Abstract
Background and purpose
Cerebral aneurysm (CA) affects 3% of the population and is associated with hemodynamic stress and inflammation. Myeloperoxidase (MPO), a major oxidative enzyme associated with inflammation, is increased in CA patients, but whether MPO contributes to CA is not known. We tested the hypotheses that MPO is increased within human CA and is critical for formation and rupture of CA in mice.
Methods
Blood was drawn from the lumen of CAs and femoral arteries of 25 patients who underwent endovascular coiling of CA, and plasma MPO concentrations were measured with ELISA. Effects of endogenous MPO on CA formation and rupture were studied in MPO knockout (KO) mice and wild-type (WT) mice using an angiotensin II-elastase induction model of CA. In addition, effects of MPO on inflammatory gene expression in endothelial cells were analyzed.
Results
Plasma concentrations of MPO were 2.7-fold higher within CA than in femoral arterial blood in CA patients. MPO-positive cells were increased in aneurysm tissue compared with superficial temporal artery of CA patients. Incidence of aneurysms and subarachnoid hemorrhage was significantly lower in MPO KO than in WT mice. In cerebral arteries, proinflammatory molecules including TNFα, COX2, CXCL1, MMP8, CD68 and MMP13, and leukocytes were increased, and α-smooth muscle actin was decreased, in WT but not in MPO KO mice after induction of CA. MPO per se increased expression of VCAM1 and ICAM1 in endothelial cells.
Conclusions
These findings suggest that MPO may contribute importantly to formation and rupture of CA.
Keywords: cerebral aneurysm, MPO, inflammation, neutrophil, VCAM1, ICAM1, SAH
INTRODUCTION
Cerebral aneurysm (CA), typically with enlargement at an arterial bifurcation, affects 3% of the population.1 Subarachnoid hemorrhage, following rupture of CA, is a major cause of death or disability in these patients.1 The etiology of CA involves hemodynamic stress and inflammation, with similarities and important differences to abdominal aortic aneurysms (AAA).1–4 Treatment for both unruptured and ruptured CA is surgical, with coiling and clipping to prevent rupture and re-rupture; there is no pharmacological treatment.5,6
Myeloperoxidase (MPO) belongs to the heme peroxidase family, which includes vascular peroxidase 1 (VPO1), a plasma peroxidase produced by endothelial cells.7 MPO is a major oxidative enzyme produced by activated neutrophils, monocytes, and macrophages.8–10 MPO is increased in blood of CA patients during SAH and vasospasm.11 It is not known, however, whether MPO is increased locally within CA. It is also not known whether MPO contributes to CA formation and rupture, although a study suggests that MPO presence in human CA tissue is positively correlated with 5-year aneurysm rupture risk.12
In the present study, we hypothesized that MPO is increased locally in the CA sac and endogenous MPO may contribute to formation and rupture of aneurysms in a mouse model.
METHODS
Plasma levels of MPO in patients
The study was performed using a protocol approved by the University of Iowa Institutional Review Board. Blood was drawn from the lumen of CA and femoral artery from 25 patients who underwent endovascular coiling of CA. Findings in a sub-cohort (13 with unruptured and 4 with ruptured CA) were described previously.13 The present study added one patient with a ruptured aneurysm and 7 patients with unruptured aneurysms. Plasma MPO was measured by ELISA (Abcam, ab119605), and values are reported as the average of 2 measurements using 2 ELISA kits. As a control for MPO, plasma concentrations of vascular peroxidase 1 (VPO1), a homolog of MPO that is a plasma peroxidase secreted by endothelial cells,7 were measured with immunoblotting against a panel of known standards.
Analyses of human tissue samples
Tissues were collected from CA and superficial temporal artery (STA) from 12 patients who underwent microsurgical clipping of CA. Samples were fixed in paraformaldehyde, and embedded in paraffin. Following antigen retrieval (by microwaving in pH6.0 citrate buffer), sections were incubated with anti-MPO antibody (ab45977, Abcam), followed by HRP-conjugated reagent (Boost, #8114, Cell Signaling) with DAB reaction (Vector Labs). Slides were stained with hematoxylin, dehydrated, and permanently mounted. Images were taken at 200x magnification with an Olympus BX61 microscope.
