Abstract
Background:
Hepatocellular carcinoma is one of the most common cancers and a lethal disease. In view of the limited treatment and a grave prognosis of liver cancer, preventive control has been emphasized.
Materials and Methods:
The methanolic extract of roots of Cynodon dactylon was screened for its hepato-protective activity in diethyl nitrosamine (DEN) induced liver cancer in Swiss albino mice. The plant extract at a dose of 50 mg/kg was administered orally once a week, up to 30 days after DEN administration. The animals were sacrificed; blood sample and liver tissue were collected and used for enzyme assay such as, asparatate amino transferase (AST), alanine aminotransferase (ALT), catalase (CAT), glutathione peroxidase (GPx) and glutathione-S-transferase (GST). The liver marker enzymes AST and ALT produced significant results in the protective action.
Results:
The antioxidant enzyme assay results concerning the improved activity of GPx, GST and CAT. These results concluded that enhanced levels of antioxidant enzyme and reduced amount of serum amino transaminase, which are suggested to be the major mechanisms of C. dactylon root extract in protecting the mice from hepatocarcinoma induced by DEN. These biochemical observations were supplemented by histopathological examination of liver sections.
Conclusion:
The methanolic extract of C. dactylon possesses significant anticancer properties
Keywords: Anticancer, Cynodon dactylon L, marker enzymes, N-diethyl nitrosamine
Introduction
Cancer is one of the leading causes of death in the world. Its high incidence and mortality and lack of effective treatment have spurred extensive research on chemoprevention. In fact, this is the second leading cause of death after cardiovascular diseases in India. The cancers such as lung, breast, colon, and stomach are the most common incidences worldwide.[1] Most commonly cancers associated with diet include esophageal, stomach, colon, liver and prostate. Furthermore, this is one of the most dreaded diseases of the 20th century and spreading with continuance and increasing incidence in 21st century. In the United States, as the leading cause of death, it accounts for 25% of all the deaths in humans.[2]
Liver cancer affects nearly 22,000 people in the United States and more than 18,000 deaths. Until recently, the U.S. Food and Drug Administration (FDA) had not approved any medications specifically for liver cancer to treat. The drug sorafenib (Nexavar) was approved by the FDA for people with hepatocellular carcinoma (HCC) (Lowell Anthony, MD). HCC is the fifth most common cancer and the third leading cause of cancer mortality in the world.[3] Approximately 5.6 lakh new cases are diagnosed each year and around 5.5 lakh deaths due to liver cancer occur mostly in developing countries.[4] Although 80% of new cases are detected primarily in developing countries, the prevalence of liver cancer is also rising in Japan, Western Europe, and the United states. It has been estimated that there will be 21,370 new cases and 18,410 deaths in the US in 2008 due to liver cancer.[5] Liver cancer consists of several histological different primary hepatic malignancies, such as cholangiocarcinoma, hepatoblastoma and hemangiosarcoma, but HCC is by the most common type, accounting for 70–85% of cases.[6,7] Medicinal plants are frequently used by traditional healers to treat a variety of ailments and symptoms including diabetes and cancer. According to world health organization, over 80% of the world's populations rely upon such traditional plant-based systems of medicine to provide them with primary healthcare. Cynodon dactylon L. is a perennial grass that has a variety of medicinal properties.[8] Twenty-two compounds were found in C. dactylon. Hydroquinone (69.49%), levoglucosenone (2.72%), furfural (6.0%), were found to be the most abundant components among the 20 characterized compounds in C. dactylon.[9] It is cultivated throughout the tropics and subtropics. Whole herb and its root stalk are practiced for medicinal use.[10] The present study was carried out to determine the anticancer potential of C. dactylon L. root extracts using diethyl nitrosamine (DEN)-induced mice model.
Materials and Methods
Plant materials
Cynodon dactylon L., roots were collected from in and around Salem and Vellore district. The roots were washed thoroughly with running tap water. Then the same was shade dried for 2–3 weeks and was grounded to moderately coarse powder.
