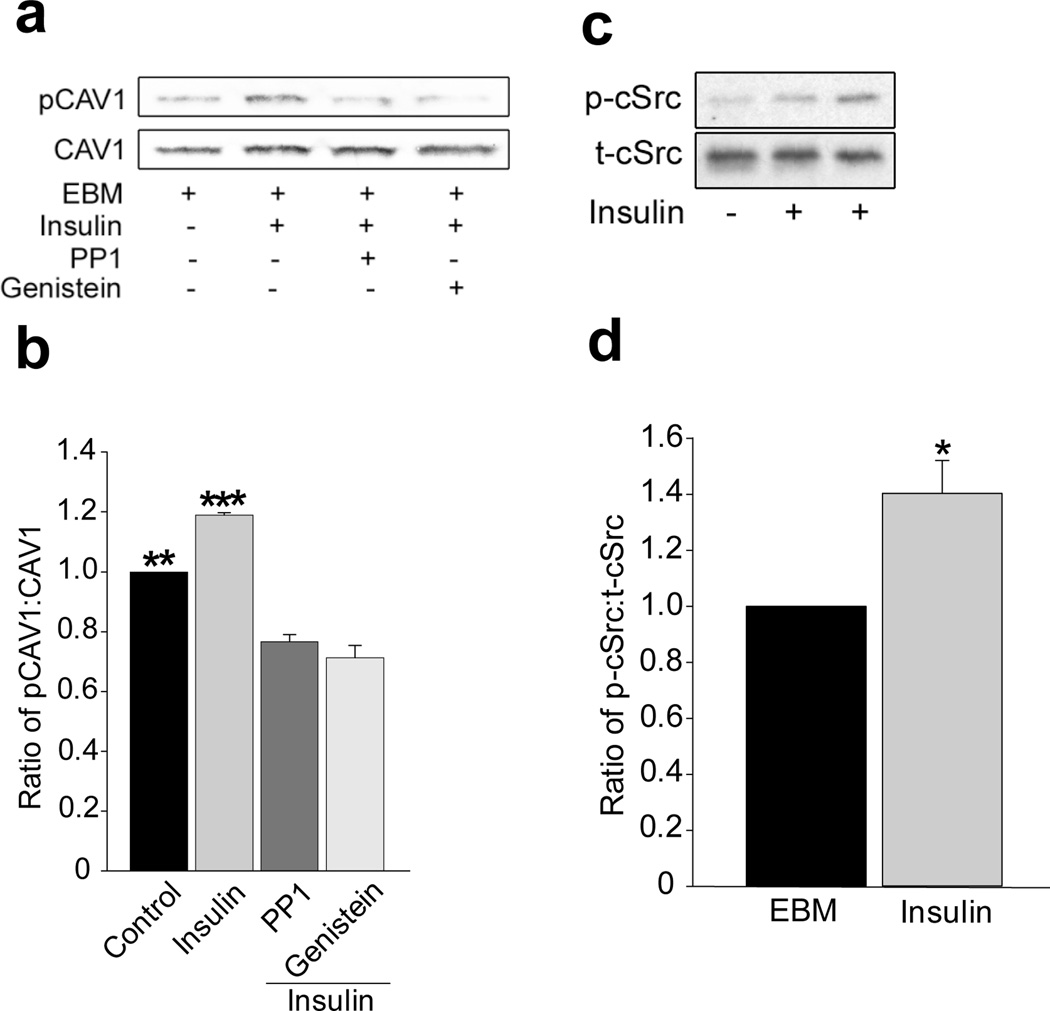
We first examined whether insulinstimulated caveolin-1 phosphorylation at Tyr14 and whether the cSrc inhibitor PP1 affected Tyr14-caveolin-1 phosphorylation and FITC-insulin uptake by freshly-harvested rat aortic ECs (rAECs). FITC-insulin treatment increased Tyr14-caveolin-1 phosphorylation and pre-treating ECs with PP1 inhibited both Tyr14-caveolin-1 phosphorylation and FITC-insulin uptake (~50% each; Fig. 1a–c). Next, we examined the effects of insulin signalling on caveolin-1 phosphorylation at Tyr14 in cultured bovine aortic endothelial cell (bAECs). Figure 2a and 2b show that 30 min of insulin treatment significantly increased Tyr14-caveolin-1 phosphorylation, and both PP1 and genistein not only completely abolished insulin-stimulated caveolin-1 phosphorylation at Tyr14 but also significantly inhibited the basal Tyr14-caveolin-1 phosphorylation. Inhibiting insulin signalling through phosphoinositide 3-kinase (PI3-K; wortmannin) had no effect (ESM Fig. 1).
Fig. 1.
Effects of Src inhibitor PP1 on FITC-insulin uptake and caveolin-1 phosphorylation at Tyr14. Rat ECs were pre-treated with either PP1 or DMSO (vehicle control) ex vivo before FITC-insulin treatment then stained for phospho-caveolin-1 (pCAV1; green) and FITC (red). (a) Representative confocal images from two independent experiments (two duplicates for each experiments). Histograms indicating the quantification of (b) FITC-insulin and (c) pCAV1. (A total 16 microscopic fields were randomly selected for quantification of fluorescent intensities for each group). *p<0.05 compared with remaining groups. Scale bar 10 µm
Fig. 2.
Effects of insulin or kinase inhibitors on insulin-induced Tyr14-caveolin-1 or Tyr416-cSrc phosphorylation. Cultured bAECs were serum-starved for 24 h then treated with insulin in the presence or absence of either PP1 or genistein (GENI). (a) A representative blot from three independent experiments. (b) Mean values for the ratio of phospho-caveolin-1 (pCAV1) to total caveolin-1 (CAV1). (c) A representative blot from three independent experiments. (d) Mean values for the ratio of phospho-cSrc (p-cSrc) to total cSrc (t-cSrc). *p<0.05 compared with control group; **p<0.01 compared with PP1 or GENI; ***p<0.001 compared with PP1 or GENI and p<0.01 compared with control
We also found that insulin stimulated cSrc phosphorylation at Tyr416 in its activation loop (Fig. 2c,d). This phosphorylation has been reported to stabilise the enzyme in its active state [25] and has been used as a surrogate measure for Src-kinase activity [26].