Mouse model of intracranial aneurysm
The angiotensin II-elastase mouse model of cerebral aneurysm was used as previously described.14,15 Twelve each of MPO KO (C57Bl/6J genetic background) and control C57Bl/6J mice (Jackson Laboratories), 17–18 weeks of age, were implanted with an osmotic mini-pump for continuous delivery of angiotensin II (1000 ng/kg/min), and injected with 35 mU elastase (Sigma) into the right basal cistern. Systolic arterial pressure was monitored by tail cuff, and neurological function was assessed daily. Mice were considered symptomatic and sacrificed if one or more of the following deficits were observed: decreased activity and hunched posture, leaning or circling to one side, or decreased food and water intake leading to weight loss of >20% of baseline. Mice without neurological signs were sacrificed 17–19 days post CA induction, and the presence of CA and subarachnoid hemorrhage (SAH) was assessed.
Gene expression in mouse cerebral arteries
Cerebral arteries (anterior and posterior cerebral arteries, anterior and posterior communicating arteries, middle cerebral arteries, and basilar arteries) were isolated and dissolved in TriZol (Life Technologies). Untreated mice (6 male WT and 6 male MPO KO) were used as baseline controls. qRT-PCR was used to quantify mRNA levels as described previously,16 with both primers for the gene of interest (FAM fluoror, Life Technologies or IDT) and the house-keeping β-actin (VIC fluoror, Life Technologies, for normalization) in the same reaction.17 All TaqMan primers/probes used are listed in Table I in the online-only Data Supplement.
Mouse brain histology and immunohistochemistry for MPO
Additional WT and MPO KO mice (n=3 and 4 respectively) underwent CA induction as described above. Brains were fixed in paraformaldehyde, paraffin-embedded, and 10 μm-thick sections were cut. Sections were stained with hematoxylin and eosin (H&E). Leukocytes in cerebral arteries of comparable sizes were counted. Immunohistochemistry for MPO was performed as described for human tissues except that a different anti-MPO antibody was used for detecting mouse MPO (ab9535, Abcam).
Effects of MPO on gene expression in endothelial cells
Primary human aortic endothelial cells were purchased from Lonza (CC-2535) and cultured according to instructions by Lonza (CC-3162). Passage 4 cells were incubated with human MPO (30 nM) (Planta Natural Products) in the absence or presence of MPO inhibitor ABAH (4-amino-benzoic acid hydrazide, 50 μM, Sigma) for 6 hours. qRT-PCR was performed as described above. Two independent experiments were performed with n of 4 per group.
Statistical analyses
All data are expressed as mean±SE. Student’s t test was used for comparisons in the patient study. Fisher’s exact test was used for incidence of aneurysms and subarachnoid hemorrhage in mice. ANOVA with Student-Newman-Keuls multiple comparisons post-test was used for gene expression. A p<0.05 was considered significantly different.
RESULTS
Plasma MPO in patients with CA
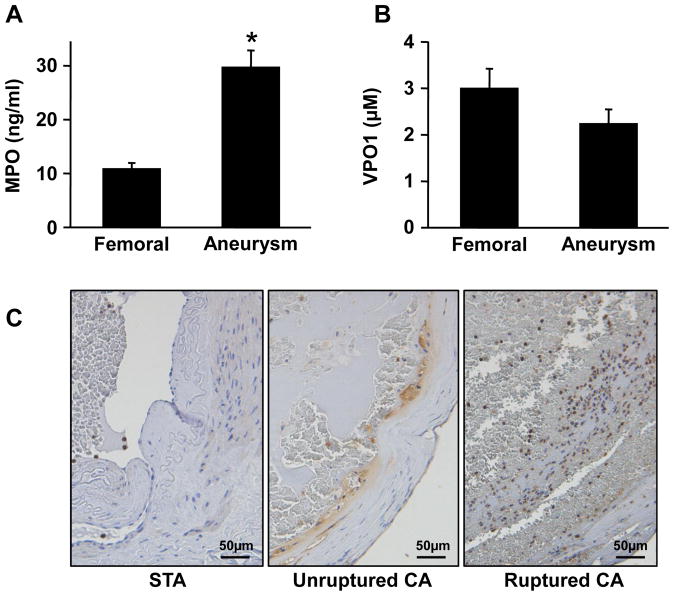
MPO concentrations were 2.7-fold higher in plasma from the CA sac (30±3 ng/ml) (mean±SE) than from femoral artery (11±1); p<0.001) (Figure 1A). Among the 25 patients, 5 patients with ruptured aneurysms demonstrated similar values of femoral (11±3) vs. aneurysm (35±10, p<0.05) concentrations of MPO. No difference was found in 5 male (femoral 15±5; aneurysm 41±9; p<0.05) vs. 20 female (femoral 15±2; aneurysm 33±4; p<0.05) patients. MPO concentrations of 7 healthy volunteers in blood drawn from an arm vein were 7.6±0.6 ng/ml. In contrast, concentrations of VPO1, a homolog of MPO that is secreted to plasma by endothelial cells,7 were not different in blood from CA (2.3±0.3 μM) and femoral arteries of the patients (3.0±0.4; p=0.12) (Figure 1B). These findings demonstrate for the first time that the local concentration of MPO within the CA sac was elevated in comparison to circulating concentrations.
