Abstract
Outbreaks of measles have been reported over the past 5 years, particularly affecting children between the ages of 1 and 5 years. Most of these children are younger than the age recommended by the Advisory Committee on Immunization Practices for the second dose of measles-mumps-rubella (MMR) vaccine. Question may arise as to whether strict adherence to the scheduled second dose is required or whether there is opportunity for earlier immunization under special circumstances (e.g., traveling abroad, poor response as evidenced by titer levels). The history of measles, its characteristics, and its evolving past and current immunization policies will be reviewed, focusing on the original intent of the recommended schedule and presenting a case in which deviating from current practice could be justified.
INDEX TERMS: immunization, measles-mumps-rubella vaccine, vaccination
THERAPEUTIC DILEMMA
A concerned mother posed the following question to her pharmacist. She had recently taken her 15-month-old son to his pediatrician for his well-child check-up. He had received his first dose of measles-mumps-rubella (MMR) vaccine at 12 months of age. The physician had discussed with the mother the possibility of offering her son his second dose at 15 months, instead of waiting until 4 to 5 years of age as recommended by the Advisory Committee on Immunization Practices (ACIP). She said her pediatrician told her the vaccine does not wear off, but the second dose is given because some children are non-responders when the vaccine is initially administered at 11 or 12 months of age.
The only way to tell whether the child is a non-responder would be to draw blood for an antibody titer. The physician explained that if her son were to experience a needle-stick, the child might as well receive the vaccination. His rationale for offering the vaccine at an earlier time than currently scheduled is based on an outbreak of measles in 2011. Thirteen cases were reported in Utah, where 9 children older than 5 years had not been vaccinated and had contracted measles by traveling abroad and importing the infection home or through contact at home or school with a child returning from travel.1 The physician noted that this scenario is not uncommon; therefore, he offers the second MMR earlier to provide appropriate protection in case the child is a non-responder and happens to be exposed during the interval between the first and second vaccinations. The concerned mother declined the second dose and sought another opinion. She told the pharmacist that her son had other immunizations scheduled in the upcoming months and wanted to know whether it would be appropriate for him to get the second MMR dose sooner than the typical school-entry immunization. How would you respond to the mother's queries, and what recommendations would you make regarding deviation from the ACIP guidelines?
OVERVIEW OF MEASLES AND ITS VACCINATION HISTORY
Measles is an acute, highly contagious illness caused by a virus in the family paramyxovirus, genus Morbillivirus.2 Measles presents with symptoms of fever (as high as 105°F), malaise, cough, coryza, and conjunctivitis, followed by the characteristic maculopapular rash (Figure 1) and Koplik spots (Figure 2).2 The disease was nearly universal during childhood in the United States prior to vaccination.3 Approximately 500,000 cases and 500 deaths were reported annually in the United States before a vaccine was available.3 In 1963, a live vaccine was licensed for use as a single dose at age 9 months. With the exception of oral poliovirus strains, complication rates during the first year of life had been higher for other diseases when live vaccines were used, compared to complication rates when the live vaccines were given after the first birthday.4 For this reason, in 1965, the schedule was moved to administer the vaccine at 12 months of age.5
Figure 1.
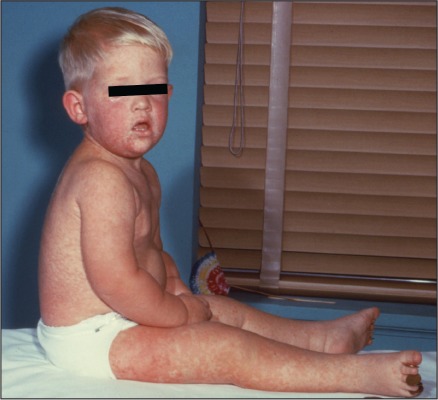
This child with measles is displaying a characteristic red blotchy pattern on his body during the third day of the rash. Image (4498) courtesy of the CDC – National Center for Infectious Disease (http://phil.cdc.gov).
Figure 2.
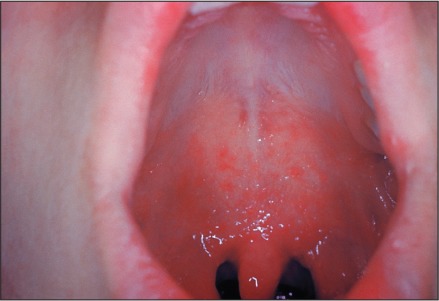
This patient shows Koplik's spots on the palate due to pre-eruptive measles on day 3 of the illness. Image (3187) courtesy of the CDC – National Center for Infectious Disease (http://phil.cdc.gov).
