Abstract
The progressive nature of colorectal cancer (CRC) and poor prognosis associated with the metastatic phase of the disease create an urgent need for the development of more efficacious strategies targeting colorectal carcinogenesis. Cumulative evidence suggests that the redox-sensitive transcription factor Nrf2 (nuclear factor-E2-related factor 2), a master regulator of the cellular antioxidant defence, represents a promising molecular target for CRC chemoprevention. Recently, we have identified cinnamon, the ground bark of Cinnamomum aromaticum (cassia cinnamon) and Cinnamomum verum (Ceylon cinnamon), as a rich dietary source of the Nrf2 inducer cinnamaldehyde (CA) eliciting the Nrf2-regulated antioxidant response in human epithelial colon cells, conferring cytoprotection against electrophilic and genotoxic insult. Here, we have explored the molecular mechanism underlying CA-induced Nrf2 activation in colorectal epithelial cells and have examined the chemopreventive potential of CA in a murine CRC model comparing Nrf2+/+ and Nrf2−/− mice. In HCT116 cells, CA caused a Keap1-C151-dependent increase in Nrf2 protein half-life via blockage of ubiquitination with upregulation of cytoprotective Nrf2 target genes and elevation of cellular glutathione. After optimizing colorectal Nrf2 activation and target gene expression by dietary CA-supplementation regimens, we demonstrated that CA suppresses AOM/DSS-induced inflammatory colon carcinogenesis with modulation of molecular markers of colorectal carcinogenesis. Dietary suppression of CRC using CA supplementation was achieved in Nrf2+/+ but not in Nrf2−/− mice confirming the Nrf2-dependence of CA-induced chemopreventive effects. Taken together, our data suggest feasibility of CRC suppression by dietary CA, an FDA-approved food additive derived from the third most consumed spice in the world.
Keywords: Nrf2, colon cancer, AOM-DSS, cinnamon, cinnamaldehyde, dietary chemoprevention
Introduction
Colorectal cancer (CRC) is a major cause of tumor-related morbidity and mortality worldwide (1, 2). Progression of CRC can occur over decades and involves the early development of adenomatous precursor lesions followed by invasive stages of the disease (3). Apart from other factors such as genetic predisposition involving somatic mutations of the tumor suppressor gene adenomatosis polyposis coli (APC), chronic inflammation with oxidative tissue damage is thought to contribute significantly to colorectal carcinogenesis (1, 2). Indeed, CRC risk is increased in the context of chronic inflammatory bowel disease including Crohn’s disease and ulcerative colitis (4). The progressive nature of the disease and the poor prognosis associated with metastatic CRC create an urgent need for the development of more efficacious strategies targeting colorectal carcinogenesis including chemoprevention (1, 2). Chemopreventive intervention aiming at pharmacological suppression of colon carcinogenesis has shown promise in cellular and animal studies as well as human clinical trials (1, 5–7), but more efficacious and safer molecular agents are needed (8).
Recent research strongly suggests that the redox-sensitive transcription factor Nrf2 (nuclear factor-E2-related factor 2) is a promising molecular target for chemoprevention of colorectal carcinogenesis (9–13). Numerous natural products and dietary chemopreventive factors activate Nrf2 through covalent adduction and/or oxidation of redox-sensitive thiol residues in Keap1 (Kelch-like ECH-associated protein 1), the negative regulator of Nrf2 (14–17). Inhibition of Keap1-dependent ubiquitination and subsequent proteasomal degradation of Nrf2 allows nuclear translocation, a process followed by Nrf2-dependent transcriptional activation of cytoprotective target genes containing an antioxidant response element (ARE) regulatory sequence such as GCLC (glutamate-cysteine ligase catalytic subunit), GCLM (glutamate-cysteine ligase rmodifier subunit), TXN (thioredoxin), PRDX1 (peroxiredoxin 1), SRXN1 (sulfiredoxin 1), NQO1 [NAD(P)H quinone oxidoreductase 1], and HMOX1 (heme oxygenase-1) (15, 16, 18). Consistent with its role as a master regulator of the cellular antioxidant stress response, Nrf2 has emerged as a critical factor determining susceptibility to inflammation-driven, colitis-associated CRC (9–11). Pharmacological intervention using dietary factors that activate Nrf2 may represent a promising strategy for chemoprevention of cancer (17, 19, 20), and cumulative evidence obtained from animal studies strongly suggests feasibility of Nrf2-directed suppression of colorectal carcinogenesis (9, 12).
The ground bark of Cinnamomum aromaticum (cassia) and Cinnamomum verum (Ceylon cinnamon), commonly referred to as ‘cinnamon’, together with pepper and vanilla is one of the three most consumed spices in the world (21), yet health effects of cinnamon consumption have remained mostly unexplored at the molecular level. Cinnamaldehyde (CA), the key flavor compound and predominant chemical constituent of cinnamon essential oil, is an FDA-approved flavorant and food additive with an established safety profile and extensive documentation of non-toxicity at FDA-approved oral doses (22–27). Recently, our detailed structure-activity relationship studies have identified CA as the key constituent in cinnamon powder responsible for potent induction of the Nrf2-regulated antioxidant response in human epithelial skin and colon cells, conferring cytoprotection against subsequent electrophilic, oxidative, and genotoxic insults (28, 29). Moreover, using Nrf2-wildtype versus Nrf2-knockout mice the efficacy of CA-based therapeutic intervention targeting the progression of diabetic nephropathy through systemic Nrf2-activation was demonstrated (30).
Following our published prototype studies on CA as a potent Nrf2-inducer (28–30), we have now explored the chemopreventive potential of CA-dependent Nrf2 activation in a relevant murine model of CRC. After optimizing colorectal Nrf2 activation by dietary CA, we now demonstrate for the first time the efficacy of Nrf2-dependent suppression of inflammatory colon carcinogenesis in CA-supplemented Nrf2+/+ versus Nrf2−/− mice.
Materials and Methods
Reagents
Cinnamaldehyde (CA) and azoxymethane (AOM) were purchased from Sigma-Aldrich (St. Louis, MO). Dextran sulfate sodium (DSS) was obtained from Affymetrix Inc. (Cleveland, OH). Antibodies against Nrf2, NQO1, γ-GCS, AKR1C1, AKR1C2, AKR1B10, Ki67, ODC, HA, β-actin, and all horseradish peroxidase (HRP)-conjugated secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-8-dihydro-2-deoxyguanosine (8-oxo-dG) and anti-COX-2 antibodies were from Trevigen Inc. (Gaithersburg, MD) and Cayman Chemical (Ann Arbor, MI), respectively.