Extraction procedure
The dried and powdered plant material was extracted using 95% V/V methanol in soxhlet apparatus for 6 h. The extract was concentrated using a rotary evaporator at 40–50°C under reduced pressure.
Animals
Adult male 6–8 weeks old, weight vary from 22 to 26 g Swiss albino mice were housed in polypropylene cages at Central Animal Facility, Periyar University, Salem. They were kept in an animal room with 12:12 h day-night cycle with temperature of 28°C ± 2°C and humidity of 45–60%. They were fed with a commercial pelleted mice chow and water ad libitum throughout the study. The study was conducted after obtaining institutional animal ethical committee clearance Reg: No: 1085/ac/07 CPCSEA (PUIAEC 11/02).
Chemicals
Diethyl nitrosamine was obtained from Sigma Aldrich Ltd. A dose of 20 mg/kg body weight in 0.9% w/v NaCl and was administered intraperitonially.
Sacrifice of mice
At the end of the treatment (30 days), the experimental animals [Table 1] were deprived of food overnight. Blood was collected without anticoagulant was used for serum separation; finally animals were sacrificed by cervical dislocation. Liver were carefully dissected out and washed with ice-cold saline and preserved in 10% w/v formalin solution for the enzymatic analysis and histological studies. 1.0 g of the liver tissue was homogenized in 0.2 M phosphate buffer, pH 7.0 at 4°C.
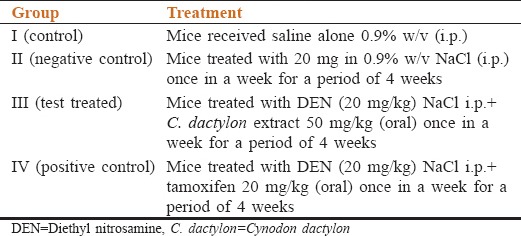
Table 1.
Experimental groups and protocol
Protein estimation
The homogenates were centrifuged at 6500 × g for 10 min at 4°C. The supernatant was used for enzymatic assay. Biochemical assessments were performed with the supernatant of liver homogenate and serum. Total protein concentration was determined by the method of Lowry et al.[11] The protein value was expressed as mg/g of tissue sample.
Marker enzyme assays
Marker enzymes were assayed by standard methods. Catalase (CAT) was assayed by the method described by Luck.[12] Glutathione peroxidase (GPx) activity was assayed by the method of Rotruck et al.[13] The enzyme activity was expressed as nano moles/min/ml. asparatate amino transferase (AST) and alanine aminotransferase (ALT) activities were measured by the methods of Bergmeyer and Bernt.[14] A standard curve was obtained using different amounts of pyruvate and serum activity was expressed as units/ml. Glutathione-S-transferase (GST) was assayed by the method of Habig et al.[15] Activity of GST was expressed as moles of 1-chloro 2,4 dinitrobenzene-glutathione (GSH) conjugate formed min/mg of protein.
Histopathological study
Liver was dissected out and washed with ice-cold saline. A part of the liver tissue was fixed in 10% formalin solution for 24–48 h and stored at room temperature. Then the tissue is removed by dehydration in series of alcohols 70, 80, 90 and 100% for a period of 1 h each. Tissue in 100% alcohol is kept for 12 h. Transfer the tissues into xylene about 2 h for clearing. Tissue was embedded in paraffin wax at 56°C temperature for 2 h. It was stored at room temperature. Fixed embedded block was sectioned using microtome, in width 7 μm. Continuous sections were floated on the warm water bath (42°C) to remove wrinkles. Picked up on a glass microscopic slide and placed on warm oven at 42°C. Dewaxing was done by running them through xylene for 2 h and series of alcohols (100, 90, 80, 70%) and to water. Then sections of liver tissues were stained with hemotoxylin and eosin. Then stained slides were examined under light microscope and photomicrographs (×400) were taken.
Statistical analysis
All statistical analysis was conducted using one-way ANOVA with Dunnett's posttest using Graph Pad InStat version 3.00 for Windows, GraphPad Software, San Diego, CA, USA.
