Abstract
The cochlear implant is considered one of the most successful neural prostheses to date, which was made possible by visionaries who continued to develop the cochlear implant through multiple technological and clinical challenges. However, patients without a functional auditory nerve or implantable cochlea cannot benefit from a cochlear implant. The focus of the paper is to review the development and translation of a new type of central auditory prosthesis for this group of patients, which is known as the auditory midbrain implant (AMI) and is designed for electrical stimulation within the inferior colliculus. The rationale and results for the first AMI clinical study using a multi-site single-shank array will be presented initially. Although the AMI has achieved encouraging results in terms of safety and improvements in lip-reading capabilities and environmental awareness, it has not yet provided sufficient speech perception. Animal and human data will then be presented to show that a two-shank AMI array can potentially improve hearing performance by targeting specific neurons of the inferior colliculus. Modifications to the AMI array design, stimulation strategy, and surgical approach have been made that are expected to improve hearing performance in the patients implanted with a two-shank array in an upcoming clinical trial funded by the National Institutes of Health. Positive outcomes from this clinical trial will motivate new efforts and developments toward improving central auditory prostheses for those who cannot sufficiently benefit from cochlear implants.
Keywords: auditory brainstem implant, brain machine interface, cochlear implant, deep brain stimulation, inferior colliculus, neuromodulation
1. INTRODUCTION
There are hundreds of thousands of individuals implanted with a neural device for restoring sensory, motor, or autonomic function as well as for treating neurological and psychiatric disorders (Johnson et al., 2013; Konrad et al., 2010; Navarro et al., 2005). These devices interface with the peripheral or central nervous system, and can be fully implanted into the body or head with wireless capabilities. One of the most successful neural prostheses is known as the cochlear implant (CI), which is designed for implantation into the cochlea for electrically stimulating nearby auditory nerve fibers for hearing restoration (Figure 1) (Wilson et al., 2008; Zeng et al., 2008). Over 320,000 patients have received a CI, with many of these individuals capable of speech perception and even the ability to converse over the telephone. Children, including infants younger than one year of age, have been implanted with a CI and have been able to integrate into mainstream schools. Therefore, the CI has been remarkably successful in restoring hearing to many deaf individuals, which in turn has guided the development of other neural prostheses for sensory or motor restoration, such as the visual prosthesis or a neural-controlled prosthetic limb (Weber et al., 2012; Weiland et al., 2011). The monumental achievements of the CI are attributed to the continuous efforts of several visionaries including André Djourno, William House, Blair Simmons, and the 2013 Lasker~DeBakey Clinical Medical Research Awardees – Graeme Clark, Ingeborg Hochmair, and Blake Wilson (Eisenberg, 2014; Lenarz, 1998; Mudry et al., 2013).
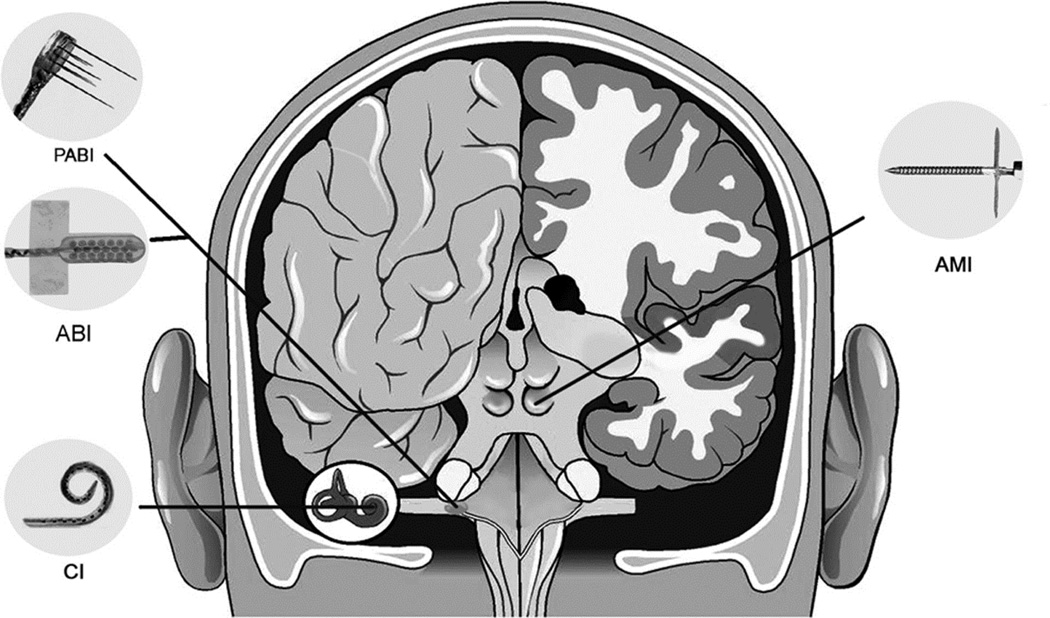
Figure 1.
Different auditory neural prosthetics used in patients for hearing restoration. CI: Cochlear Implant, which consists of an electrode array that is implanted into the cochlea and used for auditory nerve stimulation. ABI: Auditory Brainstem Implant that is used for surface stimulation of the cochlear nucleus. PABI: Penetrating Auditory Brainstem Implant that is used for penetrating stimulation of the cochlear nucleus. AMI: Auditory Midbrain Implant that is used for penetrating stimulation of the auditory midbrain (i.e., the inferior colliculus). There are several companies that build these types of implant devices. The examples shown in this figure are developed by Cochlear Limited (Australia). Figure was taken from (Lenarz et al., 2006b) and reprinted with permission from Lippincott Williams & Wilkins.
In thinking about the future of auditory prostheses, the question arises as to how hearing performance can be further improved beyond what is possible with current devices, not only for those who are implanted with a CI but also for those who do not have a functional auditory nerve or implantable cochlea. There are exciting efforts towards improving the design of CIs (e.g., new electrode arrays, and binaural or bimodal implants) and activation of the auditory nerve (e.g., current steering techniques, direct nerve stimulation, and optical activation methods) for achieving better performance in noisy environments and with more complex inputs such as music, tonal languages, and multiple talkers. Various technological, modeling, signal processing, physiology, and psychophysics research to achieve these improvements are presented in the other papers in the Lasker Award Special Issue for Hearing Research. The focus of this paper is to present the development and translation of devices for stimulation beyond the auditory nerve within more central auditory structures, particularly the inferior colliculus (IC). Central auditory implants can provide an alternative hearing option for those who cannot benefit from a CI. Furthermore, a major limitation in achieving higher performance with CIs appears to be the limited number of independent information channels available through cochlear stimulation (Friesen et al., 2001). The CI sends current through a bony modiolar wall of the cochlea with scattered flow of electrical charge to a variable distribution and reduced number of auditory neurons associated with deafness. Central auditory prostheses may also provide a way for achieving more specific activation of a greater number of frequency channels of information than is currently possible with CIs.
This review will present the rationale for the AMI and the results of the first clinical trial using a multi-site single-shank array. The animal and human studies leading to the development of a new two-shank AMI array will then be presented followed by an update on the second clinical trial.
2. RATIONALE FOR THE AMI
The CI can provide high levels of speech understanding, at least in quiet environments, for many deaf patients. However, the CI is designed for electrically activating the auditory nerve. For those patients without a functional auditory nerve (e.g., due to a head injury or tumor removal surgery, or being born without a nerve) or without an implantable cochlea to enable array insertion (e.g., due to ossification or head trauma), then the only hearing option is a central auditory implant. The first device, known as the ABI, was implanted as early as 1979 at the House Ear Institute in Los Angeles, California by William Hitselberger and William House. It consisted of two ball electrodes with a fabric backing that was built in collaboration with Douglass McCreery from the Huntington Medical Research Institutes in Pasadena, California. The ABI was positioned onto the surface of the cochlear nucleus. Further details of the development of the first ABIs are provided in (Schwartz et al., 2008; Sennaroglu et al., 2012). The ABI was initially designed and justified for patients with a genetic disease known as neurofibromatosis type 2 (NF2), which is usually associated with bilateral acoustic neuromas. Due to removal of these tumors and complete damage of the auditory nerves, the patients became bilaterally deaf and unable to benefit from CIs. Since the cochlear nucleus was already approached during tumor removal, it was then possible to place the electrodes on its surface with minimal added surgical risk. A total of 25 patients were implanted with an ABI by 1992 (Schwartz et al., 2008). Since 1992, the single channel ABI has been developed into a multi-site surface array (Figure 1) by several implant companies (e.g., Advanced Bionics Corporation, USA; Cochlear Limited, Australia; Med-El Company, Austria; MXM Digisonic, France) and implanted in over 1200 patients worldwide with etiologies no longer limited to NF2 (e.g., those with nerve aplasia/avulsion or cochlear ossification).
The current status of the ABI is that it can achieve high levels of hearing performance in some patients (Behr et al., 2014; Colletti et al., 2014; Colletti et al., 2009; Matthies et al., 2014). There appears to be certain types of deaf patients who achieve good hearing performance with an ABI. For example, one study by (Colletti et al., 2009) showed that over half of the 48 non-tumor (i.e., non-NF2) adult patients implanted with the ABI achieved reasonable speech perception with a few reaching levels comparable to the top CI patients. These non-tumor patients obtained an average score of 59% on an open-set speech test compared to an average score of 10% across 32 NF2 adult patients. Considering that similar implants, stimulation strategies, and surgical approaches were used for both patient groups in the same clinic, these findings suggested that the limited performance observed in NF2 patients may be related to tumor damage, including surgical damage, of the cochlear nucleus (Behr et al., 2014; Colletti et al., 2005). Even within the non-tumor group, it appeared that those with cochlear ossification or who lost their auditory nerve due to head trauma performed better than those who had cochlear malformations or auditory neuropathy (Colletti et al., 2009). Similar trends have also been observed in children with ABIs in which those with cochlear damage due to ossification or head trauma achieved the best performance over other groups (Colletti et al., 2014).
The fact that the ABI can provide sufficient speech understanding in some patients demonstrates that artificial electrical stimulation even within the brain can restore sufficient hearing function. The question now arises as to how we can further improve central auditory prostheses so that a majority of implanted patients can achieve sufficient hearing performance, especially those with NF2 tumors. There are recent reports indicating that a few NF2 ABI patients are able to achieve speech understanding comparable to typical CI patients (Behr et al., 2014; Colletti et al., 2012; Matthies et al., 2014). One proposed reason for these encouraging results is that the surgeons were able to minimize damage to the brainstem during tumor removal surgery and/or array implantation. In over 1000 NF2 patients with ABIs, however, only a few of them have achieved high levels of speech perception, revealing the difficulties in minimizing brainstem damage and/or accurately placing the array onto the cochlear nucleus (Colletti et al., 2005; Lenarz et al., 2002; Schwartz et al., 2008; Sennaroglu et al., 2012), assuming those are the main reasons for the limited hearing performance.
