Abstract
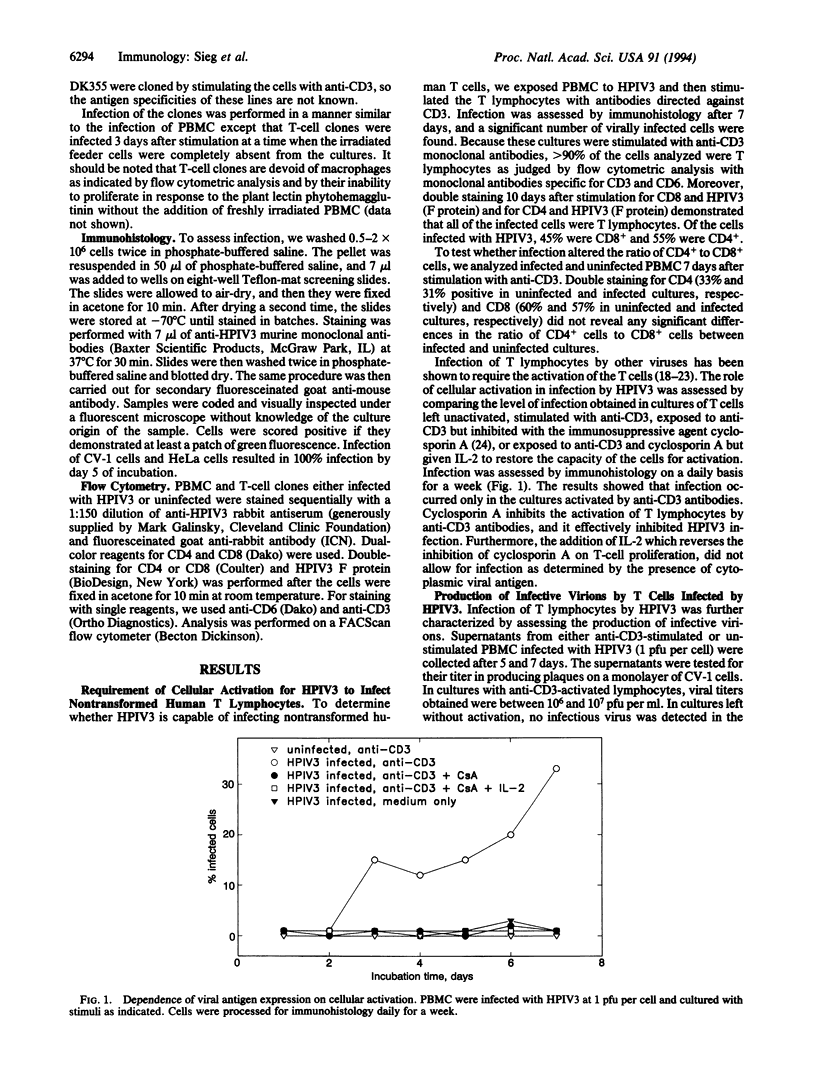
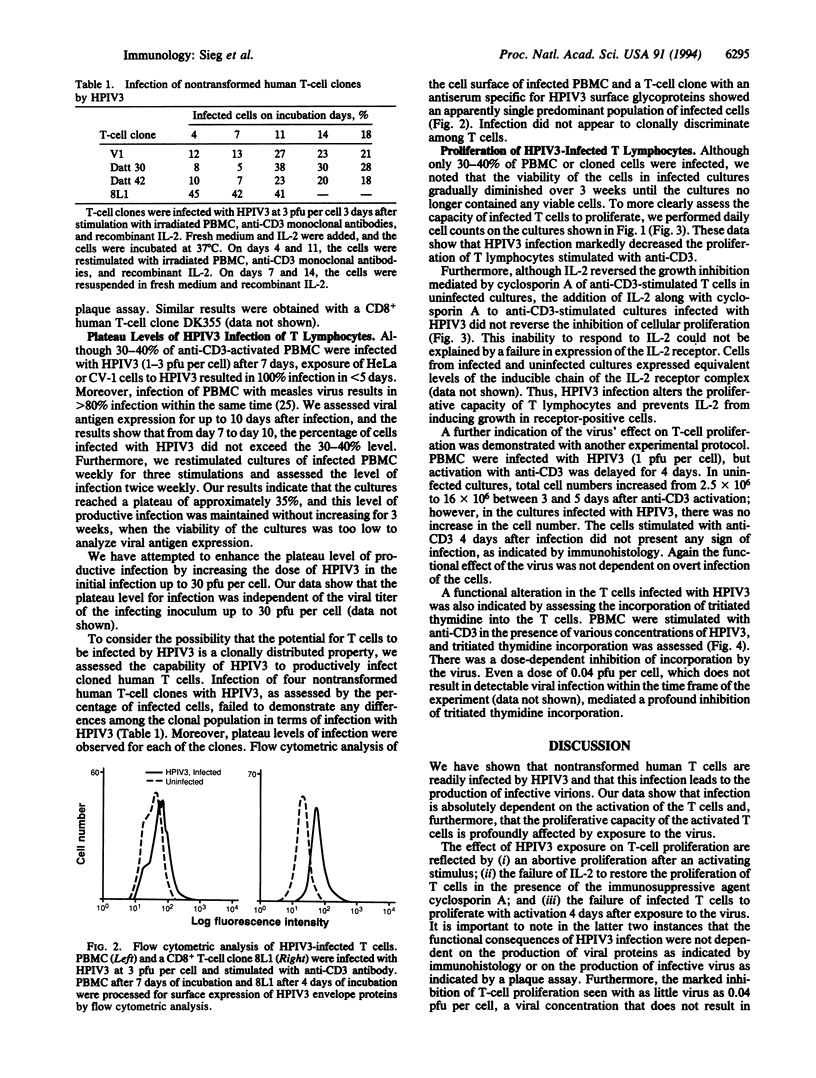
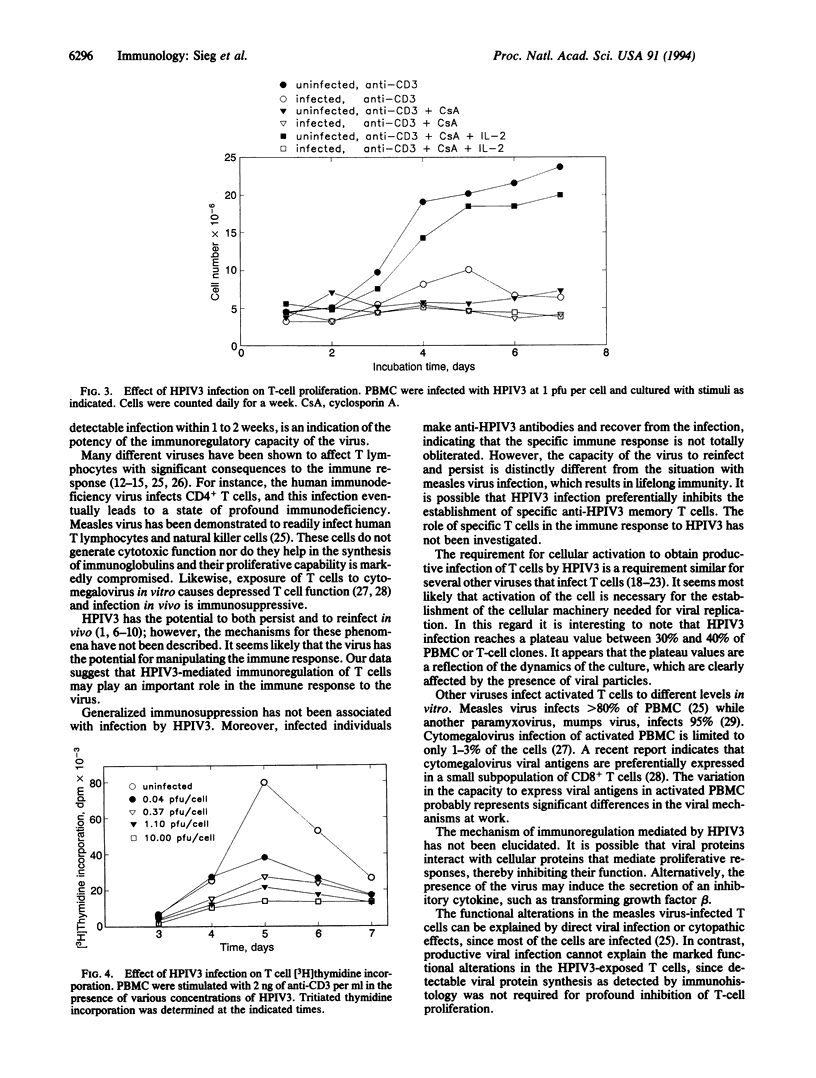
Human parainfluenza virus type 3 (HPIV3) is a major cause of disease in newborns and infants. It also has a striking potential to reinfect individuals throughout their lives, suggesting that HPIV3 does not induce lifelong immunity; however, the operative mechanism for the failure to prevent reinfection is not known. We have assessed the potential of the virus to infect nontransformed human T lymphocytes and have found that T cells are readily infected by the virus. Productive infection requires activation of the T cells and results in a marked inhibition of proliferation. Furthermore, our results indicate that exposure to the virus, even without overt expression of viral proteins as detected by immunohistology, profoundly alters the functional capacity of the T cells. The capacity of the virus to regulate T-lymphocyte function may play an important role in the failure of the virus to induce lifelong immunity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basle M. F., Russell W. C., Goswami K. K., Rebel A., Giraudon P., Wild F., Filmon R. Paramyxovirus antigens in osteoclasts from Paget's bone tissue detected by monoclonal antibodies. J Gen Virol. 1985 Oct;66(Pt 10):2103–2110. doi: 10.1099/0022-1317-66-10-2103. [DOI] [PubMed] [Google Scholar]
- Borrow P., Tishon A., Oldstone M. B. Infection of lymphocytes by a virus that aborts cytotoxic T lymphocyte activity and establishes persistent infection. J Exp Med. 1991 Jul 1;174(1):203–212. doi: 10.1084/jem.174.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R. W., Reiser H. C. Replication of human cytomegalovirus in human peripheral blood T cells. J Virol. 1986 Oct;60(1):29–36. doi: 10.1128/jvi.60.1.29-36.