Abstract
Purpose
Zoledronic acid (ZA) is being increasingly recognized for its anti-tumor properties, but the underlying functions are not well understood. In this study, we hypothesized that ZA inhibits ovarian cancer (OC) angiogenesis preventing Rac1 activation.
Experimental Design
The biological effects of ZA were examined using a series of in vitro (cell invasion, cytokine production, Rac1 activation, reverse-phase protein array and in vivo (orthotopic mouse models) experiments.
Results
There was significant inhibition of OC (HeyA8-MDR and OVCAR-5) cell invasion as well as reduced production of pro-angiogenic cytokines in response to ZA treatment. Furthermore, ZA inactivated Rac1 and decreased the levels of Pak1/p-38/matrix metalloproteinase-2 in OC cells. In vivo, ZA reduced tumor growth, angiogenesis and cell proliferation and inactivated Rac1 in both HeyA8-MDR and OVCAR-5 models. These in vivo antitumor effects were enhanced in both models when ZA was combined with nab-paclitaxel.
Conclusion
ZA has robust anti-tumor and anti-angiogenic activity and merits further clinical development as OC treatment.
Keywords: Ovarian cancer, Angiogenesis, Rac1, Zoledronic acid
Introduction
Ovarian cancer (OC) is the most common cause of death from gynecological malignancy. At present the mainstay in advanced OC is debulking surgery, chemotherapy (particularly with taxanes and platinum compounds) and radiotherapy. However, most OC patients eventually have a relapse after a median disease-free survival of 18 months (1, 2), thus highlighting the need for new avenues of therapy that target key processes in tumor progression. Angiogenesis, the formation of new blood vessels from pre-existing vasculature, is a complex and critical process for tumor growth and development of metastasis (3, 4). Current anti-angiogenic therapies are showing promise in OC, but display limited efficacy and encounter tumor resistance (5, 6). These limitations underscore the need for additional molecular targets and effective therapies for OC.
Bisphosphonates have been widely used in the treatment of patients with benign and malignant bone diseases to inhibit osteoclast-mediated bone resorption. Recently, nitrogen-containing bisphosphonates (NBPs), such as zoledronic acid (ZA) have been used in the treatment of metastatic bone disease in women with breast cancer and have been reported to possess potent antiangiogenic properties (7, 8). NBPs inhibit farnesyl pyrophosphate synthase (FPPS) and the downstream enzyme geranylgeranyl disphosphate synthase, which are required for the prenylation of small GTPases such as Rac1 (9–11). Rac1, a member of the Rho GTPase family, plays a central role in angiogenesis through the regulation of endothelial cell migration, tube formation, adhesion, invasion, and proliferation (12, 13).
In this study, we hypothesized that ZA inhibits OC angiogenesis by preventing the activation of Rac1. To test this hypothesis, we treated human OC HeyA8-MDR and OVCAR-5 cells with ZA to assess its effects on cell invasion, matrix metalloproteinase (MMP) expression and angiogenic factors (Milliplex assay). We performed reverse-phase protein array (RPPA) analysis to determine the pathway through which Rac1 inhibits angiogenesis that was validated by western blot. We also determined Rac1 activation by pull-down assay. HeyA8-MDR and OVCAR-5 orthotopic murine models of OC were assessed for expression of CD31, Ki-67 and Rac1 activation by immunohistochemistry after treatment with ZA, nab-paclitaxel, or a combination.
Materials and Methods
Reagents
ZA was purchased from Novartis Pharmaceuticals, Corporation, and nab-paclitaxel from Abraxis BioScience, Inc.
Cell culture
The human OC cell line HeyA8-MDR was maintained and propagated in RPMI 1640 medium (Invitrogen Corporation, Carlsbad, CA) supplemented with 10% FBS and 400ng/mL of paclitaxel (Bedford Laboratories). A2780-CP20 cell line was developed and maintained as previously described (14). SKOV3-TR cell line was maintained in RPMI 1640 medium (Invitrogen Corporation, Carlsbad, CA) supplemented with 10% FBS and 150ng/mL taxol. The OVCAR-5 cell line was maintained in DMEM medium (Invitrogen) with 10% FBS. The cells used are taxane sensitive and resistant. OVCAR-5 cells are cisplatin and taxane sensitive. The resistant ones are either MDR, A2780 CP20 (cisplatin resistant cells) or SKOV3-TR (taxane resistant cells).The immortalized human endothelial RF-24 (EC-RF24) cell line was maintained in MEM medium supplemented with 10% FBS, 1% MEM vitamins, 1% L-glutamine, 1% sodium pyruvate and 1% nonessential amino acids in 5% CO2/95% air at 37°C. All cell lines were kindly provided by Dr Anil K. Sood [The University of Texas MD Anderson Cancer Center (MDACC), Houston, TX] and screened for mycoplasma using a MycoAlert mycoplasma detection Kit (Lonza Rockland, Inc., Rockland, ME) as described by the manufacturer. Then cells were expanded, cryopreserved and used within 6 months after resuscitation. The authentication of all cell lines was done by the Characterized Cell Line Core Facility at The University of Texas MD Anderson Cancer Center (MDACC), Houston, TX by STR Short Tandem Repeat (STR) Method.
Invasion Assay
The invasiveness of HeyA8-MDR and OVCAR-5 cells treated with ZA was determined as previously described (15). Briefly, HeyA8-MDR and OVCAR-5 cells were collected and washed with serum-free media. Cells (4×105) were resuspended in 1mL of serum-free DMEM/F-12 medium and added onto 6-well plate transwell inserts (8µm pore-size; Fisher Scientific, Middleton, VA, USA) coated with a Matrigel basement membrane (0.7mg/mL; BD Biosciences, Bedford, MA, USA). Lower chambers were filled with 2mL of DMEM/F-12 medium supplemented with 10% FBS. HeyA8-MDR and OVCAR-5 cells in the transwell inserts were then treated with different concentrations of ZA in serum-free media. Seventy-two hours later, non-invading cells on the upper surface of the filter were removed with cotton swabs. Cells that invaded through the Matrigel onto the lower side of the filter were fixed, stained with the Hema-3 Stain System (Fisher Scientific), and photographed. For each filter, the number of invaded cells was counted in nine fields, and expressed as the mean number of cells from triplicate measurements.