Cell culture
Human colorectal carcinoma cells HCT116 obtained from the American Type Culture Collection (ATCC CCL-247™; authenticated by short-tandem repeat profiling and mycoplasma-tested by the vendor) were passaged for fewer than 6 months before experiments. Cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Cellgro, Manassas, VA) with 10% fetal bovine serum (FBS) (Cellgro) in a humidified incubator (37 °C, 5% CO2).
Human Oxidative Stress RT2 Profiler™ PCR Expression array analysis
Preparation of total cellular RNA, reverse transcription, and Human Oxidative Stress RT2 Profiler™ PCR Expression Array (SuperArray) profiling were performed as described recently (31).
Transfection of cDNA and luciferase reporter gene assay
Transfection of cDNA was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) used according to the manufacturer’s instruction. Nrf2-dependent transcriptional activity was examined as previously published (32). HCT116 were transfected with an mGST-ARE firefly luciferase reporter plasmid together with expression plasmids for Nrf2, Keap1 (or Keap1C151S), and renilla luciferase (internal control). At 24 h post-transfection, cells were treated with test compounds, and after 16 h cells were lysed for analysis of reporter gene activity. Reporter assays were performed using the dual-luciferase reporter gene assay system (Promega, Madison, WI). For each experiment, samples were run in triplicate, and the data represent the means of three independent repeats ± SD.
Immunoblot analysis, ubiquitination assay, and determination of protein half-life
Cells were harvested in sample buffer [50mM Tris-HCl (pH 6.8), 2% SDS, 10% glycerol, 100 mM DTT, and 0.1% bromophenol blue]. After sonication, cell lysates were electrophoresed through an SDS-polyacrylamide gel and subjected to immunoblot analysis. For ubiquitination assay, cells were co-transfected with expression vectors for HA-tagged ubiquitin and Nrf2, and after 24 h cells were then treated with SF or CA (5 μM, each; 4 h) along with MG132 (10 μM) (33). Cells were then harvested in buffer [2 % SDS, 150 mM NaCl, 10 mM Tris-HCl (pH 8.0), 1 mM DTT] and immediately boiled. The lysates were then diluted five-fold in buffer lacking SDS and incubated with anti-Nrf2 antibody. Immunoprecipitated proteins were analyzed by immunoblotting using an antibody directed against the HA epitope (Santa Cruz). To measure Nrf2 protein half-life, cells were either left untreated or treated with CA (10 μM, 4 h), and cycloheximide (CHX, 50 μM) was added to block protein synthesis. Total cell lysates were collected at different time points and subjected to immunoblot analysis with anti-Nrf2 antibody. The relative intensity of the bands was quantified using the ChemiDoc CRS gel documentation system and Quantity One software from BioRad (Hercules, CA).
Glutathione assay
Total intracellular glutathione in cultured cells was analyzed using the luminescent GSH-Glo™ glutathione assay (Promega, Madison, WI). Cells were harvested and then counted using a Z2 Coulter counter, and GSH was determined per 10,000 viable cells. Data represent relative levels of glutathione normalized for cell number comparing treated versus solvent controls (means ± SD; n=3).
Viability assay
Modulation of cell viability by exposure to CA was examined using flow cytometric analysis of annexinV-FITC/propidium iodide (PI) stained cells using an apoptosis detection kit according to the manufacturer’s specifications (APO-AF, Sigma) as published previously (28). Data indicate percentage viable (annexinV−/PI−) cells [comparing treated versus solvent controls (means ± SD; n=3)].
Mouse experimentation and dietary supplementation
Experimental Nrf2+/+ and Nrf2−/− C57BL/6 mice were described previously (34). Mice were housed and handled in accordance with the Institutional Animal Care policies. Animals were maintained at 12 h light/12 h dark cycles with free access to water and standard diet [global 2919 (Harlan™ Laboratories, Teklad diets, Madison, WI)]. For dietary supplementation, CA was mixed with global 2919 diet, generating the desired final CA (0.1%, 0.5%; w/w) content. Preparation and replacement of CA-supplemented 2919 diet was performed daily. Absence of CA from un-supplemented control diet and stability of CA in the food matrix were monitored using our published GC-MS methodology confirming previous reports that document the overall chemical stability of CA used as a food additive as referenced in (28).
Colon epithelial Nrf2 upregulation by dietary CA-supplementation
Eight-week-old mice (Nrf2+/+; 3 animals per group) were fed with standard diet (control) or received diet supplemented with CA (0.1% or 0.5%) for the duration of five days. On day six, animals were sacrificed, and colon tissue was processed for analysis. The entire colon was excised, cut longitudinally, rinsed with ice-cold PBS, and fixed flat between sheets of filter paper. For immunoblot analysis, colon mucosa was scraped and stored at −80 °C until use. For IHC analysis, colon tissue was fixed in 10% buffered formalin and paraffin-embedded (12).
Dietary colon cancer chemoprevention using CA-supplementation in AOM/DSS-exposed Nrf2+/+ and Nrf2−/− mice
For dietary colon cancer chemoprevention using CA-supplementation, colitis-associated carcinogenesis was modeled using an azoxymethane (AOM)/dextran sulfate sodium (DSS) exposure regimen according to a published standard procedure (35). Eight-week-old mice (Nrf2+/+ versus Nrf2−/−; 8–12 animals per group) were fed with standard diet or received diet supplemented with 0.5% CA. AOM injection (10 mg/kg, i.p.; 1 mg/ml in isotonic saline) was performed at the beginning of week 9, followed by three cycles of DSS administration (2.5 % in drinking water; one week duration, each, followed by two weeks of regular water). CA-supplementation was initiated one week before AOM injection and then maintained after AOM injection for one additional (‘CA2 AOM/DSS’) or ten weeks (‘CA11 AOM/DSS’). Eleven weeks after the initiation of the experiment, mice were sacrificed for analysis.
Histopathological scoring system
Histopathological assessment of colon mucosa in Nrf2+/+ and Nrf2−/− mice (6–12 specimens per treatment group) was performed at the end of the experiment. Histological scores were assigned following the chronic colitis scoring system (36).
Hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC)
Tissue was stained using hematoxylin and eosin (H&E) according to standard procedures. Following antigen retrieval, tissue sections were incubated with 5% normal goat serum for 30 minutes followed by 16 hours incubation using the respective antibodies. Staining was performed using the Envision System HRP-DAB kit (DAKO, Carpinteria, CA) according to manufacturer’s instructions. IHC staining was scored independently by two pathologists [intensity: four categories (from low to high): 0, 1, 2 and 3; percentage of positive cells: five categories: 0 (<10%), 1 (10–25%), 2 (25–50%), 3 (50–75%) and 4 (75–100%)] as described recently (37). A final IHC staining score (intensity score × percentage score; 6–12 specimens per treatment group) was determined.