Results and Discussion
A highly significant (P < 0.01) elevation in serum glutamate pyruvate transaminase (SGPT) activity was observed in DEN and DEN + C. dactylon groups when compared with control mice, Whereas the DEN + Tamoxifen treated animals showed the low significant alteration with DEN and DEN + C. dactylon groups [Figure 1]. A highly significant (P < 0.01) elevation in serum glutamate oxaloacetate transaminase (SGOT) activity was observed in DEN, DEN + C. dactylon and DEN + Tamoxifen mice. The saline treated did not show the any significant alteration [Figure 2].
Figure 1.
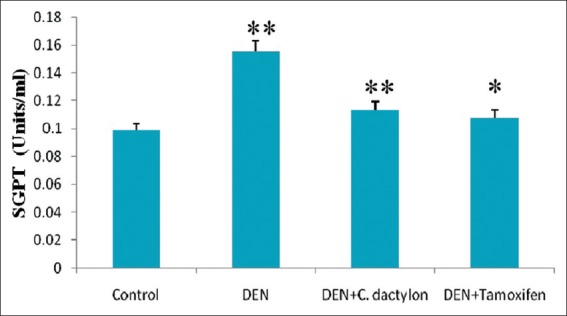
Swiss albino mice were treated with diethyl nitrosamine (20 mg/kg) and/or crude plant extract of Cynodon dactylon 50 mg/kg and tamoxifen 20 mg/kg. Saline treated animal served as control. Glutamate pyruvic transaminase values are expressed as mean ± standard deviation, n = 6 in each group, **P < 0.01, *P < 0.05 compared with control groups
Figure 2.
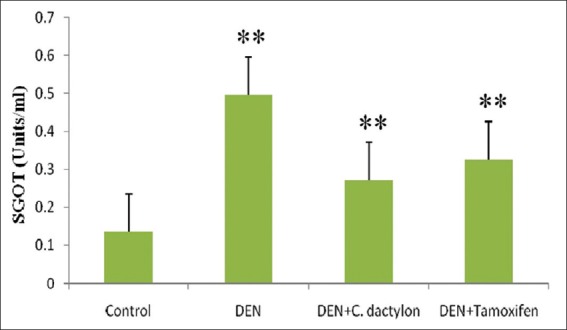
Swiss albino mice were treated with diethyl nitrosamine (20 mg/kg) and/or crude plant extract of Cynodon dactylon 50 mg/kg and tamoxifen 20 mg/kg. Saline treated animal served as control. Serum glutamate oxaloacetate transaminase values are expressed as mean ± standard deviation, n = 6 in each group, **P < 0.01, compared with control groups
Diethyl nitrosamine treated group showed low significant elevation (P < 0.05) in liver GST activity with respect to control, Whereas the control, DEN + C. dactylon, DEN + Tamoxifen treated animals did not shown any alteration [Figure 3]. A highly significant (P < 0.01) elevation in GPx activity was observed in DEN treated mice, whereas DEN + C. dactylon showed low significant alteration the same time saline and DEN + Tamoxifen treated animals did not show any significant alteration [Figure 4]. DEN, DEN + C. dactylon showed low significant depletion (P < 0.05) in liver CAT activity with respect to control, whereas saline and DEN + Tamoxifen treated animals did not showed any significant alteration [Figure 5].
Figure 3.
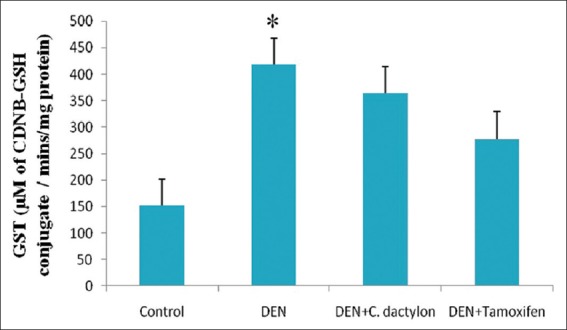
Swiss albino mice were treated with diethyl nitrosamine (20 mg/kg) and/or crude plant extract of Cynodon dactylon 50 mg/kg and tamoxifen 20 mg/kg. Saline treated animal served as control. Glutathione S-transferase values are expressed as mean ± standard deviation, n = 6 in each group, *P < 0.05 compared with control groups
Figure 4.
