Abstract
Cholinergic neurons and nicotinic acetylcholine receptors (nAChRs) in the brain participate in diverse functions: reward, learning and memory, mood, sensory processing, pain, and neuroprotection. Nicotinic systems also have well-known roles in drug abuse. Here, we review recent insights into nicotinic function, linking exogenous and endogenous manipulations of nAChRs to alterations in synapses, circuits, and behavior. We also discuss how these contemporary advances can motivate attempts to exploit nicotinic systems therapeutically in Parkinson’s disease, cognitive decline, epilepsy, and schizophrenia.
Introduction
Europeans first encountered nicotinic actions when Columbus’s crew sampled tobacco in 1492. After Jean Nicot, the French ambassador to Portugal, introduced tobacco to Paris, botanists honored him by naming the plant Nicotiana, and later its active alkaloid was named nicotine. Claude Bernard (1851) found that nicotine activates muscle when applied directly but not when applied to motor nerves; this was eventually explained by the fact that nicotine and neurally released acetylcholine activate common receptors. In 2011, we know that cholinergic actions in the brain govern various processes: cognition (attention and executive function) (Couey et al., 2007; Levin and Rezvani, 2007; Heath and Picciotto, 2009; Howe et al., 2010), learning and memory (Gould, 2006; Couey et al., 2007; Levin and Rezvani, 2007), mood (anxiety, depression) (Picciotto et al., 2008), reward (addiction, craving) (Tang and Dani, 2009), and sensory processing (Heath and Picciotto, 2009).
The discoveries of Katz and contemporaries at the nerve-muscle synapse and autonomic ganglia gave rise to the modern view that the nicotinic cholinergic synapse is an exquisite biophysical switch, specialized to function on a time scale of ~1 ms and a distance scale of < 1 μm (Wathey et al., 1979; Stiles et al., 1996). This picture did not, however, conform well to the view that acetylcholine functions in the brain as primarily a slow, more widespread modulatory transmitter, somewhat analogous to the biogenic amines. Until the mid-1980s, the “switch” versus “modulator” views were generally reconciled by assuming that nicotinic acetylcholine receptors (nAChRs) activated the dopaminergic system (thus explaining the feeling of well-being during smoking), while most cholinergic actions in the brain occur via muscarinic acetylcholine receptors. This assumption became untenable when specific nicotine binding, and cloned neuronal nAChRs, were found in many brain regions (Marks et al., 1983; Schwartz and Kellar, 1983; Heinemann et al., 1987). We now realize that acetylcholine liberated from cholinergic nerve terminals often activates both nAChRs and muscarinic receptors.
Well-characterized cholinergic projection neurons in the brain include those of the basal forebrain, the medial habenula, the striatum, and the vagal nucleus. Terminals of basal forebrain neurons radiate widely and richly innervate forebrain structures. The giant cholinergic interneurons of the striatum control several aspects of basal ganglia function (Cragg, 2006; Witten et al., 2010). Specificity within the cholinergic system arises in part through its receptors. Muscarinic and nicotinic classes comprise five and fifteen subunits, respectively. Nicotinic receptors are pentamers (Figure 1); brain nicotinic receptors can exist as heteromeric combinations of α(2–10) and β(2–4) subunits, and as α7 homopentamers (in muscle-type receptors, the non-α subunits are β1, γ or ε, and δ). Each nAChR subtype exhibits distinct biophysical and pharmacological properties. Even the precise order and stoichiometry of α and β subunits in the pentamer imposes differential response profiles. A major subtype in the brain is α4β2; the (α42β23) stoichiometry exhibits at least 10-fold-higher sensitivity than (α43β22), so that only the former has the high sensitivity (HS) that allows activation at nicotine concentrations in the 0.1–1 μM range, produced by moderate tobacco use and by the various nicotine replacement therapies. α7 nAChRs also respond to nicotine concentrations roughly an order of magnitude higher than α42β23, and α7 nAChRs have high Ca2+ permeability resembling that of NMDA receptors.