Considering the factors described above, the authors of this paper seek to improve central auditory prostheses by stimulating within the inferior colliculus (IC), particularly its central nucleus (ICC), and initially targeting those with NF2. Unlike the brainstem, the midbrain is directly visible during surgery (images are shown later in Section 4.4) and is not surrounded by distorted or damaged brain structures caused by a NF2 tumor and/or its removal (Samii et al., 2007; Vince et al., 2010). Surrounding the brainstem, there are also lower cranial nerves involved with critical functions such as breathing and swallowing that may not be easily visible during surgery. The trochlear nerve is the only nerve near the midbrain and is directly visible during surgery. In terms of function, the ICC has a well-defined tonotopic organization (De Martino et al., 2013; Lim et al., 2013; Oliver, 2005; Ress et al., 2013; Schreiner et al., 1997), which is favorable for implementing an auditory prosthesis (Shannon et al., 2004; Xu et al., 2008). The IC is also the initial converging center of the central auditory system (Casseday et al., 2002; Ehret, 1997). Once the sound information is transmitted from the auditory nerve to the brain, it gets processed across multiple structures within the brainstem through several diverging pathways (Cant et al., 2003). The ascending sound information and pathways then converge, for the most part, into the ICC en route to the thalamus and cortex. In other words, whichever neural pathways through the brainstem are involved with transmission of speech information to higher perceptual centers, it should be possible to implant electrode sites within specific regions of the ICC to access and stimulate those pathways. Whether artificial electrical stimulation of those pathways can restore sufficient speech perception needs to be assessed in future AMI patients.
There is some concern about the surgical risks associated with implanting an electrode array into the midbrain. However, several exciting developments in the field of central neural prostheses provide a positive perspective on this topic. No one could have imagined 35 years ago that the ABI would be considered safe enough to be implanted into children as young as one year old ((Sennaroglu et al., 2011); FDA recently approved children as young as two years in the United States). Continuous improvements in the safety of the surgical approach and implant technology have made this a reality. Significant progress is also occurring for the use of deep brain stimulation (DBS) to treat various neurological and psychiatric conditions, with more than 100,000 patients now implanted with a penetrating DBS array (Johnson et al., 2013). There are surgical risks with DBS surgery but it isn’t far-fetched to assume that in the future, innovative solutions will bring these complications to nearly zero. It is important to note that the standard DBS array is approximately 20 times greater in volume than an AMI shank (Figure 2A) and penetrates through several centimeters of cortical tissue to reach subcortical structures (versus the several millimeters of tissue penetration of the AMI); thus, it has significantly more risk than the AMI array yet is being implanted in an increasing number of adults and children for various brain disorders. A recent innovative technology has pushed the field of central neural prostheses even further. A 96-site, three-dimensional penetrating array was implanted into the motor cortex in people with tetraplegia to record neural signals and control assistive devices (Hochberg et al., 2012), demonstrating that micro-machined, high-density arrays can be safely implanted into the brain. These major achievements in the neural implant field provide confidence that the surgical risks of the AMI will be reduced down to nearly zero in the future and the AMI can eventually be used in a broader clinical population beyond NF2 patients.
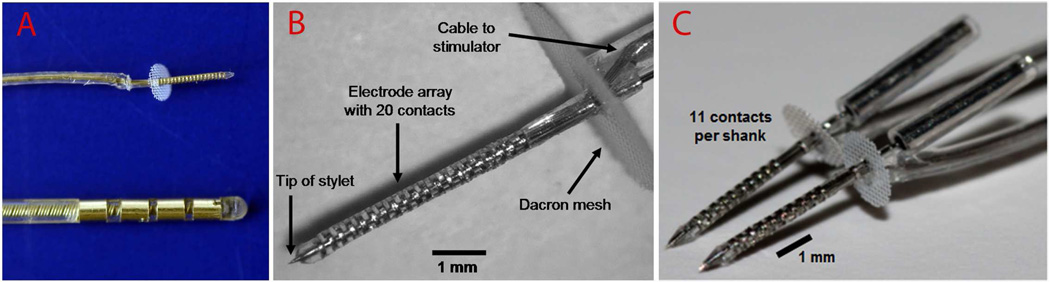
Figure 2.
AMI arrays developed by Cochlear Limited (Australia). A (top) and B show the AMI array currently implanted into humans with 20 ring sites (200 µm spacing, 200 µm thickness, 400 µm diameter) along a silicone carrier. Dacron mesh prevents over-insertion of the array into the IC and tethers it to the brain. The AMI array is much smaller than current deep brain stimulation arrays used for various neurological and psychiatric disorders in which A (bottom) shows an example array developed by Medtronic (USA). C shows the new two-shank AMI array that will be implanted into deaf patients in a second clinical trial. Each shank consists of 11 ring sites along a silicone carrier (300 µm site spacing except for one site positioned closer to the Dacron mesh for tinnitus treatment; see Section 4.5 for further details). Images in A and B were taken from (Lenarz et al., 2006b) and (Samii et al., 2007) and reprinted with permission from Lippincott Williams & Wilkins.
3. FINDINGS FROM THE FIRST CLINICAL TRIAL
3.1. Overview
The motivation for the first AMI clinical trial was to provide an alternative hearing option to the ABI in NF2 patients. AMI research and development began around 2000. Thomas Lenarz and Minoo Lenarz (currently at University of Berlin-Charité) initiated AMI developments at Hannover Medical School with James Patrick and his team from Cochlear Limited, developing an electrode array for use in humans (Figure 2A and 2B). The AMI array consists of a single shank with 20 linearly spaced sites and was designed to be aligned along the tonotopic gradient of the ICC. They collaborated with Hubert Lim and David Anderson at University of Michigan to validate this technology in animal studies, eventually obtaining sufficient evidence and approvals to begin the first clinical trial in 2006–2008 in which five adult NF2 patients were implanted with the device. Prior to the clinical trial, these researchers and clinicians had shown that ICC stimulation achieves low threshold and frequency-specific auditory activation in animals that was better or comparable to CI stimulation (Lenarz et al., 2006a; Lim et al., 2006). They also showed in a cat model that long-term implantation and stimulation of the AMI device was safe without any major side effects and induced minimal tissue damage that was comparable to other clinically approved brain implants (Lenarz et al., 2007). In terms of sound coding, multiple studies have shown that ICC neurons are capable of following the temporal modulations of acoustic stimuli up to or beyond 100 Hz and the ICC has a well-defined tonotopic organization (Geniec et al., 1971; Joris et al., 2004; Langner et al., 2002; Oliver, 2005; Rees et al., 2005; Schreiner et al., 1997). Considering that speech perception, at least in quiet backgrounds, is possible with temporal modulations as low as ~50 Hz with just ~4–8 frequency channels (Friesen et al., 2001; Shannon et al., 1995; Zeng, 2004), they envisioned that the AMI would be able to restore reasonable speech perception using a CI-based strategy. In particular, each electrode site in a specific frequency region would be presented with an amplitude modulated pulse train following the bandpass-filtered envelope extracted for the corresponding frequency channel.
After obtaining the necessary approvals, five patients were implanted with the AMI and provided with a CI-based strategy. Encouragingly, the AMI has proven to be safe in all five patients for over six years and has provided improvements in lip-reading capabilities and environmental awareness with some speech perception, comparable to the range of performance achieved by most ABI NF2 patients (Lim et al., 2009; Lim et al., 2011; Schwartz et al., 2008; Sennaroglu et al., 2012). These clinical results demonstrate that useful hearing can be provided by IC stimulation. However, the patients have not yet achieved sufficient speech perception without lip-reading cues. Therefore, there is still a critical need to improve the AMI if it is going to be considered as an alternative to the ABI.
3.2. Surgical Limitations
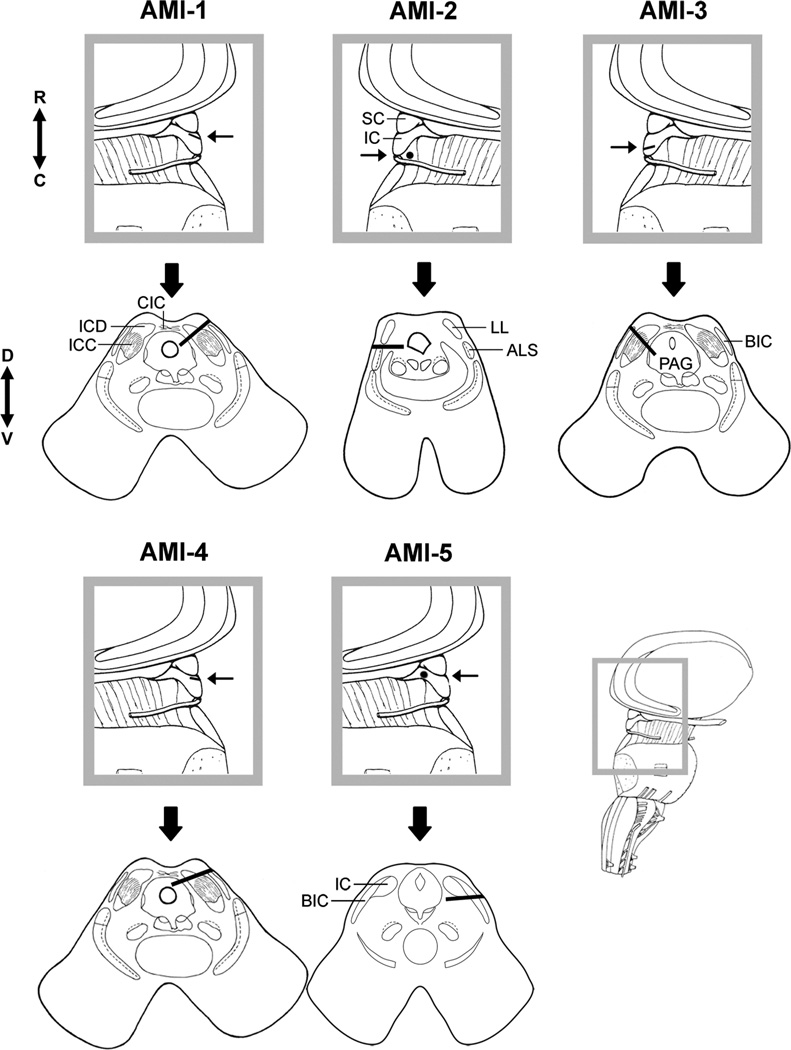
One major limitation in the first clinical trial was related to the difficulties in accurately placing the AMI array into the ICC (Figure 3). Out of five patients, only one (patient AMI-3) was implanted across the tonotopic gradient of the ICC. All other patients were implanted predominantly in other regions, including the dorsal and rostral IC, brachium of IC, and lateral lemniscus. As expected, AMI-3 exhibited the best hearing performance and a clear pitch organization across the sites (Lim et al., 2009; Lim et al., 2013) consistent with the tonotopy that is expected from animal and human studies (De Martino et al., 2013; Geniec et al., 1971; Malmierca et al., 2008; Oliver, 2005; Ress et al., 2013; Schreiner et al., 1997).
Figure 3.
Array placement across patients in the first AMI clinical trial. For each patient, the parasagittal (top, gray box) and axial (bottom, below gray box) sections show the location and orientation of the array within the midbrain. Arrow in parasagittal section points to the caudorostral location of the array and the corresponding axial section below. The black line (or dot for AMI-2 and AMI-5) representing the array in each section corresponds to the trajectory of the array across several superimposed CT-MRI slices. ALS, anterolateral system; BIC, brachium of inferior colliculus; CIC, commissure of inferior colliculus; IC, inferior colliculus; ICC, inferior colliculus central nucleus; ICD, inferior colliculus dorsal nucleus; LL, lateral lemniscus; PAG, periaqueductal gray; SC, superior colliculus. Anatomical directions: C, caudal; D, dorsal; R, rostral; V, ventral. Further details of the reconstruction technique, anatomy of the midbrain, and AMI surgery are presented in (Lim et al., 2007b; Samii et al., 2007). Only AMI-3 was properly implanted into the target region of the ICC with the array aligned along its tonotopic axis. A portion of this figure was taken from (Lim et al., 2007b) and reprinted with permission from the Society for Neuroscience.