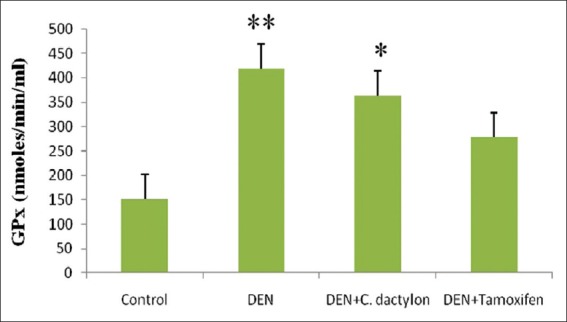
Swiss albino mice were treated with diethyl nitrosamine (20 mg/kg) and/or crude plant extract of Cynodon dactylon 50 mg/kg and tamoxifen 20 mg/kg. Saline treated animal served as control. Glutathione peroxidase values are expressed as mean ± standard deviation, n = 6 in each group, **P < 0.01, *P < 0.05 compared with control groups
Figure 5.
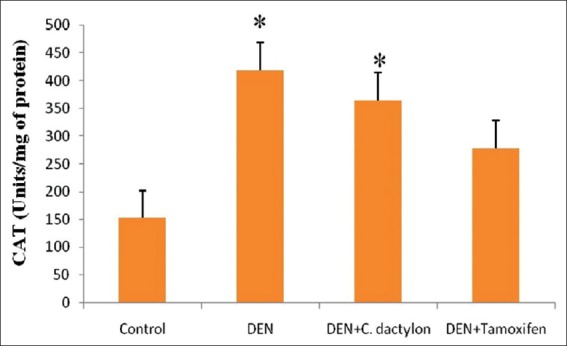
Swiss albino mice were treated with diethyl nitrosamine (20 mg/kg) and/or crude plant extract of Cynodon dactylon 50 mg/kg and tamoxifen 20 mg/kg. Saline treated animal served as control. Catalase values are expressed as mean ± standard deviation, n = 6 in each group, *P < 0.05 compared with control groups
Discussion
In Indian system of medicine, certain herbs are claimed to provide relief against liver disorders. Numerous cancer research studies have been conducted using traditional medicinal plants in an effort to discover new therapeutic agents that lack the toxic side effects associated with current chemotherapeutic agents.[16] One of the most versatile plant used is C. dactylon, a member of the poaceae family was taken for the anticancer evaluation in DEN induced mice. Elevation of the plasma levels of cytoplasmic and mitochondrial enzymes is a sensitive indicator of liver damage. Excessive antioxidants could dangerously interfere with these protective functions while temporary depletion of antioxidants can enhance anticancer effects of apoptosis.
Hepatocellular carcinoma, a highly malignant tumor with extremely poor prognosis, represents 4% of all malignant tumors and is the seventh most common cancer in man worldwide.[17] There is now agreement among oncologists that the incidence of cancer is determined by the factors in the environment and it is surprising that diet is suggested to be responsible for about 30–70% of the causes of cancers.[18]
Diethyl nitrosamine is one of the most important environmental carcinogens that primarily induce tumors in the liver, because of its relatively simple metabolic pathway and potent carcinogenic activity.[19] In experimental liver carcinogenesis, early preneoplastic foci appear which are induced by an initiating carcinogen DEN, and they exhibit moderately elevated rate of proliferation.[20,21] Diethylinitrosamine is a powerful hepatocarcinogen known to induce cancer in experimental animals.[22,23] The liver weight increased nearly two folds in those animals that received DEN. The liver weight of animals treated with DEN increased up to 90% and administration of Ethanolic extract of Indigofera aspalathoides (EIA) brought down to 35% compared to control animals[24] whereas, in the present study, the liver weight increased drastically. The weight of the liver was increased to 41%, 17%, 13% in DEN, DEN + C. dactylon, DEN + tamoxifen respectively when compared with control. When compared to all groups the DEN treated mice gained increase in body weight that may be due to high metabolic activity of liver and increased accumulation of high-density lipoproteins. The food and water intake was increased in DEN induced mice when compared to control. While in DEN induced group food and water intake slightly increased when compared to DEN + C. dactylon and DEN + Tamoxifen. The effect of C. dactylon is perhaps owing to the abundant of diterpenoids presence in it. However, the role of diterpenoids is not only limited to radical scavenging activity it may also involve in apoptosis introduction.