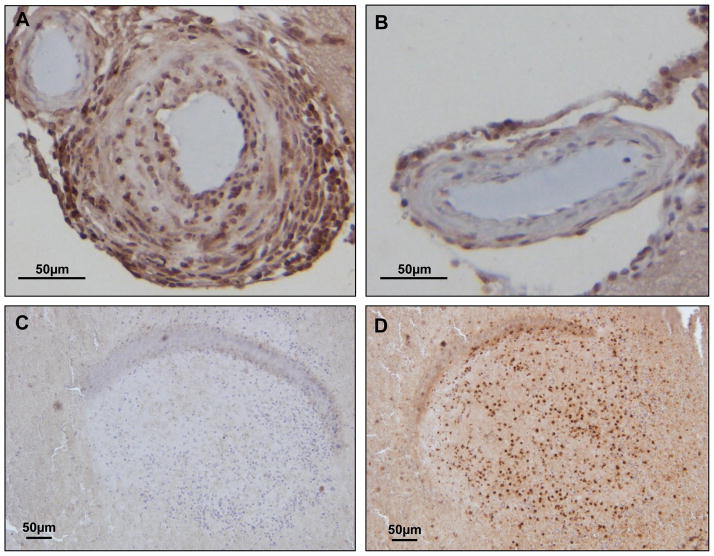
Figure 1.
Plasma concentrations of MPO (A) and VPO1 (B) in femoral artery and cerebral aneurysms of 25 patients with cerebral aneurysms. Patients ranged from 25 to 76 years of age (55±3). Values are mean ± SE, * = p<0.05 vs. femoral artery blood. C. Representative images of immunohistochemistry for MPO in superficial temporal artery (STA), unruptured and ruptured cerebral aneurysm tissue. MPO positive cells were stained brown, with negative cells blue by hematoxylin counterstaining. Scale bars indicate 50 μm.
Tissue from ruptured CA appeared to have more MPO-positive cells than unruptured CA, whereas superficial temporal arteries had no MPO-positive cells except in the area containing blood (Figure 1C). These findings suggest that the presence of more MPO-positive cells in human cerebral aneurysms could produce the local increase of MPO in the CA sac.
Endogenous MPO contributes to formation and rupture of CA in mice induced by angiotensin II and elastase
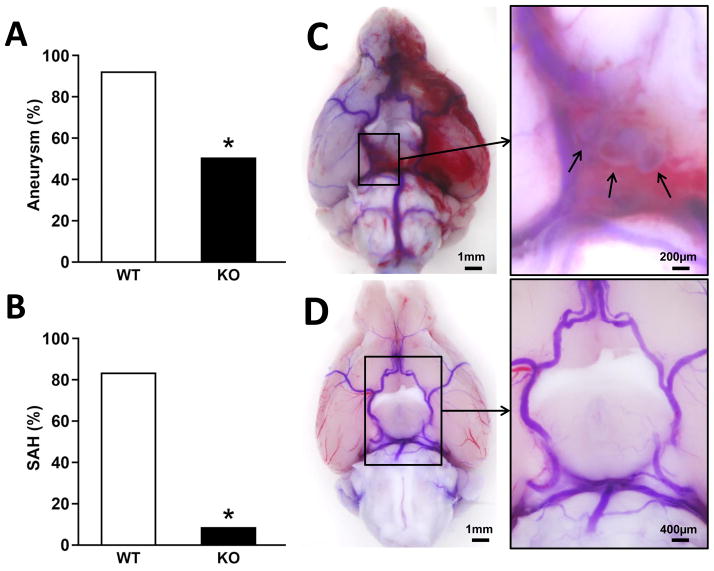
The incidence of CA was lower in MPO KO mice (50%) than in WT control (90%, p<0.05) (Figure 2A). The occurrence of subarachnoid hemorrhage also was lower in MPO KO mice (8%) than in WT control (83%, p<0.001) (Figure 2B). Systolic blood pressure increased to 140–150 mmHg from a baseline of 117 (WT) or 120 (KO) mmHg, with no difference between WT and MPO KO mice (see Figure I in the online-only Data Supplement). Mortality during the course of study was not different between WT and MPO KO mice, with 1 death among 12 mice in each group. Consequently, comparisons in gene expression analyses were not subject to influences from different survival rates between the two groups. These findings indicate that the formation and rupture of cerebral aneurysms in this experimental model of CA were attenuated in the absence of MPO.