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANOCK R. M., PARROTT R. H., JOHNSON K. M., KAPIKIAN A. Z., BELL J. A. MYXOVIRUSES: PARAINFLUENZA. Am Rev Respir Dis. 1963 Sep;88:SUPPL–166. doi: 10.1164/arrd.1963.88.3P2.152. [DOI] [PubMed] [Google Scholar]
- Casali P., Rice G. P., Oldstone M. B. Viruses disrupt functions of human lymphocytes. Effects of measles virus and influenza virus on lymphocyte-mediated killing and antibody production. J Exp Med. 1984 May 1;159(5):1322–1337. doi: 10.1084/jem.159.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Kreth H. W. Mumps virus replication in human lymphoid cell lines and in peripheral blood lymphocytes: preference for T cells. Infect Immun. 1982 Jan;35(1):25–31. doi: 10.1128/iai.35.1.25-31.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Schirmer E. C., Katsafanas G., June C. H. T-cell activation is required for efficient replication of human herpesvirus 6. J Virol. 1990 Sep;64(9):4598–4602. doi: 10.1128/jvi.64.9.4598-4602.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glezen W. P., Frank A. L., Taber L. H., Kasel J. A. Parainfluenza virus type 3: seasonality and risk of infection and reinfection in young children. J Infect Dis. 1984 Dec;150(6):851–857. doi: 10.1093/infdis/150.6.851. [DOI] [PubMed] [Google Scholar]
- Goswami K. K., Cameron K. R., Russell W. C., Lange L. S., Mitchell D. N. Evidence for the persistence of paramyxoviruses in human bone marrows. J Gen Virol. 1984 Nov;65(Pt 11):1881–1888. doi: 10.1099/0022-1317-65-11-1881. [DOI] [PubMed] [Google Scholar]
- Gross P. A., Green R. H., Curnen M. G. Persistent infection with parainfluenza type 3 virus in man. Am Rev Respir Dis. 1973 Oct;108(4):894–898. doi: 10.1164/arrd.1973.108.4.894. [DOI] [PubMed] [Google Scholar]