Details of the surgical approach for the first AMI trial are provided in (Samii et al., 2007) with its limitations described in (Lim et al., 2009). Briefly, the array implantation was performed after removing the NF2 tumor at the brainstem level using a modified lateral suboccipital approach in a semi-sitting position. The cerebellum was retracted medially to expose the tumor. After the tumor was removed, the cerebellum was allowed to drop downwards due to gravity, and the IC surface could be directly viewed through the same skull opening. The main surgical limitation in the first clinical trial was the use of a small craniotomy, which made it difficult to view several key anatomical landmarks defining the outer borders of the IC and to determine the orientation of the array relative to the surface of the IC during insertion. These landmarks include the rostral border of the IC with the superior colliculus (SC), midline between both ICs, and caudal IC edge corresponding to the exit point of the trochlear nerve. The array needs to be aligned along the tonotopic gradient of the ICC, which requires an angle of insertion of about 40° relative to the sagittal plane (see Figure 3 for the location and orientation of the frequency laminae of the ICC; (Geniec et al., 1971; Kretschmann et al., 1992)). A small craniotomy was initially used to minimize surgical risks. As described in Section 4.4, an improved surgical approach has been developed with a larger exposure up to the midline that can still access the NF2 tumor more laterally and then approach the IC more medially with complete visibility of the landmarks mentioned above.
3.3. Frequency specific activation but limited temporal coding abilities
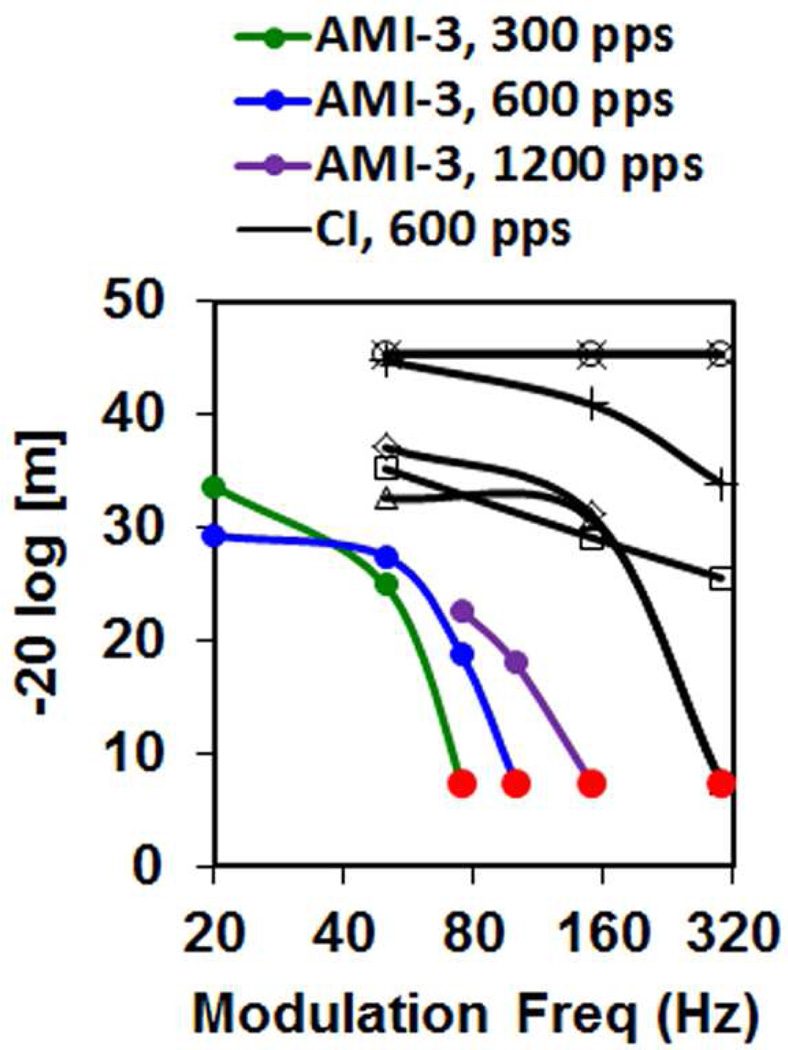
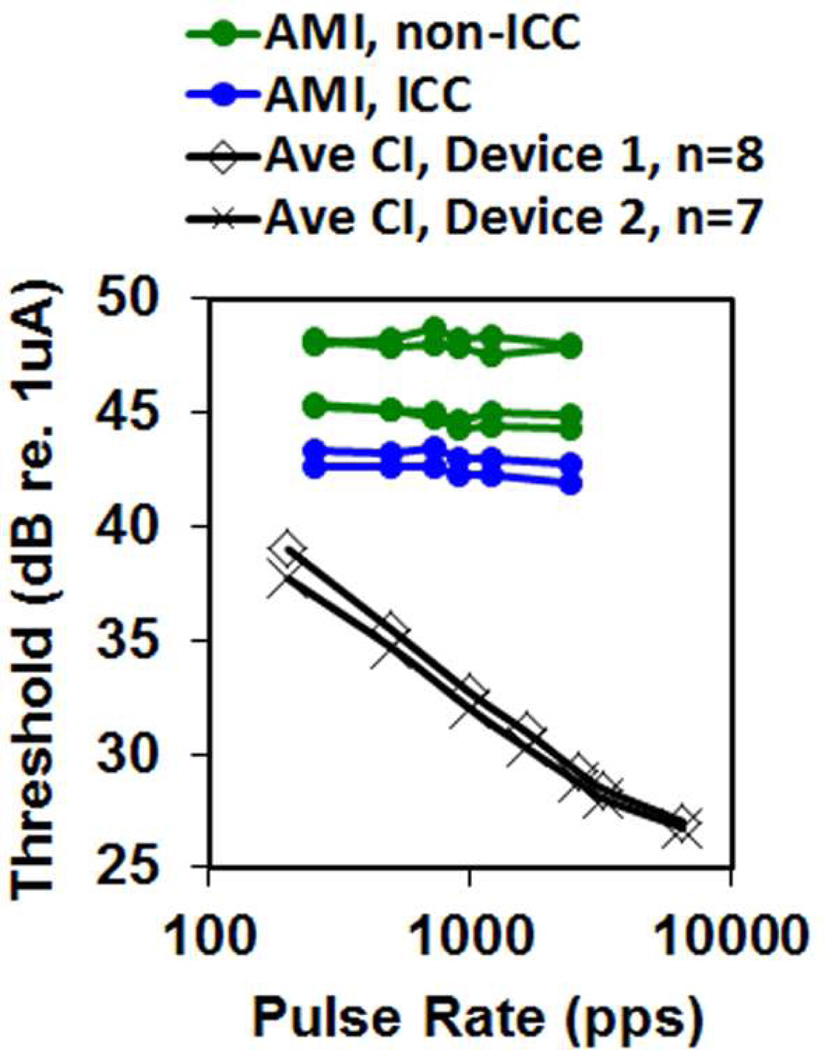
For the one patient implanted into the ICC (AMI-3), a systematic pitch organization from low to high was observed for stimulation of superficial to deeper AMI sites as expected from the tonotopic organization observed in animal and human studies (De Martino et al., 2013; Malmierca et al., 2008; Ress et al., 2013; Schreiner et al., 1997). However, poor temporal coding abilities were observed for AMI-3 (and the other AMI patients). Speech performance depends on both spectral and temporal cues (Nie et al., 2006; Shannon, 2002; Xu et al., 2008), and thus transmission of degraded temporal information may be limiting speech perception performance in the AMI patients. In particular, AMI-3 (and the other AMI patients) exhibited poor temporal modulation detection (i.e., ability to detect small changes in amplitude modulation, AM) and temporal resolution (i.e., ability to detect small temporal changes) compared to CI patients (Lim et al., 2008; McKay et al., 2013). CI users can achieve reliable AM detection beyond 150–300 Hz (Fraser et al., 2012), whereas the best AMI patient exhibited degraded capabilities even at 20–50 Hz (Figure 4) (McKay et al., 2013). What was surprising was the drastic difference between CI and AMI stimulation for shorter interval pulse trains. CI patients exhibit lower thresholds (and louder percepts) as the pulse rate increases (Figure 5) (Kreft et al., 2004; McKay et al., 1998; Shannon, 1989). This is attributed to a short-term integrator that sums the incoming activity within a short window (~5 ms) to track the fast temporal features that can contribute to speech understanding (McKay et al., 1998; Viemeister, 1979; Viemeister et al., 1991). AMI stimulation does not exhibit this short-term integration (Figure 5) (Lim et al., 2008; McKay et al., 2013).
Figure 4.
AM detection ability is lower for AMI-3 than for six typical CI users. A 3-interval task with one modulated and two non-modulated pulse trains were presented in a randomized sequence, and the subject selected the modulated interval. The modulation depth (m; higher ordinate value means less depth and better detection) for 70% correct was identified for each modulation frequency (Hz) and carrier rate (pps: pulses per second). Red circle: maximum depth used. Data for this figure was taken from (McKay et al., 2013) and replotted with permission from the Association for Research in Otolaryngology. Further details on the methods and results are presented in that publication.
Figure 5.
Detection thresholds do not decrease with higher pulse train rates for AMI as in CIs. AMI data is for individually stimulated sites within and outside of ICC and is taken from (Lim et al., 2008)) and replotted with permission from Elsevier. CI data are averages across subjects for two different devices taken from (Kreft et al., 2004) and replotted with permission from the Acoustical Society of America. Further details on the methods and results are presented in those corresponding publications. pps: pulses per second.
