Abstract
Background:
This study aimed to determine the intracellular (red blood cell (RBC)) magnesium levels in children with chronic bronchial asthma and to determine the relationship between the magnesium level and peak expiratory flow rate (PEFR), type of asthma treatment, and level of asthma control.
Methods:
A cross-sectional study was conducted at the Paediatric Clinic, Sarawak General Hospital. A total of 100 children, aged 6–12 years with chronic bronchial asthma, were recruited according to the study criteria. Venous blood samples were obtained to measure the intracellular (RBC) magnesium level using the GBC Avanta Flame Atomic Absorption Spectrophotometer.
Results:
Mean age was 8.57 (SD 1.18) years, and 63% of the participants were male. Mean duration of asthma was 62.2 (SD 32.3) months. A normal intracellular magnesium level was found in 95% of the participants, with a mean of 2.27 (SD 0.33) mmol/L. Two-thirds of the participants had a normal peak flow expiratory rate (> 80% of predicted value). About 85% were using both reliever and controller. Almost half of the participants (49%) had chronic asthma that was well-controlled. No significant relationship was found between magnesium level and age (r = –0.089, P = 0.379), gender (t = 0.64, P = 0.52), duration of asthma (r = –0.03, P = 0.74), PEFR (t = 0.41, P = 0.68), current level of asthma control (t = 0.02, P = 0.97), and current treatment (t = 0.414, P = 0.680).
Conclusion:
There was no significant intracellular magnesium deficiency in children with chronic bronchial asthma. There was no significant relationship between therapeutic medications used for treatment of children with chronic asthma and intracellular magnesium levels.
Keywords: intracellular, magnesium, asthma
Introduction
Bronchial asthma is a chronic inflammatory disease of the airways and is one of the leading causes of childhood morbidity worldwide. Currently, in the US alone, an estimated 7.1 million children under 18 years suffer from asthma, of which 4.1 million developed an asthma attack in 2011 (1). In a study conducted in Kuala Lumpur, 13.8% of local primary school children were found to be asthmatic (2).
Magnesium plays a crucial role in the regulation of bronchial smooth muscle contractility and hyper-responsiveness (3). There have been many studies on the relationship between asthmatic exacerbation and blood magnesium level, but findings and conclusions of these studies have varied. Studies in adult patients have shown that low serum magnesium levels were associated with an increased risk of asthma attacks and subsequent hospitalisations compared to the risk in patients with normal magnesium levels (4–6). Studies in children have reported significantly lower intracellular magnesium levels in children with acute bronchial asthma than nonasthmatic children (7). Other studies showed no significant difference in serum magnesium level during and in between asthmatic exacerbations in asthmatic children (8).
As magnesium is mainly an intracellular cation, the serum magnesium level reflects only a small proportion of total magnesium ions in the body (3). As such, serum magnesium measurement is not a reliable predictor of asthma severity (9). Intracellular (red blood cell(RBC)) magnesium level, on the other hand, has been documented as an important determinant of bronchial hyper-reactivity (7). Some studies in adult patients with chronic asthma have shown that there is a significant decrease in their erythrocyte magnesium level but not in their serum magnesium level (10–13). Other studies in adults, however, have shown no significant difference in intracellular magnesium levels between attacks (14,15). Therefore, it is not clear whether low magnesium level in chronic asthmatic children is associated with higher frequency of asthmatic exacerbation.
Anti-inflammatory and bronchodilator medications used in the treatment of asthma may cause magnesium depletion in humans (16). High magnesium intake is associated with better lung function and less risk of bronchial hyper-reactivity and wheezing (17). By giving oral magnesium supplementation, lung function and frequency of attacks are improved (18,19). In clinical trials, children with acute bronchial asthma were reported to have remarkable improvement in short-term pulmonary function when given high doses of intravenous magnesium sulphate for moderate to severe asthmatic exacerbation (20–24). Conversely, some studies in children and adults showed no beneficial effect of intravenous magnesium sulphate in the management of acute severe asthma (25,26). Nevertheless, meta-analyses and systematic reviews of intravenous magnesium sulphate for treating acute asthma have shown benefit in children with acute asthma (27,28).