Figure 2.
Effects of MPO deficiency on cerebral aneurysms. Incidence of aneurysms (A) and occurrence of subarachnoid hemorrhage (SAH) (B) after 17–19 days of CA induction, n=12 for each type of mice; * = p<0.05 vs. WT. Representative images show several saccular cerebral aneurysms (arrows) in the right posterior communicating artery, with dilated arteries and SAH on the left side in a WT mouse (C); and no aneurysm or hemorrhage in an MPO KO mouse (D). Scale bars are 1 mm (C) and 200 μm (enlargement) for WT, and 1 mm (D) and 400 μm (enlargement), respectively.
Gene expression in cerebral arteries of WT vs. MPO KO mice
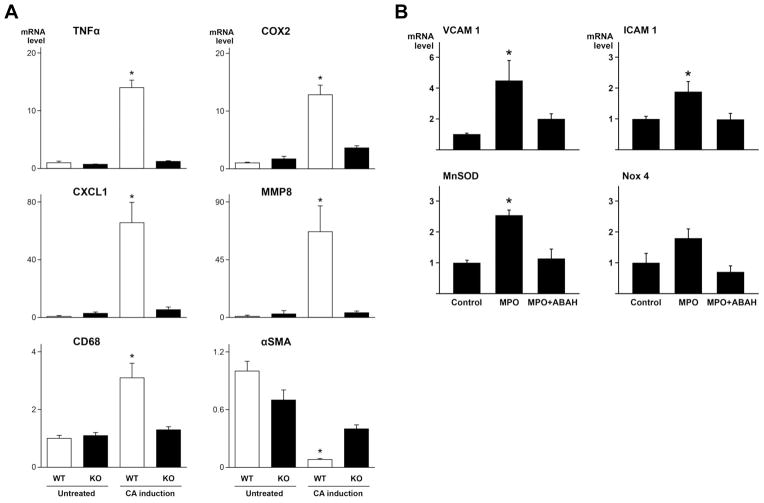
Induction of CA increased expression of the proinflammatory agents TNFα and COX2 in WT mice but not in MPO KO mice (Figure 3A). Likewise, induction of CA increased expression of CXCL1 and MMP8, mediators important for neutrophil recruitment and function, more significantly in WT, but not in MPO KO mice (Figure 3A). Furthermore, induction of CA increased expression of CD68, a macrophage marker, in WT but not in MPO KO mice (Figure 3A). In contrast, α-smooth muscle actin, a marker for vascular smooth muscle cells, was reduced in WT mice after induction of CA (Figure 3A). MPO deficiency attenuated reduction of α-smooth muscle actin in response to CA induction (Figure 3A).
Figure 3.
Gene expression of proinflammatory molecules in mouse cerebral arteries and human endothelial cells. A. Cerebral arteries were collected from untreated WT and MPO KO mice (n=6 respectively), and CA-induced WT (n=11) and MPO KO (n=10) mice after 17–19 days of CA induction. * = p<0.05 vs. the other 3 groups (p>0.05 between the 3 groups). B. Gene expression in human aortic endothelial cells 6 hours after incubation with MPO (30 nM) in the absence or presence of MPO inhibitor ABAH (50 μM). *=p<0.05 vs. the other 2 groups (p>0.05 between the 2 groups). mRNA levels were quantified using TaqMan ΔΔCt method, with normalization to β-actin in the same reaction.