The challenges seen in the first few years after the live vaccine was developed were attributed to multiple factors. Simultaneous inoculation with γ-globulin, diminished potency caused by mishandled storage conditions, and persistence of passively acquired maternal antibody, among others, were reported as causes of vaccine failure.6 In 1977, Yeager et al7 showed that children immunized at 13 to 14 months of age showed higher antibody titers compared to children who had received their measles vaccine at 12 months or earlier. Albrecht et al8 evaluated maternal transmission of measles antibodies. They conducted a prospective study of thirty-four 12-month-old children and found that maternal measles-neutralizing antibodies may persist up to and beyond 12 months of age. A direct correlation of persisting levels of maternal antibody in the infant and a decreased response to immunization was observed.8 With the growing body of evidence, the ACIP recommended that the scheduled dose of measles vaccine be changed to 15 months.6
In the mid-1980s, there still were concerns regarding maternal transmission of immunity against measles in infants, especially in young mothers who had received the vaccine. Studies showed that women born after 1956 were more likely to have vaccine-induced immunity, transferring lower titers of measles antibodies to their infants.9,10 Additionally, maternal antibodies waned earlier, causing preterm infants to become susceptible to measles at a younger age.10
Jenks et al11 evaluated measles antibody levels in children born to unvaccinated mothers at different gestational ages. Because most maternal antibodies are transferred to the fetus during the third trimester, these preterm infants are at particular risk of having low or even undetectable antibody titers by their first year of life and may benefit from earlier administration of vaccine. Public health authorities acknowledged these cases were the exception rather than the rule, therefore a change in the schedule was not warranted. They suggested that each patient should be evaluated independently, and early administration close to 12 months should be considered in those with the highest risk.
The Centers for Disease Control (CDC) reported 6282 cases of measles in 152 outbreaks from 1985 through 1986. Sixty percent of the cases occurred in appropriately immunized school-age (5–19 years) persons and in 27% of children less than 15 months of age, in whom the vaccine was not recommended at the time.12 In 101 of these outbreaks, 67% of the cases occurred in school-aged children, of whom 27% were unvaccinated and therefore considered to have preventable disease.13 Measles outbreaks during this period developed in schools with immunization rates usually ≥96%.14,15 In 1989, the Committee of Infectious Disease of the American Academy of Pediatrics (AAP) assessed trends of infection and vaccine failure for measles. Because most cases had been reported in older school-aged children, the committee recommended that a second dose be given at entrance to middle/junior high school, at approximately 11 or 12 years of age. This modification also targeted students in colleges and other institutions of higher education, health care workers, and international travelers.13,16 It should be noted that the second dose was not intended to be considered a booster dose.16 The measles vaccine is about 95% effective and provides long-term protection. Most of the 5% of vaccinated persons who do not seroconvert following the first dose will develop an immune response to the second dose. In addition, this change in policy helped reduce the number of individuals with an inadequate response to the initial vaccination, provided the opportunity to vaccinate those who had no previous immunization, and ensured vaccination of those lacking proper documentation.
At the same time, the ACIP recommended that the second dose be administered upon entrance to kindergarten or first grade, between the ages of about 4 and 6 years.16 This recommendation was based primarily on administrative considerations. The childhood immunization scheduled diphtheria-tetanus-pertussis and poliovirus vaccinations before school entry. Simultaneous provision of the MMR vaccination therefore reduced the number of office visits required. Also, by vaccinating at this age, school officials could identify and track children with incomplete immunizations.16
According to the CDC, between 1989 and 1990, more than 55,000 cases of measles were reported in the United States. Almost 25% of cases reported during this time occurred in children ≤15 months (the recommended age for vaccination); 9% of the cases were in children 12 to 15 months of age.17 It is known that natural disease results in higher antibody titers than vaccine induced immunity, but few studies have evaluated the effectiveness of revaccination.9 Markowitz et al18 evaluated seroconversion in students in 6th and 10th grades using a more sensitive method, plaque reduction neutralization (PRN), instead of the conventional hemagglutination-inhibition antibody used in previous studies. Seven students (18%) had undetectable PRN levels and 25 students (63%) had low PRN levels; however, 3 weeks after revaccination, all children exhibited rises in PRN antibody