Studies both using extracellular and intracellular magnesium level as determination of the magnesium level in the body have been reported; however, intracellular magnesium has been demonstrated to be a better measure of magnesium status in patients than serum magnesium level (13,29). This is because serum magnesium reflects short-term variations in magnesium intake and is not representative of the total body magnesium stores.
Although there have been substantial studies on the relationship of blood magnesium level and bronchial asthma, there have been few such studies in paediatric patients. The aim of this study was to investigate the intracellular (RBC) magnesium level in children with chronic bronchial asthma and to determine its relationship with peak expiratory flow rate (PEFR), duration of asthma, type of asthma treatment, and level of asthmatic control.
Materials and Methods
This was a cross-sectional study conducted at the Paediatric Clinic of Sarawak General Hospital in Kuching, Malaysia from 1st August 2011 to 31st May 2012. Kuching, the capital city of Sarawak, is the fourth largest urban area in Malaysia. With an estimated population of 579 900 people, Kuching has a diverse population consisting of Chinese, Malay, and indigenous groups such as Iban, Bidayuh, Melanau, and Orang Ulu, amongst others.
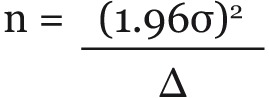
The required sample size was calculated based on estimation of a mean in the population.
Using the standard deviation of 0.1 mmol/L (7), estimating the true mean to within 0.02 SD with 95% confidence, the minimum sample size required was 90. As a 10% attrition rate was anticipated, the final minimum sample size was 100. All asthmatic patients who came for their regular follow-up at the Paediatric Clinic at Sarawak General Hospital during the period of data collection were recruited. Once identified, these patients were assigned to the researchers’ consultation room for a check-up followed by interview. Patients with chronic bronchial asthma (defined as having bronchial asthma duration of ≥ 1 year) were recruited for the study. In accordance with the Global Initiatives for Asthma (GINA), diagnosis of bronchial asthma was made based on clinical history and ≥ 15% reversibility of lung function test (PEFR) with bronchodilator administration or a diurnal variation in PEFR of ≥ 20%. PEFR reversibility is mainly based on the patient’s follow-up clinical note (20). Patients with chronic bronchial asthma who were less than six years of age, those with other associated diseases that could potentially cause magnesium deficiency (namely, kidney disease, bone disease, gastrointestinal disease, cardiovascular disease, hormonal disorder, metabolic disorder, recent infection), and those who were taking calcium antagonists, diuretics, digoxin, laxatives, or vitamin D were excluded from this study.
Informed consent was obtained from the parents. Participants were interviewed and clinically evaluated, and PEFR was measured. For classification into controlled and uncontrolled asthma, patients were assessed based on attack frequency over the previous three months, and their current level of asthma control was based on frequency of daytime symptoms, nocturnal symptoms or awakening because of asthma, frequency of using reliever treatment, reduced lung function (PEFR), and any limitation of daily activities including exercise. Controlled asthma was defined as no (≤ 2 times/week) daytime symptoms, no limitation of daily activities including exercise, no nocturnal symptoms or awakening because of asthma, no (≤ 2 times/week) need for reliever treatment, normal or near normal lung function results, and no exacerbation. Both partly controlled and uncontrolled asthma were categorised as uncontrolled asthma. The patient was diagnosed with partly controlled asthma if any of the following were experienced: daytime symptoms more than twice per week, using rescue treatment more than twice per week, any limitation of activity, nighttime cough, and reduced lung function < 80% of predicted or personal best (30). If the patient experienced three or more of the above criteria, the condition was defined as uncontrolled asthma. Information on the patient’s age, gender, duration of bronchial asthma, and current treatment was obtained from each subject and checked against their case notes. Height was measured to determine the predicted normal PEFR value.
Venous blood samples were collected from participants using vacuum tubes. The intracellular (RBC) magnesium concentration was measured using the GBC Avanta Flame Atomic Absorption Spectrophotometer with absorption at 285.2 nm (GBC Scientific, Australia). The normal intracellular Mg value measured in the laboratory was 1.7 to 2.9 mmol/L.