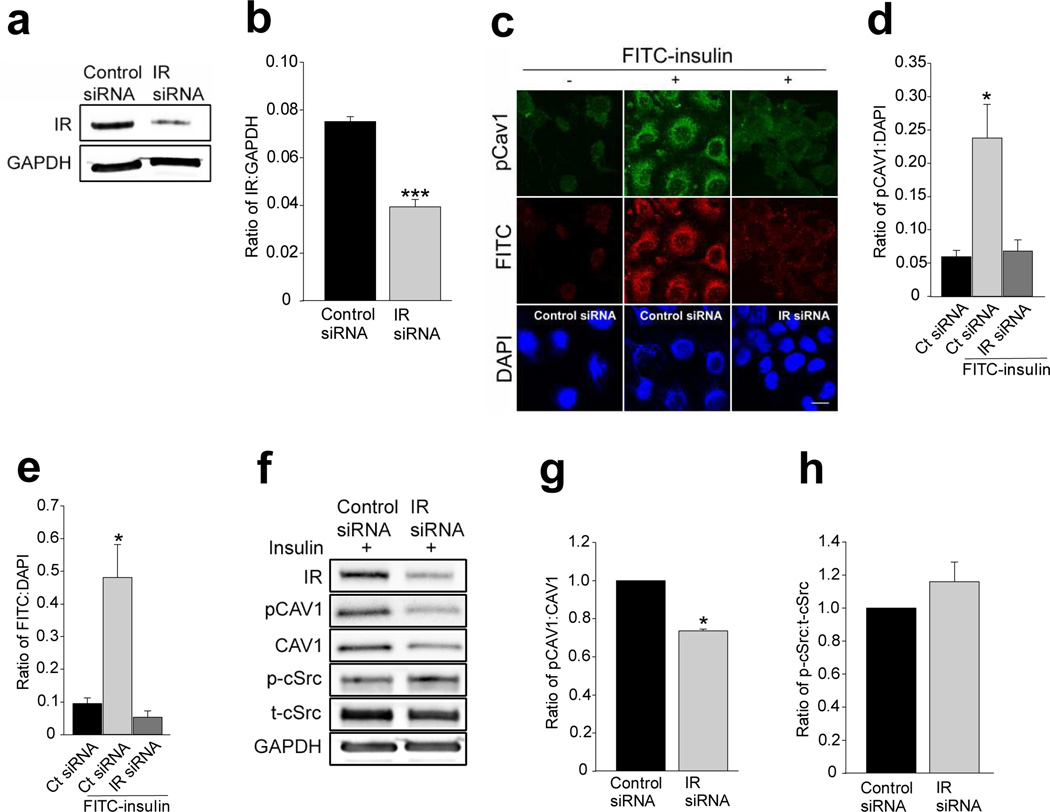
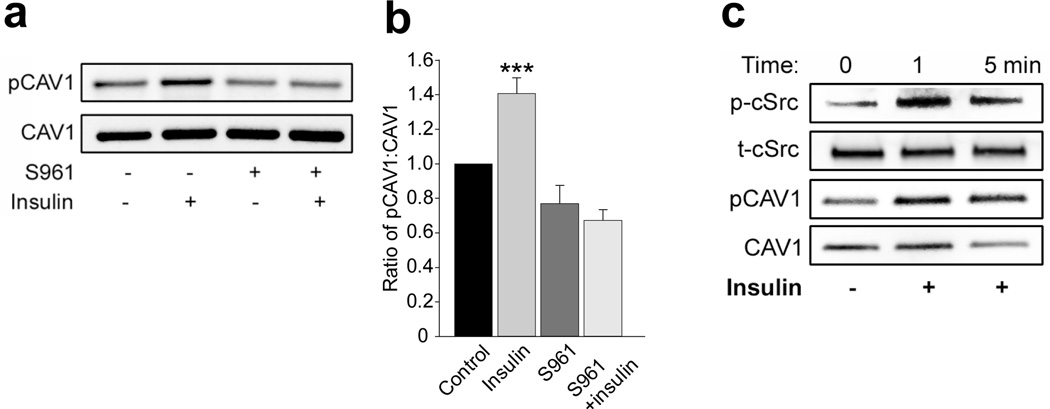
We then examined the effects of siRNA knockdown of the IR on insulin-stimulated Tyr14-caveolin-1 phosphorylation. Compared with scrambled siRNA, transfection of cells with specific siRNAs against the IR reduced EC IR abundance by~50% (Fig. 3a,b) and eliminated the immunofluorescence of insulin-stimulated Tyr14-caveolin-1 phosphorylation and FITC-insulin uptake (Fig. 3c–f) but did not affect insulin-stimulated cSrc Tyr416 phosphorylation (Fig. 3f,h). Knockdown of IR appeared to affect caveolin-1 protein content and the ratio of total caveolin-1 to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was reduced by ~30% compared with the control siRNA group (p>0.05, n=4; Fig. 3f). However, knockdown of IR significantly reduced the ratio of phosphorylated caveolin-1 to total caveolin-1 by ~30% (p<0.05, n=4; Fig. 3f,g). We further examined the effect of directly inhibiting IR activation on insulin-stimulated Tyr14-caveolin-1 phosphorylation using a specific peptide IR antagonist, S961 [27,28]. Compared with controls, S961 treatment significantly inhibited insulin-stimulated Tyr14-caveolin-1 phosphorylation (Fig. 4a,b).
Fig. 3.
Effects of siRNA knockdown of IR on caveolin-1 expression and insulin-stimulated caveolin-1 Tyr14 phosphorylation. Cultured rAECs were transfected with either scrambled siRNAs or specific siRNAs against IR for 72 h with or without 3 h serum starvation, followed by insulin treatment for 30 min before being processed for either western blots or immunocytochemiacal staining. (a) A representative blot from three independent experiments. (b) Mean values for the ratio of IR to GAPDH. (c) Representative confocal images from three independent experiments, scale bar 10 µm. Histograms indicate the quantification of immunofluorescence of (d) Tyr14-phosphorylated caveolin-1 (pCAV1) and (e) FITC-insulin. (f) A representative blot from three independent experiments (g) mean values for the ratio of pCAV1 to total caveolin-1 (CAV1). (h) Mean values for the ratio of Tyr416-phosphorylated cSrc (p-Src) to total cSrc (t-Src). * p<0.05 compared with controls; ***p<0.001 compared with controls
Fig. 4.