Effects of MPO on gene expression in endothelial cells
To investigate whether MPO exerts effects in the absence of MPO-producing cells, human aortic endothelial cells were incubated with human MPO (30 nM) in the absence or presence of its inhibitor ABAH (4-amino-benzoic acid hydrazide) for 6 hours. Among >20 genes measured with qRT-PCR, adhesion molecules VCAM1 and ICAM1 and oxidative stress marker SOD2 (MnSOD) were increased significantly by MPO (p<0.05) (Figure 3B). The increase was eliminated by ABAH, suggesting that the catalytic activity of MPO was required for the effects (Figure 3B). Interestingly, increased SOD2 and NOX4 (with a trend, p=0.05) may increase hydrogen peroxide, a substrate for MPO enzyme.
Leukocytes in cerebral arteries
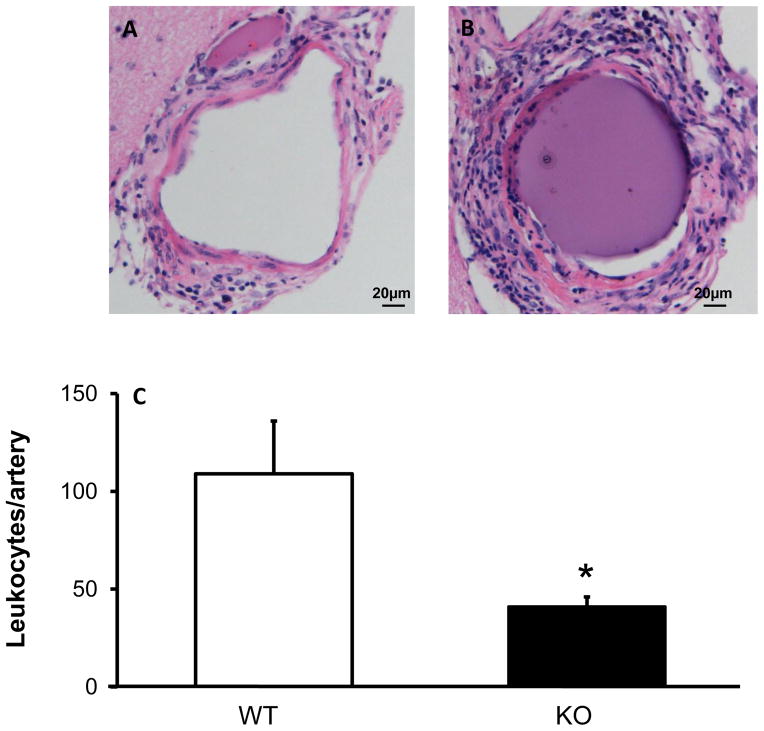
The number of leukocytes (based on morphology) in cerebral arteries of comparable sizes was decreased in MPO KO mice compared to that in WT mice, after induction of CA (Figure 4). MPO-positive cells appeared fewer in MPO KO than WT mice (Figure 5). These findings are in concordance with gene expression data which suggested decreased recruitment of neutrophils and perhaps macrophages in cerebral arteries of MPO KO mice.
Figure 4.
There are fewer leukocytes (H&E staining) in cerebral arteries in MPO KO mice than WT mice. Representative images of WT (A) and MPO KO (B) cerebral arteries stained with H&E, with quantification of cell numbers per artery (C). n=3–4 mice in each group. * = p<0.05 vs. WT.
Figure 5.
Immunohistochemistry for MPO in WT (A) and MPO KO (B) cerebral artery after CA induction. An isolated aneurysm containing blood inside the sac was used for negative control (without anti-MPO antibody, C) and positive control (D) for MPO.