The PEFR was determined using Wright’s peak flow meter (Ferraris Medical Ltd, Edmonton, London, UK). The peak flow rate was measured with the subject standing. The mouthpiece of the meter was placed in the subject’s mouth, and lips were sealed around the mouthpiece. As PEFR measurements depend significantly on the subject’s effort and technique, instructions for proper use were given and followed by a demonstration of technique prior to measurement. PEFR measurements can be accurately performed by most patients older than five years (31). Each subject was asked to perform the test three times, and the highest of the three readings was used as the recorded value of PEFR. This highest recorded PEFR value was compared to the values on the predicted curves for Malaysian children (32).
This study protocol was approved by the Ethics Committee of Ministry of Health Malaysia (NMRR-11-317-8642). Data was entered and analysed using SPSS statistical package version 20 (IBM Corporation, New York). For descriptive data, the percentage, mean, and standard deviation were used. Normality testing of the data was carried out using boxplot and the Kolmogorov-Smirnov test. If the data were found to be normally distributed, a parametric test such as Pearson correlation was used to test the association between intracellular (RBC) magnesium level, age, and duration of asthma. The independent t test was used for the association between intracellular (RBC) magnesium level, gender, and PEFR. For all tests, a P value of less than 0.05 was significant.
Results
A total of 100 children were studied; 63% were male, and 37% were female. The mean age was 8.57 years (SD 1.18). The mean duration of asthma was 62.2 months (SD 32.3) (ranging from 12 to 132 months). The mean intracellular (RBC) magnesium level was 2.27 mmol/L (SD 0.33) (range 1.5–3.54 mmol/L). Normal intracellular (RBC) magnesium levels were found in 95% of children. Abnormal PEFR (< 80% of predicted value) was found in 24% of children. The majority of children in this study (85%), were using both reliever and controller medications, whereas a smaller proportion (15%) were using only reliever medications. Asthma was well-controlled in 49% of children (Table 1).
Table 1.
Demographic characteristic of the 100 children with asthma
| Variable | N (%) | Mean (SD) | Range |
|---|---|---|---|
| Age (year) | 8.57 (1.181) | 6–13 | |
| Gender | |||
| Male | 63 (63) | ||
| Female | 37 (37) | ||
| Duration of asthma (months) | 62.2 (32.3) | 12–132 | |
| Intracellular Mg level (mmol/L) | 2.27 (0.331) | 1.5–3.54 | |
| Normal | 95 (95) | ||
| Abnormal | 5 (5) | ||
| Peak Expiratory Flow Rate | |||
| Normal (> 80%) | 76 (76) | ||
| Abnormal (80% and below) | 24 (24) | ||
| Current level of asthma control | |||
| Controlled | 49 (49) | ||
| Uncontrolled | 51 (51) | ||
| Current treatment | |||
| Reliever | 15 (15) | ||
| Reliever & controller | 85 (84) |
Both boxplot and Kolmogorov-Smirnov tests showed data to be normally distributed. Statistical analysis (Table 2) revealed that there was no significant relationship between intracellular level of magnesium and age (r = -0.089, P = 0.379), or the duration of asthma (r = -0.033, P = 0.741). There was no significant difference in mean intracellular (RBC) magnesium level between male and female children (t = 0.644, P = 0.521). No significant relationship was demonstrated between PEFR (t = 0.412, P = 0.681), current level of asthma control (t = 0.027, P = 0.978), and the current treatment (t = 0.414, P = 0.680) (Table 3).
Table 2.
Correlationa between intracellular Mg level with age and duration of having asthma (N = 100)
| Mg level | |
|---|---|
| Age | –0.089 |
| Duration of asthma | –0.033 |
aPearson correlation test, significant at P ≤ 0.05.