Statistics
All results were analyzed using the GraphPad Prism software (version 6.0; Graphpad, San Diego, CA; www.graphpad.com). Results obtained in vitro are presented as the means ± SD of at least three independent experiments performed in duplicate or triplicate each. Data were analyzed employing the two-sided Student’s t-test, and selected data sets were analyzed employing one-way analysis of variance (ANOVA) with Tukey’s post hoc test; means without a common letter differ. Where indicated, nonparametric data analysis of murine experimentation was performed using the Mann-Whitney test. Differences between groups were considered significant at p < 0.05.
Results
Cinnamaldehyde increases Nrf2 protein half-life via blockage of ubiquitination causing Nrf2 target gene expression and elevation of glutathione in HCT116 cells
First, in order to comprehensively monitor the antioxidant response gene expression upregulated by CA in HCT116 cells, oxidative Stress RT2 Profiler™ PCR Expression Array analysis was performed (CA, 20 μM, 24h; Fig. 1A). Genes upregulated in response to CA treatment by at least threefold included HMOX1, SRXN1, SLC7A11, AKR1C2, GCLM, SQSTM1, GCLC, FTH1, TXNRD1, GPX2, GLA, ALOX12, NQO1, GSR, PTGR1, and AOX1, all of which are established Nrf2-target genes. In contrast, expression of the Nrf2-encoding gene NFE2L2 was not upregulated at the mRNA level. Increased protein levels of Nrf2 and Nrf2 targets (HO-1, GCS, NQO1, SRXN1, FTH1, SQSTM1/p62; Fig. 1B) were observed in response to CA treatment, employing CA exposure regimens (1–10 μM, 24 h) that did not impair cellular viability as assessed using flow cytometric analysis of annexinV-FITC/PI-stained cells (Fig. 1C), findings consistent with our earlier report that low micromolar concentrations of CA cause Nrf2 upregulation in the absence of cytotoxic effects (28). Consistent with upregulation of the intracellular antioxidant response including genes involved in glutathione biosynthesis and function (SLC7A11, GCLM, GCLC, GPX2, GSR), total glutathione levels were elevated by almost two-fold in HCT116 cells exposed to CA, displaying a dose response relationship in the low micromolar range (5–20 μM; 24 h; Fig. 1D).
Figure 1.
Molecular mechanism underlying CA-modulation of Nrf2 activity in HCT116 cells. A, For Oxidative Stress RT2 Profiler™ PCR expression array analysis, cells were exposed to CA (20 μM, 24h) followed by gene expression analysis. upper panel: scatter blot depiction of CA-induced gene expression (versus untreated); cut-off lines: threefold up- or down-regulation; the insert shows the chemical structure of cinnamaldehyde; bottom panel: numerical expression changes [n=3, mean ± SD; (p<0.05)]. B, Upregulation of Nrf2 target gene-encoded proteins in response to CA exposure was examined in HCT116 cells treated with CA (1–20 μM; 24 h), and cell lysates were used for immunoblot analysis. Equal loading was assessed by immunodetection of β-actin. In addition, Nrf2 protein levels were examined (CA, 1–20 μM; 4h). C, Viability was monitored in HCT116 cells exposed to CA (0–20 μM; 24 h) using flow cytometric analysis as detailed in Materials and Methods. D, Intracellular total glutathione levels relative to untreated control were determined in HCT116 cells exposed to CA (1–20 μM; 24 h) using the luminescent GSH-Glo™ glutathione assay as detailed in Materials and Methods. Data are expressed as means ± SD. Means without a common letter differ (ANOVA with Tukey’s post hoc test; *p < 0.05; n = 3). E, CA-modulation of ARE-luciferase reporter gene expression was assessed employing Keap1-C151S and Keap1-WT cotransfections. In addition, a Keap1-siRNA against the 3′-untranslated region was cotransfected to suppress endogenous Keap1. The transfected cells were then treated with CA (10 μM), As(III) (10 μM), SF (5 μM), or tBHQ (50 μM) for 16 h, before the measurement of firefly and Renilla luciferase activities. Data are expressed as means ± SD (*p < 0.05 control vs. compound treatment; #p < 0.05 Keap1-WT vs. Keap1-C151S). An aliquot of cell lysates was used for immunoblot analysis. F, CA-modulation of Nrf2-ubiquitination was assessed by cotransfecting cells with plasmids encoding the indicated proteins (Nrf2, HA-Ub). Cells were then treated with SF, CA, or tBHQ (5 μM; 4 h) along with MG132 (10 μM; 4 h) before cell lysates were collected for the ubiquitination assay. For detection of ubiquitin-conjugated Nrf2, anti-Nrf2 immunoprecipitates were analyzed by immunoblotting using an anti-HA antibody. G, CA-modulation of Nrf2 protein half-life. After cells were left untreated or treated with CA (10 μM; 4 h), cycloheximide (50 μM) was added and cells were harvested at the indicated time points (0–60 min). Cell lysates were subjected to immunoblot analysis using anti-Nrf2 and anti-tubulin antibodies. Band intensity was quantified using Quantity One software and plotted against the time after cycloheximide treatment.
Next, the molecular mechanism underlying CA-induced Nrf2 activation in HCT116 cells was explored. Using Keap1-C151S versus Keap1-wt cotransfection (in addition to Keap1-siRNA directed against the 3′-untranslated region to suppress endogenous Keap1; Fig. 1E, top panel) in an ARE-luciferase activity assay, it was observed that CA-induced Nrf2 activation depends on Keap1-C151. This observation is consistent with prior findings indicating that this cysteine residue represents an important redox sensor for CA-related electrophilic Michael acceptor pharmacophores including 4-hydroxynonenal and acrolein (38). Similar results were obtained using other Keap1-Cys151-dependent Nrf2 inducers including tBHQ and SF, whereas As (III) known to activate Nrf2 independently of Keap1-Cys151 served as a negative control (39). These results were also confirmed when CA-modulation of Nrf2 protein levels was monitored by immunoblot analysis revealing that CA-induction of Nrf2 was strongly attenuated in Keap1 C151S-transfectants (Fig. 1E; bottom panel). Next, since it has been demonstrated previously that established Nrf2 inducers such as SF and tBHQ cause Nrf2 activation through inhibition of the Keap1-mediated ubiquitination of Nrf2, a cellular ubiquitination assay was performed (Fig. 1F). HCT116 cells were first cotransfected with expression vectors for Nrf2 and hemagglutinin-tagged (HA)-ubiquitin and then exposed to Nrf2 activator test compound (CA, SF, tBHQ) or left untreated, followed by immunoprecipitation of Nrf2 and immunodetection of ubiquitinated protein. In cells treated with CA, ubiquitination of Nrf2 decreased dramatically compared to untreated control. Likewise, SF or tBHQ also decreased Nrf2 ubiquitination, consistent with the known attenuation of ubiquitination by these Nrf2 inducers (40, 41). Following these experiments, the half-life of endogenous Nrf2 protein and its modulation by CA exposure was measured in HCT116 cells (Fig. 1G). The half-life of Nrf2 under untreated conditions (control) was 17.6 minutes, whereas Nrf2 half-life more than doubled (40.7 minutes) in response to CA treatment (5 μM).