Tube Formation Assay
EC-RF-24 cells were treated with different concentrations of ZA and nab-paclitaxel for 72h. Forty-eight-well plates were coated with 200µL of BD Matrigel (BD Biosciences) and incubated at 37°C for 30 min. EC-RF24 cells were trypsinized, adjusted at 2 × 104 cells and plated onto coated plates and incubated for an additional 6h at 37°C. Experiments were performed in triplicate. Using an Olympus IX81 inverted microscope, five images per well were taken at 100× magnification. The number of nodes (defined as when at least three cells formed a single point) per image were quantified as previously described (16).
Measurement of angiogenic factors and matrix metalloproteinases
Angiopoietin-2, BMP-9, EGF, endoglin, endothelin-1, FGF-1, FGF-2, follistatin, G-CSF, HB-EGF, HGF, IL-8, leptin, PLGF, VEGF-A, VEGF-C, VEGF-D, MMP-1, MMP-10, MMP-2, MMP-7 and MMP-9 levels were detected in the supernatant of HeyA8-MDR and OVCAR-5 cells treated with ZA respectively by multiplex bead immunoassay using a Luminex kit (Millipore Corp., Billerica, MA).
Active Rac1 pull-down
HeyA8-MDR and OVCAR-5 cells were treated with different concentrations of ZA for 72h. After cell incubation, the Active Rac1 Pull-down and Detection Kit was used according to the manufacturer’s instructions (Thermo Scientific, Pittsburgh PA).
siRNA transfection
EC-RF24 cells were plated at a density of 1.5×104 cells/well in a 6-well plate. The next day, cells were transfected with 5µg control or Rac1 siRNA (Sigma Aldrich) using HiPerFect® transfection reagent (Qiagen, Valencia, CA, USA) for 48h.
Reverse-phase protein array (RPPA)
The RPPA is a high-throughput antibody-based technique that measures levels of protein expression, as well as protein modifications such as phosphorylation. The RPPA was done by the MD Anderson Cancer Center RPPA Core Facility. Briefly, HeyA8-MDR was treated with ZA for 72 h. After protein extraction and denaturalization by 1% SDS, serial dilutions of each sample were performed. Samples were arrayed on nitrocellulose-coated slides. The slides were probed with 161 validated antibodies detected by 3,3-diaminobenzidine (DAB) colorimetric reaction. The slides were scanned, analyzed and quantified using a customized-software MicroVigene (VigeneTech Inc) to generate spot intensity. The fitted curve for each dilution was generated using the super curve fitting, a logistic model developed by the Department of Bioinformatics and Computational Biology at MD Anderson Cancer Center. The fitted curve was plotted with the signal intensities on y-axis and log2-concentration of proteins on the x-axis. The protein concentrations were normalized using median polish, that was corrected across samples using linear expression values and the median expression levels of all antibody experiments to calculate a loading correction factor for each sample.
Western Blotting
Lysates were centrifuged, supernatants were collected, and protein concentration was determined using a DC Protein Assay (Bio-Rad Laboratories, Hercules, CA). Samples were electrophoresed using 4–15% gradient polyacrylamide gels (Bio-Rad) and then transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked, rinsed and incubated with primary antibodies against unprenylated Rap1A (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), p-P38, total P38, p-Pak1, total Pak1 (Cell Signaling Technology, Inc., Danvers, MA), cleaved PARP-1 (Cell Signaling) and cleaved caspase-3 (eBioscience). After overnight incubation at 4°C, membranes were washed and incubated with their corresponding secondary antibody conjugated with horseradish peroxidase (HRP). Protein bands were detected with an enhanced chemiluminescence detection kit (GE Healthcare, Piscataway, NJ). β-Actin (Sigma Aldrich) and vinculin (Santa Cruz) were used as loading controls.
Orthotopic tumor implantation and drug treatment
Female athymic nude mice (NCr-nu, 8 to 12 weeks old.) were purchased from Taconic (Hudson, NY, USA). HeyA8-MDR (1×106 cells/0.2 mL Hanks’ Balanced Salt Solution [HBSS; Gibco, Carlsbad, CA]) and OVCAR-5 (1×106 cells/0.2 mL HBSS) cells were injected into the peritoneal cavity. One week after cell implantation, mice bearing HeyA8-MDR and OVCAR-5 tumors were randomly divided into 4 treatment groups (N=5 per group): (a) saline solution (200µL i.p.), (b) ZA alone (1mg/kg body weight (BW)/once per week/i.p.), (c) nab-paclitaxel alone (10mg/kg BW/once per week/i.v.), or (d) a combination of ZA (1mg/kg BW/once per week/i.p.) and nab-paclitaxel (10 mg/kg BW/once per week/i.v.). Mice were sacrificed the fourth week after treatment onset. Tumor weight and number of tumor nodules were determined. Tumor tissue was either fixed in formalin for paraffin embedding or frozen in optimal cutting temperature (OCT) media to prepare frozen slides. The animal experiments were conducted in accordance with the American Association for Laboratory Animal Science (AALAS) regulations and with the approval of the MD Anderson Cancer Center Institutional Animal Care and Use Committee. All drugs were used in a clinically relevant doses, based on the equivalent surface area dosage conversion factors described by Freireich EJ, et al (17) and in the Drug Guidances by the U.S. Food and Drug Administration (18).
Immunohistochemical analysis
The immunohistochemical analysis was perfomed as previously described (19). Briefly, unstained sections of mouse tissues were deparaffinized and rehydrated. Antigen retrieval was performed with DAKO antigen retrieval solution (DAKO, North America Inc., Carpinteria, CA). Endogenous peroxidase was blocked by hydrogen peroxide (3%). For protein blocking, IgG blocking from a Vector M.O.M. kit (Vector Laboratories, Inc., Bulingame, CA) was applied for 1h (for active Rac1-GTP) or 5% normal horse serum and 1% normal goat serum in PBS were used (for Ki67 and CD31). Primary antibodies against active Rac1-GTP (Neweast Biosciences, King of Prussia, PA), Ki67 (Thermo/Lab Vision) and anti-CD31 (Pharmingen, San Diego,CA) were incubated overnight at 4°C. For active Rac1-GTP, a M.O.M. kit anti-mouse biotinylated secondary antibody (Vector) was incubated for 30 minutes. Slides were then incubated with Vectastain elite ABC solution (Vector) for 30 minutes. For Ki67, goat anti-rabbit HRP secondary antibody and for CD31, goat anti-rat HRP-conjugated secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) diluted in blocking solution were added and incubated for 1h at room temperature.