Table 3.
t testa results comparing gender, PEFR, asthma control and treatment on intracellular Mg level (N = 100)
| Mg level Mean (SD) mmol/L | Mean difference (95% CI) | P value | |
|---|---|---|---|
| Gender | 0.521 | ||
| Male | 2.28 (0.331) | 0.044 (–0.09,0.18) | |
| Female | 2.24 (0.333) | 0.044 (–0.09,0.18) | |
| PEFR | 0.681 | ||
| Normal | 2.27 (0.343) | 0.032 (–1.12, 0.19) | |
| Abnormal | 2.24 (0.293) | 0.032 (–1.12, 0.18) | |
| Current level of asthma control | 0.978 | ||
| Uncontrolled | 2.27 (0.371) | 0.002 (–0.13,0.13) | |
| Controlled | 2.26 (0.291) | 0.002 (–0.13,0.13) | |
| Current treatment | 0.680 | ||
| Reliever | 2.30 (0.339) | 0.04 (–0.15,0.23) | |
| Reliever & Controller | 2.26 (0.331) | 0.04 (–0.17, 0.24) |
aIndependent t test, significant at P < 0.05.
Discussion
The majority (95%) of the participants in this study were found to have normal intracellular (RBC) magnesium levels. A high intracellular magnesium level was found in 4% of the children, and the prevalence of hypomagnesaemia was 1%. The low prevalence of hypomagnesaemia in our study is similar to other previous studies in adults (14,15) which demonstrated no significant difference in intracellular (RBC) magnesium in asthmatic patients between attacks and stable asthma. Another study in patients from Palermo, Italy, however, showed a higher prevalence of hypomagnesaemia in asthmatic patients (10) than seen in our study. Our low prevalence of hypomagnesaemia may be attributed to the type of food in Asian diets, which consist of rice and rice products as the main staple food, along with green vegetables and soya bean. These foods have been found to be rich in magnesium, shown in a study by Wang JL et al (33) in Taiwan.
In patients with chronic, stable asthma, the intracellular (RBC) magnesium levels may not be affected compared to levels obtained during an asthma attack. This is because during an acute asthma attack, bronchoconstriction induced by histamine challenge or exacerbation leads to a significant fall in erythrocyte magnesium level (34). It is postulated, that, when bronchoconstriction occurs, magnesium is forced out of the intracellular space, thus leading to depletion in the erythrocyte magnesium level (12).
The age of the patients in this study ranged from 6–12 years. Most previous studies on intracellular (RBC) have been carried out in an older adult population. As it has been shown that the intracellular magnesium level varies markedly with age (35), the difference in age range of patients from different studies could explain the discrepancies in results reported.
A recent published study showed no significant effect of regular controller therapy usage on magnesium level (12,15). This is in accordance with our study where there was no significant difference in erythrocyte magnesium level in either treatment group.
In our study, mean erythrocyte magnesium level was slightly higher in boys than girls. This was different from the finding in a previous study (8). We also found that there was no significant association between duration of asthma and intracellular magnesium level. It is thought that redistribution of magnesium mainly takes place during an acute asthma attack, and this leads to a significant fall in intracellular magnesium level (36,37).
The limitations of this study are that it did not include healthy children without asthma, and the study population was relatively small. Moreover, we only used PEFR for a lung function test, and we did not enquire about the dietary history of the children. The children’s magnesium-rich diet might have explained why there was no significant difference in the level of magnesium in these 2 cohorts.
Conclusion
In this study, we found that there was no significant difference in intracellular magnesium levels between uncontrolled and controlled chronic asthmatic patients. Moreover, medications used for asthma had no effect on intracellular magnesium levels. Further studies with larger numbers of children are required to verify that there is no relationship between intracellular magnesium and asthma control.
Acknowledgments
This study was done under UNIMAS grant number 01(S74)/809/2011. The authors would like to thank Mr. Joseph Tau Katip for his assistance.
Footnotes
Conflict of interest
None.
Funds
Unimas Grant 01(S74)/809/2011.