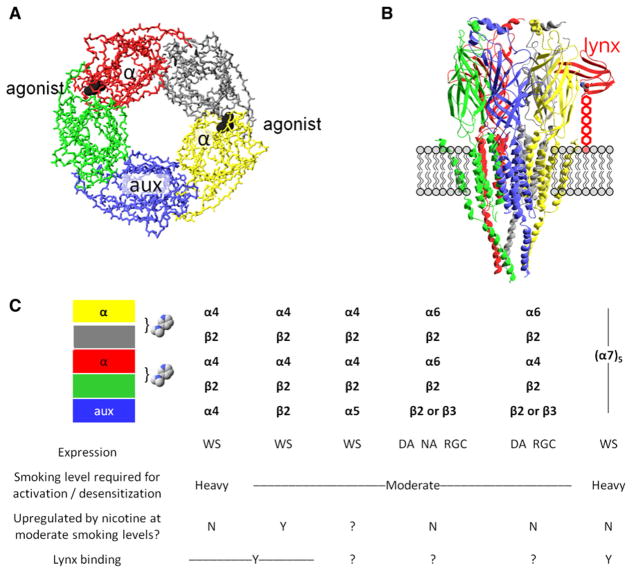
Figure 1. Major Characteristics of Some nAChRs.
(A) A diagram of the symmetric or pseudosymmetric pentameric extracellular binding region, modeled by the acetylcholine receptor binding protein AChBP. The eyepoint is the cytosol; the side chains and transmembrane domains do not appear. The exemplar agonist (nicotine) is represented in black; two agonist binding sites form at the interface between subunits. The open state of the ion channel is more likely to occur when agonist molecules bind at both interfaces than at a single interface. An α subunit (red and yellow) always participates in the binding interface; the other participants are either α subunits (in α7 homopentameric nAChRs) or non-α subunits (in heteropentameric nAChRs such as α4β2*); (see the table in C). The auxiliary subunit (aux, in blue) does not participate in an agonist binding site.
(B) Depiction of a nAChR molecule in the membrane. The eyepoint is a neighboring nAChR. The receptor is Unwin’s model for the Torpedo electric organ muscle-type AChR (Unwin, 2005). The model depicts the full extracellular region (mostly β sheets), which strongly resembles the AChBP structure shown in (A). Ribbons depict the structural elements, whereas neither backbone nor side-chain atoms appear. The model includes the full transmembrane region (mostly α-helical) and only part of the intracellular domains. The schematic also imagines a lynx molecule (red) bound at an α/non-α interface, positioned as in structures of snake α-toxins bound to AChBP (Hansen et al., 2005) or to the muscle nAChR (Dellisanti et al., 2007). Lynx binding, as independently proposed in a recent study (Lyukmanova et al., 2011), occurs at the agonist site shown in (A). The lynx molecule, unlike toxins, is tethered to the membrane by a GPI linkage, here stretched to nearly its full extent and depicted as five hexagons.
(C) Some major nAChR subtypes found in brain. Each column represents the composition of a single pentameric receptor. The table shows our best present knowledge about the properties of detailed stoichiometries. The colored boxes correspond to the subunits of (A) and (B). The bracket and the nicotine molecules show the agonist-binding interfaces between individual subunits. Expression of each receptor subtype is wide-spread (WS), or restricted in the case of α6* nAChRs, confined largely to dopaminergic neurons (DA), noradrenergic neurons (NA), or retinal ganglion cells (RGC).
Most brain HS nAChRs reside on presynaptic terminals, where they stimulate neurotransmitter release (Gotti et al., 2006; Albuquerque et al., 2009). Such presynaptic nAChR activation influences synaptic efficacy and synaptic plasticity (Mansvelder and McGehee, 2000; Dani et al., 2001), spike-timing-dependent plasticity (Couey et al., 2007), frequency-dependent filtering (Exley and Cragg, 2008; Tang and Dani, 2009; Zhang et al., 2009), and overall signal-to-noise ratio in cortex (Disney et al., 2007). Many studies also reveal the presence of somatodendritic nAChRs, but there are relatively few classically defined somatodendritic cholinergic synapses (Aznavour et al., 2005). The “volume transmission” hypothesis states that ACh released from presynaptic terminals spreads to more distant areas, reaching concentrations < 1 μM (Descarries et al., 1997), but that multiple presynaptic impulses produce enough summed release to activate receptors (Lester, 2004). In most regions that receive cholinergic innervation, the high density of acetylcholinesterase (which can hydrolyze ACh at a rate of one per 100 μs!) might vitiate the volume transmission mechanism. In the interpeduncular nucleus, the acetylcholinesterase density is sufficiently low to rationalize long-awaited, recent evidence that 20–50 Hz presynaptic stimulation eventually generates a postsynaptic response via volume transmission (Ren et al., 2011). As we will see below, the mystery of somatodendritic nAChRs can also be resolved by the sensitivity of α7 nAChRs to constant levels of another agonist, choline.