- Hambor J. E., Tykocinski M. L., Kaplan D. R. Functional consequences of anti-sense RNA-mediated inhibition of CD8 surface expression in a human T cell clone. J Exp Med. 1988 Oct 1;168(4):1237–1245. doi: 10.1084/jem.168.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson F. W. Pulmonary infections with respiratory syncytial virus and the parainfluenza viruses. Semin Respir Infect. 1987 Jun;2(2):112–121. [PubMed] [Google Scholar]
- Joseph B. S., Lampert P. W., Oldstone M. B. Replication and persistence of measles virus in defined subpopulations of human leukocytes. J Virol. 1975 Dec;16(6):1638–1649. doi: 10.1128/jvi.16.6.1638-1649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Griffith R., Braciale V. L., Braciale T. J. Influenza virus-specific human cytotoxic T cell clones: heterogeneity in antigenic specificity and restriction by class II MHC products. Cell Immunol. 1984 Oct 1;88(1):193–206. doi: 10.1016/0008-8749(84)90064-9. [DOI] [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Arya S. K., Wong-Staal F., Gallo R. C., Waldmann T. A., Greene W. C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolick J. B., Volkman D. J., Folks T. M., Fauci A. S. Amplification of HTLV-III/LAV infection by antigen-induced activation of T cells and direct suppression by virus of lymphocyte blastogenic responses. J Immunol. 1987 Mar 15;138(6):1719–1723. [PubMed] [Google Scholar]
- McChesney M. B., Oldstone M. B. Viruses perturb lymphocyte functions: selected principles characterizing virus-induced immunosuppression. Annu Rev Immunol. 1987;5:279–304. doi: 10.1146/annurev.iy.05.040187.001431. [DOI] [PubMed] [Google Scholar]
- Monto A. S. The Tecumseh study of respiratory illness. V. Patterns of infection with the parainfluenzaviruses. Am J Epidemiol. 1973 May;97(5):338–348. doi: 10.1093/oxfordjournals.aje.a121514. [DOI] [PubMed] [Google Scholar]