Slides were developed with DAB substrate (Vector Labs) and counterstained with Gill’s no. 3 hematoxylin solution. For active Rac1, the slides were imaged by an ACIS III image analysis system (DAKO) and the % of active Rac1 intensity was quantified in five random fields per slide (one slide per mouse, 5 slides per group). The Rac1 staining was reviewed by Dr. Huamin Wang [Pathologist at The University of Texas MD Anderson Cancer Center (MDACC), Houston, TX]. To quantify Ki67 and CD31 expression, the number of positive (DAB-stained) cells was counted in five random fields per slide (one slide per mouse, 5 slides per group) a 200× magnification, and the percentage of cells that were Ki67 and CD31 positive was calculated for each group. A single microvessel was defined as a discrete cluster or single cell stained positive for CD31, and the presence of a lumen was required for scoring as a microvessel (20). Cell apoptosis was determined by immunohistochemical analysis as described previously (19).
Statistical analysis
Statistical analyses were performed in R and the statistical significance was set to 0.05. The Shapiro-Wilk test was applied to verify if the data follows a normal distribution. Accordingly, Student’s t-test and ANOVA test together with Post- Hoc Tukey test, or the nonparametric test Kruskal-Wallis test followed by a Nemenyi post-hoc test, was applied to assess the relationship between groups.
Results
Effect of ZA and nab-paclitaxel on angiogenesis, cell proliferation and apoptosis
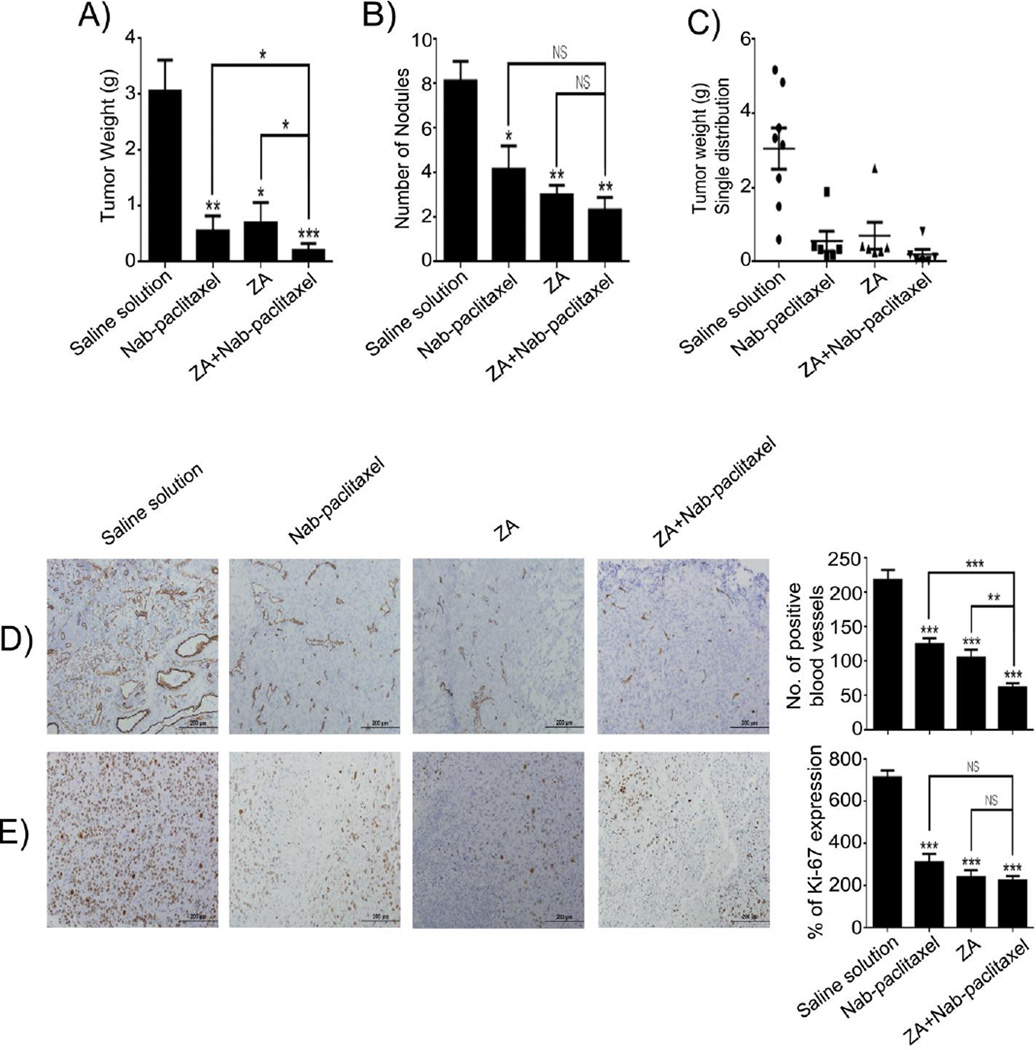
First, we examined the effects of ZA using orthotopic mouse models of OC. In the OVCAR-5 model, four weeks after treatment onset mice were sacrificed and tumor weight and number of tumor nodules were quantified. We found a significant decrease in tumor weight in mice treated with ZA (P<.05) alone or nab-paclitaxel (P<.05) alone and enhanced activity when the drugs were combined (P<.001) (Fig. 1A) compared with saline solution group. A decrease in the number of nodules was found in the groups treated with ZA alone (P<.001), nab-paclitaxel alone (P<.001), and a combination of both (P<.001) (Fig. 1B). The tumor weight single distribution showed a low variance (Fig. 1C). The number of CD31-positive cells resulted in a significant reduction after treatment with ZA or nab-paclitaxel and the combination of ZA with nab-paclitaxel (P<.0001 for each treatment group) (Fig. 1D), compared with saline solution group. Cell proliferation was also decrease significantly in mice treated with ZA (P<.0001) or nab-paclitaxel (P<.0001) alone and the combination of these agents further decreased proliferation (P<.0001) (Fig. 1E), compared with the group that received saline solution only. We found a significant decrease in Ki67 expression when nab-paclitaxel was combined with ZA (P<.05 and P<.001) compared with nab-paclitaxel alone and ZA alone respectively. Moreover, we observed that ZA, nab-paclitaxel and the combination of both agents induced apoptosis in OVCAR-5 in vivo model (Supplementary Fig 1A).
Figure 1.