Authors’ contributions
Conception and design: HHS, KJL, JSLN, MAS, CWL
Analysis and interpretation of the data: HHS, CWL
Drafting of the article: HHS, KJL, AR, JSLN, MAS, CWL
Critical revision of the article for the important intellectual content: HHS, KJL, AR, JSLN
Final approval of the article: HHS, KJL, JSLN, CWL
Provision of study materials or patient: HHS, AR, JSLN
Statistical expertise, collection and assembly of data: CWL
Obtaining of funding, administrative, technical or logistic support: HHS
References
- 1.Centers for Disease Control and Prevention . America (AS): Centers for Disease Control and Prevention; Year of publication unknown. National Center for Health Statistics. National Health Interview Survey Raw Data, 1997-2011. Analysis performed by American Lung Association Research and Health Education Division using SPSS and SUDAAN software. [Google Scholar]
- 2.Omar AH. Respiratory symptoms and asthma in primary school children in Kuala Lumpur. ACTA Paediatr Japan. 1990;32:183–187. doi: 10.1111/j.1442-200x.1990.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 3.Reinhart RA. Magnesium metabolism. A review with special reference to the relationship between intracellular content and serum levels. Arch Intern Med. 1988;148(11):2415–2420. doi: 10.1001/archinte.148.11.2415. doi: 10.1001/archinte.148.11.2415 . [DOI] [PubMed] [Google Scholar]
- 4.Alamoudi OSB. Hypomagnesemia in chronic stable asthmatics: prevalence, colleration with severity and hospitalization. Euro Respir J. 2000;16:427–431. doi: 10.1034/j.1399-3003.2000.016003427.x. [DOI] [PubMed] [Google Scholar]
- 5.Khosrow A, Hamid Reza JD. Blood serum magnesium values in chronic stable asthmatic patients: A case - control study. Tanaffos. 2005;4(13):27–32. [Google Scholar]
- 6.Sibes KD, Arup KH, Indranath G, Samirendra KS, Anirban D, Saurabh B. Serum Magnesium and stable asthma. Lung India. 2010;27(4):205–208. doi: 10.4103/0970-2113.71944. doi: 10.4103/0970-2113.71944 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedighi M, Pourpak Z, Bavarian B, Safaralizadeh R, Zare A, Moin M. Low magnesium concentration in erythrocytes of children with acute asthma. Iran J Allergy Asthma Immunol. 2006;5(4):183–186. [PubMed] [Google Scholar]
- 8.Kakish KS. Serum Magnesium level in asthmatic children during and between exacerbations. Arch Paediatr Adolesc Med. 2001;155(2):181–183. doi: 10.1001/archpedi.155.2.181. doi: 10.1001/archpedi.155.2.181 . [DOI] [PubMed] [Google Scholar]
- 9.Falkner D, Glauser J, Allen M. Serum magnesium levels in asthmatic patients during acute exacerbations of asthma. Am J Emerg Med. 1992;10(1):1–3. doi: 10.1016/0735-6757(92)90114-d. doi: 10.1016/0735-6757(92)90114-D . [DOI] [PubMed] [Google Scholar]
- 10.Dominguez LJ, Barbagallo M, Di Lorenzo G, Drago A, Scola S, Morici G, Caruso C. Bronchial reactivity and intracellular magnesium. Clin Sci (Lond) 1998;95(2):137–142. [PubMed] [Google Scholar]
- 11.Emelyanov A, Fedoseev G, Barnes PJ. Reduced intracellular magnesium concentrations in asthmatic patients. Eur Respir J. 1999;13(1):38–40. doi: 10.1183/09031936.99.13103899. doi: 10.1183/09031936.99.13103899 . [DOI] [PubMed] [Google Scholar]
- 12.Zervas E, Loukides S, Papatheodorou G, Psathakis K, Tsindiris K, Panagou P, Kalogeropoulos N. Magnesium levels in plasma and erythrocyte before and after histamine challenge. Eur Respir J. 2000;16(4):621–625. doi: 10.1034/j.1399-3003.2000.16d09.x. doi: 10.1034/j.1399-3003.2000.16d09.x . [DOI] [PubMed] [Google Scholar]