Taken together, these results indicate that CA is a Keap1-C151-dependent Nrf2-activator that attenuates Nrf2 ubiquitination and stabilizes Nrf2 at the protein level upregulating antioxidant stress response gene expression.
Dietary CA-supplementation elevates Nrf2 target gene expression in mouse colon epithelial cells
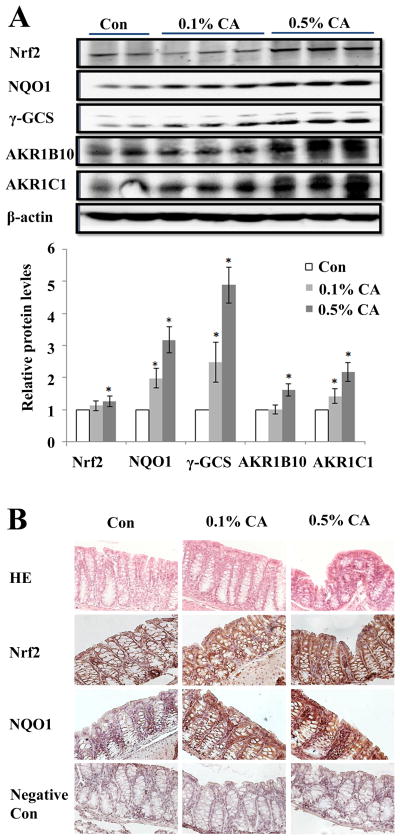
Next, we tested feasibility of Nrf2 induction in vivo examining the effects of CA dietary supplementation in murine colon epithelial cells (Fig. 2). Eight-week-old mice were fed with standard diet or received diet supplemented with 0.1% or 0.5% CA for 5 days followed by examination of Nrf2 and Nrf2 target gene expression at the protein level. This specific dose range was chosen based on prior murine studies examining toxicological effects of chronic dietary CA supplementation [up to 33,000 ppm (i.e. 3.3%) in standard feed)] administered over an extended period (between three months and two years) demonstrating the absence of carcinogenic effects at high dose levels (22–24). It was observed that 0.5% CA supplementation displayed superior efficacy causing upregulation of Nrf2 and Nrf2 target protein levels including NQO1 (approximately three fold), γ-GCS (almost five fold) and others (AKR1B10, AKR1C1). Remarkably, even at lower doses (0.1%) CA supplementation displayed significant Nrf2-modulatory efficacy as confirmed by immunoblot (Fig. 2A) and immunohistochemical analysis (Fig. 2B). Based on the extensive Nrf2-enhancing effects observed with 0.5% CA supplementation that occurred in the absence of any adverse effects this dose was chosen for the subsequent chemoprevention experimentation.
Figure 2.
Dietary CA-supplementation upregulates murine colon epithelial Nrf2 levels. A, Eight-week-old mice (Nrf2+/+) were fed with standard diet or received supplemented diet (CA 0.1%; CA 0.5%; three mice, each). After five days of dietary supplementation, animals were sacrificed on day 6, colon tissue was harvested, and Nrf2 and Nrf2 target gene expression at the protein level was examined by immunoblot analysis. The intensity of the bands was quantified using Quantity One software (mean ± SD; * p< 0.05). B, Colon tissue was subjected to H&E and IHC (Nrf2, NQO1) analysis confirming supplementation-induced Nrf2 and NQO1 upregulation. Per treatment group, one representative specimen is depicted (400 x magnification). As a negative control, staining was performed with omission of primary antibody.
Dietary colon cancer chemoprevention using CA-supplementation in AOM/DSS-exposed Nrf2+/+ and Nrf2−/− mice
In order to explore the Nrf2-dependent chemopreventive potential of CA targeting CRC we employed a standard model of inflammatory carcinogenesis performed in Nrf2+/+ and Nrf2−/− mice (Fig. 3). Eight-week-old mice (Nrf2+/+ versus Nrf2−/−; 8–12 animals per group), fed a standard diet or diet supplemented with 0.5% CA, received AOM injection and DSS administration in drinking water as specified (Fig. 3A). CA supplementation started one week before AOM injection and was then maintained for another week (two weeks total, ‘CA2 AOM/DSS’). Since it has recently been demonstrated that after initiation has occurred Nrf2 upregulation may promote tumorigenic progression, we also included an additional prolonged feeding regimen in order to assess if extended CA supplementation (10 more weeks after AOM, 11 weeks total; ‘CA11 AOM/DSS’) may enhance tumorigenesis (16).
Figure 3.
Dietary CA supplementation decreases AOM/DSS-induced colitis in Nrf2+/+ but not Nrf2−/− mice. A, Eight-week-old Nrf2+/+ and Nrf2−/− mice were fed with standard diet or received diet supplemented with 0.5% CA. AOM injection was performed at the beginning of week 9, followed by three cycles of DSS (2.5 %) administration in drinking water. CA-supplementation was initiated one week before AOM injection and then maintained after AOM injection for one (CA2) or ten (CA11) weeks. Eleven weeks after the initiation of the experiment, mice were sacrificed for analysis. B, CA supplementation significantly decreased loss of crypts and maintained mucosal crypt organization in Nrf2+/+ mice only, as demonstrated by H&E staining (100 x magnification). C, Histopathological assessment of colon mucosa in Nrf2+/+ and Nrf2−/− mice was performed following the chronic colitis scoring system described in Materials and Methods. Horizontal lines indicate median values (*p<0.05; Mann-Whitney test); N.S.: not significant. D, AOM/DSS-induced weight loss was significantly attenuated by CA11 supplementation in Nrf2+/+ mice. The graph indicates average body weight (mean + SD) per treatment group at the beginning and at the end of the experimental regimen (eleven weeks total duration; *p < 0.05 versus control; #p < 0.05 versus AOM/DSS;). E–F, AOM/DSS-induced body weight loss in Nrf2+/+ versus Nrf2−/− mice was analyzed as a function of CA supplementation. At the end of the treatment regimen, the relative weight loss was compared between treatment groups; the difference between AOM/DSS versus untreated control was numerically defined as 1 (*p < 0.05).