DISCUSSION
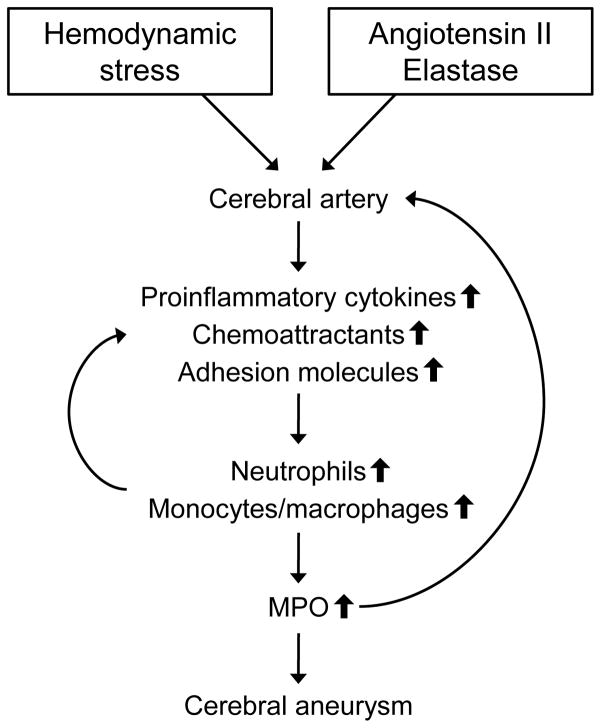
To our knowledge, the present study is the first to demonstrate localized increase of MPO concentration in the CA sac of patients, which may be produced by MPO-positive cells (neutrophils and macrophages) in CA. The localized MPO may contribute to formation, progression and rupture of CA in at least four ways. First, MPO may attract neutrophils, and perhaps other leukocytes, to endothelium of cerebral arteries, with negative charges on their surfaces, by physical forces due to strong positive charges on the surface of MPO.18 Our finding of an increase in leukocytes in aneurysm tissue of patients as well as cerebral arteries of mice is consistent with this mechanism. Second, MPO may enter into endothelial cells through direct cell-cell contact from neutrophils to cause impairment of endothelial function,19 which is a common characteristic in an early stage of CA as well as AAA.20 Third, MPO may transcytose through endothelium into the arterial media to catalyze oxidation reactions in the extracellular matrix,21 thereby reducing tensile strength of the wall of cerebral arteries. Fourth, MPO per se may induce inflammation by increasing expression of adhesion molecules in endothelial cells, promoting leukocyte adherence and inflammation. See Figure 6 for a summary of our findings and interpretations.
Figure 6.
Summary of the findings and interpretations in this study.
MPO deficiency may protect against CA through profound reduction in expression of TNFα in cerebral arteries of MPO KO compared to WT mice, in response to induction of CA. In cerebral arteries, TNFα may recruit leukocytes and increase inflammatory cytokines and chemokines, injure endothelial cells, produce phenotypic transformation of smooth muscle cells, and upregulate MMPs that deteriorate integrity of cerebral arteries.22,23
CXCL1 is an important chemoattractant that recruits neutrophils to endothelium at the site of inflammation.24–26 A greater than 10-fold reduction of CXCL1 in cerebral arteries of MPO KO mice compared to WT mice after induction of CA was associated with a 17-fold decrease of MMP8 (collagenase-2), an MMP expressed specifically in neutrophils.27 Thus, in addition to a reduction in recruited leukocytes, deficiency of MPO may inhibit cellular production of proinflammatory molecules.
Both leukocytes and vascular cells express matrix metalloproteinases.28 MMP9 has been linked to CA, using MMP9 KO mice.29 In the present study, we found not only a 4-fold decrease in gene expression of MMP9, but also a significant reduction of both MMP3 (3-fold) and MMP13 (18-fold) (see Figure II in the online-only Data Supplement), which are strong activators of MMP9.29,30 Thus, we speculate that there may be a significant decrease in MMP9 activity. The role of MMP3 and MMP13 has not been studied in CA. Thus, findings in the present study provide a rationale for future studies of the role of MMP3 and MMP13 in CA.
Smooth muscle differentiation molecules, including α-smooth muscle actin, are decreased in cerebral smooth muscle cells in response to TNFα in culture,30 in aorta of abdominal aortic aneurysms in mice,31 and after other vascular injuries.32 Thus, our finding of preserved expression of α-smooth muscle actin (also positive in myofibroblast) in MPO KO mice after induction of CA suggests that MPO deficiency attenuates formation and rupture of CA via a mechanism that involves protection of smooth muscle cells from injury.
A limitation in the present study is that mechanistic findings to explain attenuation of cerebral aneurysm by MPO deficiency are based on quantification of mRNA levels, not by protein or activity levels. This limitation resulted from very limited quantities of cerebral arteries in mice. On the other hand, qRT-PCR produces the most accurate quantification with limited material.
In summary, in patients with cerebral aneurysms, circulating MPO concentrations are increased locally in the CA sac, which is associated with increased neutrophils and other MPO-positive cells in aneurysm tissues. In a mouse model of CA, MPO deficiency reduced expression in cerebral arteries of proinflammatory molecules, preserved expression of α-smooth muscle actin, decreased the number of leukocytes, and attenuated formation and rupture of cerebral aneurysms. MPO itself increased expression of adhesion molecules in endothelial cells. These findings suggest that MPO, which is increased locally in the CA sac of patients, contributes importantly to the pathophysiology of CA in a mouse model. Because MPO deficiency is common in human population,33,34 it would be of interest to examine the incidence of cerebral aneurysms in patients with MPO deficiency.