In vivo therapeutic efficacy of ZA and nab-paclitaxel. Mice bearing OVCAR-5 ovarian tumor and treated with ZA (1 mg/kg BW/i.p.), nab-paclitaxel (10 mg/kg BW/i.v.), or both for 5 weeks exhibited lower tumor weight (A), number of nodules (B), Tumor weight single distribution (C) and CD31 positive cells (D) and Ki67 index (E) compared with treatment with saline solution. Means ± SD. *, P<.05; **, P<.001; ***, P<.0001; NS, no significant versus saline solution, n= 10 mice per group.
Given that many patients will develop chemotherapy-resistant disease, we also tested the effects of ZA in the HeyA8-MDR model. After four weeks treatment onset mice were sacrificed and tumor weight and number of tumor nodules were quantified. We found that compared with saline solution group, tumor weight was lower in the mice treated with ZA alone (P<.05) or nab-paclitaxel alone (P<.001) and these effects were further enhanced after treatment with both drugs (P<.0001) (Figure 2A). The same decrease was found in the number of nodules, ZA alone (P<.001), nab-paclitaxel alone (P<.05), and their combination (P<.001) (Fig. 2B). The tumor weight single distribution is shown in Fig. 2C. Angiogenesis was analyzed by determining the number of CD31-positive cells. Compared with results in the saline solution-treated group, ZA alone, nab-paclitaxel alone or a combination of the two resulted in a significant decrease in the number of CD31-positive cells, (P<.0001 for each treatment group) (Fig. 2D). Cell proliferation was determined by staining for the nuclear marker Ki-67. ZA (P<.0001) and nab-paclitaxel (P<0001) each induced a significant decrease in the Ki-67 index as single agent; the combination of these agents further decreased proliferation (P<.0001) (Fig. 2E). Also, we found a significant comparison of nab-paclitaxel alone or ZA alone with the combination of both agents in tumor weight and CD31-positive cells. There was a significant reduction of tumor weight in the combination treatment (P<.05) as compared with either nab-pacliatxel or ZA alone. Blood vessel density was significantly decreased in the combination group (P<.0001 and P<.001) as compared to either nab-paclitaxel or ZA alone respectively. Additionally, we observed that ZA, nab-paclitaxel and the combination of both agents induced apoptosis in HeyA8-MDR in vivo model (Supplementary Fig 1B) and in vitro (Supplementary Fig S1C–D).
Figure 2.
In vivo therapeutic efficacy of ZA and nab-paclitaxel. Mice bearing HeyA8-MDR ovarian tumor and treated with ZA (1 mg/kg BW/i.p.), nab-paclitaxel (10 mg/kg BW/i.v.), or both for 5 weeks exhibited lower tumor weight (A), number of nodules (B), Tumor weight single distribution (C) and CD31 positive cells (D) and Ki67 index (E) compared with treatment with saline solution. Means ± SD. *, P<.05; **, P<.001; ***, P<.0001; NS, no significant versus saline solution, n= 10 mice per group.
ZA prevents activation of Rac1 in vivo
To address whether ZA prevents Rac1 activation in vivo, we assessed immunohistochemistry using an active Rac1-GTP antibody. In OVCAR-5 cells (Figure 3A), we observed a significant (P<.0001) decrease in the expression of active Rac1 in the groups treated with ZA alone compared with results in the mice treated with saline solution. In HeyA8-MDR cells (Figure 3B), we observed the same results. Moreover, we found that when we combined ZA and nab-paclitaxel the prevention of Rac1 activation was enhanced. These results suggest that ZA inhibits in vivo angiogenesis and thus cell proliferation by preventing activation of Rac1. Additionally, in both in vivo models a significant (P<.0001) decreased in the expression of active Rac1 was observed when we compared the mice treated with the combination of ZA and nab-paclitaxel with nab-paclitaxel alone, but not in the mice treated with ZA alone, suggesting that the prevention of Rac1 activation occurs through the effect of ZA treatment.
Figure 3.
Rac1 inactivation by ZA in vivo. Representative images of immunohistochemistry staining for active Rac1, showing inactivation of Rac1 by ZA in OVCAR-5 (A) and HeyA8-MDR (B) in vivo models. Means ± SD. ***, P<.0001; NS, no significant versus saline solution, n= 10 mice per group.
ZA inhibits tube formation and invasion in vitro
To examine the effect of ZA on angiogenesis in vitro, we performed tube formation and invasion assays as well as luminex assay to determine the levels of MMP’s and angiogenic factors. To assess tube formation, EC-RF24 cells were treated with 5, 10, 50 and 100nM ZA for 72 h. After 6h of incubation in Matrigel, we observed a significant dose-dependent decrease in the number of nodes in the endothelial cells treated with ZA compared with the number of nodes in the untreated cells (Fig 4A), indicating that ZA inhibits in vitro angiogenesis. Since we also observed a reduction on angiogenesis in vivo by nab-paclitaxel, we examined the effect of nab-paclitaxel on angiogenesis in vitro by tube formation assay. EC-RF24 cells were treated with 5, 10, 50 and 100nM nab-paclitaxel for 72 h. After 6h of incubation in Matrigel, we observed a significant dose-dependent decrease in the number of nodes in the cells treated with nab-paclitaxel compared with the number of nodes in the untreated cells (Supplementary Fig S2), indicating that nab-paclitaxel inhibits in vitro angiogenesis.
Figure 4.
ZA inhibits tube formation and cell invasion. EC-RF24 cells were treated with ZA for 72 h and then incubated in Matrigel 6 h. (A) The number of nodes were significantly lower in the cells treated with ZA compared with the untreated cells. Invasion assay was assessed to determine the effect of ZA on the invasiveness of (B) HeyA8-MDR and (C) OVCAR-5 cells. The number of invaded cells was significantly lower in the cells treated with ZA compared with the untreated cells. Means ± SD. *, P<.05; **, P<.001; ***, P<.0001.
To determine the effect of ZA on OC invasion, we treated HeyA8-MDR cells with 10µM and 50µM ZA and OVCAR-5 cells with 60µM and 100µM ZA. After 72 h, cells were harvested and counted for the invasion assay. In HeyA8-MDR cells, we observed that at 10µM and 50µM of ZA the percentages of invading cells were 40.8% (P<.0001), and 10.2% (P<.0001), respectively, compared with untreated cells (Fig. 4B). In the presence of 60µM and 100µM ZA, the percentages of invading OVCAR-5 cells were 36.5% (P<.0001), and 17.9% (P<.0001), respectively compared with the untreated cells (Fig. 4C). These results indicate that ZA decreases the invasiveness of both OC cell lines in a dose-dependent manner.