levels.18 This response persisted even after 6 years, although some children, originally with low levels, showed decreased titers. Watson et al19 evaluated efficacy in children at school entry (4–6 years) with enzyme-linked immunosorbent assay microneutralization. Six hundred seventy-nine children were tested, of whom 37 (5.4%) were seronegative.19 Thirty-six of these children showed detectable immunoglobulin M levels and/or increases in neutralizing antibody titers, indicating primary immune response, upon revaccination.19 In 1994, the ACIP and AAP convened to unify the differing immunization schedules for the public and private sectors. They addressed changes in infant hepatitis B, oral poliovirus, and MMR vaccinations. It was recommended that the first dose of measles vaccine be administered at 12 to 15 months.17 The second dose was deferred to state-specific laws, allowing for administration at 4 to 6 years of age (kindergarten) or 11 to 12 years of age (middle school/junior high).17
In 1998, the ACIP, the AAP and the American Academy of Family Physicians jointly adopted 4 to 6 years as the recommended age for the second dose in the MMR schedule20 (Table). This age range was chosen to facilitate “reaching” the greatest number possible of unvaccinated children in a controlled setting where there was access to vaccinations, such as entry into the school system. Since 2009, fewer than 80 cases per year have been reported in the United States, with the exception of 2011, in which 222 cases were distributed over 17 different outbreaks.21 Of these cases, 196 were U.S. residents: 27 (14%) were aged <12 months, 51 (26%) were aged 1 to 4 years, 42 (21%) were aged 5 to 19 years, and 76 (39%) were aged =20 years.22 The single largest outbreak since 1996 was reported in the first 8 months of 2013 (58 cases). Later, it was reported that the year's total reached 159 cases, of which 131 (82%) were in persons who were unvaccinated, while 15 cases (9%) were in those with unknown vaccination status.23 Thirteen (8%) of the 159 cases had been in vaccinated persons, including 2 infants =6 months of age who had traveled abroad and only received one dose of MMR.23
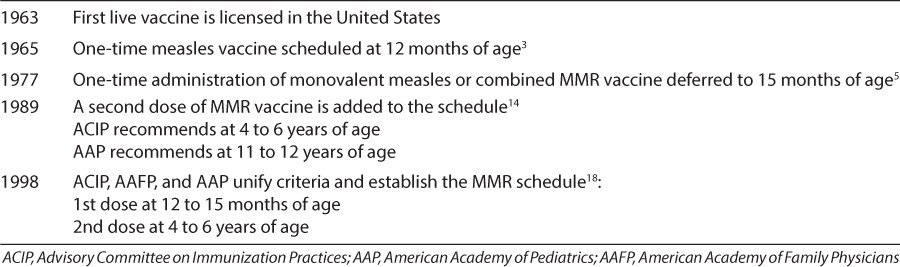
Table.
Timeline of MMR vaccine development and administration schedule.
In the case of a missed MMR dose at 12 to 15 months, the ACIP currently recommends that a dose be administered as soon as possible. The interval before the next dose should be no less than 28 days to complete the series. If only the second dose is missing, a single MMR dose should be given.
RESPONSE TO THERAPEUTIC DILEMMA
As in the case described at the beginning of this article, a child might present having received only one dose of measles vaccine and be offered the second dose prior to turning 4 years of age. Some children will develop normal antibody titers in response to the initial dose of MMR vaccine and develop higher antibody titers after the second dose of the vaccine. Typically, these increased antibody levels do not persist.18 No medical evidence has suggested that harm would result from administration of a second dose earlier than recommended, while it may provide benefit if the child was a poor responder to the first dose. An earlier second dose may be considered if the child is indigenous to a high-risk area for developing measles or has to relocate to one (including international travel).16 During an outbreak, control activities should not be delayed by pending laboratory results. Persons who cannot readily provide proper documentation with administration dates of a 2-dose measles immunity should be vaccinated or excluded from the setting.16 In the case of a school-based or daycare-based outbreak, revaccination efforts should involve all students, their siblings, and all school personnel born in or after 1957 who cannot document immunity as indicated earlier.16 Current ACIP recommendations state that no additional dose is required if a child has received 2 doses of MMR vaccine at least 1 month apart and if the first dose was administered after the child's first birthday.
Current practice calls for the administration of 2 doses of MMR, the first after the first birthday (12 to 15 months) and the second at school entry (4 to 6 years). The recommendation for the second dose at this age range is based on administrative considerations. MMR can be administered at an earlier age as long as the interval between the doses is 28 days or longer. Caregivers are advised that early administration of the second dose is not common and may not be recognized by providers or school officials, who may not be familiar with this approach. These children may require an additional dose at school entry. As long as documentation of a second dose of measles vaccination is provided, compliance with current immunization policies will be achieved.
ABBREVIATIONS
- ACIP
Advisory Committee on Immunization Practices
- CDC
Centers for Disease Control
- MMR
measles-mumps-rubella
- PRN
plaque reduction neutralization
Footnotes
Disclosure Dr Herrera, Dr Thornton, and Dr Helms declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. Dr Foster is on an advisory board for Pfizer and the speakers' bureau for Merck, MedImmune, and Sanofi-Pasteur, for which he receives honoraria.