First, histopathological assessment of non-tumorous colon mucosa in Nrf2+/+ and Nrf2−/− mice at the end of the experiment was performed employing the chronic colitis scoring system described in Materials and Methods (Fig. 3B and C). Remarkably, CA supplementation significantly attenuated histological damage (with maintenance of crypt organization in normal mucosa) only in Nrf2+/+ mice and decreased the chronic colitis histological score (p<0.05). Specifically, in colon tissue adjacent to tumors, histopathological changes were evident from epithelial erosion, loss of goblet cells, and massive lymphocytic infiltration, inflammatory alterations typical of AOM/DSS-induced damage that were suppressed by CA administration in Nrf2+/+ but not in Nrf2−/− mice. Moreover, it was observed that AOM/DSS-induced weight loss was significantly attenuated by CA supplementation (p<0.05), a finding observed only in the group receiving the extended CA regimen (‘CA11 AOM/DSS’ group; Fig. 3D). Furthermore, it was observed that AOM/DSS-induced suppression of weight increase is significantly antagonized by dietary CA-supplementation in Nrf2+/+ mice (Fig. 3E), whereas no such effect was observable in Nrf2−/− mice (Fig. 3F), demonstrating the Nrf2-dependence of CA-antagonism of AOM/DSS-induced weight loss.
Next, it was observed that AOM/DSS-induced colon carcinogenesis was suppressed significantly by dietary CA-supplementation in Nrf2+/+ but not in Nrf2−/− mice (Fig. 4 and table 1). Remarkably, both CA-supplementation regimens significantly suppressed tumor multiplicity by up to 45%, but no significant difference was observed between the ‘CA 2 AOM/DSS’ and ‘CA11 AOM/DSS’ treatment groups. Importantly, a chemopreventive effect of CA supplementation was observable only in Nrf2+/+ but not in Nrf2−/− mice demonstrating that CA-mediated protection is Nrf2-dependent. When stratified by tumor colonic location and size, cancer chemopreventive efficacy of CA was most pronounced suppressing the occurrence of proximal (irrespective of size) and large (≥ 2 mm diameter) middle and distal colonic tumors (table 1). Remarkably, in Nrf2+/+ mice large tumors (> 5 mm diameter) located in the middle section were observed exclusively in the ‘CA11’ supplementation group whereas none were detected in the ‘CA2’ supplementation group (data not shown), an observation consistent with Nrf2-dependent enhancement of tumor growth once initiation has occurred (16). Moreover, pathological grading of distal tumors (from table 1) indicated that CA supplementation caused a significant change in adenoma/adenocarcinoma ratio per treatment group: In Nr2+/+ mice, CA supplementation increased the adenoma/adenocarcinoma ratio from 1.25 (‘AOM/DSS only’) to 2.6 (‘CA2’) or 4.0 (‘CA11’) suggesting a supplementation-induced attenuation of tumorigenic progression. This trend was not observed in Nrf2−/− mice where CA supplementation did not significantly change the adenoma/adenocarcinoma ratio observed in the ‘AOM/DSS only’ group (1.3).
Figure 4.

Dietary CA-supplementation suppresses AOM/DSS-induced colon carcinogenesis in Nrf2+/+ but not Nrf2−/− mice. At the end of the treatment regimen, total tumor number (distal, middle, proximal combined) per mouse was determined as a function of treatment regimen. Horizontal lines indicate median values for every treatment group. CA-supplementation regimens (CA2 and CA11) caused a significant suppression of tumor multiplicity in Nrf2+/+ but not in Nrf2−/− mice (*p < 0.05; Mann-Whitney test; N.S.: not significant).
Table 1.
Data indicate median tumor number per mouse (including range from minimum to maximum) as a function of size (diameter in mm) and anatomical location (distal, middle, proximal) per experimental group (*p < 0.05 compared to ‘Nrf2+/+ AOM/DSS’ group; Mann-Whitney test).
| Groups | Nrf2 +/+
|
Nrf2 −/−
|
|||||
|---|---|---|---|---|---|---|---|
| AOM/DSS | CA2 AOM/DSS | CA11 AOM/DSS | AOM/DSS | CA2 AOM/DSS | CA11 AOM/DSS | ||
| Distal | <2mm | 4.0 (2–7) | 3.0 (3–5) | 3.0 (1–9) | 5.0 (4–6) | 6.0 (5–8) | 5.5 (4–8) |
| ≥2mm | 3.0 (2–8) | 1.0 (1–3)* | 1.0 (1–2)* | 2.0 (2–3) | 2.0 (2–2) | 2.5 (0–4) | |
| Middle | <2mm | 2.0 (0–5) | 3.0 (2–4) | 2.5 (0–5) | 3.5 (2–5) | 3.0 (2–4) | 3.0 (2–5) |
| ≥2mm | 2.0 (0–3) | 1.0 (0–2)* | 1.0 (0–2)* | 2.0 (1–3) | 2.0 (1–4) | 2.0 (0–3) | |
| Proximal | <2mm | 3.0 (0–4) | 0.5 (0–3) | 0.0 (0–1)* | 1.5 (0–3) | 2.0 (0–2) | 2.0 (0–3) |
| ≥2mm | 1.0 (0–3) | 0.0 (0–0)* | 0.0 (0–0)* | 0.0 (0–2) | 0.0 (0–2) | 0.5 (0–2) | |
| Total | 14.5 (12–20) | 10.0 (8–12)* | 9.0 (4–12)* | 14.5 (13–18) | 16.0 (12–20) | 15.0 (12–19) | |
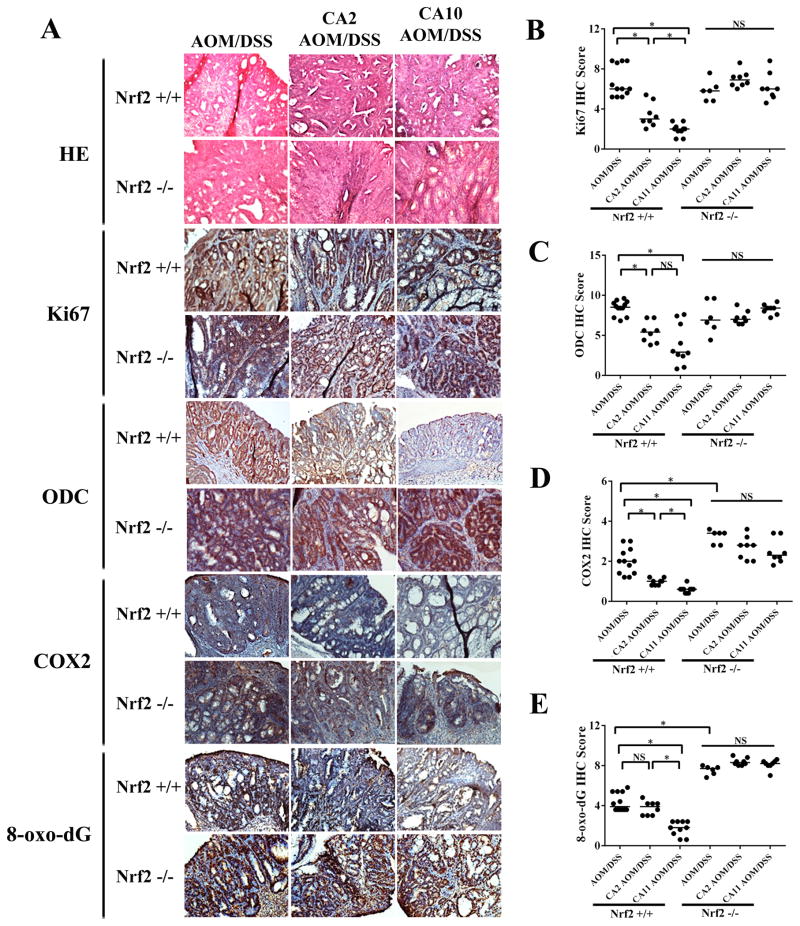