Although researchers have located the cholinergic neurons and the nicotinic receptors, the problem remains: how can changes in biophysical switches lead to widespread modulation? A series of explanations arise, because nicotinic systems are tightly balanced through a multilayered hierarchy of control mechanisms. Acetylcholinesterase efficiently hydrolyzes acetylcholine, both turning off cholinergic signaling and also reducing the likelihood of receptor desensitization. In addition, changes in subunit composition and stoichiometry can influence receptor desensitization, ligand affinity profiles, and conductance. Mutations in nicotinic receptor subunits are linked to human disease, α4 and β2 in some epilepsies, α7 in schizophrenia, and α5 in nicotine addiction; and each mutation ultimately manifests itself as an imbalance in the properties of neuronal circuits. Hyperactivating mutations in nAChR subunits have revealed the existence of previously underappreciated cholinergic mechanisms (Fonck et al., 2005; Drenan et al., 2008). Furthermore, posttranslational mechanisms such as upregulation can play a part in modifying the response properties of nAChRs and may underlie susceptibility toward nicotine dependence. Finally, nAChRs exist in complexes in the brain; interacting proteins engage in complexes with nAChRs and aid in the assembly and trafficking of nAChR to the plasma membrane; examples are RIC-3 (Lansdell et al., 2005), 14-3-3 proteins (Jeanclos et al., 2001), neurexins (Cheng et al., 2009), and VILIP-1 (Lin et al., 2002).
The challenge of explaining the modulation of behavior in terms of the microscopic properties of all-or-none synapses occupies much of neuroscience; but one expects studies on nicotinic systems to lead the way, if only because of their venerability. Within the control hierarchy, especially sensitive points of regulation can have important sequelae. This review discusses three emerging hypotheses about ways that the nicotinic system can be modulated. First is the role played by lynx modulators as molecular brakes over the cholinergic system in stabilizing neural plasticity and circuitry. A second example is a critical time in neurodevelopment that controls the maturation of inhibition; misregulation of α7 nAChR function may lead to increased risk of schizophrenia. Lastly, we discuss how chronic nicotine exposure due to smoking leads to nicotine dependence—and also to two inadvertent therapeutic effects.
Neuromodulation through Lynx Protein Modulators of nAChR Function
Maintaining the levels and function of nAChRs during development and in adulthood is critical for proper circuit function. An inverted U-shape characterizes an organism’s response to cholinergic activators. On the extremes of this range, underactivation is associated with lower cognitive performance and dementias (Hasselmo and Sarter, 2011), whereas overactivation may be linked to epilepsy (Bertrand et al., 2002) and, in even more extreme cases, to neurodegeneration (Schwarz et al., 2006). Apparently, tight control over cholinergic systems, operating at several levels, can counteract such imbalances at both extremes. Proteins that engage nAChRs within stable complexes, such as lynx family members, provide a homeostatic influence over nicotinic receptor systems. Through functionally driven regulation of lynx expression, the inhibition exerted over the system can be released or enhanced selectively within neuronal circuits.
The Lynx Family Acts as Nicotinic Receptor Modulators
The lynx genes belong to the ly-6/PLAUR superfamily, which shares a marked structural similarity with elapid snake venom proteins such as α-bungarotoxin; all have a characteristic three-looped motif. These α-neurotoxins are secreted proteins with sub-nM affinity for nAChRs (Tsetlin et al., 2009) and other receptors (Auer et al., 2010). α-neurotoxins interact on the extra-cellular face of the nAChR near ligand binding sites (Figure 1B), in contrast to most other nAChR-interacting proteins, which bind to the intracellular loops. Extrapolating from these interactions, the structurally similar lynx proteins may bind at such sites as well (Lyukmanova et al., 2011). Five interfaces occur in each nAChR pentamer (Figure 1); we do not yet know which, if any, interfaces form the binding sites for various lynx paralogs (Hansen and Taylor, 2007). Most previous studies of lynx have emphasized interactions at the plasma membrane. As GPI-anchored proteins can bind to transmembrane receptors intracellularly, the interactions of lynx with nAChRs could potentially alter receptor trafficking, stoichiometry, and surface number (Lester et al., 2009).