- 13.Zervas E, Papatheodorou G, Psathakis K, Panagou P, Georgatou N, Loukides S. Reduced intracellular Mg concentrations in patients with acute asthma. Chest. 2003;123(1):113–118. doi: 10.1378/chest.123.1.113. doi: 10.1378/chest.123.1.113 . [DOI] [PubMed] [Google Scholar]
- 14.de Valk HW, Kok PT, Struyvenberg A, van Riji HJ, Haaloom JR, Kreukniet J, Lammers JW. Extracellular and intracellular magnesium concentrations in asthmatic patients. Euro Resp J. 1993;6(8):1122–1125. [PubMed] [Google Scholar]
- 15.Fantidis P, Ruiz CJ, Marin M, Madero JR, Solera J, Herrero E. Intracellular (polymorphonuclear) magnesium content in patients with bronchial asthma between attacks. J R Soc Med. 1995;88:441–445. doi: 10.1177/014107689508800806 . [PMC free article] [PubMed] [Google Scholar]
- 16.Alamoudi OS. Electrolyte disturbances in patients with chronic, stable asthma: effect of therapy. Chest. 2001;120(2):431–436. doi: 10.1378/chest.120.2.431. doi: 10.1378/chest.120. 2.431 . [DOI] [PubMed] [Google Scholar]
- 17.Britton J, Pavord I, Richards K, Wisniewski A, Knox A, Lewis S, et al. Dietary magnesium, lung function, wheezing, and airway hyper-reactivity in a random adult population sample. Lancet. 1994;344(8919):357–362. doi: 10.1016/s0140-6736(94)91399-4. doi: 10.1016/S0140-6736(94)91399-4 . [DOI] [PubMed] [Google Scholar]
- 18.Gontijo-Amaral C, Ribeiro MA, Gontijo LS, Condino-Neto A, Ribeiro JD. Oral magnesium supplementation in asthmatic children: a double-blind randomized placebo-controlled trial. Eur J Clin Nutr. 2007;61:54–60. doi: 10.1038/sj.ejcn.1602475. doi: 10.1038/sj.ejcn.1602475 . [DOI] [PubMed] [Google Scholar]
- 19.Kazaks AG, Uriu-Adams JY, Albertson TE, Shenoy SF, Stern JS. Effect of oral magnesium supplementation on measures of airway resistance and subjective assessment of asthma control and quality of life in men and women with mild to moderate asthma. J Asthma. 2010;47(1):83–92. doi: 10.3109/02770900903331127. doi: 10.3109/02770900903331127 . [DOI] [PubMed] [Google Scholar]
- 20.Ciarallo L, Brousseau D, Reinert S. Higher-dose intravenous magnesium therapy for children with moderate to severe acute asthma. Arch Pediatr Adolesc Med. 2000;154(10):979–983. doi: 10.1001/archpedi.154.10.979. doi: 10.1001/archpedi.154.10.979 . [DOI] [PubMed] [Google Scholar]
- 21.Bloch H, Silverman R, Mancherje N, Grant S, Jagminas L, Scharf SM. Intravenous magnesium sulfate as an adjunct in the treatment of acute asthma. Chest. 1995;107(6):1576–1581. doi: 10.1378/chest.107.6.1576. doi: 10.1378/chest.107.6.1576 . [DOI] [PubMed] [Google Scholar]
- 22.Ciarallo L, Sauer AH, Shanon MW. Intravenous magnesium therapy for moderate to severe paediatric asthma. J Pediatr. 1996;129(6):809–814. doi: 10.1016/s0022-3476(96)70023-9. doi: 10.1016/S0022-3476(96)70023-9 . [DOI] [PubMed] [Google Scholar]
- 23.Divi PR, Kumar L, Singhi SC, Prasad R, Singh M. Intravenous magnesium sulphate in acute severe asthma not responding to conventional therapy. Indian Pediatr. 1997;34(5):389–397. [PubMed] [Google Scholar]