ZA inhibits OC angiogenesis through the prevention of Rac-1 activation in vitro
RPPA analysis was used to study proteins that were affected in HeyA8-MDR after treatment with 10µM and 50µM ZA. For assessing the significance of protein expression change, t-test was performing. Significantly changed proteins were analyzed with Ingenuity Pathway Analysis (IPA) to find pathways and molecular functions. The RPPA showed a significant decrease in phospho-p-38 (P<.05) in treated cells compared with non-treated cells (Figure 5A). There was also a significant decrease in the levels of proteins related with angiogenesis such as paxillin, PI3K and EGFR (P<.05) and proteins related to apoptosis such as cleaved caspase-7 and 9 as it is shown in (Supplementary Fig S3). However, IPA showed that p-38 is related to the Rac1 pathway (Figure 5B). P-38 MAPK (mitogen-activated protein kinase) plays a role in invasion, migration (21, 22) and angiogenesis (23, 24). Based on these data, we focused and started to validate by western blot the proteins involved in the Rac1/p38 pathway shown in blue color in figure 5B. First, we performed an active Rac1 pull-down in HeyA8-MDR cells. We found the absence of active Rac1 in the samples treated with GDP (negative control) and the presence of active Rac1 in the samples to which we added GTP (positive control). In the samples without any control, we found a decrease in active Rac1 in HeyA8-MDR (Fig. 6A). Then we detected the levels of p21-activated kinase 1 (Pak1), a potential downstream mediator of Rac1 (12, 25). We observed a decrease in the levels of phospho-Pak1 in the HeyA8-MDR cell line after treatment with ZA (Figure 6B). Pak1 has been reported to activate p-38 MAPK pathway (25, 26). We next determined the levels of phospho-p-38 (Figure 6C) and we found a decrease in the levels of phospho-p38. P-38 is reported to mediate MMP-2 activity (27, 28). We finally, determined by Luminex assay MMP-2 secretion in HeyA8-MDR treated with ZA. We found a reduction in the secretion of MMP-2 in HeyA8-MDR (Figure 6D). These data suggest that ZA inhibits the production of angiogenetic factors in HeyA8-MDR by preventing the activation of the Rac1/Pak1/p-38/MMP-2 pathway.
Figure 5.
RPPA analysis in HeyA8-MDR treated with ZA. (A) RPPA showing decrease in phospho-p38. Red color shows upregulation of proteins in response to ZA and green color downregulation. IPA network showing the Rac1/Pak1/p38/MMP-2 pathway (B). Pink color of molecules indicates upregulation of proteins, red color represents higher levels of protein upregulation, whereas the protein that were not analized are in white.
Figure 6.
ZA inhibits Rac1/Pak1/p38/MMP-2 in HeyA8-MDR. (A) Active Rac1 pull-down, ZA reduced the inactivation of Rac1 in HeyA8-MDR. Immunoblotting of Pak1(B), phospho-p38 (C) and MMP-2 levels in HeyA8-MDR cells determined by Luminex (D).
The most differentially expressed proteins in HeyA8-MDR cells after treatment with ZA have their biological functions in cellular development, growth and proliferation; cell death and survival; cell cycle; cellular movement, assembly and organization; cellular function, maintenance and cell morphology (Supplementary Fig S4).
ZA inhibits angiogenic factors in vitro
Angiogenic factors were analyzed by Luminex assay, ZA significantly decreased the levels of VEGF-C in HeyA8-MDR cells (Supplementary Fig S5A) and in OVCAR-5 cells significantly decreased the levels of VEGF-C, HGF, IL-8, G-CSF, follistatin and PLGF (Supplementary Fig S5B). These data suggest that ZA decreases the secretion of important angiogenic factors in HeyA8-MDR and OVCAR-5 cells.
Detection of increased levels of unprenylated Rap1A is a surrogate measurement of NBP uptake by cells in vitro and of inhibition of prenylation (29–31). To confirm that the inhibition of angiogenesis occurs through the effect of ZA, we measured the levels of unprenylated Rap1A by western blot. The level of unprenylated Rap1A in HeyA8-MDR increased as ZA concentration increased (by 497.9% at 10µM and by 608% at 50µM compared with untreated cells); similar increases were obtained in OVCAR-5 cells (by 436.1% at 60µM ZA and by 666.6% at 100µM compared with untreated cells) (Supplementary Fig S5C).
In order to demonstrate the relevance of ZA-mediated prenylation inhibition we studied two additional chemotherapy resistant cell lines A2780 CP20 and SKOV3-TR, cisplatin and taxol resistant cells respectively. We first demonstrated the inhibition of prenylation by ZA in a dose dependent manner in both cell lines (Supplementary Fig S5D). Decreased invasion was observed at similar concentrations in both cell lines (Supplementary Fig. 6A–B). These data demonstrated that inhibition of prenylation is a key factor in reduction of invasiveness in chemoresistant cells.
To examine the effect of Rac1 on angiogenesis in vitro, we transfected EC-RF-24 cells with control and Rac1 siRNA for 48 h to perform tube formation. After 6 h incubation of cells in Matrigel, we observed a significant decrease in the number of nodes in the endothelial cells treated with Rac1 siRNA compared with the control cells (Supplementary Fig S6C), indicating that Rac1 is required for endothelial cell tube formation. Western blot was performed to verify Rac1 silencing (Supplementary Fig S6D).
Discussion
The key findings from this study are that ZA has potent anti-tumor and anti-angiogenic effects in OC models and that these effects are mediated by the Rac1/Pak1/p-38/MMP-2 pathway in HeyA8-MDR OC cells. Pak1 has been reported as an upstream kinase that activates p-38 (25, 32), those reports are consistent with our RPPA and western blot findings that ZA decreased levels of Pak1 and p-38.
Matrix metalloproteinases (MMP’s) are a family of proteolytic enzymes that degrade components of the extracellular matrix (33, 34). MMP’s have been implicated in cancer cell growth, differentiation, apoptosis, migration and invasion, and angiogenesis (33). In the angiogenesis process, MMP’s not only play an important role by degrading basement membrane and other ECM components, facilitating endothelial cell migration, but also by releasing ECM-bound proangiogenic factors (b-FGF, VEGF and TGF-β) (35). Endogenous and synthetic MMP inhibitors have been shown to be potent inhibitors of angiogenesis both in vivo and in vitro (33, 36–38). It has been reported that MMP-2 is required for the switch to the angiogenic phenotype during the development of chondrosarcoma (36). Also, it has been shown that MMP-2 is produced by endothelial cells during tube formation and the exogenous addition of MMP-2 enhance this process (39). Moreover, it was found that in a Mmp2-deficient mice the tumor angiogenesis and growth were reduced compared with wild type mice (40). All of these published data support our data and the important role of MMP-2 on angiogenesis.