4. ANIMAL AND HUMAN STUDIES TOWARDS A SECOND CLINICAL TRIAL
4.1. Improving neural activation and possibly temporal coding
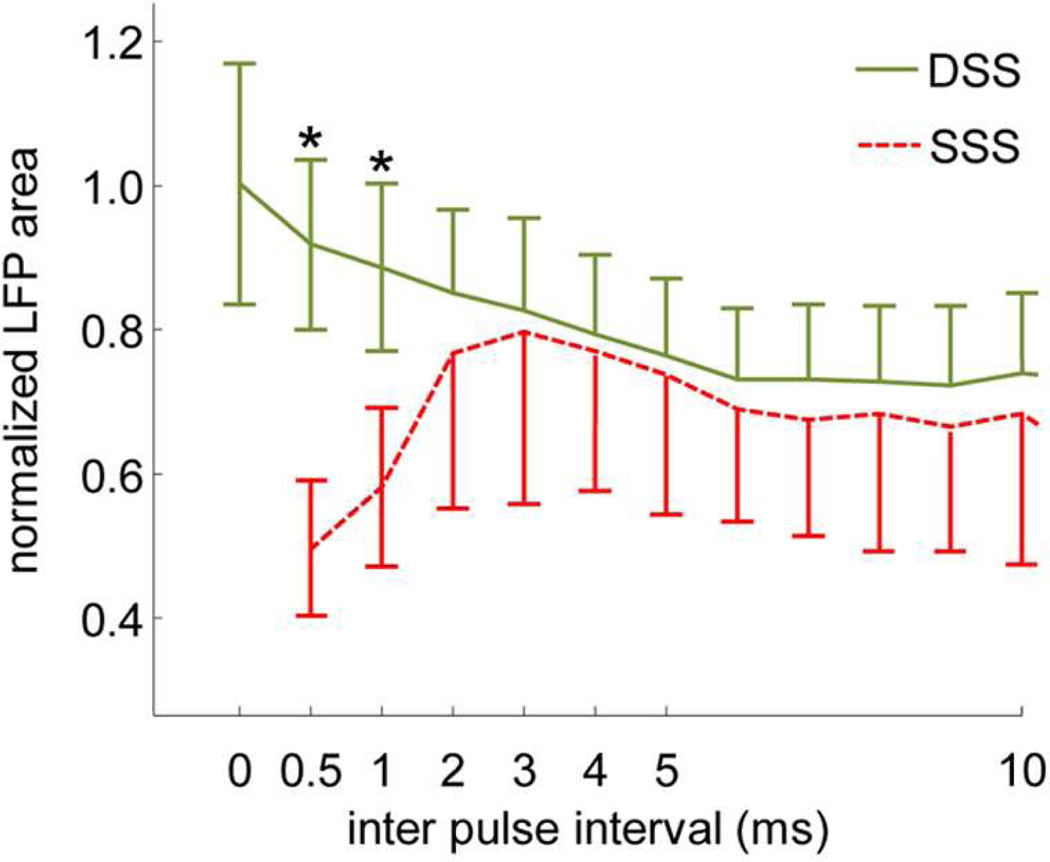
To better understand what may be limiting short-term integration and AM detection abilities in the first AMI patients, a previous study performed ICC stimulation experiments in six ketamine-anesthetized guinea pigs (Calixto et al., 2012). Two single-shank AMI arrays were implanted parallel to each other (1.5 mm apart) with sites aligned along the tonotopic axis of the ICC. Two electrical biphasic pulses (200 µs/phase), either on one site or between two sites with varying inter-pulse delays (0–100 ms) were presented. The two sites were positioned into a similar ICC lamina to assess how stimulation of one versus two regions along an isofrequency lamina affected auditory cortical activity. The neural activity was recorded in the primary auditory cortex (A1) in a similar frequency region as the stimulated ICC lamina. The study discovered that stimulation of a single site with two pulses elicits strong refractory effects for shorter inter-pulse intervals approaching full refractory below an interval of 2 ms (Figure 6, SSS). In other words, AMI stimulation with more pulses with short intervals contributes little or no additional A1 activity than that of a single pulse, consistent with Figure 5 in which there was no decrease in threshold (or increase in loudness) with increasing pulse rates using the AMI. This is in contrast to CI stimulation that achieves increased activity, lower thresholds, and louder percepts for shorter inter-pulse intervals or higher pulse rates ((McKay et al., 1998; Middlebrooks, 2004; Shannon, 1985); e.g., lower thresholds are shown in Figure 5 for CI stimulation).
Figure 6.
Strong suppressive effects are minimized with a two-shank AMI array. Local field potential (LFP) recordings from primary auditory cortex in response to two pulse ICC stimulation with varying inter pulse intervals either on one site (SSS) or across two sites (DSS, in same lamina). Ordinate is LFP activity to two pulses divided by the linear sum of activity to each pulse. For SSS, 0.5 means no contribution of activity for the second pulse (full refractory). Mean and standard deviation bars are shown for data across six animals (SSS: n=41, DSS: n=72; Asterisks: p<1e-10). Two single-shank AMI arrays (shown in Figure 2B) separated by 1.5 mm were used for these experiments. Figure was taken from (Calixto et al., 2012) and reprinted with permission from the American Physiological Society.
The ICC is a three-dimensional structure with two-dimensional isofrequency laminae that have shown to code for varying temporal features of sound across different neurons (Ehret, 1997; Langner et al., 2002; Rees et al., 2005). Unlike stimulation of the cochlea, stimulation of a single site within a given frequency region in the ICC may not sufficiently activate higher centers with repeated electrical pulses. Instead, multi-site stimulation within a lamina may be needed to achieve improved temporal activation. Encouragingly, the study by (Calixto et al., 2012) showed that stimulation of two sites within an ICC lamina elicits enhanced A1 activity with shorter intervals and overcomes the strong refractory effects observed for SSS (Figure 6, DSS). This type of enhanced activity cannot be simply achieved by activating more sites across different isofrequency laminae but requires activation of multiple sites within the same lamina (Straka et al., 2013). Therefore, stimulating at least two sites along a lamina may restore short-term integration and could improve hearing performance. Additionally, the same study discovered that stimulation of only a single site in a lamina elicits strong suppressive effects that last beyond 100 ms in which activity to a second pulse is significantly reduced due to the activity to the first pulse. In fact, activity to the second pulse could be completely suppressed even beyond 100 ms (i.e., 4–100% suppression at 100 ms, n=41). In contrast, stimulation of two sites could exhibit full recovery and even enhanced activity to the second pulse by 100 ms (i.e., 77% suppression up to 214% enhancement, n=72), exhibiting patterns closer to what is observed for two-click acoustic stimulation with varying delays (Brosch et al., 1999; Eggermont et al., 1995; Wehr et al., 2005). The significant suppressive effects exceeding 100 ms for single site stimulation within an ICC lamina is likely limiting AM detection abilities in which activated neurons cannot sufficiently follow the envelope fluctuations. These findings suggest that AMI stimulation of at least two sites along an ICC lamina could greatly improve temporal coding abilities on a short (<5 ms) and long (beyond 100 ms) scale, which in turn could improve speech understanding.
4.2. Can a CI-based strategy still work in the ICC?
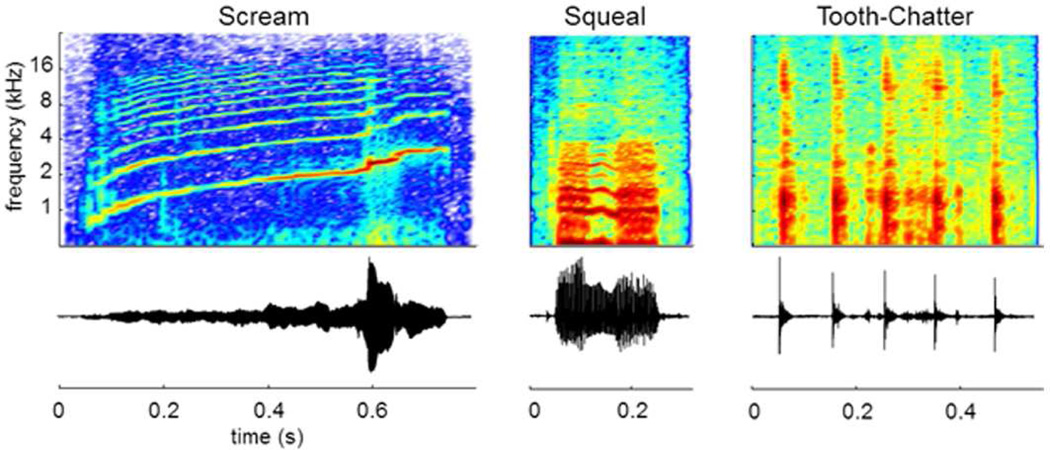
For neural prostheses, it is challenging to develop completely new hardware and software since considerable testing and approvals are needed before using them in humans. Instead, it is favorable to use components and algorithms already approved for human use, such as those in CIs (Patrick et al., 2006). Several studies have shown that ICC neurons can follow envelope modulations of simple and natural stimuli up to a few hundred hertz (Krishna et al., 2000; Langner et al., 2002; Rees et al., 1987; Suta et al., 2003; Woolley et al., 2006). One particular study performed experiments in 10 ketamine-anesthetized guinea pigs to further assess if a CI type of strategy could potentially be effective for the AMI (Rode et al., 2013). Natural vocalizations (i.e., guinea pig speech; Figure 7), which exhibit similar temporal and spectral patterns to human speech, were presented to the left ear of the animals and neural spiking activity was recorded across the right ICC using 32-site arrays in multiple locations per animal.
Figure 7.
Guinea pig vocalizations. BOTTOM: Normalized waveforms. TOP: Spectrograms (jet color map from Matlab - blue: minimum, red: maximum). Three different types of vocalizations were used as acoustic stimuli: a temporally and spectrally varying stimulus (Scream), a broadband with harmonic components stimulus (Squeal), and a broadband and transient components stimulus (Tooth-Chatter). All three cover a wide spectral range but differ in their spectral and temporal characteristics. The vocalizations presented to the animals were bandpass filtered (500 Hz – 40 kHz) and calibrated with respect to the speaker-ear interface. Figure was taken from (Rode et al., 2013) and reprinted with open access rights by the authors.
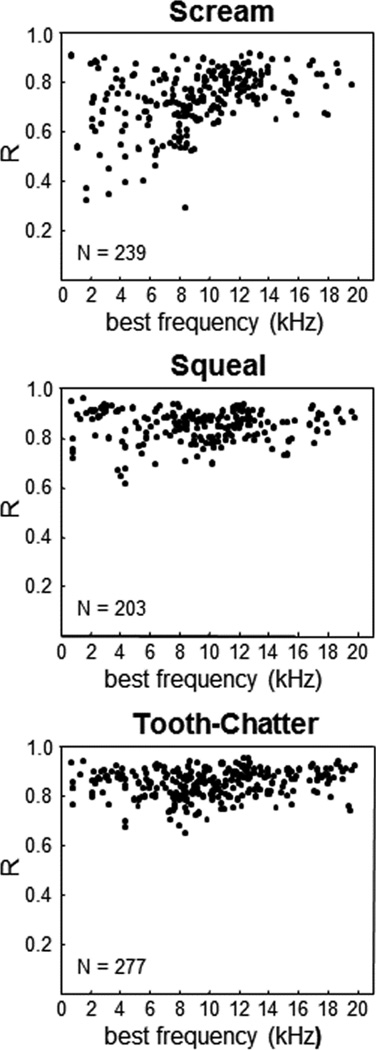
The unique aspect of that study was that a peripheral ear model was used to obtain an estimate for the true envelope pattern of the sound stimuli that reaches the basilar membrane of the cochlea. Sound travels through the ear drum and middle ear components to reach inside the fluid-filled cochlea. The fluid vibrations then cause the basilar membrane within the cochlea to fluctuate, which in turn activates hair cells and the corresponding auditory nerve fibers going to the brain. Previous studies have not typically accounted for this pre-processing that occurs from the ear drum to the basilar membrane when characterizing the effects of speech sounds on different neurons within the ICC. Fortunately, there is already a reasonably accurate mathematical model of the peripheral ear in the guinea pig, which is known as the dual resonance nonlinear (DNRL) model (Meddis et al., 2001; Sumner et al., 2003). The vocalizations were inputted into the DRNL model to obtain the output that is observed at a given frequency region along the basilar membrane. More specifically, the envelope of the output signal was extracted (up to ~100 Hz) since speech perception has been strongly correlated with the ability to transmit sufficient envelope cues to the brain (Shannon et al., 1995). What is important about this pre-processing is that it resembles the type of pre-processing already implemented in CI stimulation strategies, which electrically stimulate each electrode site with the envelope pattern of the bandpass filtered components of the original inputted sound signal. The advantage of using the DRNL model is that it is extracting the frequency components using what is believed to be more natural processing steps compared to arbitrary bandpass filters as used in previous studies. Cross-correlation analysis can then be performed between each of those envelope signals and the temporal spiking pattern of ICC neurons (i.e., smoothed post-stimulus time histograms (PSTHs)) located in the same frequency region corresponding to the envelope signals.