Immunohistochemical analysis of molecular markers associated with tumorigenic progression and inflammatory tissue damage confirmed CA-suppression of AOM/DSS-induced carcinogenesis (Fig. 5A). CA-supplementation caused pronounced downregulation of Ki67 (Fig. 5B), a nuclear proliferative factor known to be upregulated in CRC (42), and Ki67-directed effects of the ‘CA11’ supplementation regimen were more pronounced than ‘CA2’-induced changes. Likewise, attenuation of the expression levels of the polyamine-synthesis enzyme ornithine decarboxylase (ODC; Fig. 5C), and pronounced suppression of the AOM/DSS-induced inflammatory enzyme cyclooxygenase 2 (COX-2; Fig. 5D), important key factors in CRC progression, was observed in response to CA supplementation (5, 43). In addition, AOM/DSS-induced oxidative damage targeting nuclear DNA was attenuated by CA supplementation as indicated by reduced staining for 8-oxodG, an effect that was observed only in the ‘CA11’ supplementation group (Fig. 5E). Interestingly, in the ‘AOM/DSS only’ groups, IHC scores for COX-2 and 8-oxodG were elevated in Nrf2−/− versus Nrf2+/+ mice (Fig. 5D–E), consistent with an impaired antioxidant tissue defence due to the absence of functional Nrf2. Strikingly, as observed with CA effects on AOM/DSS-induced carcinogenesis, CA-modulation of CRC progression markers was confined to Nrf2+/+ mice only (Fig. 5B–E), suggesting the strict Nrf2-dependence of the molecular effects of CA supplementation.
Figure 5.
Dietary CA-supplementation modulates tissue markers of AOM/DSS-induced colon tumorigenesis. A, At the end of the experiment, colon tissue was harvested. Tumor tissue was processed for H&E staining and immunohistochemical analysis. Per treatment group, one representative specimen out of at least ten tumors is depicted (200 x magnification). B–E, IHC analysis of tumor specimens from Nrf2+/+ and Nrf2−/− mice was performed following the scoring system described in Materials and Methods. Horizontal lines indicate median values of markers of proliferation (Ki67), oxidative DNA damage (8-oxodG), inflammatory dysregulation (COX-2), and polyamine synthesis (ODC); (*p < 0.05; Mann-Whitney test; N.S.: not significant)
Discussion
This preclinical prototype study has examined the feasibility of dietary chemoprevention of CRC by the Nrf2 activator CA, a flavor compound and FDA-approved food additive contained abundantly in cinnamon, the ground bark of Cinnamomum aromaticum (cassia) and Cinnamomum verum (Ceylon cinnamon), the third most consumed spice in the world after pepper and vanilla (21, 44). Apart from its role as a dietary constituent of global importance, ethno-pharmacological evidence indicates a long history of use as a traditional medicine, and cinnamon has recently emerged as a CAM (complementary and alternative medicine) dietary supplement (25–27). Earlier studies performed in cell culture and murine models have demonstrated antimicrobial, antioxidant, anti-inflammatory, anti-hyperglycemic and anti-diabetic, and cancer-suppressive activities of cinnamon, but only a small number of mechanistic studies has attributed these cellular effects to specific molecular key constituents including CA and phenolic proanthocyanidins (28, 29, 31).
In this preclinical chemoprevention study, we first elucidated the molecular mechanism underlying Nrf2 activation by CA in human colon epithelial cells (Fig. 1). Prior research has demonstrated that pharmacological upregulation of Nrf2 may result from impairment of Keap1-dependent ubiquitination and subsequent proteasomal Nrf2 protein degradation, and genetic studies involving site-directed mutagenesis of critical Keap1 cysteine residues have identified adduction/oxidation of specific sensor thiol-groups (including Cys151) by electrophiles (14, 45). This key mechanism underlying Nrf2-activation has been observed with many chemopreventive food factors including broccoli-derived sulforaphane, turmeric-derived curcumin, and grape-derived resveratrol (14, 15, 38, 45). Our data indicate that CA-induced Nrf2 activation largely depends on Keap1-Cys151 status, an observation consistent with the prior demonstration that Keap1-Cys151 functions as an electrophile sensor that can be adducted by the ‘enone’-type Michael acceptor pharmacophore contained in cinnamaldehyde.
Next, preclinical efficacy of CA for dietary chemoprevention was demonstrated in an established two-stage colon tumorigenesis model mimicking colitis-associated colon carcinogenesis (Fig. 3–5) (9, 46). Because of its high reproducibility and ability to rapidly recapitulate the aberrant crypt foci-adenoma-carcinoma progressive sequence that occurs in human CRC, the AOM/DSS is a widely accepted model for colon carcinogenesis and chemoprevention studies implemented using C57BL/6J mice before (10, 12, 46, 47), and chemopreventive efficacy of Nrf2-activators administered orally has been demonstrated (12). In our murine model, a significant CA-dependent attenuation of AOM/DSS colon tumorigenesis was observed, and the crucial involvement of Nrf2-mediated mechanisms underlying CA-induced anti-tumorigenic effects was evident from the observation that CA chemopreventive effects were only detectable in Nrf2+/+ mice (Fig. 3–5).