In this study, SGOT and SGPT values were decreased in test group when compared to Tamoxifen and DEN treated groups. This is due to high cancerous cell present in the DEN treated groups and thereby high liver marker enzyme synthesis. These results coincide with the results obtained by Jahan et al.[25]
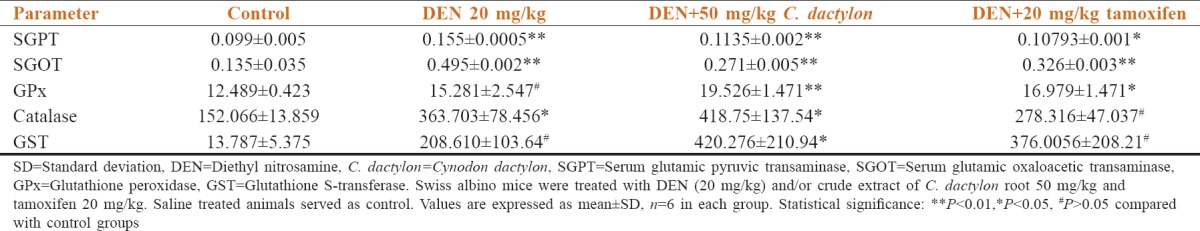
A high level of GST occurs in neoplastic and preneoplastic lesions induced by hepatic chemical carcinogens. The low level of GST in animals receiving the extract + DEN indicates the ability of the EIA to inhibit tumor progression.[26,27] In our result, increased level of GSH has been observed in DEN treated mice [Table 2]. The obtained results in the current investigation concerning GPx and GST enzyme activities were increased in the (test) C. dactylon treated groups when compared to tamoxifen (positive control) treatment. When compared to an earlier study,[26,27] GST shows low significance in DEN and did not show any alteration in saline, DEN + C. dactylon and DEN + tamoxifen treated groups. GPx shows high significance in DEN, low depletion in DEN + C. dactylon and did not show any alteration in saline and DEN + tamoxifen treated groups. DEN induced group may have increased level of lipid peroxidation, that is confirmed by high level of GST and GPx.
Table 2.
Effect of C. dactylon root extract on liver marker enzymes and enzymatic antioxidants in DEN induced liver cancer in mice
Catalase activity in the liver of some transplantable hepatoma cells and cultured hepatoma cell lines have found to be decreased due to the depression of enzyme biosynthesis, which in turn is because of depression of CAT gene expression.[28,29,30] When Compared with the above study CAT activity in DEN, DEN + C. dactylon showed low significance when compared to control group, whereas DEN + tamoxifen group did not show any alteration.
The histochemical results of this study revealed the disarrangement of normal hepatic cells with intense centrilobular necrosis in DEN intoxicated liver [Figure 6]. A global hepato-protective effect of chrysin administration was evidenced by a marked reduction in vacuolization and disappearance of binucleated cells in animals posttreated with oral dose of chrysin. The protective effect of chrysin produced significant decrease in GST-Pi positive foci and PCNA staining in the neoplastic nodules. GST-Pi positive cells are considered precursors of preneoplastic foci that frequently occur during early stages of experimental carcinogenesis.[31] Our present histopathology results showed normal hepatocytes in control animals, whereas DEN induced group showed hepatocytes with hyperchromatic nuclei, prominent nucleoli and marked fat accumulation within the liver cells. In test group animals, proper arrangement of normal hepatic cells was observed.