Effects of S961 on insulin-stimulated caveolin-1 phosphorylation at Tyr14. rAECs were serum-starved for 6 h before insulin treatment (10 nmol/l) with or without S961 (20 nmol/l) for 30 min. (a) Representative blots from three independent experiments. (b) Mean values for the ratio of phospho-caveolin-1 (pCAV1) to total caveolin-1 (CAV1). (c) A representative blot from three independent experiments indicating early changes in insulin-stimulated cSrc Tyr416 phosphorylation and caveolin-1 Tyr14 phosphorylation. p-Src, Tyr416-phosphorylated cSrc; t-Src total cSrc. *** p<0.001 compared with remaining groups
These data indicate that in vascular ECs insulin can stimulate Tyr14-caveolin-1 phosphorylation and this process requires intact IR activity. Insulin-induced activation of Src-kinase also contributes to this process, which is consistent with findings in other cell types [19]. Since we have previously observed that EC insulin uptake reaches plateau within ~10 min both in vivo and in vitro [13,29], we further examined the effects of insulin treatment on both Tyr14-caveolin-1 and Tyr416-cSrc phosphorylation in the early phase of the treatment. Figure 4c shows that insulin stimulated both Tyr14-caveolin-1 and Tyr416-cSrc phosphorylation within 1 min and these effects lasted for at least 30 min (Fig. 4a–c; Fig. 2c,d).
Mutation of Tyr14 of cavolin-1 inhibits EC insulin uptake
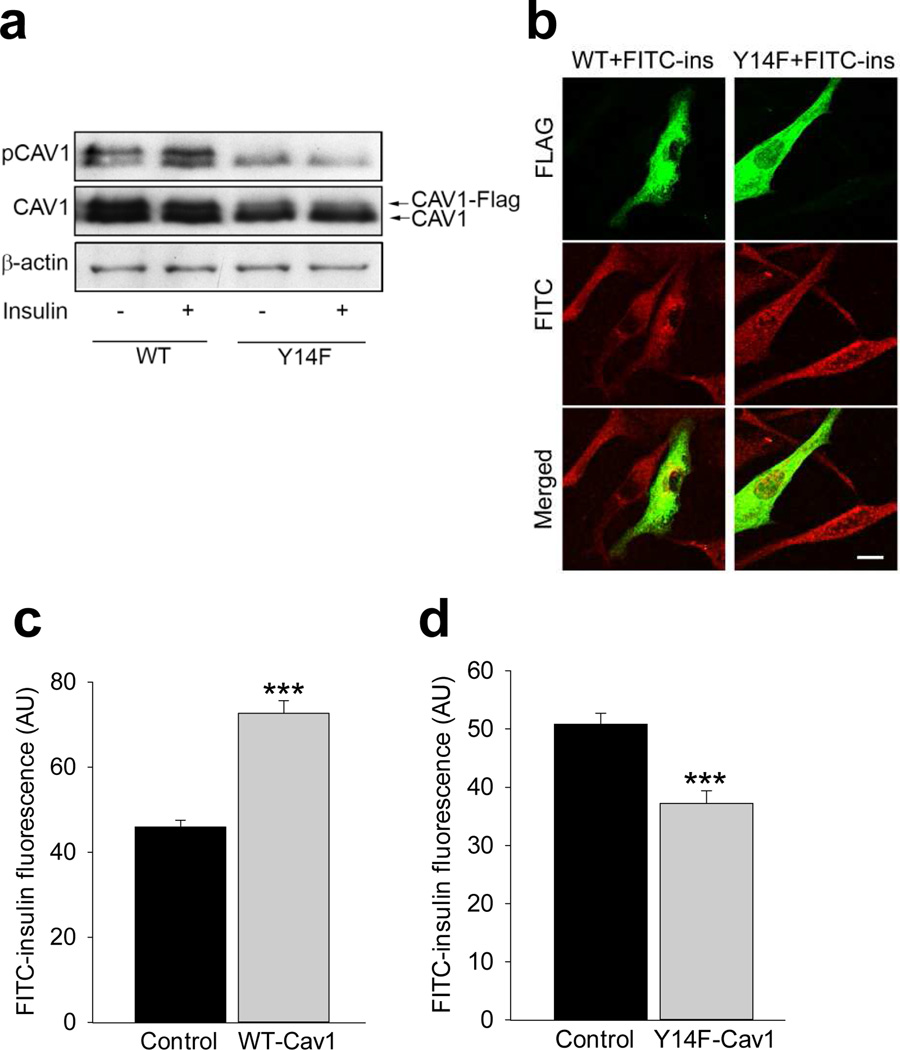
Since Tyr14-caveolin-1 phosphorylation corresponded to EC FITC-insulin uptake (Fig. 1a–c) and as we have previously shown that overexpression of WT caveolin-1 significantly increased FITC-insulin uptake by bAECs [14], we further examined the effects of overexpression of a FLAG-tagged caveolin-1 mutant at Tyr14, that is caveolin-1Y14F on FITC-insulin uptake by bAECs, and compared the results with that in the cells overexpressing WT caveolin-1.
Figure 5a shows that insulin (10 nmol/l for 30 min) increased both endogenous and FLAG-tagged WT caveolin-1 phosphorylation at Tyr14. By contrast, insulin failed to stimulate either FLAG-tagged caveolin-1Y14F or endogenous caveolin-1 Tyr14 phosphorylation in the mutant-transfected cells. In addition, FITC-insulin uptake significantly increased by ~40% in the bAECs transfected with WT caveolin-1 (Fig. 5b,c), while in the cells transfected with caveolin-1Y14F (Fig. 5b,d) FITC-insulin uptake decreased significantly by ~30% compared with control cells, that is adjacent cells not expressing FLAG. This indicates a regulatory role of insulin-stimulated Tyr14-caveolin-1 phosphorylation in insulin uptake.
Fig. 5.