Figure 8 plots the correlation coefficient (R) values across all recording sites in the ICC from 10 animals and different frequency laminae. There were multiple neurons that exhibited high correlation values close to 1 for all three vocalizations, and thus for a wide range of spectral and temporal sound patterns. Based on visual inspection of all the raw data, R values ≥0.85 corresponded to neurons that accurately followed the stimulus envelope (for further examples and justifications of this criterion, see (Rode et al., 2013)). The high 0.85 criterion was achieved by 15%, 60%, and 58% of neurons for scream, squeal, and tooth-chatter, respectively. It can also be seen from Figure 8 that the majority of cases still had a moderately high R value above ~0.70. These results demonstrate that ICC neurons can follow the envelope structure of natural stimuli across different frequencies. This is an important finding because it indicates that a CI-based strategy may potentially be used for the AMI to restore sufficient speech perception as long as the right neurons are being activated (further discussed in the next section). Combined with the findings from Section 4.1, these results suggest that improved activation of the auditory system may be achieved with the AMI by using a CI-based strategy except that the pulse patterns for each frequency channel would be presented in an alternating or time-varying sequence across two sites (i.e., using a two-shank array) instead of just one site in each ICC lamina. Although it may be possible to insert more than two shanks into the ICC, there would be greater surgical risks and significant technological challenges in making an AMI array with smaller dimensions to safely implant multiple shanks into the ICC.
Figure 8.
Envelope correlation coefficient (R) values for ICC neurons located in different frequency regions. R is calculated between the neuron’s spiking pattern and the envelope of the vocalization for the best-matched frequency component outputted from the DRNL model. Further details on the DRNL model and correlation analysis are provided in Section 4.2. N: total number of neurons (i.e., multi-unit sites) per vocalization. Figure was taken from (Rode et al., 2013) and reprinted with open access rights by the authors.
4.3. A specific midbrain pathway that may improve speech perception with the AMI
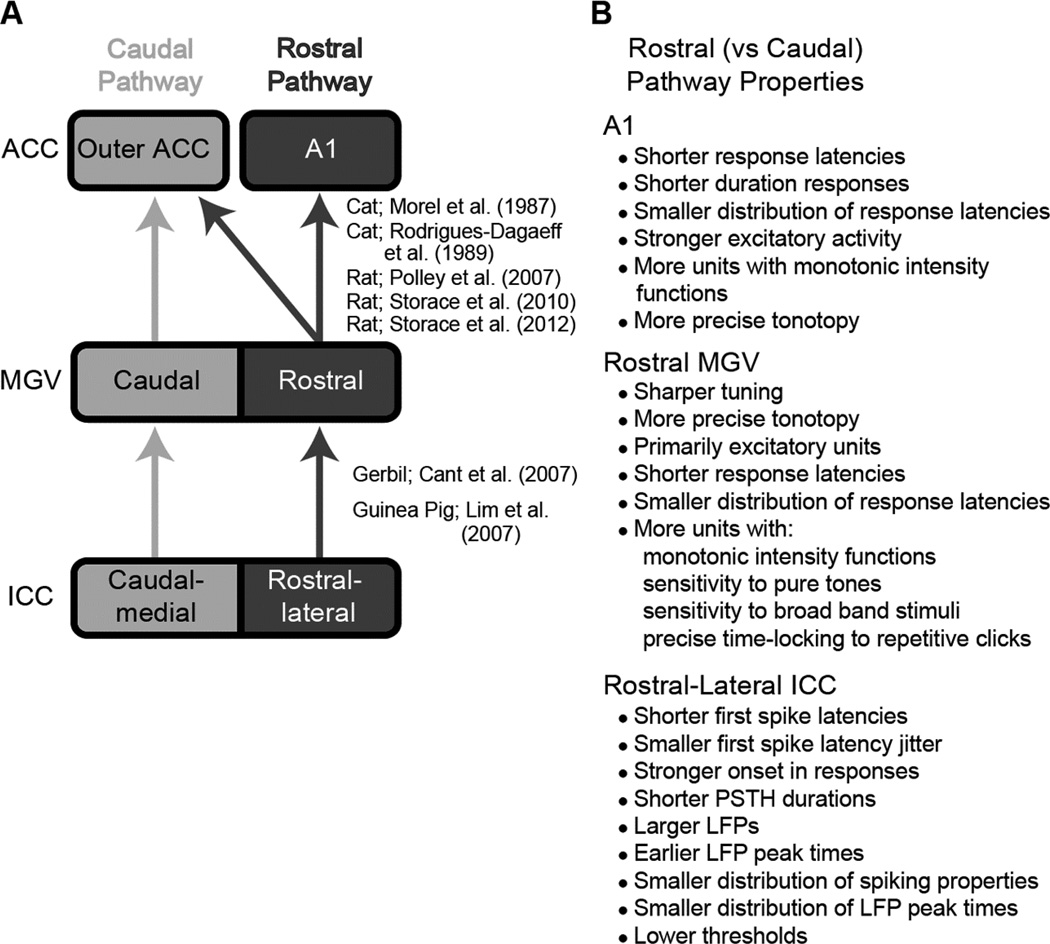
One caveat to the findings presented in the previous section is that not all ICC neurons had high R values greater than 0.85. If the neurons with the highest R values are located in a specific region within each ICC lamina, then it may be possible to insert a two-shank AMI array into that region (i.e., the two shanks cross each lamina to position two electrodes in that region) and systematically activate those neurons with a CI-based strategy. However, if the high R values are scattered throughout the ICC, then positioning only two shanks within the ICC may not sufficiently access enough of those high R-valued neurons. A study systematically investigating how the R values change as a function of location across an isofrequency lamina of the ICC still needs to be performed to answer that question. However, there are a few studies suggesting that there may be a better region within the ICC for AMI stimulation. One recent study performed experiments in 12 ketamine-anesthetized guinea pigs in which multi-site arrays were used to position sites fully across a given isofrequency lamina of the ICC. Details of the methods and results are provided in (Straka et al., 2014a). This study discovered that along a given ICC lamina, there exists two subregions: a rostral-lateral region and a caudal-medial region (Figure 9A). The rostral-lateral ICC exhibited more precise temporal firing, shorter latencies, stronger activity, lower thresholds, and greater spatial synchrony across neurons in response to acoustic stimuli compared to the caudal-medial ICC, as listed in Figure 9B. In other words, there appears to exist a dual lemniscal organization within the ICC in which one pathway may be designed for more robustly transmitting sound cues to higher centers.
Figure 9.
Schematic of anatomical projections and physiological responses for the dual lemniscal pathways hypothesis. A: The rostral and caudal ascending pathways show spatially segregated anatomical projections from the ICC up to ACC. Overlapping projections between the two pathways are not shown. B: In contrast to the caudal pathway, the rostral pathway also shows different responses to acoustic stimuli in A1 (Phillips et al., 1995; Polley et al., 2007; Storace et al., 2012; Wallace et al., 2000), the rostral MGV (Rodrigues-Dagaeff et al., 1989) and the rostral-lateral ICC (Straka et al., 2014a). Figure was taken from (Straka et al., 2014a) and reprinted with permission from the American Physiological Society.
The concept of a dual lemniscal organization was first revealed in the 1980s (Morel et al., 1987; Rodrigues-Dagaeff et al., 1989), specifically for projections from the ventral division of the medial geniculate nucleus (MGV) up to the core/primary auditory cortex regions (ACC) in a cat model. The dual lemniscal pathway hypothesis was further expanded in 2006 to 2007 to include pathways from the brainstem up through the ICC, MGV, and ACC across several species, including gerbil, rat, and guinea pig (Cant et al., 2006; Cant et al., 2007; Lim et al., 2007a; Polley et al., 2007). Together, these studies revealed two segregated anatomical and functional pathways through the ICC (caudal-medial versus rostral-lateral regions), MGV (caudal versus rostral regions), and ACC (A1 versus core regions outside of A1). Figure 9A provides a simplified schematic summarizing the dual lemniscal pathways. The differences in coding properties between the rostral versus caudal MGV, demonstrated by Rodrigues-Dagaeff (1989) in cat and listed in Figure 9B, suggest that the rostral pathway is designed for stronger excitatory activation and more temporally and spectrally precise transmission of information up to higher centers. Many of these differences in coding properties between the dual pathways have also been shown in ACC (Phillips et al., 1995; Polley et al., 2007; Schreiner et al., 2011; Storace et al., 2012; Wallace et al., 2000) and more recently in ICC (Straka et al., 2014a), as listed in 9B.
The high R values discussed in the previous section may correspond to neurons within this “rostral” pathway (i.e., rostral-lateral portion of a given isofrequency lamina of the ICC) and if targeted with the AMI, could enable high levels of speech perception. This is not to claim that speech information is only coded in this pathway but to suggest that artificial stimulation with modulated electrical pulse trains may somehow activate this pathway to provide sufficient speech understanding. Previous studies in guinea pigs have already shown that electrical stimulation of the rostral-lateral versus caudal-medial ICC achieves lower activation thresholds, stronger responses, smaller discriminable level steps, shorter response latencies, and more temporally precise firing within A1 (Lim et al., 2007a; Neuheiser et al., 2010). Stimulation of this rostral-lateral ICC region with two sites within a given lamina can also minimize or overcome the strong suppressive effects described in Section 3.1, which is not typically or sufficiently achieved for stimulation of more caudal-medial ICC locations (Straka et al., 2014b). In future AMI patients and by targeting the rostral-lateral ICC, there will be a unique opportunity to test if these findings in animals also occur in humans and if they relate to better speech understanding.
4.4. Improving the surgical approach for array implantation
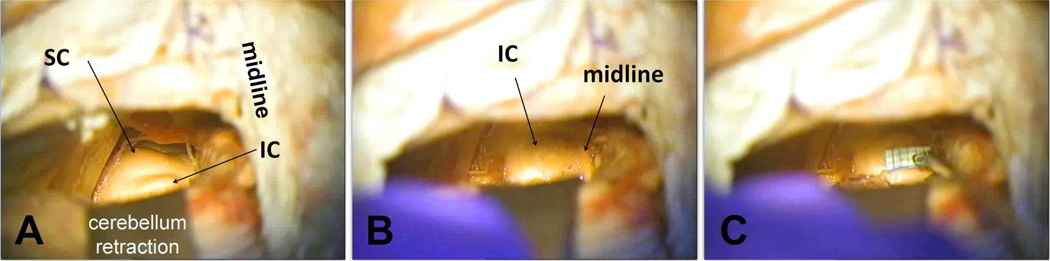
As described in Section 3.2, the main surgical limitation in the first clinical trial was the use of a small craniotomy, through which it was not possible to clearly identify key anatomical landmarks surrounding the IC. A new surgical approach for the second clinical trial was developed based on cadaver studies. This new approach still uses a modified lateral suboccipital exposure (Samii et al., 2007), except that the skull opening is extended up to the midline (Figure 10A). This type of paramedian exposure has been safely used in the neurosurgical field for operating on lesions in the IC, SC, superior and middle cerebellar peduncles, and quadrangular lobules of the cerebellum (Ogata et al., 1997), and can be used for AMI implantation (Vince et al., 2010). With the expanded exposure, the NF2 tumor can still be accessed more laterally and then the IC can be approached more medially. In patients not requiring tumor removal, a traditional paramedian approach without the lateral exposure can be used so as to minimize the opening of the skull (Colletti et al., 2007; Ogata et al., 1997; Vince et al., 2010). Once the dura is opened along the sinuses and the cerebellum is retracted downwards, the IC and SC surfaces can be seen after pushing aside the overlying arachnoid and blood vessels (Figure 10A). The viewed structures are confirmed to be the IC and SC using CT-MRI guided brain navigation (using the systems from Brainlab AG, Germany and Fiagon GmbH, Germany). Then the IC-SC border (rostral edge of IC), midline (medial edge of IC), and exit point of the trochlear nerve (caudal edge of IC; not shown) can be identified through the expanded craniotomy (Figures 10A and 10B), which is in contrast to the previous surgical approach in which these landmarks were not clearly visible due to the limited view of the midbrain. The direct view of the midline of the brain also provides a frame of reference for determining the sagittal plane and inserting the AMI array into the IC at an angle of 40° relative to that plane to align it along the tonotopic gradient of the ICC.