Supplementary Material
Acknowledgments
We thank Dr. William M. Nauseef for helpful discussions and suggestions. We thank Ms. Teresa Ruggle for assistance in preparation of the figures, and the Central Microscopy Core Facility for use of equipments.
SOURCES OF FUNDING: NIH grants NS082363 and HL62984. American Association of Neurological Surgeons-Neurosurgery Research and Education Foundation.
Footnotes
DISCLOSURES: None.
References
- 1.Etminan N, Buchholz BA, Dreier R, Bruckner P, Torner JC, Steiger HJ, et al. Cerebral aneurysms: formation, progression, and developmental chronology. Transl Stroke Res. 2014;5:167–173. doi: 10.1007/s12975-013-0294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. 2013;44:3613–3622. doi: 10.1161/STROKEAHA.113.002390. [DOI] [PubMed] [Google Scholar]
- 3.Turjman AS, Turjman F, Edelman ER. Role of fluid dynamics and inflammation in intracranial aneurysm formation. Circulation. 2014;129:373–382. doi: 10.1161/CIRCULATIONAHA.113.001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van ‘t Hof FNG, Ruigrok YM, Baas AF, Kiemeney ALM, Vermeulen SH, Uitterlinden AG, et al. Impact of inherited genetic variants associated with lipid profile, hypertension, and coronary disease on the risk of intracranial and abdominal aortic aneurysms. Circ Cardiovasc Genet. 2013;6:264–270. doi: 10.1161/CIRCGENETICS.113.000022. [DOI] [PubMed] [Google Scholar]
- 5.Brown RD, Jr, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014;13:393–404. doi: 10.1016/S1474-4422(14)70015-8. [DOI] [PubMed] [Google Scholar]
- 6.Shivashankar R, Miller TR, Jindal G, Simard JM, Aldrich EF, Gandhi D. Treatment of cerebral aneurysms – surgical clipping or endovascular coiling: the guiding principles. Semin Neurol. 2013;33:476–487. doi: 10.1055/s-0033-1364217. [DOI] [PubMed] [Google Scholar]
- 7.Cheng G, Salerno JC, Cao Z, Pagano PJ, Lambeth JD. Identification and characterization of VPO1, a new animal heme-containing peroxidase. Free Radic Biol Med. 2008;45:1682–1694. doi: 10.1016/j.freeradbiomed.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukocyte Biol. 2013;93:185–198. doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bos A, Wever R, Dirk R. Characterization and quantification of the peroxidase in human monocytes. Biochim Biophys Acta. 1978;525:37–44. doi: 10.1016/0005-2744(78)90197-3. [DOI] [PubMed] [Google Scholar]
- 10.Daugherty A, Duun JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim M, Bower RS, Wang Y, Sims L, Bower MR, Camara-Quintana J, et al. The predictive value of serum myeloperoxidase for vasospasm in patients with aneurismal subarachnoid hemorrhage. Neurosurg Rev. 2012;35:413–419. doi: 10.1007/s10143-012-0375-4. [DOI] [PubMed] [Google Scholar]
- 12.Gounis MJ, Vedantham S, Weaver JP, Puri AS, Brooks CS, Wakhloo AK, et al. Myeloperoxidase in human intracranial aneurysms: preliminary evidence. Stroke. 2014;45:1474–1477. doi: 10.1161/STROKEAHA.114.004956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chalouhi N, Points L, Pierce GL, Ballas Z, Jabbour P, Hasan D. Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke. 2013;44:2594–2597. doi: 10.1161/STROKEAHA.113.002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344. doi: 10.1161/HYPERTENSIONAHA.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pena Silva RA, Kung DK, Mitchell IJ, Alenina N, Bader M, Santos RA, et al. Angiotensin 1–7 reduces mortality and rupture of intracranial aneurysms in Mice. Hypertension. 2014;64:362–368. doi: 10.1161/HYPERTENSIONAHA.114.03415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu Y, Heistad DD, Knudtson KL, Lamping KG, Faraci FM. Quantification of mRNA for endothelial NO synthase in mouse blood vessels by real-time polymerase chain reaction. Arterioscler Thromb Vasc Biol. 2002;22:611–616. doi: 10.1161/01.atv.0000012663.85364.fa. [DOI] [PubMed] [Google Scholar]