Earlier research has already generated strong genetic evidence supporting the protective role of Nrf2 against inflammatory colorectal carcinogenesis (9). Importantly, using Nrf2−/− versus Nrf2+/+ mice, the crucial role of Nrf2 in determining susceptibility to inflammatory induction of colitis and colitis-driven colorectal carcinogenesis has been established (9–11, 13). Consistent with the antioxidant, anti-inflammatory, and cytoprotective cellular effects downstream of Nrf2 transcriptional activation, recent research demonstrates feasibility of chemoprevention targeting colitis-associated colorectal carcinogenesis in the murine AOM/DSS model by oral administration of experimental agents including dibenzoylmethane, phenyl isothiocyanate, zerumbone, and 2,3′,4,4′,5′-pentamethoxy-trans-stilbene known to induce Nrf2 activity (12, 48, 49).
In our search for potent and safe dietary Nrf2 activators we recently focused on the cinnamon-derived dietary factor CA. Remarkably, CA is the only α,β-unsaturated aldehyde which is FDA-approved for use in foods (21 CFR § 182.60) and given the ‘Generally Recognized As Safe’ status by the ‘Flavor and Extract Manufacturers’ Association (FEMA) in the United States (FEMA no. 2286) (22–24, 50). Moreover, murine toxicity data, published in the context of safety profiling supporting FDA-approval of CA as food additive, indicate non-toxicity of oral CA administration at supplementation levels as employed in our pilot study (22–24, 44, 50). It is therefore reasonable to speculate that a potential chemopreventive use of this dietary factor contained in cinnamon powder may be achievable with an acceptable safety profile, an important prerequisite for the development of novel chemopreventive interventions administered to healthy or genetically-predisposed cancer-prone individuals (8). CA content in food ranges from trace amounts (e.g. in orange juice) to 12.2 mg/100 g (122 ppm) in apple cinnamon cereals and 31.1 mg/100 g (311 ppm) for cinnamon swirl bread, suggesting that significant dietary intake of this potent Nrf2 inducer occurs in large populations (44). Future preclinical studies will aim at optimizing CA dosing regimens for dietary Nrf2 activation and will also test the feasibility of using the third most consumed spice in the world, cinnamon powder, as a widely accessible source of this unique and safe Nrf2 inducer for dietary cancer chemoprevention.
Acknowledgments
Supported in part by grants from the National Institutes of Health [2R01ES015010, R01CA154377 (D.D. Zhang); Arizona Cancer Center Support Grant CA023074, ES007091, ES06694, R21CA166926 (G.T. Wondrak; D.D. Zhang)], the State Scholarship Fund of China (201207610022; M. Long), the National Natural Science Foundation of China (81228023; D.D. Zhang) and Chongqing Science Foundation (cstc2013jcsfC10001-5; M. Long).
Footnotes
Disclosure of Potential Conflicts of Interest: The authors have no conflict of interest to disclose.
References
- 1.Half E, Arber N. Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother. 2009;10:211–9. doi: 10.1517/14656560802560153. [DOI] [PubMed] [Google Scholar]
- 2.Marshall JR. Prevention of colorectal cancer: diet, chemoprevention, and lifestyle. Gastroenterol Clin North Am. 2008;37:73–82. vi. doi: 10.1016/j.gtc.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka T. Colorectal carcinogenesis: Review of human and experimental animal studies. J Carcinog. 2009;8:5. doi: 10.4103/1477-3163.49014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 5.Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 2008;1:32–8. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudolf E, Andelova H, Cervinka M. Polyphenolic compounds in chemoprevention of colon cancer - targets and signaling pathways. Anticancer Agents Med Chem. 2007;7:559–75. doi: 10.2174/187152007781668670. [DOI] [PubMed] [Google Scholar]
- 7.Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170–81. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Kummar S, Doroshow JH. Phase 0 trials: expediting the development of chemoprevention agents. Cancer Prev Res (Phila) 2011;4:288–92. doi: 10.1158/1940-6207.CAPR-11-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saw CL, Kong AN. Nuclear factor-erythroid 2-related factor 2 as a chemopreventive target in colorectal cancer. Expert Opin Ther Targets. 2011;15:281–95. doi: 10.1517/14728222.2011.553602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khor TO, Huang MT, Prawan A, Liu Y, Hao X, Yu S, et al. Increased susceptibility of Nrf2 knockout mice to colitis-associated colorectal cancer. Cancer Prev Res (Phila Pa) 2008;1:187–91. doi: 10.1158/1940-6207.CAPR-08-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osburn WO, Karim B, Dolan PM, Liu G, Yamamoto M, Huso DL, et al. Increased colonic inflammatory injury and formation of aberrant crypt foci in Nrf2-deficient mice upon dextran sulfate treatment. Int J Cancer. 2007;121:1883–91. doi: 10.1002/ijc.22943. [DOI] [PubMed] [Google Scholar]
- 12.Cheung KL, Khor TO, Huang MT, Kong AN. Differential in vivo mechanism of chemoprevention of tumor formation in azoxymethane/dextran sodium sulfate mice by PEITC and DBM. Carcinogenesis. 2010;31:880–5. doi: 10.1093/carcin/bgp285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khor TO, Huang MT, Kwon KH, Chan JY, Reddy BS, Kong AN. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006;66:11580–4. doi: 10.1158/0008-5472.CAN-06-3562. [DOI] [PubMed] [Google Scholar]
- 14.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M. Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol. 2004;24:10941–53. doi: 10.1128/MCB.24.24.10941-10953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18:1779–91. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 16.Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–91. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwak MK, Kensler TW. Targeting NRF2 signaling for cancer chemoprevention. Toxicol Appl Pharmacol. 2010;244:66–76. doi: 10.1016/j.taap.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT. Cancer chemoprevention mechanisms mediated through the keap1-nrf2 pathway. Antioxid Redox Signal. 2010;13:1713–48. doi: 10.1089/ars.2010.3221. [DOI] [PubMed] [Google Scholar]
- 19.Kundu JK, Surh YJ. Nrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis. Pharm Res. 2010;27:999–1013. doi: 10.1007/s11095-010-0096-8. [DOI] [PubMed] [Google Scholar]
- 20.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–5. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.FAOSTAT. Food and Agricultural commodities, Figures. FAOSTAT; 2008. Food and Agricultural Organization of the United Nations. available at: http://faostat.fao.org. [Google Scholar]