Figure 10.
A refined surgical approach for AMI array implantation. A: Modified lateral suboccipital approach (left side of the head in this image) with the craniotomy extended to the midline. The tentorium is located immediately above the skull opening. The cerebellum is retracted downwards to expose the midbrain. The IC and SC are clearly visible through this exposure. B: The midline and caudal edge of the IC (at the exit point of the trochlea nerve; not shown) can also be identified through this exposure. C: Measurements can be made along the IC surface relative to the different anatomical landmarks to identify the location for inserting the AMI array. Further details on the surgical approach and AMI implantation are provided in Section 4.4.
A major advantage of this new surgical approach is that the distances relative to these anatomical landmarks can be measured during surgery to identify the locations along the IC surface for inserting each shank of the AMI array (Figure 10C). Based on ICC stimulation studies in animals (Lim et al., 2007a; Neuheiser et al., 2010; Straka et al., 2014b), anatomical and functional data of the IC in humans (De Martino et al., 2013; Geniec et al., 1971; Lim et al., 2009; Lim et al., 2013; Ress et al., 2013), and IC surgical studies in cadavers (unpublished observations), the authors of this paper have determined coordinates for inserting a two-shank AMI array into the ICC, particularly its rostral-lateral portion based on the findings presented in Section 4.3. The first shank will be inserted at a position of 0.25 caudally from the IC-SC border (rostral-to-caudal location normalized to the total distance between the IC-SC border and the exit point of trochlear nerve). Using a normalized location minimizes errors associated with variations in brain size across patients. Since there is no visible lateral landmark, the first shank will be inserted at a position of 7 mm from the midline. During surgery, electrical stimulation with a bipolar electrode along the surface of the IC and noninvasive neural recordings of the corresponding auditory cortical activity (i.e., middle-latency responses) may provide a way to identify a lateral landmark for more accurate array placement for each patient. Preliminary data and descriptions for this intraoperative technique is described in (Lim et al., 2009) and will be further explored in future AMI surgeries. The second shank will then be inserted 1.5 mm diagonally towards the caudal and medial direction relative to the first shank. These new steps for positioning the AMI array into the IC are expected to improve placement of the electrode sites along the tonotopic gradient of the ICC compared to what was possible in the first clinical trial. Furthermore, these steps should enable placement of the arrays into the rostral-lateral portion of the ICC.
4.5. Two-shank AMI clinical trial
Based on the encouraging animal and human findings described above, the authors of this paper collaborated with Cochlear Limited (led by James Patrick) to design a new AMI device that consists of two shanks with 11 sites along each shank (Figure 2C; note that 22 sites is the channel limit of the stimulator developed by Cochlear Limited). The shanks will be individually inserted into the ICC as described in Section 4.4. The previous single-shank array was able to obtain a reasonable range of pitches with 11 sites (i.e., ~2-mm spatial span with a site spacing of 200 µm; (Lim et al., 2013)). To sufficiently span the tonotopic axis of the ICC with the new two-shank AMI array, each shank was designed with a site spacing of 300 µm for 10 of the 11 sites (i.e., ~2.7-mm spatial span). The site spacing may be slightly larger than the ~200 µm thickness of each ICC lamina (Geniec et al., 1971; Oliver, 2005). However, the current level can be increased on each site to access adjacent laminae. This site spacing across the frequency dimension is considerably finer than what is possible with the CI, which still achieves high performance levels (Shannon, 2002; Wilson et al., 2008). As shown in Figure 2C, the 11th site on each shank is positioned closer to the Dacron mesh. Since some of the patients implanted with the AMI will also have tinnitus, activation of the outer regions of the IC with those superficial sites may provide a way to suppress tinnitus using stimulation strategies derived from animal experiments (Offutt et al., 2014).
The second AMI clinical trial is currently underway and is funded by the National Institutes of Health (Grant Number U01DC013030). The clinical trial will be performed at Hannover Medical School in collaboration with Cochlear Limited and University of Minnesota for implanting the two-shank AMI device in five adult NF2 patients who cannot sufficiently benefit from a CI or ABI. The clinical study is occurring from April 2014 to March 2019. The primary objectives of this study are to demonstrate the safety and reliability of the new two-shank AMI array and the ability to consistently position the array into the ICC across patients. The secondary objectives are to show that the two-shank AMI can achieve hearing performance greater than what is typically achievable with the single-shank AMI and ABI devices used in NF2 deaf patients. Success with these initial patients will open up opportunities for expanding the use of the AMI to a larger patient population and in clinics within different countries including the United States.
5. CONCLUSIONS
The first AMI clinical study demonstrated that implantation and stimulation of a single-shank electrode array within the midbrain can be safe and provide useful hearing on a daily basis. However, there were difficulties in accurately placing the array into the IC in which only one out of five patients had sites properly aligned along the tonotopic gradient of the ICC. Based on psychophysical testing in the implanted AMI patients and experiments in animals, stimulation of individual sites on the single-shank array produces strong refractory and suppressive effects within the auditory pathway, which likely contributes to the poor temporal coding abilities and limited speech perception observed for the AMI patients. To address these two issues, animal experiments were performed to identify ways to minimize the refractory and suppressive effects and cadaver studies were performed to improve the surgical approach for implanting the AMI array into the ICC. At least in animals, it appears that stimulation of two sites within each ICC lamina can sufficiently overcome these refractory and suppressive effects, especially when stimulating within the rostral-lateral portion of each lamina. Using a modified lateral suboccipital approach that is extended to the midline and identifying several key anatomical landmarks, it also appears that the AMI array can be consistently inserted into the rostral-lateral portion of the ICC.
Considering the encouraging findings described above, a new two-shank AMI array was developed in collaboration with Cochlear Limited that will target the rostral-lateral region of the ICC in a second clinical trial funded by the National Institutes of Health. This new array design will have two shanks aligned along the tonotopic gradient of the ICC with two sites positioned within each isofrequency lamina. Based on additional animal studies, a CI-based stimulation strategy within the ICC will initially be used in the patients except that the pulse pattern for each frequency channel will be distributed across two sites in each ICC lamina with varying delays between the pulses. Speech performance tests and various psychophysical measurements will be performed in the implanted AMI patients to evaluate this modified CI strategy while also developing other types of stimulation patterns for improving hearing performance. Demonstrating the safety and reliability of the AMI in this second clinical trial as well as achieving better speech perception performance with the AMI compared to current ABI devices will revive research interests and discussions in using penetrating electrode arrays for central auditory prostheses.
One major limitation in achieving significant improvements in hearing performance with current ABI and CI devices appears to be the limited number of independent information channels possible with these implants (Friesen et al., 2001; Kuchta et al., 2004). The CI sends current through a bony modiolar wall of the cochlea with scattered flow of electrical charge to a variable distribution and reduced number of auditory neurons associated with deafness. The ABI is placed on the surface of the brainstem, resulting in high stimulation levels and broad current spread to activate the appropriate neurons within deeper regions. Therefore, development of new types of central auditory prostheses such as the AMI, penetrating auditory brainstem implant (Figure 1; (McCreery, 2008)), or auditory thalamic implant (Atencio et al., 2014) may eventually lead to innovative solutions for achieving hearing performance beyond what is possible with current technologies.
Highlights.
A central auditory prosthesis (AMI) was developed for stimulation of the midbrain
The AMI was safe and provided useful hearing in a clinical trial from 2006 to 2008
A new array, stimulation strategy, and surgical approach was developed for the AMI
These improvements may enable sufficient speech perception in the upcoming patients
ACKNOWLEDGEMENTS
Minoo Lenarz from University of Berlin-Charité (Germany) and James Patrick from Cochlear Limited (Australia) both played a critical part in the initiation and progress of the AMI research and clinical study. David Anderson from University of Michigan (USA) played a critical role in the initiation and progress of the AMI animal research. Gert Joseph, Urte Rost, Joerg Pesch, Nicole Neben, Thilo Rode, and Rolf-Dieter Battmer contributed to the fitting and testing of the AMI patients at Hannover Medical School (Germany). Thilo Rode, Roger Calixto, Anke Neuheiser, Tanja Hartmann, Günter Reuter, Uta Reich, Gerrit Paasche, Verena Scheper, and Andrej Kral contributed to the AMI animal studies at Hannover Medical School, while Malgorzata Straka and Sarah Offutt performed AMI animal studies at University of Minnesota (USA). The AMI surgeries were performed together with Madjid Samii and Amir Samii at the International Neuroscience Institute (Germany). The improved surgical approach in cadaver studies was developed together with Amir Samii, Omid Majdani, Markus Pietsch, Peter Erfurt, and Sven Balster at Hannover Medical School. The engineers and scientists at Cochlear Limited including James Patrick, Frank Risi, Godofredo (JR) Timbol, Peter Gibson, Brett Swanson, and Jason Leavens developed the AMI devices and software. Colette McKay from Bionics Institute (Australia) and Robert Shannon from University of Southern California (USA) helped with AMI and ABI psychophysical studies. Ray Meddis (University of Essex, UK) as well as Christian Sumner and Mark Steadman (Nottingham University, UK) provided and helped with the code for the DRNL model. Funding was provided by Cochlear Limited, German Research Foundation (SFB 599, Cluster of Excellence Hearing4All), Germany Ministry of Research and Education (01GQ0816), funds from University of Minnesota, and National Institutes of Health (P41EB2030, R03DC011589, U01DC013030).
ABBREVIATIONS
- A1
primary auditory cortex
- ABI
auditory brainstem implant
- ACC
core/primary auditory cortex regions
- AM
amplitude modulation
- AMI
auditory midbrain implant
- CI
cochlear implant
- CT
computed tomography (imaging)
- DRNL
dual-resonance nonlinear (model)
- DSS
dual-site stimulation (within an ICC lamina)
- IC
inferior colliculus
- ICC
central nucleus of inferior colliculus
- LFP
local field potential
- MGV
ventral division of medial geniculate nucleus
- MRI
magnetic resonance imaging
- NF2
neurofibromatosis type 2
- PABI
penetrating auditory brainstem implant
- PSTH
post-stimulus time histogram
- R
correlation coefficient
- SC
superior colliculus
- SSS
single-site stimulation (within an ICC lamina)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Hubert H. Lim, Email: hlim@umn.edu.
Thomas Lenarz, Email: lenarz.thomas@mh-hannover.de.