Effects of overexpression of FLAG-tagged WT caveolin-1 or FLAG-tagged caveolin-1 Y14F mutant in bAECs on FITC-insulin uptake. (a) Representative blots of three independent experiments. (b) Representative confocal images of three independent experiments staining for FITC (red) and FLAG (green), scale bar 10 µm. (c, d) Average fluorescent intensity of individual cells from three independent experiments; total four groups; FLAG-tagged WT caveolin-1 transfected group (c), FLAG-tagged caveolin-1Y14F mutant-transfected group (d) and non-transfected (no FLAG labelling) groups in both (c) and (d) serving as the control (n=16 for each group). In the FLAG-tagged WT CAV1 or FLAG-tagged caveolin-1Y14F mutant-transfected group, only strongly FLAG-positive cells (FLAG+) were selected for counting, and only those cells near the FLAG+ cells in which no FLAG labelling was seen were defined as non-transfected cells and selected for counting. ***p<0.001 compared with the control group. pCAV1, phospho-caveolin-1; WTCAV1, FLAG-tagged WT caveolin-1; Y14F-CAV1, FLAG-tagged caveolin-1Y14F mutant
Proinflammatory cytokines in vitro and HFD feeding in vivo inhibit endothelial insulin-stimulated Tyr14-caveolin-1 phosphorylation and insulin uptake
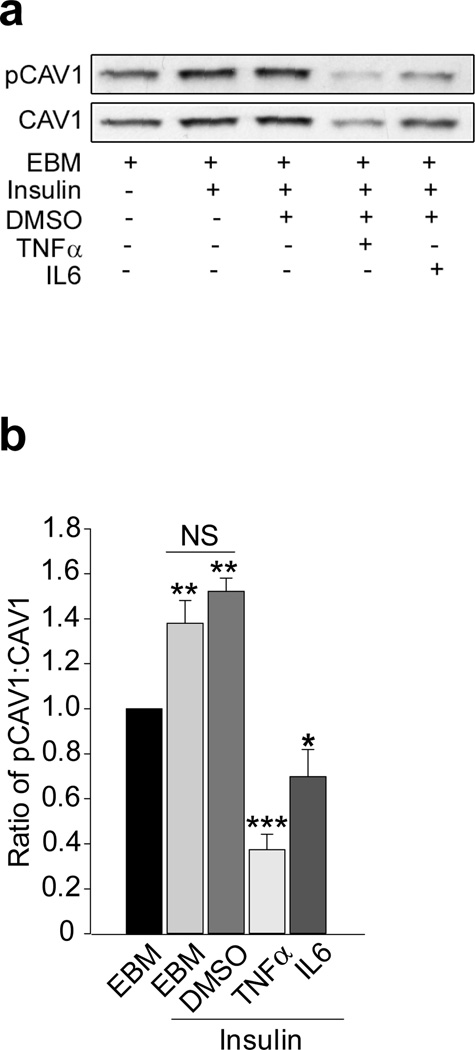
We have previously shown that treating bAECs with 5 ng/ml TNF-α for just 6 h reduced insulin signalling and uptake [15,18]. In addition, incubating bAECs with either TNF-α or IL-6 (20 ng/ml each) for 24 h significantly decreased both caveolin-1 expression and FITC-insulin uptake without affecting cell viability [14]. Here, we examined the effects of 24 h TNF-α or IL-6 treatment (20 ng/ml for each) on insulin-stimulated Tyr14-caveolin-1 phosphorylation. Compared with control bAECs, either TNF-α or IL-6 inhibited both caveolin-1 protein expression and caveolin-1 phosphorylation at Tyr14 as shown by the ratio of phospho-caveolin-1 to total caveolin-1 (Fig. 6a, b), suggesting that these cytokines may target multiple sites within insulin signalling pathways [30].
Fig. 6.
Effects of cytokines on Tyr14-caveolin-1 phosphorylation. bAECs with or without IL-6 or TNF-α or DMSO (vehicle) were incubated in EBM for 24 h before insulin treatment. (a) A representative western blot from four independent experiments. (b) Quantitative data for the ratio of phospho-caveolin-1 (pCAV1) to total caveolin-1 (CAV1). *p<0.05 compared with EBM alone; **p<0.01 compared with EBM alone and †††p<0.001 compared with TNF-α+insulin or IL-6+insulin; ***p<0.001 compared with EBM alone and p<0.01 compared with IL-6+insulin
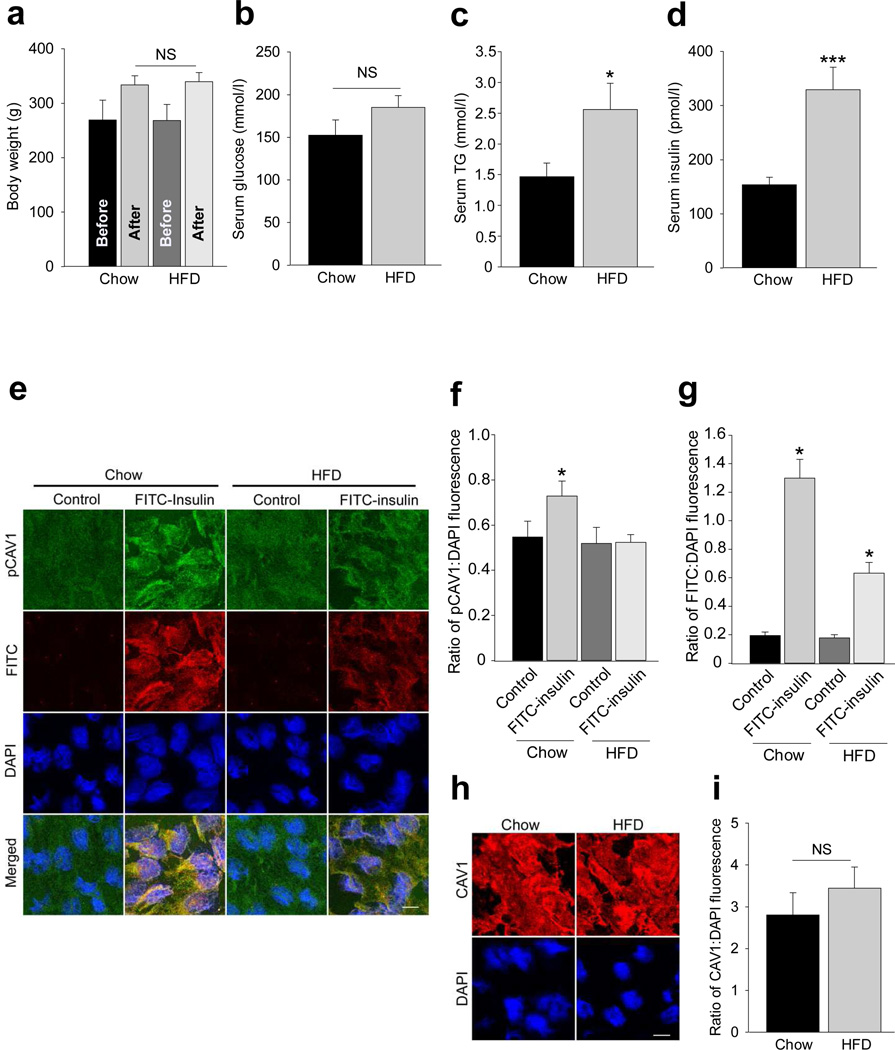
Since 1 week of HFD feeding has been shown to induce insulin resistance in both rodents and young healthy men [20,21], we next examined the effect of HFD feeding on insulin-stimulated Tyr14-caveolin-1 phosphorylation and FITC-insulin uptake by freshlyisolated rat aortic ECs. Figure 7a–d shows that, compared with the chow diet, HFD feeding for 1 week significantly increased serum TG and insulin concentrations, while no significant changes were observed in body weight or serum glucose level in HFD rats. Interestingly, in chow-fed rats FITC-insulin significantly stimulated caveolin-1 phosphorylation at Tyr14 accompanied by