ZA has anti-tumor and anti-angiogenic effects, but its underlying mechanisms are not fully understood. However, inhibition of the prenylation of GTPases is thought to be the main molecular mechanism involved directly in the antitumor and antiangiogenic effects of NBPs. Therefore, we carried out the blocking of prenylation by ZA. Our results showed that ZA increased the levels of unprenylated Rap1A protein and prevents the activation of Rac1 in HeyA8-MDR and OVCAR-5 cell lines; our findings were consistent with findings reported earlier on the ZA-mediated inhibition of prenylation of small GTPases such as Rac1, process that is required for the activation of GTPases (9, 41, 42).
Rac1 activation requires prenylation and eventual membrane localization (41, 42). Prenylation is a posttranslational modification of the C-terminal CAAX motif (where C is cysteine, A is any aliphatic amino acid and X is any amino acid) by the addition of farnesyl or geranygeranyl isoprenoid lipid tail to the cysteine residue. This modification increases protein hydrophobicity facilitating membrane association (41, 42). In our in vitro and in vivo results, we observed that ZA decreased the expression levels of active Rac1 protein. We didn’t observed changes in the cellular location of Rac1 protein between control group and the groups treated with ZA because we used an antibody that specifically recognized the active form of Rac1 (GTP-Rac1), thus the changes occur just in the expression level of Rac1, suggesting that although there are two distinct and additional sequence elements upstream of the CAAX motif necessary to the membrane association and activation of the GTPases (42) the inhibition of prenylation prevents the activation of GTPases like Rac1.
In vivo, we observed that ZA decreased the activation of Rac1 and decreased angiogenesis and cell proliferation. The inhibition of cell proliferation could be the consequence of, a) angiogenesis inhibition because angiogenesis is absolutely required for tumor growth; b) consequence of the inhibition of Rac1 which is also involved in cell proliferation (43) or c) a consequence of ZA treatment, we previously showed that ZA not only has anti-angiogenic activity but also anti-proliferative and pro-apoptotic effects (19). In this study, we observed that ZA induces apoptosis in OVCAR-5 in vivo model (Supplementary Fig S1A) and in HeyA8-MDR both in vitro and in vivo (Supplementary Fig S1B–D).
When we silenced Rac1 expression, we observed less effect on endothelial tube formation that observed with ZA treatment. An explanation for this finding could be that ZA works through several mechanisms including prevention of Rac1 activation, which maybe predominant, however ZA also inhibits other proteins involved in angiogenesis like paxillin, PI3K and EGFR as shown in the heatmap from the RPPA analysis in supplementary Fig S3.
Rac1 is a GTPase involved in tumorigenesis, angiogenesis, invasion and metastasis (12, 13), and is emerging as a potential target for angiogenesis. It has been shown that NSC23766, an inhibitor of Rac1, inhibits migration and reverse cellular phenotypes of trastuzumab resistance in lung (44) and breast cancers (45). However, studies about the efficacy and toxicity of NSC23766 are not available.
Nevertheless, ZA, the most potent of the NBPs, was already approved by the FDA to treat patients with multiple myeloma or patients with bone metastases from breast cancer or other solid tumors (46, 47). NBP’s are associated with side effects such as nephrotoxicity and osteonecrosis, however we have previously shown that neither toxicity or overt deleterious clinical effects were present after prolonged administration of ZA in mice (13).
Nab-paclitaxel, an albumin-bound paclitaxel-nanoparticle has a better therapeutic window than paclitaxel in metastatic breast cancer, non–small lung cancer and pancreatic cancer, where this agent has been approved by the Food and Drug Administration (FDA) (48, 49). In a phase II evaluation, weekly administration of nab-paclitaxel in highly resistant ovarian cancer patients showed better efficacy than weekly administration of paclitaxel (49). Other agents such as gossypol have been combined with ZA, enhancing the induce of apoptosis and the downregulation of mRNA of angiogenic factors such as FGF, PDGF, and VEGF (50) in ovarian cancer.
Our data support the important role of ZA-mediated inhibition on Rac1 activity and of the anti-angiogenesis pathway. These findings open the door for the therapeutic use of a nab-paclitaxel plus ZA combination in OC and other solid tumors.
Supplementary Material
Translational Relevance.
Ovarian cancer (OC) is the most common cause of death from gynecological malignancy. Although current anti-angiogenic therapies are showing promise in OC, they display limited efficacy and resistance. This highlights the need for additional molecular targets and effective therapies. In this study, we found that ZA reduced tumor growth, angiogenesis, cell proliferation and inactivated Rac1, in OC models, showing a potent anti-tumor and antiangiogenic effects that were enhanced with the combination of ZA and nab-paclitaxel. We also found that these effects are mediated by Rac1/Pak1/p-38/MMP-2 pathway in HeyA8-MDR OC cells. These findings open the door for the use of the combination of nab-paclitaxel with ZA as a novel therapeutic approach in OC and other solid tumors.
Acknowledgments
Financial Support
This study was supported by Grants to G. Lopez-Berestein, NCI-U54CA096300, NCI-P50PKCA093459, CA151668, CA180145, NCI-CA151668, NCI-UH2TR00943; A.K. Sood, Department of Defense grant W81XWH-09-1-0212; NIH grants U54CA143837, U54CA151668, and P50 CA083639; the CPRIT grant RP121071 from the State of Texas; the RGK Foundation; the Gilder Foundation; the Betty Anne Asche Murray Distinguished Professorship; and the John Q. Gaines Foundation Professorship for Cancer Research.
Footnotes
Conflict of interests
The authors declare no competing financial interests.