REFERENCES
- Atencio CA, Shih JY, Schreiner CE, Cheung SW. Primary auditory cortical responses to electrical stimulation of the thalamus. J Neurophysiol. 2014;111:1077–1087. doi: 10.1152/jn.00749.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr R, Colletti V, Matthies C, Morita A, Nakatomi H, Dominique L, Darrouzet V, Brill S, Shehata-Dieler W, Lorens A, Skarzynski H. New Outcomes With Auditory Brainstem Implants in NF2 Patients. Otol Neurotol. 2014;35:1844–1851. doi: 10.1097/MAO.0000000000000584. [DOI] [PubMed] [Google Scholar]
- Brosch M, Schulz A, Scheich H. Processing of sound sequences in macaque auditory cortex: response enhancement. J Neurophysiol. 1999;82:1542–1559. doi: 10.1152/jn.1999.82.3.1542. [DOI] [PubMed] [Google Scholar]
- Calixto R, Lenarz M, Neuheiser A, Scheper V, Lenarz T, Lim HH. Coactivation of different neurons within an isofrequency lamina of the inferior colliculus elicits enhanced auditory cortical activation. J Neurophysiol. 2012;108:1199–1210. doi: 10.1152/jn.00111.2012. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60:457–474. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Organization of the inferior colliculus of the gerbil (Meriones unguiculatus): differences in distribution of projections from the cochlear nuclei and the superior olivary complex. J Comp Neurol. 2006;495:511–528. doi: 10.1002/cne.20888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Multiple topographically organized projections connect the central nucleus of the inferior colliculus to the ventral division of the medial geniculate nucleus in the gerbil, Meriones unguiculatus. J Comp Neurol. 2007;503:432–453. doi: 10.1002/cne.21391. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Fremouw T, Covey E. The inferior colliculus: A hub for the central auditory system. In: Oertel D, Fay RR, Popper AN, editors. Springer Handbook of Auditory Research: Integrative Functions in the Mammalian Auditory Pathway (Vol. 15) New York: Springer-Verlag; 2002. pp. 238–318. [Google Scholar]
- Colletti L, Shannon R, Colletti V. Auditory brainstem implants for neurofibromatosis type 2. Curr Opin Otolaryngol Head Neck Surg. 2012;20:353–357. doi: 10.1097/MOO.0b013e328357613d. [DOI] [PubMed] [Google Scholar]
- Colletti L, Shannon RV, Colletti V. The Development of Auditory Perception in Children after Auditory Brainstem Implantation. Audiol Neurootol. 2014;19:386–394. doi: 10.1159/000363684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletti V, Shannon RV. Open set speech perception with auditory brainstem implant? Laryngoscope. 2005;115:1974–1978. doi: 10.1097/01.mlg.0000178327.42926.ec. [DOI] [PubMed] [Google Scholar]
- Colletti V, Shannon R, Carner M, Veronese S, Colletti L. Outcomes in Nontumor Adults Fitted With the Auditory Brainstem Implant: 10 Years' Experience. Otol Neurotol. 2009;30:614–618. doi: 10.1097/MAO.0b013e3181a864f2. [DOI] [PubMed] [Google Scholar]
- Colletti V, Shannon R, Carner M, Sacchetto L, Turazzi S, Masotto B, Colletti L. The first successful case of hearing produced by electrical stimulation of the human midbrain. Otol Neurotol. 2007;28:39–43. doi: 10.1097/01.mao.0000247808.47712.02. [DOI] [PubMed] [Google Scholar]
- De Martino F, Moerel M, van de Moortele PF, Ugurbil K, Goebel R, Yacoub E, Formisano E. Spatial organization of frequency preference and selectivity in the human inferior colliculus. Nature communications. 2013;4:1386. doi: 10.1038/ncomms2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermont JJ, Smith GM. Synchrony between single-unit activity and local field potentials in relation to periodicity coding in primary auditory cortex. J Neurophysiol. 1995;73:227–245. doi: 10.1152/jn.1995.73.1.227. [DOI] [PubMed] [Google Scholar]
- Ehret G. The auditory midbrain, a "shunting yard" of acoustical information processing. In: Ehret G, Romand R, editors. The Central Auditory System. New York: Oxford University Press, Inc.; 1997. pp. 259–316. [Google Scholar]
- Eisenberg LS. The contributions of William F. House to the field of implantable auditory devices. Hear Res. 2014 doi: 10.1016/j.heares.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Fraser M, McKay CM. Temporal modulation transfer functions in cochlear implantees using a method that limits overall loudness cues. Hear Res. 2012;283:59–69. doi: 10.1016/j.heares.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen LM, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am. 2001;110:1150–1163. doi: 10.1121/1.1381538. [DOI] [PubMed] [Google Scholar]
- Geniec P, Morest DK. The neuronal architecture of the human posterior colliculus. A study with the Golgi method. Acta Otolaryngol Suppl. 1971;295:1–33. [PubMed] [Google Scholar]
- Hochberg LR, Bacher D, Jarosiewicz B, Masse NY, Simeral JD, Vogel J, Haddadin S, Liu J, Cash SS, van der Smagt P, Donoghue JP. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Lim HH, Netoff TI, Connolly AT, Johnson N, Roy A, Holt A, Lim KO, Carey JR, Vitek JL, He B. Neuromodulation for brain disorders: challenges and opportunities. IEEE Trans Biomed Eng. 2013;60:610–624. doi: 10.1109/TBME.2013.2244890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiol Rev. 2004;84:541–577. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Konrad P, Shanks T. Implantable brain computer interface: challenges to neurotechnology translation. Neurobiology of disease. 2010;38:369–375. doi: 10.1016/j.nbd.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Kreft HA, Donaldson GS, Nelson DA. Effects of pulse rate on threshold and dynamic range in Clarion cochlear-implant users. J Acoust Soc Am. 2004;115:1885–1888. doi: 10.1121/1.1701895. [DOI] [PubMed] [Google Scholar]
- Kretschmann HJ, Weinrich W. Cranial Neuroimaging and Clinical Neuroanatomy: Magnetic Resonance Imaging and Computed Tomography. 2nd ed. New York: Thieme Medical Publishers, Inc.; 1992. [Google Scholar]
- Krishna BS, Semple MN. Auditory temporal processing: responses to sinusoidally amplitude-modulated tones in the inferior colliculus. J Neurophysiol. 2000;84:255–273. doi: 10.1152/jn.2000.84.1.255. [DOI] [PubMed] [Google Scholar]
- Kuchta J, Otto SR, Shannon RV, Hitselberger WE, Brackmann DE. The multichannel auditory brainstem implant: how many electrodes make sense? J Neurosurg. 2004;100:16–23. doi: 10.3171/jns.2004.100.1.0016. [DOI] [PubMed] [Google Scholar]
- Langner G, Albert M, Briede T. Temporal and spatial coding of periodicity information in the inferior colliculus of awake chinchilla (Chinchilla laniger) Hear Res. 2002;168:110–130. doi: 10.1016/s0378-5955(02)00367-2. [DOI] [PubMed] [Google Scholar]
- Lenarz M, Lim HH, Patrick JF, Anderson DJ, Lenarz T. Electrophysiological validation of a human prototype auditory midbrain implant in a guinea pig model. J Assoc Res Otolaryngol. 2006a;7:383–398. doi: 10.1007/s10162-006-0056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarz M, Matthies C, Lesinski-Schiedat A, Frohne C, Rost U, Illg A, Battmer RD, Samii M, Lenarz T. Auditory brainstem implant part II: subjective assessment of functional outcome. Otol Neurotol. 2002;23:694–697. doi: 10.1097/00129492-200209000-00015. [DOI] [PubMed] [Google Scholar]
- Lenarz M, Lim HH, Lenarz T, Reich U, Marquardt N, Klingberg MN, Paasche G, Reuter G, Stan AC. Auditory midbrain implant: histomorphologic effects of long-term implantation and electric stimulation of a new deep brain stimulation array. Otol Neurotol. 2007;28:1045–1052. doi: 10.1097/MAO.0b013e318159e74f. [DOI] [PubMed] [Google Scholar]
- Lenarz T, editor. Cochlea-Implantat. Berlin Heidelberg: Springer; 1998. [Google Scholar]
- Lenarz T, Lim HH, Reuter G, Patrick JF, Lenarz M. The auditory midbrain implant: a new auditory prosthesis for neural deafness-concept and device description. Otol Neurotol. 2006b;27:838–843. doi: 10.1097/01.mao.0000232010.01116.e9. [DOI] [PubMed] [Google Scholar]
- Lim HH, Anderson DJ. Auditory cortical responses to electrical stimulation of the inferior colliculus: implications for an auditory midbrain implant. J Neurophysiol. 2006;96:975–988. doi: 10.1152/jn.01112.2005. [DOI] [PubMed] [Google Scholar]
- Lim HH, Anderson DJ. Spatially distinct functional output regions within the central nucleus of the inferior colliculus: Implications for an auditory midbrain implant. J Neurosci. 2007a;27:8733–8743. doi: 10.1523/JNEUROSCI.5127-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Lenarz M, Lenarz T. Auditory midbrain implant: a review. Trends in amplification. 2009;13:149–180. doi: 10.1177/1084713809348372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Lenarz M, Lenarz T. Midbrain Auditory Prostheses. In: Zeng FG, Fay RR, Popper AN, editors. Auditory Prostheses: New Horizons, Vol. 39. New York: Springer Science+Business Media, LLC; 2011. pp. 207–232. [Google Scholar]
- Lim HH, Lenarz M, Joseph G, Lenarz T. Frequency representation within the human brain: Stability versus plasticity. Sci Rep. 2013;3:1474. doi: 10.1038/srep01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HH, Lenarz T, Joseph G, Battmer RD, Patrick JF, Lenarz M. Effects of phase duration and pulse rate on loudness and pitch percepts in the first auditory midbrain implant patients: Comparison to cochlear implant and auditory brainstem implant results. Neuroscience. 2008;154:370–380. doi: 10.1016/j.neuroscience.2008.02.041. [DOI] [PubMed] [Google Scholar]
- Lim HH, Lenarz T, Joseph G, Battmer RD, Samii A, Samii M, Patrick JF, Lenarz M. Electrical stimulation of the midbrain for hearing restoration: insight into the functional organization of the human central auditory system. J Neurosci. 2007b;27:13541–13551. doi: 10.1523/JNEUROSCI.3123-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmierca MS, Izquierdo MA, Cristaudo S, Hernandez O, Perez-Gonzalez D, Covey E, Oliver DL. A discontinuous tonotopic organization in the inferior colliculus of the rat. J Neurosci. 2008;28:4767–4776. doi: 10.1523/JNEUROSCI.0238-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies C, Brill S, Varallyay C, Solymosi L, Gelbrich G, Roosen K, Ernestus RI, Helms J, Hagen R, Mlynski R, Shehata-Dieler W, Muller J. Auditory brainstem implants in neurofibromatosis Type 2: is open speech perception feasible? J Neurosurg. 2014;120:546–558. doi: 10.3171/2013.9.JNS12686. [DOI] [PubMed] [Google Scholar]
- McCreery DB. Cochlear nucleus auditory prostheses. Hear Res. 2008;242:64–73. doi: 10.1016/j.heares.2007.11.014. [DOI] [PubMed] [Google Scholar]
- McKay CM, McDermott HJ. Loudness perception with pulsatile electrical stimulation: the effect of interpulse intervals. J Acoust Soc Am. 1998;104:1061–1074. doi: 10.1121/1.423316. [DOI] [PubMed] [Google Scholar]