increased EC FITC-insulin uptake. By contrast, HFD feeding completely abolished insulin-stimulated Tyr14-caveolin-1 phosphorylation and inhibited EC FITC-insulin uptake by ~50% (Fig. 7e–g) compared with chow-fed rats. However, HFD did not cause any significant changes in rat EC caveolin-1 content (Fig. 7h,i). These data further support that insulin-stimulated Tyr14-caveolin-1 phosphorylation can regulate endothelial insulin uptake and suggest that proinflammatory cytokines and HFD feeding may reduce EC insulin uptake, at least in part, by inhibition of insulin-stimulated Tyr14-caveolin-1 phosphorylation.
Fig. 7.
Effects of high fat feeding on endothelial caveolin-1 phosphorylation at Tyr14 and FITC-insulin uptake in rats. Rat ECs from either HFD or chow-fed rats were stained for phospho-caveolin-1 (pCAV1; green) and FITC (red). Metabolic characteristics of the rats on either HFD or regular chow diet (n=7 for each group): (a) body weight, (b) serum glucose concentration, (c) serum TG, *p<0.05 compared with the chow group; (d) serum insulin concentrations, ***p<0.001 compared with the chow group. (e) Representative confocal images. Histograms indicate quantification of (f) pCAV1 or (g) FITC-insulin. *p<0.05 compared with remaining groups (f and g). (h) Representative confocal images. (i) Histograms indicate the quantification of CAV1 (n=4 for each group). Scale bars, 10 µm
Discussion
Our findings indicate a significant role of Tyr14-caveolin-1 phosphorylation in the process of EC insulin uptake. The observation that HFD for 1 week completely eliminated insulin-stimulated EC Tyr14-caveolin-1 phosphorylation and significantly inhibited FITC-insulin uptake supports the physiologic/clinical significance of this pathway. Importantly, transfection of ECs with WT caveolin-1 significantly increased EC FITC-insulin uptake while transfection with the caveolin-1 Y14F mutant inhibited EC insulin uptake by ~30%. These data, to our knowledge, provide the first direct evidence indicating that EC insulin uptake involves Tyr14-caveolin-1 phosphorylation.
We also found that the Src inhibitor PP1 dramatically reduced rat EC Try14-caveolin-1 phosphorylation and comparably decreased EC FITC-insulin uptake ex vivo, indicating a crucial role of cSrc kinase in regulation of this process. We found that insulin stimulated cSrc phosphorylation at Tyr416 in its activation loop [25], which can directly increase Src activity [26]. Caveolin-1 is a major tyrosine-phosphorylated substrate of Src-kinase [31,32]. In both 3T3L1 adipocytes and fibroblasts insulin can stimulate caveolin-1 phosphorylation at Try14 [19]. Both insulin-stimulated Tyr14-caveolin-1 and Tyr416-cSrc phosphorylation occurred very rapidly (within 1 min) upon insulin treatment and lasted for at least 30 min, which is in parallel with the dynamics of EC insulin uptake both in vivo and in vitro (which reaches a plateau within 10 min) [13,29]. Moreover, Try14-caveolin-1 phosphorylation is induced by the inhibition of tyrosine phosphatase and is dramatically reduced by the inhibition of Src-kinase; however, it is not affected by the inhibition either of EGF and platelet-derived growth gactor receptor tyrosine kinases [19] or p42/44 mitogen-activated protein kinase [33].