- Muchmore H. G., Parkinson A. J., Humphries J. E., Scott E. N., McIntosh D. A., Scott L. V., Cooney M. K., Miles J. A. Persistent parainfluenza virus shedding during isolation at the South Pole. Nature. 1981 Jan 15;289(5794):187–189. doi: 10.1038/289187a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular anatomy of viral persistence. J Virol. 1991 Dec;65(12):6381–6386. doi: 10.1128/jvi.65.12.6381-6386.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Viral persistence. Cell. 1989 Feb 24;56(4):517–520. doi: 10.1016/0092-8674(89)90573-4. [DOI] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Naz N., Willy M., Riggs N. Outbreak of parainfluenza virus type 3 in a neonatal nursery. Pediatr Infect Dis J. 1990 Jan;9(1):31–33. doi: 10.1097/00006454-199001000-00007. [DOI] [PubMed] [Google Scholar]
- Söderberg C., Larsson S., Bergstedt-Lindqvist S., Möller E. Definition of a subset of human peripheral blood mononuclear cells that are permissive to human cytomegalovirus infection. J Virol. 1993 Jun;67(6):3166–3175. doi: 10.1128/jvi.67.6.3166-3175.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong-Starksen S. E., Luciw P. A., Peterlin B. M. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6845–6849. doi: 10.1073/pnas.84.19.6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verini M. A., Lief F. S. Interaction between 6/94 virus, a parainfluenza type 1 strain, and human leukocytes. Infect Immun. 1979 Jun;24(3):734–741. doi: 10.1128/iai.24.3.734-741.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welliver R., Wong D. T., Choi T. S., Ogra P. L. Natural history of parainfluenza virus infection in childhood. J Pediatr. 1982 Aug;101(2):180–187. doi: 10.1016/s0022-3476(82)80113-3. [DOI] [PubMed] [Google Scholar]