Figure 6.
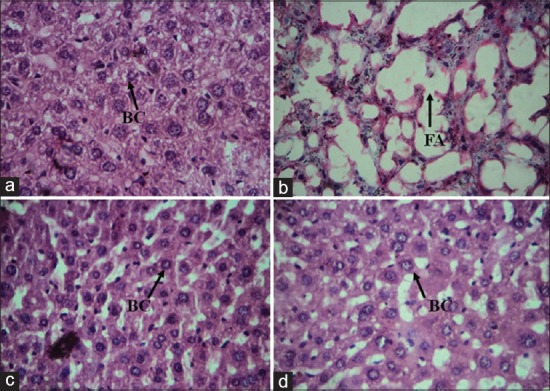
Microscopic evaluation of hepatic tissue (a) control showing normal hepatocytes, (b) diethyl nitrosamine (DEN) treated group showing high accumulation of fat with in the hepatocytes, (c) DEN + Cynodon dactylon 50 mg/kg treated group and (d) DEN + tamoxifen 20 mg/kg treated groups showing recovery of normal hepatocytes (H and E, ×400). FA = Fat accumulation, BC = Binucleated cells
Conclusion
The present study concludes C. dactylon treatment was observed to exhibit anti cancerous effect as demonstrated by enhanced activities of antioxidant enzymes (AST, ALT, GST, GPx and CAT). It may be due to the presence of antioxidant property. This gives an opportunity to find out the active compound which is present in C. dactylon responsible for the anticancer activity.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Bingham S, Riboli E. Diet and cancer – The European Prospective Investigation into Cancer and Nutrition. Nat Rev Cancer. 2004;4:206–15. doi: 10.1038/nrc1298. [DOI] [PubMed] [Google Scholar]
- 2.Balachandran P, Govindarajan R. Cancer – An ayurvedic perspective. Pharmacol Res. 2005;51:19–30. doi: 10.1016/j.phrs.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: Diagnosis and treatment. Gastroenterology. 2002;122:1609–19. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 4.McGlynn KA, London WT. Epidemiology and natural history of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2005;19:3–23. doi: 10.1016/j.bpg.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Cancer Facts and Figures 2008. [Last accessd on 2013 Dec 12]. Available from: http://www.cancer.org .
- 6.American Cancer Society, Inc; 2007. American Cancer Society. Global Cancer Facts and Figures. [Google Scholar]
- 7.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: From genes to environment. Nat Rev Cancer. 2006;6:674–87. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 8.Singh SK, Rai PK, Mehta S, Singh RK, Watal G. Curative effect of Cynodon dactylon against STZ induced hepatic injury in diabetic rats. Indian J Clin Biochem. 2009;24:410–3. doi: 10.1007/s12291-009-0073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shabi MM, Gayathri K, Venkatalakshmi R, Sasikal C. Chemical constituents of hydro alcoholic extract and phenolic fraction of Cynodon dactylon. Int J Chemtech Res. 2010;2:149–54. [Google Scholar]
- 10.Kritikar KK, Basu BD. In: Indian Medicinal Plants. 2nd ed. Dehradun: International Book Distributors; 1980. C. dacylon; p. 2650. [Google Scholar]
- 11.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 12.Luck H. In: Methods in enzymatic analysis. Bergmeyer. 2nd Ed. New York: Academic Press; 1974. p. 885. [Google Scholar]
- 13.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 14.Bergmeyer HU, Bernt E. In: Methods of Enzymatic Analysis. Vol. 2. New York and London: Verlag Chemie Weinheim/Academic Press, Inc; 1974. p. 735. [Google Scholar]
- 15.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 16.Jeyachandran R, Mahesh A, Cindrella L. DEN-induced cancer and its alleviation by anisomeles malabarica (L.) R. Br. Ethanolic leaf extract in male albino mice. Int J Cancer Res. 2007;3:174–9. [Google Scholar]