In the present study, we also show that blocking PI3-K activity did not affect insulin-stimulated Try14-caveolin-1 phosphorylation. However, PI3-K activity does inhibit insulin uptake and TET [22], consistent with similar finding in adipocytes [34]. Of note, we found that either knockdown of IR by using a specific siRNA or inhibition of IR activity by S961 eliminates insulin-induced Tyr14-caveolin-1 phosphorylation. Previously, it has been reported that blocking the direct interaction between caveolin-1 and IR by mutation of caveolin-1 scaffolding domain prevents insulin-stimulated Tyr14-caveolin-1 phosphorylation in adipocytes [34]. These data suggest that insulin-induced caveolin-1 phosphorylation is IR or IR-activity-dependent. In the present study, we also found that insulin stimulated Tyr416-cSrc phosphorylation. Inhibiting Src-kinase by PP1 significantly inhibited insulin-induced Tyr14-caveolin-1 phosphorylation, suggesting that Src-kinase contributed to insulin-stimulated caveolin-1 phosphorylation [19]. Knockdown of IR did not affect Tyr416-cSrc phosphorylation but completely eliminated both insulin-stimulated caveolin-1 phosphorylation and insulin uptake, suggesting that intact insulin signalling is essential for this process. The exact mechanism for this selective effect is unclear. It has been reported that enhanced protein phosphatase 1B activity, induced by reduced IRs [35], causes cSrc dephosphorylation at Tyr527 leading to increased cSrc auto-phosphorylation at Tyr416 [36], and may contribute to the selective effect induced by knockdown of IR. Recently, it has been reported that cSrc activation by a calcium and nitric oxide-dependent mechanism stimulates Tyr14-caveolin-1 phosphorylation in human umbilical vein ECs [37]. Early studies have shown that binding of protein macromolecules to their receptors induced a caveola-mediated endocytosis of these proteins in rat liver cells [38], Hela cells [6] and ECs [7]. This caveola-mediated endocytosis was Src-dependent as knockdown of Src caused caveola clustering and defective endocytosis of macromolecules [6,39]. In studies of albumin TET, binding of albumin to its receptor (gp60) was found to activate cSrc kinase and induce Tyr14-caveolin-1 phosphorylation [10]. The resultant increase in EC albumin uptake was prevented by inhibition of protein tyrosine kinase [10] and by expression of dominant negative Src [40]. Thus, our data are consistent with previous findings that Src tyrosine kinase is responsible for insulin-stimulated Tyr14-caveolin-1 phosphorylation in ECs and plays an important role in the regulation of EC insulin uptake.
The exact mechanism by which insulin-stimulated Tyr14-caveolin-1 phosphorylation enhances EC insulin uptake is not clear. Early studies demonstrate that insulin TET is saturable and mediated by IRs both in vitro and in vivo [12,41]. However, this appeared not to be the case when supraphysiological insulin doses were applied in vivo [42]. Given that vascular ECs also possess insulin-like growth factor-1 receptors (IGF-1Rs), which are ~10-times more abundant than IRs with a much lower affinity for insulin binding [43,44], we found that both IGF-1 peptide and a neutralising antibody against IGF-1R significantly inhibited insulin uptake and TET when a pharmacologic insulin concentration (50 nmol/l) was used [13]. This finding provides an alternative explanation for the seemingly conflicting data regarding the saturability of insulin transport into muscle; specifically, that at physiological insulin concentrations insulin TET is mediated predominantly by IRs, but at supraphysiologic insulin concentrations both IR and IGF-1R (and IR/IGF-1R hybrid receptors) contribute to insulin TET [45,46]. In addition, for unclear reasons, vascular EC-specific IR knockout mice are only insulin resistant when on a low-salt diet [47] (compensation by IGF-1R may play a role). However, a recent in vivo study has shown that inhibiting EC insulin signalling by endothelium-specific knockout of insulin receptor substrate (IRS)-2 reduces EC endothelial nitric oxide synthase (eNOS) activity and reduces insulin delivery to muscle interstitium [48]. Consistent with this finding, we have recently demonstrated that nitric oxide directly promotes endothelial insulin uptake and TET [16].
Src-mediated Tyr14-caveolin-1 phosphorylation has been shown to affect caveolin-1 association with, and caveola targeting of, both signalling molecules (e.g. eNOS, dynamin-2) and kinases such as active cSrc [37,49,50]. It has been reported that in ECs, albumin binding to its receptor induced cSrc activation that simultaneously increased the tyrosine phosphorylation of both dynamin-2 (a large GTPase that is required for the fission of plasmalemmal caveolae for transcytosis) and Tyr14-caveolin-1 accompanied by increased association of these two tyrosine-phosphorylated proteins and caveola-mediated endocytosis of albumin [49]. Either expression of kinase-defective Src or inhibition of Src-kinase activity by PP2 interrupted the association between dynamin-2 and caveolin-1 and prevented albumin uptake [49]. Interestingly, a recent study found that increased interaction of phosphorylated caveolin-1 with eNOS mediated by cSrc activation was determined by Tyr14-caveolin-1 phosphorylation but not by phosphorylation of eNOS at Ser1177 [37]. This suggests that Tyr14-phosphorylated caveolin-1 may serve as a specific form of scaffold to recruit and organise multiple molecular components of endothelial caveolar transcytotic machinery [51].
Moreover, increasing EC Tyr14-caveolin-1 phosphorylation by inhibition of protein tyrosine phosphatase promotes caveolar vesicles moving away from the cell surface into the cytoplasm [11], supporting the important role of Tyr14-caveolin-1 phosphorylation in the regulation of EC caveola-mediated macromolecule transport. Further studies are needed to clarify the exact mechanism by which insulin-stimulated Tyr14-caveolin-1 phosphorylation enhances EC insulin uptake.
We have previously shown that TNF-α or IL-6 can inhibit EC insulin uptake through inhibition of EC caveolin-1 expression [14]. Here, we observed that TNF-α or IL-6 treatment strikingly diminished the insulin-stimulated Tyr14-caveolin-1 phosphorylation in ECs. This is consistent with previous findings in both 3T3L1 adipocytes and fibroblasts [19]. In addition, TNF-α treatment has been found to inhibit insulin-stimulated IR autophosphorylation, insulin-stimulated IRS-1 tyrosine phosphorylation and IR protein expression (at a dose >5 ng/ml) in adipocytes, indicating that it blocks insulin action by direct interference to IR signalling [52]. Similarly, both acute and chronic exposure to IL-6 has been shown to cause hepatic insulin resistance in vitro and in vivo through significant inhibition of IR autophosphorylation and IRS-1/2 tyrosine phosphorylation [53]. These data suggest that these cytokines target multiple sites of the molecular machinery governing caveola-mediated macromolecule endocytosis and profoundly impair EC insulin uptake [14].
Interestingly, 1 week of HFD similarly almost completely eliminated EC insulin-stimulated Tyr14-caveolin-1 phosphorylation and strongly inhibited EC insulin uptake, though caveolin-1 expression was not significantly affected by the HFD. Serum insulin level doubled following 1 week of HFD feeding compared with the chow-fed rats. It has been shown that HFD feeding can elicit liver insulin resistance within 3 days in rats [20] and 5 days in young healthy men [21]. Of note, HFD-fed mice had impaired insulin signalling in aortic tissue much earlier (within 1 week of HFD feeding) than in liver, muscle or adipose tissue [54]. The early impairment of insulin-stimulated Tyr14-caveolin-1 phosphorylation and aortic EC insulin uptake in HFD-fed rats seen in the present study is consistent with the findings in HFD-fed mice [54]. These data are consistent with ECs being an early responder to the HFD insult that can profoundly impair EC insulin uptake.