References
- 1.Yap TA, Carden CP, Kaye SB. Beyond chemotherapy: targeted therapies in ovarian cancer. Nat Rev Cancer. 2009;9:167–181. doi: 10.1038/nrc2583. [DOI] [PubMed] [Google Scholar]
- 2.Kim A, Ueda Y, Naka T, Enomoto T. Therapeutic strategies in epithelial ovarian cancer. J Exp Clin Cancer Res. 2012;31:14. doi: 10.1186/1756-9966-31-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez-Raposo C, Mendiola M, Barriuso J, Casado E, Hardisson D, Redondo A. Angiogenesis and ovarian cancer. Clin Transl Oncol. 2009;11:564–571. doi: 10.1007/s12094-009-0406-y. [DOI] [PubMed] [Google Scholar]
- 4.Spannuth WA, Sood AK, Coleman RL. Angiogenesis as a strategic target for ovarian cancer therapy. Nat Clin Pract Oncol. 2008;5:194–204. doi: 10.1038/ncponc1051. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raja FA, Hook JM, Ledermann JA. Biomarkers in the development of anti-angiogenic therapies for ovarian cancer. Cancer Treat Rev. 2012;38:662–672. doi: 10.1016/j.ctrv.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Stresing V, Fournier PG, Bellahcene A, Benzaid I, Monkkonen H, Colombel M, et al. Nitrogen-containing bisphosphonates can inhibit angiogenesis in vivo without the involvement of farnesyl pyrophosphate synthase. Bone. 2011;48:259–266. doi: 10.1016/j.bone.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 8.Wood J, Bonjean K, Ruetz S, Bellahcene A, Devy L, Foidart JM, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. Journal of Pharmacology and Experimental Therapeutics. 2002;302:1055–1061. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- 9.Misso G, Porru M, Stoppacciaro A, Castellano M, De Cicco F, Leonetti C, et al. Evaluation of the in vitro and in vivo antiangiogenic effects of denosumab and zoledronic acid. Cancer Biol Ther. 2012;13:1491–1500. doi: 10.4161/cbt.22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knight LA, Kurbacher CM, Glaysher S, Fernando A, Reichelt R, Dexel S, et al. Activity of mevalonate pathway inhibitors against breast and ovarian cancers in the ATP-based tumour chemosensitivity assay. BMC Cancer. 2009;9:38. doi: 10.1186/1471-2407-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clezardin P. Insights into the Antitumor Effects of Bisphosphonates from Preclinical Models and Potential Clinical Implications. International bone and Mineral Society. 2009;6:210–217. [Google Scholar]
- 12.Bid HK, Roberts RD, Manchanda PK, Houghton PJ. RAC1: an emerging therapeutic option for targeting cancer angiogenesis and metastasis. Mol Cancer Ther. 2013;12:1925–1934. doi: 10.1158/1535-7163.MCT-13-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan W, Palmby TR, Gavard J, Amornphimoltham P, Zheng Y, Gutkind JS. An essential role for Rac1 in endothelial cell function and vascular development. FASEB J. 2008;22:1829–1838. doi: 10.1096/fj.07-096438. [DOI] [PubMed] [Google Scholar]
- 14.Moreno-Smith M, Halder JB, Meltzer PS, Gonda TA, Mangala LS, Rupaimoole R, et al. ATP11B mediates platinum resistance in ovarian cancer. J Clin Invest. 2013;123:2119–2130. doi: 10.1172/JCI65425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Villasana V, Nieves-Alicea R, McMurtry V, Gutierrez-Puente Y, Tari AM. Programmed cell death 4 inhibits leptin-induced breast cancer cell invasion. Oncol Rep. 2012;27:861–866. doi: 10.3892/or.2011.1600. [DOI] [PubMed] [Google Scholar]
- 16.Pecot CV, Rupaimoole R, Yang D, Akbani R, Ivan C, Lu CH, et al. Tumour angiogenesis regulation by the miR-200 family. Nature Communications. 2013;4 doi: 10.1038/ncomms3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–244. [PubMed] [Google Scholar]
- 18.Administration FaD. Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. [Acces Date, 2005]; http://www.fda.gov/downloads/Drugs/Guidances/UCM078932.pdf.
- 19.Gonzalez-Villasana V, Rodriguez-Aguayo C, Arumugam T, Cruz-Monserrate Z, Fuentes-Mattei E, Deng D, et al. Bisphosphonates inhibit stellate cell activity and enhance antitumor effects of nanoparticle albumin-bound Paclitaxel in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2014;13:2583–2594. doi: 10.1158/1535-7163.MCT-14-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu C, Kamat AA, Lin YG, Merritt WM, Landen CN, Kim TJ, et al. Dual targeting of endothelial cells and pericytes in antivascular therapy for ovarian carcinoma. Clin Cancer Res. 2007;13:4209–4217. doi: 10.1158/1078-0432.CCR-07-0197. [DOI] [PubMed] [Google Scholar]
- 21.del Barco Barrantes I, Nebreda AR. Roles of p38 MAPKs in invasion and metastasis. Biochem Soc Trans. 2012;40:79–84. doi: 10.1042/BST20110676. [DOI] [PubMed] [Google Scholar]
- 22.Liu XY, Zhang L, Wu J, Zhou L, Ren YJ, Yang WQ, et al. Inhibition of elongation factor-2 kinase augments the antitumor activity of Temozolomide against glioma. PLoS One. 2013;8:e81345. doi: 10.1371/journal.pone.0081345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu G, Luo J, Rana JS, Laham R, Sellke FW, Li J. Involvement of COX-2 in VEGF-induced angiogenesis via P38 and JNK pathways in vascular endothelial cells. Cardiovasc Res. 2006;69:512–519. doi: 10.1016/j.cardiores.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Khan AA, Dace DS, Ryazanov AG, Kelly J, Apte RS. Resveratrol regulates pathologic angiogenesis by a eukaryotic elongation factor-2 kinase-regulated pathway. Am J Pathol. 2010;177:481–492. doi: 10.2353/ajpath.2010.090836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Han J, Sells MA, Chernoff J, Knaus UG, Ulevitch RJ, et al. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]
- 26.Kumar R, Gururaj AE, Barnes CJ. p21-activated kinases in cancer. Nat Rev Cancer. 2006;6:459–471. doi: 10.1038/nrc1892. [DOI] [PubMed] [Google Scholar]
- 27.Zhong J, Gencay MM, Bubendorf L, Burgess JK, Parson H, Robinson BW, et al. ERK1/2 and p38 MAP kinase control MMP-2, MT1-MMP, and TIMP action and affect cell migration: a comparison between mesothelioma and mesothelial cells. J Cell Physiol. 2006;207:540–552. doi: 10.1002/jcp.20605. [DOI] [PubMed] [Google Scholar]