The high level of conservation with toxins implies that lynx genes are prototoxins—evolutionary antecedents to α-neurotoxins (Miwa et al., 1999; Chimienti et al., 2003; Dessaud et al., 2006; Arredondo et al., 2007; Hruska et al., 2009). The lynx family occurs in other species, including C. elegans (Chou et al., 2001) and Drosophila (Wu et al., 2010)—and in nonvenomous snakes, where it is distinct from the neurotoxin genes. We note that, in several cases, snake toxins employ functional mimicry of proteins in normal physiological processes. Often, virulent gene variants distort endogenous pathways at sensitive or rate-limiting steps. Therefore, the evolutionary relationship between lynx modulators and the α-neurotoxins agrees with the view that lynx modulators govern critical control points in the pathway of nicotinic receptor signaling.
Lynx1, the first discovered member of this family expressed in the brain (Miwa et al., 1999), has an overall inhibitory effect on nAChR function. In an α4β2* nAChR-expressing cell, coexpression of lynx1 results in reduced agonist sensitivity, accelerated onset of desensitization, and slower recovery from desensitization (Ibañez-Tallon et al., 2002). Each lynx paralog has a relative binding specificity and modulatory capability on α4β2 (Miwa et al., 1999; Ibañez-Tallon et al., 2002; Levitin et al., 2008), α3 (Arredondo et al., 2006), and α7 (Chimienti et al., 2003; Levitin et al., 2008; Hruska et al., 2009) nAChR subtypes; some interactions actually enhance nicotinic responses (Chimienti et al., 2003; Levitin et al., 2008), or their Ca2+ components (Darvas et al., 2009). The actions of lynx family proteins manifest themselves at both circuit (Hruska et al., 2009) and network levels (Pfeffer et al., 2009) on nicotinic systems. The blunting effect of lynx proteins could be responsible for the paucity of synaptically driven nicotinic responses recorded in brain tissue despite the rich cholinergic innervation, as well as the different response properties in brain tissue as compared with heterologous expression systems (Quick and Lester, 2002).
Lynx Acts as a Molecular Brake on Cholinergic-Dependent Plasticity
Removal of the molecular brake provided by lynx proteins can lead to nicotinic receptor hypersensitivity—larger direct nicotinic responses, slowed desensitization kinetics (Miwa et al., 2006), and enhanced sensitivity of the EPSC frequency in the cortex to nicotine (Tekinay et al., 2009). As a consequence of nAChR hypersensitivity, lynx1 knockout mice display increased levels of Ca2+ in neurons, enhancements in synaptic efficacy, and improved learning and memory functions (Miwa et al., 2006; Darvas et al., 2009; Tekinay et al., 2009). Studies on such hyper-active nicotinic receptors can reveal cholinergic-dependent processes with increased clarity. For instance, adult lynx1KO mice display heightened ocular dominance plasticity after the normal close of the critical period (Morishita et al., 2010). While the role of the cholinergic system during visual processing (Disney et al., 2007) and development has been appreciated (Bear and Singer, 1986), it has been a mystery why the critical period closes in late postnatal development and remains closed despite heavy cholinergic innervation of the visual system. These findings indicate that suppression of the cholinergic system by lynx proteins stabilizes neural circuitry. Indeed, cholinergic enhancement (via cholinesterase inhibition) reopens the critical period for visual acuity in adult wild-type mice (Morishita et al., 2010), indicating that cellular mechanisms for robust plasticity are maintained in adulthood through the cholinergic system but are suppressed by the action of lynx.
Top-Down Control over the Cholinergic System through Lynx: What Regulates the Regulator?