- 17.Chu Y, Lund DD, Weiss RM, Brooks RM, Doshi H, Hajj GP, et al. Pioglitazone attenuates valvular calcification induced by hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2013;33:523–532. doi: 10.1161/ATVBAHA.112.300794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klinke A, Nussbaum C, Kubala L, Friedrichs K, Rudolph TK, Rudolph V, et al. Myeloperoxidase attracts neutrophils by physical forces. Blood. 2011;117:1350–1358. doi: 10.1182/blood-2010-05-284513. [DOI] [PubMed] [Google Scholar]
- 19.Jerke U, Rolle S, Purfürst B, Luft FC, Nauseef WM, Kettritz R. β2 integrin-mediated cell-cell contact transfers active myeloperoxidase from neutrophils to endothelial cell. J Biol Chem. 2013;288:12910–12919. doi: 10.1074/jbc.M112.434613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:1–106. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldus S, Eiserich JP, Mani A, Castro L, Figueroa M, Chumley P, et al. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starke RM, Raper DM, Ding D, Chalouhi N, Owens GK, Hasan DM, et al. Tumor necrosis factor-α modulates cerebral aneurysm formation and rupture. Transl Stroke Res. 2014;5:269–277. doi: 10.1007/s12975-013-0287-9. [DOI] [PubMed] [Google Scholar]
- 23.Ali MS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. TNF-α induces phenotypic modulation in cerebral vascular smooth muscle cells: implications for cerebral aneurysm pathology. J Cereb Blood Flow Metab. 2013;33:1564–1573. doi: 10.1038/jcbfm.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao L, Yago T, Shao B, Liu Z, Silasi-Mansat R, Setiadi H, et al. Elevated CXCL1 expression in gp130-deficient endothelial cells impairs neutrophil migration in mice. Blood. 2013;122:3832–3842. doi: 10.1182/blood-2012-12-473835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, et al. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood. 2013;121:4930–4937. doi: 10.1182/blood-2013-02-486217. [DOI] [PubMed] [Google Scholar]
- 26.Nencioni A, da Silva RF, Fraga-Silva RA, Steffens S, Fabre M, Bauer I, et al. Nicotinamide phosphoribosyltransferase inhibition reduces intraplaque CXCL1 production and associated neutrophil infiltration in atherosclerotic mice. Thromb Haemost. 2014;111:308–322. doi: 10.1160/TH13-07-0531. [DOI] [PubMed] [Google Scholar]
- 27.Balbín M, Fueyo A, Tester AM, Pendás AM, Pitiot AS, Astudillo A, et al. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- 28.Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit Rev Biochem Mol Biol. 2013;48:222–272. doi: 10.3109/10409238.2013.770819. [DOI] [PubMed] [Google Scholar]
- 29.Nuki Y, Tsou TL, Kurihara C, Kanematsu M, Kanematsu Y, Hashimoto T. Elastase-induced intracranial aneurysms in hypertensive mice. Hypertension. 2009;54:1337–1344. doi: 10.1161/HYPERTENSIONAHA.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vempati P, Karagiannis ED, Popel AS. A biochemical model of matrix metalloproteinase 9 activation and inhibition. J Biol Chem. 2007;282:37585–37596. doi: 10.1074/jbc.M611500200. [DOI] [PubMed] [Google Scholar]
- 31.Salmon M, Johnston WF, Woo A, Pope NH, Su G, Upchurch GR, et al. KLF4 regulates abdominal aortic aneurysm morphology and deletion attenuates aneurysm formation. Circulation. 2013;128:S163–S174. doi: 10.1161/CIRCULATIONAHA.112.000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 33.Nauseef WM, Root RK, Malech HL. Biochemical and immunologic analysis of hereditary myeloperoxidase deficiency. J Clin Invest. 1983;71:1297–1307. doi: 10.1172/JCI110880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dinauer MC, Nauseef WM, Newburger PE. Inherited disorders of phagocyte killing. In: Scriver CR, Vogelstein B, Kinzler KW, Childs B, Valle D, editors. The metabolic and molecular bases of inherited diseases. 8. New York, NY: McGraw-Hill; 2001. pp. 4857–4887. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.