The present results suggest that insulin-stimulated Tyr14-caveolin-1 phosphorylation plays a significant role in the regulation of EC insulin uptake. However, we are mindful that insulin uptake by cells transfected with caveolin-1Y14F mutant decreased only modestly (~30%). This estimate does not correct for background EC fluorescence and may, therefore, underestimate the inhibition of insulin uptake. Thus, Tyr14-caveolin-1 phosphorylation may facilitate, but may not be absolutely required, for insulin uptake by ECs. Alternatively, the residual endogenous caveolin-1 may have been sufficient to support insulin uptake in the mutant-transfected cells.
In summary, EC insulin uptake occurs via a process involving IR binding, activation of EC insulin signalling and membrane trafficking via caveolae [13–16. 45]. The findings presented here provide the first evidence indicating that insulin may facilitate its own EC transport in part by stimulating Tyr14-caveolin-1 phosphorylation in an IR-dependent fashion. Insulin-stimulated activation of cSrc contributes to this process. This phosphorylation, as well as EC insulin uptake, is inhibited in vivo by HFD for a short time and by TNF-α and IL-6 in vitro.
Supplementary Material
Acknowledgments
Funding
This work was supported by research grants from the NIH (DK057878 and DK073059) and ADA 11-BS6 to EJB.
Abbreviations
- bAEC
Bovine aortic endothelial cell
- cSrc
Cellular Src-kinase
- EBM
Endothelial basal medium
- ECs
Endothelial cells
- eNOS
Endothelial nitric oxide synthase
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HFD
High fat diet
- IGF-1R
Insulin-like growth factor-1 receptor
- IR
Insulin receptor
- IRS
Insulin receptor substrate
- PI3-K
Phosphoinositide 3-kinase
- rAEC
Rat aortic endothelial cell
- siRNA
Small interfering RNA
- TET
Transendothelial transport
- TG
Triacylglycerol
- Tyr
Tyrosine
- WT
Wild-type
- Y14F
Mutation of tyrosine 14 to phenylalanine
Footnotes
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
HW designed the study, conducted experiments, performed data analyses and wrote the manuscript. AW and KA contributed to data acquisition and discussion for drafting the article. EB contributed to discussion for designing the study and interpreting the data, and reviewed and edited the manuscript. HW is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version of the manuscript to be published.
References
- 1.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J.Clin.Invest. 1989;84:1620–1628. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miles PD, Li S, Hart M, et al. Mechanisms of insulin resistance in experimental hyperinsulinemic dogs. J.Clin.Invest. 1998;101:202–211. doi: 10.1172/JCI1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sjostrand M, Gudbjornsdottir S, Holmang A, Lonn L, Strindberg L, Lonnroth P. Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes. 2002;51:2742–2748. doi: 10.2337/diabetes.51.9.2742. [DOI] [PubMed] [Google Scholar]
- 4.Simionescu M, Simionescu N, Palade GE. Morphometric data on the endothelium of blood capillaries. J.Cell Biol. 1974;60:128–152. doi: 10.1083/jcb.60.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Predescu D, Palade GE. Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium. Am.J.Physiol. 1993;265:H725–H733. doi: 10.1152/ajpheart.1993.265.2.H725. [DOI] [PubMed] [Google Scholar]
- 6.Pelkmans L, Zerial M. Kinase-regulated quantal assemblies and kiss-and-run recycling of caveolae. Nature. 2005;436:128–133. doi: 10.1038/nature03866. [DOI] [PubMed] [Google Scholar]
- 7.Oh P, Borgstrom P, Witkiewicz H, et al. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat.Biotechnol. 2007;25:327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drab M, Verkade P, Elger M, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing "preassembled signaling complexes" at the plasma membrane. J.Biol.Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 10.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J.Biol.Chem. 1997;272:25968–25975. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 11.Aoki T, Nomura R, Fujimoto T. Tyrosine phosphorylation of caveolin-1 in the endothelium. Experimental Cell Research. 1999;253:629–636. doi: 10.1006/excr.1999.4652. [DOI] [PubMed] [Google Scholar]
- 12.King GL, Johnson SM. Receptor-mediated transport of insulin across endothelial cells. Science. 1985;227:1583–1586. doi: 10.1126/science.3883490. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Liu Z, Li G, Barrett EJ. The vascular endothelial cell mediates insulin transport into skeletal muscle. Am.J.Physiol Endocrinol.Metab. 2006;291:E323–E332. doi: 10.1152/ajpendo.00047.2006. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Wang AX, Barrett EJ. Caveolin-1 is required for vascular endothelial insulin uptake. Am.J.Physiol Endocrinol.Metab.2011. 2011;300:E134–E144. doi: 10.1152/ajpendo.00498.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, Wang AX, Liu Z, Barrett EJ. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes. 2008;57:540–547. doi: 10.2337/db07-0967. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Wang AX, Aylor K, Barrett EJ. Nitric oxide directly promotes vascular endothelial insulin transport. Diabetes. 2013;62:4030–4042. doi: 10.2337/db13-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Wheatley CM, Richards SM, Barrett EJ, Clark MG, Rattigan S. TNF-alpha acutely inhibits vascular effects of physiological but not high insulin or contraction. Am.J.Physiol Endocrinol.Metab. 2003;285:E654–E660. doi: 10.1152/ajpendo.00119.2003. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Barrett EJ, Barrett MO, Cao W, Liu Z. Tumor necrosis factor-alpha induces insulin resistance in endothelial cells via a p38 mitogen-activated protein kinase-dependent pathway. Endocrinology. 2007;148:3356–3363. doi: 10.1210/en.2006-1441. [DOI] [PubMed] [Google Scholar]
- 19.Lee H, Volonte D, Galbiati F, et al. Constitutive and growth factor-regulated phosphorylation of caveolin-1 occurs at the same site (Tyr-14) in vivo: identification of a c-Src/Cav-1/Grb7 signaling cassette. Mol.Endocrinol. 2000;14:1750–1775. doi: 10.1210/mend.14.11.0553. [DOI] [PubMed] [Google Scholar]
- 20.Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 1991;40:1397–1403. doi: 10.2337/diab.40.11.1397. [DOI] [PubMed] [Google Scholar]