- McKay CM, Lim HH, Lenarz T. Temporal processing in the auditory system : insights from cochlear and auditory midbrain implantees. J Assoc Res Otolaryngol. 2013;14:103–124. doi: 10.1007/s10162-012-0354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meddis R, O'Mard LP, Lopez-Poveda EA. A computational algorithm for computing nonlinear auditory frequency selectivity. J Acoust Soc Am. 2001;109:2852–2861. doi: 10.1121/1.1370357. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC. Effects of cochlear-implant pulse rate and inter-channel timing on channel interactions and thresholds. J Acoust Soc Am. 2004;116:452–468. doi: 10.1121/1.1760795. [DOI] [PubMed] [Google Scholar]
- Morel A, Imig TJ. Thalamic projections to fields A, AI, P, and VP in the cat auditory cortex. J Comp Neurol. 1987;265:119–144. doi: 10.1002/cne.902650109. [DOI] [PubMed] [Google Scholar]
- Mudry A, Mills M. The early history of the cochlear implant: a retrospective. JAMA otolaryngology--head & neck surgery. 2013;139:446–453. doi: 10.1001/jamaoto.2013.293. [DOI] [PubMed] [Google Scholar]
- Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. Journal of the peripheral nervous system : JPNS. 2005;10:229–258. doi: 10.1111/j.1085-9489.2005.10303.x. [DOI] [PubMed] [Google Scholar]
- Neuheiser A, Lenarz M, Reuter G, Calixto R, Nolte I, Lenarz T, Lim HH. Effects of pulse phase duration and location of stimulation within the inferior colliculus on auditory cortical evoked potentials in a guinea pig model. J Assoc Res Otolaryngol. 2010;11:689–708. doi: 10.1007/s10162-010-0229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie K, Barco A, Zeng FG. Spectral and temporal cues in cochlear implant speech perception. Ear Hear. 2006;27:208–217. doi: 10.1097/01.aud.0000202312.31837.25. [DOI] [PubMed] [Google Scholar]
- Offutt SJ, Ryan KJ, Konop AE, Lim HH. Suppression and facilitation of auditory neurons through coordinated acoustic and midbrain stimulation: investigating a deep brain stimulator for tinnitus. J Neural Eng. 2014;11:066001. doi: 10.1088/1741-2560/11/6/066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata N, Yonekawa Y. Paramedian supracerebellar approach to the upper brain stem and peduncular lesions. Neurosurgery. 1997;40:101–104. doi: 10.1097/00006123-199701000-00023. discussion 104-5. [DOI] [PubMed] [Google Scholar]
- Oliver DL. Neuronal organization in the inferior colliculus. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer Science+Business Media, Inc.; 2005. pp. 69–114. [Google Scholar]
- Patrick JF, Busby PA, Gibson PJ. The development of the Nucleus Freedom Cochlear implant system. Trends in amplification. 2006;10:175–200. doi: 10.1177/1084713806296386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DP, Semple MN, Kitzes LM. Factors shaping the tone level sensitivity of single neurons in posterior field of cat auditory cortex. J Neurophysiol. 1995;73:674–686. doi: 10.1152/jn.1995.73.2.674. [DOI] [PubMed] [Google Scholar]
- Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. J Neurophysiol. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- Rees A, Moller AR. Stimulus properties influencing the responses of inferior colliculus neurons to amplitude-modulated sounds. Hear Res. 1987;27:129–143. doi: 10.1016/0378-5955(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Rees A, Langner G. Temporal coding in the auditory midbrain. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. New York: Springer Science+Business Media, Inc.; 2005. pp. 346–376. [Google Scholar]
- Ress D, Chandrasekaran B. Tonotopic organization in the depth of human inferior colliculus. Frontiers in human neuroscience. 2013;7:586. doi: 10.3389/fnhum.2013.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode T, Hartmann T, Hubka P, Scheper V, Lenarz M, Lenarz T, Kral A, Lim HH. Neural representation in the auditory midbrain of the envelope of vocalizations based on a peripheral ear model. Front Neural Circuits. 2013;7:166. doi: 10.3389/fncir.2013.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Dagaeff C, Simm G, De Ribaupierre Y, Villa A, De Ribaupierre F, Rouiller EM. Functional organization of the ventral division of the medial geniculate body of the cat: evidence for a rostro-caudal gradient of response properties and cortical projections. Hear Res. 1989;39:103–125. doi: 10.1016/0378-5955(89)90085-3. [DOI] [PubMed] [Google Scholar]
- Samii A, Lenarz M, Majdani O, Lim HH, Samii M, Lenarz T. Auditory midbrain implant: a combined approach for vestibular schwannoma surgery and device implantation. Otol Neurotol. 2007;28:31–38. doi: 10.1097/01.mao.0000247819.16325.7d. [DOI] [PubMed] [Google Scholar]
- Schreiner C, Froemke R, Atencio C. Spectral Processing in Auditory Cortex. In: Winer JA, Schreiner CE, editors. The Auditory Cortex. US: Springer; 2011. pp. 275–308. [Google Scholar]
- Schreiner CE, Langner G. Laminar fine structure of frequency organization in auditory midbrain. Nature. 1997;388:383–386. doi: 10.1038/41106. [DOI] [PubMed] [Google Scholar]
- Schwartz MS, Otto SR, Shannon RV, Hitselberger WE, Brackmann DE. Auditory brainstem implants. Neurotherapeutics. 2008;5:128–136. doi: 10.1016/j.nurt.2007.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sennaroglu L, Ziyal I. Auditory brainstem implantation. Auris, nasus, larynx. 2012;39:439–450. doi: 10.1016/j.anl.2011.10.013. [DOI] [PubMed] [Google Scholar]
- Sennaroglu L, Colletti V, Manrique M, Laszig R, Offeciers E, Saeed S, Ramsden R, Sarac S, Freeman S, Andersen HR, Zarowski A, Ziyal I, Sollmann WP, Kaminsky J, Bejarano B, Atas A, Sennaroglu G, Yucel E, Sevinc S, Colletti L, Huarte A, Henderson L, Wesarg T, Konradsson K. Auditory brainstem implantation in children and non-neurofibromatosis type 2 patients: a consensus statement. Otol Neurotol. 2011;32:187–191. doi: 10.1097/MAO.0b013e318206fc1e. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Threshold and loudness functions for pulsatile stimulation of cochlear implants. Hear Res. 1985;18:135–143. doi: 10.1016/0378-5955(85)90005-x. [DOI] [PubMed] [Google Scholar]
- Shannon RV. A model of threshold for pulsatile electrical stimulation of cochlear implants. Hear Res. 1989;40:197–204. doi: 10.1016/0378-5955(89)90160-3. [DOI] [PubMed] [Google Scholar]
- Shannon RV. The relative importance of amplitude, temporal, and spectral cues for cochlear implant processor design. Am J Audiol. 2002;11:124–127. doi: 10.1044/1059-0889(2002/013). [DOI] [PubMed] [Google Scholar]
- Shannon RV, Fu QJ, Galvin J., 3rd The number of spectral channels required for speech recognition depends on the difficulty of the listening situation. Acta Otolaryngol Suppl. 2004:50–54. doi: 10.1080/03655230410017562. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Storace DA, Higgins NC, Chikar JA, Oliver DL, Read HL. Gene expression identifies distinct ascending glutamatergic pathways to frequency-organized auditory cortex in the rat brain. J Neurosci. 2012;32:15759–15768. doi: 10.1523/JNEUROSCI.1310-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka MM, Schendel D, Lim HH. Neural integration and enhancement from the inferior colliculus up to different layers of auditory cortex. J Neurophysiol. 2013;110:1009–1020. doi: 10.1152/jn.00022.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka MM, Schmitz S, Lim HH. Response features across the auditory midbrain reveal an organization consistent with a dual lemniscal pathway. J Neurophysiol. 2014a;112:981–998. doi: 10.1152/jn.00008.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka MM, McMahon M, Markovitz CD, Lim HH. Effects of location and timing of co-activated neurons in the auditory midbrain on cortical activity: implications for a new central auditory prosthesis. J Neural Eng. 2014b;11:046021. doi: 10.1088/1741-2560/11/4/046021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner CJ, O'Mard LP, Lopez-Poveda EA, Meddis R. A nonlinear filter-bank model of the guinea-pig cochlear nerve: rate responses. J Acoust Soc Am. 2003;113:3264–3274. doi: 10.1121/1.1568946. [DOI] [PubMed] [Google Scholar]
- Suta D, Kvasnak E, Popelar J, Syka J. Representation of species-specific vocalizations in the inferior colliculus of the guinea pig. J Neurophysiol. 2003;90:3794–3808. doi: 10.1152/jn.01175.2002. [DOI] [PubMed] [Google Scholar]
- Viemeister NF. Temporal modulation transfer functions based upon modulation thresholds. J Acoust Soc Am. 1979;66:1364–1380. doi: 10.1121/1.383531. [DOI] [PubMed] [Google Scholar]
- Viemeister NF, Wakefield GH. Temporal integration and multiple looks. J Acoust Soc Am. 1991;90:858–865. doi: 10.1121/1.401953. [DOI] [PubMed] [Google Scholar]
- Vince GH, Herbold C, Coburger J, Westermaier T, Drenckhahn D, Schuetz A, Kunze E, Solymosi L, Roosen K, Matthies C. An anatomical assessment of the supracerebellar midline and paramedian approaches to the inferior colliculus for auditory midbrain implants using a neuronavigation model on cadaveric specimens. J Clin Neurosci. 2010;17:107–112. doi: 10.1016/j.jocn.2009.06.034. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Rutkowski RG, Palmer AR. Identification and localisation of auditory areas in guinea pig cortex. Exp Brain Res. 2000;132:445–456. doi: 10.1007/s002210000362. [DOI] [PubMed] [Google Scholar]
- Weber DJ, Friesen R, Miller LE. Interfacing the somatosensory system to restore touch and proprioception: essential considerations. Journal of motor behavior. 2012;44:403–418. doi: 10.1080/00222895.2012.735283. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Synaptic mechanisms of forward suppression in rat auditory cortex. Neuron. 2005;47:437–445. doi: 10.1016/j.neuron.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Weiland JD, Cho AK, Humayun MS. Retinal prostheses: current clinical results and future needs. Ophthalmology. 2011;118:2227–2237. doi: 10.1016/j.ophtha.2011.08.042. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Dorman MF. Cochlear implants: a remarkable past and a brilliant future. Hear Res. 2008;242:3–21. doi: 10.1016/j.heares.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SM, Gill PR, Theunissen FE. Stimulus-dependent auditory tuning results in synchronous population coding of vocalizations in the songbird midbrain. J Neurosci. 2006;26:2499–2512. doi: 10.1523/JNEUROSCI.3731-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Pfingst BE. Spectral and temporal cues for speech recognition: implications for auditory prostheses. Hear Res. 2008;242:132–140. doi: 10.1016/j.heares.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG. Trends in cochlear implants. Trends in amplification. 2004;8:1–34. doi: 10.1177/108471380400800102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG, Rebscher S, Harrison W, Sun X, Feng H. Cochlear implants: system design, integration, and evaluation. IEEE Rev Biomed Eng. 2008;1:115–142. doi: 10.1109/RBME.2008.2008250. [DOI] [PMC free article] [PubMed] [Google Scholar]