- 24.Gurkan F, Haspolat K, Bosnak M, Dikici B, Derman O, Ece A. Intravenous magnesium sulphate in the management of moderate to severe acute asthmatic children non-responding to conventional therapy. Eur J Emreg Med. 1996;6(3):201–205. doi: 10.1097/00063110-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Scarfone RJ, Loiselle JM, Joffe MD, Mull CC, Stiller S, Thompson K, et al. A randomized trial of magnesium in the emergency department treatment of children with asthma. Ann Emerg Med. 2000;36(6):572–578. doi: 10.1067/mem.2000.111060. doi: 10.1067/mem.2000.111060 . [DOI] [PubMed] [Google Scholar]
- 26.Bradshaw TA, Matusiewicz SP, Crompton GK, Innes JA, Greening AP. Intravenous magnesium sulphate provides no additive benefit to standard management in acute asthma. Respir Med. 2008;102(1):143–149. doi: 10.1016/j.rmed.2007.07.022. doi: 10.1016/j.rmed.2007.07.022 . [DOI] [PubMed] [Google Scholar]
- 27.Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Blitz S, Camargo CA. Magnesium sulfate for treating exacerbations of acute asthma in the emergency department. Cochrane Database Syst Rev. 2000;1:CD0011490. doi: 10.1002/14651858.CD001490. doi: 10.1002/14651858.CD0011490 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheuk DK, Chau TC, Lee SL. A meta-analysis on intravenous magnesium sulphate for treatingacuteasthma. Arch Dis Child. 2005;90(1):74–77. doi: 10.1136/adc.2004.050005. doi: 10.1136/adc.2004.050005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saris NE, Mervaala E, Karppanen H, Khawaja JA, Lewenstam A. Magnesium. An update on physiological, clinical and analytical aspects. Clin Chim Acta. 2000;294(1–2):1–26. doi: 10.1016/s0009-8981(99)00258-2. doi: 10.1016/S0009-8981(99)00258-2 . [DOI] [PubMed] [Google Scholar]
- 30.Global Initiative For Asthma (GINA) United States (US): The Global Initiatove for Asthma; 2013. Global Strategy for Asthma Management and Prevention Updated 2013. [Google Scholar]
- 31.Eid N, Yandell B, Howell L, Eddy M, Sheikh S. Can peak expiratory flow predict airflow obstruction in children with asthma. Pediatrics. 2000;105(2):354–358 . doi: 10.1542/peds.105.2.354. doi: 10.1542/peds.105.2.354 . [DOI] [PubMed] [Google Scholar]
- 32.Azizi O, Henry RL. Peak expiratory rate (PEFR) of Malaysian children. Med J Malaysia. 1991;46(1):82–87. [PubMed] [Google Scholar]
- 33.Wang JL, Shaw NS, Kao MD. Magnesium deficiency and its lack of association with asthma in Taiwanese elementary school children. Asia Pac J Clin Nutr. 2007;16(Suppl 2):579–584. [PubMed] [Google Scholar]
- 34.Mircetic RN, Dodig S, Raos M, Petres B, Cepelak I. Magnesium concentration in plasma, leukocytes and urine of children with intermittent asthma. Clin Chim Acta. 2001;312(1–2):197–203. doi: 10.1016/s0009-8981(01)00622-2. doi: 10.1016/S0009-8981(01)00622-2 . [DOI] [PubMed] [Google Scholar]
- 35.Barbagallo M, Dominguez LJ, Putignano E, Barbagallo-Sangiorgi G, Resnick LM. Effect of aging on intracellular divalent cation metabolism: a link to the increased incidence of hypertension and non-insulin dependent diabetes mellitus in the elderly? Arch Gerontol Geriatr. 1996;22(Suppl 1):233–238. doi: 10.1016/0167-4943(96)86943-3. doi: 10.1016/0167-4943(96)86943-3 . [DOI] [PubMed] [Google Scholar]
- 36.Barnes PJ. Reactive oxygen species and airway inflammation. Free Radic Biol Med. 1990;9(3):235–243. doi: 10.1016/0891-5849(90)90034-g. doi: 10.1016/0891-5849(90)90034-G . [DOI] [PubMed] [Google Scholar]
- 37.Gunther T, Vormann J, Forster RM. Effect of oxygen free radicals on Mg2+ efflux from erythrocytes. Eur J Clin Chem Biochem. 1994;32(4):273–277. doi: 10.1515/cclm.1994.32.4.273. doi: org/10.1515/cclm.1994.32.4.273 . [DOI] [PubMed] [Google Scholar]