- 17.Parkin DM, Stjernswärd J, Muir CS. Estimates of the worldwide frequency of twelve major cancers. Bull World Health Organ. 1984;62:163–82. [PMC free article] [PubMed] [Google Scholar]
- 18.Doll R. Symposium on diet and cancer. Proc Nutr Soc. 1990;49:119–31. doi: 10.1079/pns19900018. [DOI] [PubMed] [Google Scholar]
- 19.Archer MC. Mechanisms of action of N-nitroso compounds. Cancer Surv. 1989;8:241–50. [PubMed] [Google Scholar]
- 20.Rabes HM, Scholze P, Jantsch B. Growth kinetics of diethylnitrosamine-induced, enzyme-deficient “preneoplastic” liver cell populations in vivo and in vitro. Cancer Res. 1972;32:2577–86. [PubMed] [Google Scholar]
- 21.Schulte-Hermann R, Ohde G, Schuppler J, Timmermann-Trosiener I. Enhanced proliferation of putative preneoplastic cells in rat liver following treatment with the tumor promoters phenobarbital, hexachlorocyclohexane, steroid compounds, and nafenopin. Cancer Res. 1981;41:2556–62. [PubMed] [Google Scholar]
- 22.Sreepriya M, Bali G. Chemopreventive effects of embelin and curcumin against N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in Wistar rats. Fitoterapia. 2005;76:549–55. doi: 10.1016/j.fitote.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Björkhem-Bergman L, Torndal UB, Eken S, Nyström C, Capitanio A, Larsen EH, et al. Selenium prevents tumor development in a rat model for chemical carcinogenesis. Carcinogenesis. 2005;26:125–31. doi: 10.1093/carcin/bgh290. [DOI] [PubMed] [Google Scholar]
- 24.Rajkapoor B, Murugesh N, Chodon D, Sakthisekaran D. Chemoprevention of N-nitrosodiethylamine induced phenobarbitol promoted liver tumors in rat by extract of Indigofera aspalathoides. Biol Pharm Bull. 2005;28:364–6. doi: 10.1248/bpb.28.364. [DOI] [PubMed] [Google Scholar]
- 25.Jahan MS, Vani G, Shyamaladevi CS. Anti-carcinogenic effect of solanum trilobatum in diethylnitrosame induced and Phenobarbital promoted heaptocarcinogenesis in rats. Asian J Biochem. 2011;6:74–81. [Google Scholar]
- 26.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–20. [PubMed] [Google Scholar]
- 27.Satoh K, Hatayama I, Tsuchida S, Sato K. Biochemical characteristics of a preneoplastic marker enzyme glutathione S-transferase P-form (7-7) Arch Biochem Biophys. 1991;285:312–6. doi: 10.1016/0003-9861(91)90365-p. [DOI] [PubMed] [Google Scholar]
- 28.Bozzi A, Mavelli I, Agro AF, Strom R, Wolf AM, Mondovi B, et al. Twenty five years of research on medicinal plants in Latin America: A personal review. J Ethnopharmacol. 2005;100:131–4. doi: 10.1016/j.jep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Kashiwagi K, Tobe T, Higashi T, Warabioka K. Impaired synthesis of liver catalase in tumor-bearing rats. Gann. 1972;63:57–62. [PubMed] [Google Scholar]
- 30.Sato K, Ito K, Kohara H, Yamaguchi Y, Adachi K, Endo H. Negative regulation of catalase gene expression in hepatoma cells. Mol Cell Biol. 1992;12:2525–33. doi: 10.1128/mcb.12.6.2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Satoh K, Hatayama I. Anomalous elevation of glutathione S-transferase P-form (GST-P) in the elementary process of epigenetic initiation of chemical hepatocarcinogenesis in rats. Carcinogenesis. 2002;23:1193–8. doi: 10.1093/carcin/23.7.1193. [DOI] [PubMed] [Google Scholar]