- 22.NATIONAL-TOXICOLOGY-PROGRAM. NTP TR 514 NIH Publication No 04-4448. 2004. NTP Technical Report on the Toxicology and Carcinogenesis Studies of trans-Cinnamaldehyde. [Google Scholar]
- 23.WHO. Evaluation of certain food additives and contaminants. Thirty-fifth report of the Joint FAO/WHO Expert Committee on Food Additives. Tech Rep Ser. 1990;789:1–48. [PubMed] [Google Scholar]
- 24.Hooth MJ, Sills RC, Burka LT, Haseman JK, Witt KL, Orzech DP, et al. Toxicology and carcinogenesis studies of microencapsulated trans-cinnamaldehyde in rats and mice. Food Chem Toxicol. 2004;42:1757–68. doi: 10.1016/j.fct.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Gruenwald J, Freder J, Armbruester N. Cinnamon and health. Crit Rev Food Sci Nutr. 2010;50:822–34. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- 26.Otto AD. Cinnamon as a supplemental treatment for impaired glucose tolerance and type 2 diabetes. Curr Diab Rep. 2010;10:170–2. doi: 10.1007/s11892-010-0106-6. [DOI] [PubMed] [Google Scholar]
- 27.Kirkham S, Akilen R, Sharma S, Tsiami A. The potential of cinnamon to reduce blood glucose levels in patients with type 2 diabetes and insulin resistance. Diabetes Obes Metab. 2009;11:1100–13. doi: 10.1111/j.1463-1326.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- 28.Wondrak GT, Villeneuve NF, Lamore SD, Bause AS, Jiang T, Zhang DD. The cinnamon-derived dietary factor cinnamic aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells. Molecules. 2010;15:3338–55. doi: 10.3390/molecules15053338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wondrak GT, Cabello CM, Villeneuve NF, Zhang S, Ley S, Li Y, et al. Cinnamoyl-based Nrf2-activators targeting human skin cell photo-oxidative stress. Free Radical Biol Med. 2008;45:385–95. doi: 10.1016/j.freeradbiomed.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, et al. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011;60:3055–66. doi: 10.2337/db11-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabello CM, Bair WB, 3rd, Lamore SD, Bause AS, Azimian S, Wondrak GT. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic Biol Med. 2009;46:220–31. doi: 10.1016/j.freeradbiomed.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang XJ, Sun Z, Chen W, Li Y, Villeneuve NF, Zhang DD. Activation of Nrf2 by arsenite and monomethylarsonous acid is independent of Keap1-C151: enhanced Keap1-Cul3 interaction. Toxicol Appl Pharmacol. 2008;230:383–9. doi: 10.1016/j.taap.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Z, Zhang S, Chan JY, Zhang DD. Keap1 controls postinduction repression of the Nrf2-mediated antioxidant response by escorting nuclear export of Nrf2. Mol Cell Biol. 2007;27:6334–49. doi: 10.1128/MCB.00630-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moi P, Chan K, Asunis I, Cao A, Kan YW. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc Natl Acad Sci U S A. 1994;91:9926–30. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thaker AI, Shaker A, Rao MS, Ciorba MA. Modeling colitis-associated cancer with azoxymethane (AOM) and dextran sulfate sodium (DSS) J Vis Exp. 2012 doi: 10.3791/4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chinen T, Komai K, Muto G, Morita R, Inoue N, Yoshida H, et al. Prostaglandin E2 and SOCS1 have a role in intestinal immune tolerance. Nat Commun. 2011;2:190. doi: 10.1038/ncomms1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hernandez JM, Farma JM, Coppola D, Hakam A, William JF, Chen D-T, et al. Expression of the antiapoptotic protein survivin in colon cancer. Clin Colorectal Cancer. 2011;10:188–93. doi: 10.1016/j.clcc.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMahon M, Lamont DJ, Beattie KA, Hayes JD. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc Natl Acad Sci U S A. 2010;107:18838–43. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau A, Zheng Y, Tao S, Wang H, Whitman SA, White E, et al. Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner. Mol Cell Biol. 2013;33:2436–46. doi: 10.1128/MCB.01748-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tao S, Justiniano R, Zhang DD, Wondrak GT. The Nrf2-inducers tanshinone I and dihydrotanshinone protect human skin cells and reconstructed human skin against solar simulated UV. Redox Biol. 2013;1:532–41. doi: 10.1016/j.redox.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tao S, Zheng Y, Lau A, Jaramillo MC, Chau BT, Lantz RC, et al. Tanshinone I activates the Nrf2-dependent antioxidant response and protects against As(III)-induced lung inflammation in vitro and in vivo. Antioxid Redox Signal. 2013;19:1647–61. doi: 10.1089/ars.2012.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estevez-Garcia P, Lopez-Calderero I, Molina-Pinelo S, Munoz-Galvan S, Salinas A, Gomez-Izquierdo L, et al. Spinophilin loss correlates with poor patient prognosis in advanced stages of colon carcinoma. Clin Cancer Res. 2013;19:3925–35. doi: 10.1158/1078-0432.CCR-13-0057. [DOI] [PubMed] [Google Scholar]
- 43.Benbrook DM, Guruswamy S, Wang Y, Sun Z, Mohammed A, Zhang Y, et al. Chemoprevention of colon and small intestinal tumorigenesis in APC(min/+) mice by SHetA2 (NSC721689) without toxicity. Cancer Prev Res (Phila) 2013;6:908–16. doi: 10.1158/1940-6207.CAPR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman M, Kozukue N, Harden LA. Cinnamaldehyde content in foods determined by gas chromatography-mass spectrometry. J Agric Food Chem. 2000;48:5702–9. doi: 10.1021/jf000585g. [DOI] [PubMed] [Google Scholar]
- 45.Zhang DD, Hannink M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol. 2003;23:8137–51. doi: 10.1128/MCB.23.22.8137-8151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. doi: 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- 47.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, et al. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim M, Miyamoto S, Yasui Y, Oyama T, Murakami A, Tanaka T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int J Cancer. 2009;124:264–71. doi: 10.1002/ijc.23923. [DOI] [PubMed] [Google Scholar]
- 49.Li H, Wu WK, Li ZJ, Chan KM, Wong CC, Ye CG, et al. 2,3′,4,4′,5′-Pentamethoxy-trans-stilbene, a resveratrol derivative, inhibits colitis-associated colorectal carcinogenesis in mice. Br J Pharmacol. 2010;160:1352–61. doi: 10.1111/j.1476-5381.2010.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.European-Food-Safety-Authority. Flavouring Group Evaluation 214: alpha, beta-Unsaturated aldehydes and precursors from chemical subgroup 3.1 of FGE.19: Cinnamyl derivatives. The EFSA Journal. 2009;880:1–27. [Google Scholar]