- 28.Lin ML, Lu YC, Chung JG, Wang SG, Lin HT, Kang SE, et al. Down-regulation of MMP-2 through the p38 MAPK-NF-kappaB-dependent pathway by aloe-emodin leads to inhibition of nasopharyngeal carcinoma cell invasion. Mol Carcinog. 2010;49:783–797. doi: 10.1002/mc.20652. [DOI] [PubMed] [Google Scholar]
- 29.Michailidou M, Brown HK, Lefley DV, Evans A, Cross SS, Coleman RE, et al. Microvascular endothelial cell responses in vitro and in vivo: modulation by zoledronic acid and paclitaxel? J Vasc Res. 2010;47:481–493. doi: 10.1159/000313876. [DOI] [PubMed] [Google Scholar]
- 30.Holen I, Coleman RE. Anti-tumour activity of bisphosphonates in preclinical models of breast cancer. Breast Cancer Res. 2010;12:214. doi: 10.1186/bcr2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakchoure S, Merrell MA, Aldrich W, Millender-Swain T, Harris KW, Triozzi P, et al. Bisphosphonates inhibit the growth of mesothelioma cells in vitro and in vivo. Clin Cancer Res. 2006;12:2862–2868. doi: 10.1158/1078-0432.CCR-05-2766. [DOI] [PubMed] [Google Scholar]
- 32.Zarubin T, Han J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 33.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 34.Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: a moving target for therapeutic intervention. J Clin Invest. 1999;103:1237–1241. doi: 10.1172/JCI6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rundhaug JE. Matrix metalloproteinases, angiogenesis, and cancer: commentary re: A.C. Lockhart et al., Reduction of wound angiogenesis in patients treated with BMS-275291, a broad spectrum matrix metalloproteinase inhibitor. Clin. Cancer Res.,9: 00-00, 2003. Clin Cancer Res. 2003;9:551–554. [PubMed] [Google Scholar]
- 36.Fang J, Shing Y, Wiederschain D, Yan L, Butterfield C, Jackson G, et al. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci U S A. 2000;97:3884–3889. doi: 10.1073/pnas.97.8.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li H, Lindenmeyer F, Grenet C, Opolon P, Menashi S, Soria C, et al. AdTIMP-2 inhibits tumor growth, angiogenesis, and metastasis, and prolongs survival in mice. Hum Gene Ther. 2001;12:515–526. doi: 10.1089/104303401300042429. [DOI] [PubMed] [Google Scholar]
- 38.Gatto C, Rieppi M, Borsotti P, Innocenti S, Ceruti R, Drudis T, et al. BAY 12-9566, a novel inhibitor of matrix metalloproteinases with antiangiogenic activity. Clin Cancer Res. 1999;5:3603–3607. [PubMed] [Google Scholar]
- 39.Schnaper HW, Grant DS, Stetler-Stevenson WG, Fridman R, D'Orazi G, Murphy AN, et al. Type IV collagenase(s) and TIMPs modulate endothelial cell morphogenesis in vitro. J Cell Physiol. 1993;156:235–246. doi: 10.1002/jcp.1041560204. [DOI] [PubMed] [Google Scholar]
- 40.Itoh T, Tanioka M, Yoshida H, Yoshioka T, Nishimoto H, Itohara S. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 41.Zhou Y, Johnson JL, Cerione RA, Erickson JW. Prenylation and membrane localization of Cdc42 are essential for activation by DOCK7. Biochemistry. 2013;52:4354–4363. doi: 10.1021/bi301688g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts PJ, Mitin N, Keller PJ, Chenette EJ, Madigan JP, Currin RO, et al. Rho Family GTPase modification and dependence on CAAX motif-signaled posttranslational modification. J Biol Chem. 2008;283:25150–25163. doi: 10.1074/jbc.M800882200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guo X, Wang M, Jiang J, Xie C, Peng F, Li X, et al. Balanced Tiam1-rac1 and RhoA drives proliferation and invasion of pancreatic cancer cells. Mol Cancer Res. 2013;11:230–239. doi: 10.1158/1541-7786.MCR-12-0632. [DOI] [PubMed] [Google Scholar]
- 44.Gastonguay A, Berg T, Hauser AD, Schuld N, Lorimer E, Williams CL. The role of Rac1 in the regulation of NF-kappaB activity, cell proliferation, and cell migration in non-small cell lung carcinoma. Cancer Biol Ther. 2012;13:647–656. doi: 10.4161/cbt.20082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dokmanovic M, Hirsch DS, Shen Y, Wu WJ. Rac1 contributes to trastuzumab resistance of breast cancer cells: Rac1 as a potential therapeutic target for the treatment of trastuzumab-resistant breast cancer. Mol Cancer Ther. 2009;8:1557–1569. doi: 10.1158/1535-7163.MCT-09-0140. [DOI] [PubMed] [Google Scholar]
- 46.Major P. The use of zoledronic acid, a novel, highly potent bisphosphonate, for the treatment of hypercalcemia of malignancy. Oncologist. 2002;7:481–491. doi: 10.1634/theoncologist.7-6-481. [DOI] [PubMed] [Google Scholar]
- 47.Kellinsalmi M, Monkkonen H, Monkkonen J, Leskela HV, Parikka V, Hamalainen M, et al. In vitro comparison of clodronate, pamidronate and zoledronic acid effects on rat osteoclasts and human stem cell-derived osteoblasts. Basic Clin Pharmacol Toxicol. 2005;97:382–391. doi: 10.1111/j.1742-7843.2005.pto_176.x. [DOI] [PubMed] [Google Scholar]
- 48.Cucinotto I, Fiorillo L, Gualtieri S, Arbitrio M, Ciliberto D, Staropoli N, et al. Nanoparticle albumin bound Paclitaxel in the treatment of human cancer: nanodelivery reaches prime-time? J Drug Deliv. 2013;2013:905091. doi: 10.1155/2013/905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coleman RL, Brady WE, McMeekin DS, Rose PG, Soper JT, Lentz SS, et al. A phase II evaluation of nanoparticle, albumin-bound (nab) paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecologic Oncology. 2011;122:111–115. doi: 10.1016/j.ygyno.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Atmaca H, Gorumlu G, Karaca B, Degirmenci M, Tunali D, Cirak Y, et al. Combined gossypol and zoledronic acid treatment results in synergistic induction of cell death and regulates angiogenic molecules in ovarian cancer cells. Eur Cytokine Netw. 2009;20:121–130. doi: 10.1684/ecn.2009.0159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.