- 21.Brons C, Jensen CB, Storgaard H, et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J.Physiol. 2009;587:2387–2397. doi: 10.1113/jphysiol.2009.169078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Wang AX, Barrett EJ. Insulin-induced endothelial cell cortical actin filament remodeling: a requirement for trans-endothelial insulin transport. Mol.Endocrinol. 2012;26:1327–1338. doi: 10.1210/me.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Wang AX, Liu Z, Chai W, Barrett EJ. The trafficking/interaction of eNOS and caveolin-1 induced by insulin modulates endothelial nitric oxide production. Mol.Endocrinol.2009. 2009;23:1613–1623. doi: 10.1210/me.2009-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Pozo MA, Balasubramanian N, Alderson NB, et al. Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nat.Cell Biol.2005. 2005;7:901–908. doi: 10.1038/ncb1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem.Biophys.Res.Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 27.Schaffer L, Brand CL, Hansen BF, et al. A novel high-affinity peptide antagonist to the insulin receptor. Biochem.Biophys.Res.Commun. 2008;376:380–383. doi: 10.1016/j.bbrc.2008.08.151. [DOI] [PubMed] [Google Scholar]
- 28.Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic beta cell proliferation. Cell. 2013;153:747–758. doi: 10.1016/j.cell.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Genders AJ, Frison V, Abramson SR, Barrett EJ. Endothelial cells actively concentrate insulin during its transendothelial transport. Microcirculation. 2013;20:434–439. doi: 10.1111/micc.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J.Biol.Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 31.Glenney JR., Jr Tyrosine phosphorylation of a 22-kDa protein is correlated with transformation by Rous sarcoma virus. J.Biol.Chem. 1989;264:20163–20166. [PubMed] [Google Scholar]
- 32.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 33.Volonte D, Galbiati F, Pestell RG, Lisanti MP. Cellular stress induces the tyrosine phosphorylation of caveolin-1 (TyR14)) via activation of p38 mitogen-activated protein kinase and c-Src kinase. Evidence for caveolae, the actin cytoskeleton, and focal adhesions as mechanical sensors of osmotic stress. J.Biol.Chem. 2001;276:8094–8103. doi: 10.1074/jbc.M009245200. [DOI] [PubMed] [Google Scholar]
- 34.Kimura A, Mora S, Shigematsu S, Pessin JE, Saltiel AR. The insulin receptor catalyzes the tyrosine phosphorylation of caveolin-1. J.Biol.Chem. 2002;277:30153–30158. doi: 10.1074/jbc.M203375200. [DOI] [PubMed] [Google Scholar]
- 35.Tao J, Malbon CC, Wang HY. Insulin stimulates tyrosine phosphorylation and inactivation of protein-tyrosine phosphatase 1B in vivo. J.Biol.Chem. 2001;276:29520–29525. doi: 10.1074/jbc.M103721200. [DOI] [PubMed] [Google Scholar]
- 36.Dadke S, Chernoff J. Protein-tyrosine phosphatase 1B mediates the effects of insulin on the actin cytoskeleton in immortalized fibroblasts. J.Biol.Chem. 2003;278:40607–40611. doi: 10.1074/jbc.M306772200. [DOI] [PubMed] [Google Scholar]
- 37.Chen Z, Bakhshi FR, Shajahan AN, et al. Nitric oxide-dependent Src activation and resultant caveolin-1 phosphorylation promote eNOS/caveolin-1 binding and eNOS inhibition. Mol.Biol.Cell. 2012;23:1388–1398. doi: 10.1091/mbc.E11-09-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montesano R, Roth J, Robert A, Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982;296:651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- 39.Pelkmans L, Fava E, Grabner H, et al. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 40.Minshall RD, Tiruppathi C, Vogel SM, et al. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J.Cell Biol. 2000;150:1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herkner H, Klein N, Joukhadar C, et al. Transcapillary insulin transfer in human skeletal muscle. Eur.J.Clin.Invest. 2003;33:141–146. doi: 10.1046/j.1365-2362.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- 42.Steil GM, Ader M, Moore DM, Rebrin K, Bergman RN. Transendothelial insulin transport is not saturable in vivo. No evidence for a receptor-mediated process. J.Clin.Invest. 1996;97:1497–1503. doi: 10.1172/JCI118572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bar RS, Boes M, Dake BL, Booth BA, Henley SA, Sandra A. Insulin, insulin-like growth factors, and vascular endothelium. Am.J.Med. 1988;85:59–70. doi: 10.1016/0002-9343(88)90398-1. [DOI] [PubMed] [Google Scholar]
- 44.Li G, Barrett EJ, Wang H, Chai W, Liu Z. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology. 2005;146:4690–4696. doi: 10.1210/en.2005-0505. [DOI] [PubMed] [Google Scholar]
- 45.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am.J.Physiol Endocrinol.Metab. 2011;301:E252–E263. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kolka CM, Bergman RN. The barrier within: endothelial transport of hormones. Physiology.(Bethesda.) 2012;27:237–247. doi: 10.1152/physiol.00012.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vicent D, Ilany J, Kondo T, et al. The role of endothelial insulin signaling in the regulation of vascular tone and insulin resistance. J.Clin.Invest. 2003;111:1373–1380. doi: 10.1172/JCI15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubota T, Kubota N, Kumagai H, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13:294–307. doi: 10.1016/j.cmet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 49.Shajahan AN, Timblin BK, Sandoval R, Tiruppathi C, Malik AB, Minshall RD. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J.Biol.Chem. 2004;279:20392–20400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- 50.Gottlieb-Abraham E, Shvartsman DE, Donaldson JC, et al. Src-mediated caveolin-1 phosphorylation affects the targeting of active Src to specific membrane sites. Mol.Biol.Cell. 2013;24:3881–3895. doi: 10.1091/mbc.E13-03-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J.Biol.Chem. 1995;270:14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- 52.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc.Natl.Acad.Sci.U.S.A. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52:2784–2789. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- 54.Kim F, Pham M, Maloney E, et al. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler.Thromb.Vasc.Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.