REFERENCES
- 1.CDC. Two measles outbreaks after importation—Utah, March–June 2011. MMWR Morb Mortal Wkly Rep. 2013;62(12):222–225. [PMC free article] [PubMed] [Google Scholar]
- 2.Strebel P, Papania MJ, Dayan GH . Measles vaccine. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia, PA: WB Saunders; 2008. pp. 353–398. [Google Scholar]
- 3.Atkinson W, Wolfe C, Hamborsky J, editors. Epidemiology and Prevention of Vaccine-Preventable Diseases. 12th ed. Atlanta, GA: Centers for Disease Control and Prevention; 2012. Measles; pp. 173–191. [Google Scholar]
- 4.Wehrle PF. Immune response with particular reference to the use of multiple antigens. Calif Med. 1968;109(6):452–457. [PMC free article] [PubMed] [Google Scholar]
- 5.Orenstein WA, Markowitz L, Preblud SR et al. Appropriate age for measles vaccination in the United States. Dev Biol Stand. 1986;65:13–21. [PubMed] [Google Scholar]
- 6.Krugman S. Measles immunization: new recommendation. JAMA. 1977;237(4):366. doi: 10.1001/jama.237.4.366. [DOI] [PubMed] [Google Scholar]
- 7.Yeager AS, Davis JH, Ross LA et al. Measles immunization. Successes and failures. JAMA. 1977;237(4):347–351. [PubMed] [Google Scholar]
- 8.Albrecht P, Ennis FA, Saltzman EJ et al. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J Pediatr. 1977;91(5):715–718. doi: 10.1016/s0022-3476(77)81021-4. [DOI] [PubMed] [Google Scholar]
- 9.Krugman S, Giles JP, Friedman H et al. Studies on Immunity to Measles. J Pediatr. 1965;66:471–488. doi: 10.1016/s0022-3476(65)80112-3. [DOI] [PubMed] [Google Scholar]
- 10.Lennon JL, Black FL. Maternally derived measles immunity in era of vaccine-protected mothers. J Pediatr. 1986;108(5 Pt 1):671–676. doi: 10.1016/s0022-3476(86)81039-3. [DOI] [PubMed] [Google Scholar]
- 11.Jenks PJ, Caul EO, Roome AP. Maternally derived measles immunity in children of naturally infected and vaccinated mothers. Epidemiol Infect. 1988;101(2):473–476. doi: 10.1017/s095026880005442x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markowitz LE, Preblud SR, Orenstein WA et al. Patterns of transmission in measles outbreaks in the United States, 1985–1986. N Engl J Med. 1989;320(2):75–81. doi: 10.1056/NEJM198901123200202. [DOI] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics Committee on Infectious Diseases. Measles: reassessment of the current immunization policy. Pediatrics. 1989;84(6):1110–1113. [PubMed] [Google Scholar]
- 14.Gustafson TL, Lievens AW, Brunell PA et al. Measles outbreak in a fully immunized secondary-school population. N Engl J Med. 1987;316(13):771–774. doi: 10.1056/NEJM198703263161303. [DOI] [PubMed] [Google Scholar]
- 15.Nkowane BM, Bart SW, Orenstein WA et al. Measles outbreak in a vaccinated school population: epidemiology, chains of transmission and the role of vaccine failures. Am J Public Health. 1987;77(4):434–438. doi: 10.2105/ajph.77.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. Measles prevention. MMWR Morb Mortal Wkly Rep. 1989;38(Suppl 9):1–18. [PubMed] [Google Scholar]
- 17.CDC. Recommended childhood immunization schedule—United States, 1995. MMWR Recomm Rep. 1995;44(RR-5):1–9. [PubMed] [Google Scholar]
- 18.Markowitz LE, Albrecht P, Orenstein WA et al. Persistence of measles antibody after revaccination. J Infect Dis. 1992;166(1):205–208. doi: 10.1093/infdis/166.1.205. [DOI] [PubMed] [Google Scholar]
- 19.Watson JC, Pearson JA, Markowitz LE et al. An evaluation of measles revaccination among school-entry-aged children. Pediatrics. 1996;97(5):613–618. [PubMed] [Google Scholar]
- 20.Watson JC, Hadler SC, Dykewicz CA et al. Measles, mumps, and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1998;47(RR-8):1–57. [PubMed] [Google Scholar]
- 21.CDC. Measles: United States, January—May 20, 2011. MMWR Morb Mortal Wkly Rep. 2011;60(20):666–668. [PubMed] [Google Scholar]
- 22.CDC. Measles—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:253–257. [PubMed] [Google Scholar]
- 23.CDC. Measles—United States, January 1–August 24, 2013. MMWR Morb Mortal Wkly Rep. 2013;62(36):741–743. [PMC free article] [PubMed] [Google Scholar]