Abolishing receptor function through null mutations or pharmacological blockers of nAChRs abolished some of the gain-of-function phenotypes in lynx mouse models, indicating that nAChRs are necessary for the expression of lynx perturbations (Miwa et al., 2006). This indicates that lynx proteins exist, genetically, as upstream modulators of nicotinic receptor function and cholinergic signaling and can exert control over cholinergic-dependent processes. Because excess activation of nAChRs damages neuronal health and brain function, organisms have a clear need to restrict the degree of nAChR activation. Yet specific enhancement of cholinergic activity in functional circuits would benefit many processes, as described above. Therefore, regulation of lynx function that would allow the sensitivity of the cholinergic system to shift in response to environmental changes would be critical. Partial, transient, or local reductions in lynx function may produce an optimal balance; moderate cholinergic signaling would enhance synaptic plasticity, yet still protect against hyperactivation that could make neurons susceptible to excitotoxic damage. What, then, regulates the regulator? Evidence thus far indicates that the lynx family is regulated in response to relatively strong perturbations: downregulation in NKCC1 knockout mice (Pfeffer et al., 2009), in adenylyl cyclase mutant mice (Wieczorek et al., 2010), and by α7 nAChR blockade (Hruska et al., 2009), whereas it is upregulated at the close of the critical period in the visual cortex, and by nicotine in the lung (Sekhon et al., 2005). Through functionally driven regulation of lynx expression, cholinergic systems have the ability to exert top-down influences on circuits underlying relevant behavior via coordinated regulation of nicotinic receptors subsets. While genetic linkages of lynx family members to neurological disorders have not been found, evidence for cholinergic dysregulation has been linked to a lynx family member expressed in nonneuronal tissues and involved in human disease (Chimienti et al., 2003), and as such, alterations in lynx dosage may be useful in ameliorating cognitive decline associated with neuropsychiatric disorders.
Lynx Modulators and the Neurodevelopmental Program
The synaptic pruning of neuronal circuits takes place late in the developing brain, after a period of early sculpting of neuronal number through programmed cell death. Nicotinic receptor systems have been implicated at both these stages and evidence suggests an involvement with lynx prototoxins as well. For instance, early expression of lynx1 family member, PSCA, prevents programmed cell death of parasympathetic neurons (Hruska et al., 2009). Neuronal maturation and loss of synaptic lability appear to be correlated with the onset of lynx1 expression. In the majority of cases, circuit stability would provide an adaptive advantage once sculpting of circuitry has been influenced by the patterned activity of experience. Temporal coherence of information is critical for creating a stable internal representation of our environment and provides the background for salient information to reach our attention. But what happens in cases when that program goes awry? Lynx1 is downregulated in NKCC1 KO mice (Pfeffer et al., 2009), a strain that has a delayed developmental program of GABAergic neurons, diminished inhibition, and less spontaneous network activity. The neurodevelopmental program depends in part on α7 signaling (Liu et al., 2006). Lynx1 upregulation during a critical neurodevelopmental period, the switch in the sign of GABAergic signaling, and coexpression of lynx with GABAergic subsets all indicate a possible role of lynx mediating the timing of such developmental transitions. Nicotinic receptor control over GABAergic neuronal development and mature activity may represent a point of convergence for diseases such as schizophrenia (see next section), some amblyopias (Bavelier et al., 2010), and some epilepsies (Klaassen et al., 2006), which distort the excitatory-inhibitory balance in general and implicate GABAergic signaling defects in particular. In such cases, interventions through lynx could be useful for reestablishing the robust plasticity of youth exhibited prior to the close of the critical period, for instance in cases of amblyopia or brain repair in stroke. Further, manipulations of lynx activity could help to restore proper inhibitory-excitatory imbalance. Developmental changes in nAChR functions may play a role in nicotine addiction, as a central question in tobacco control is young adult smokers’ marked sensitivity to developing nicotine dependence (DSM-V Nicotine Workgroup, 2010; DiFranza et al., 2000; Difranza, 2010). Molecules, such as lynx, which have direct contacts with nAChRs are promising candidates for the control of such phenomena and sensitive periods.
An Emerging Role for α7 nAChRs in Schizophrenia: Pharmacotherapeutic and Developmental Perspectives
Individuals with schizophrenia have a number of elementary psychophysiological abnormalities in filtering sensory stimuli that have been hypothesized to underlie their characteristic hallucinations and delusions (Venables, 1967). Their hallucinated voices and paranoid suspicions sometimes can be triggered by background noises in the environment that most other people can ignore. For example, a common hallucination in schizophrenia is a voice from the television, perhaps combined with the paranoid delusion that the television is commanding certain actions. The breakthrough of background noises into hallucinations and delusions can be considered a nonspecific manifestation of disorganized thinking, but increasingly it has been conceptualized as more specific evidence for failure in elementary inhibitory processes that the brain uses to regulate the amount of sensory stimuli that it processes. In many persons with schizophrenia, cerebral evoked potential recording shows diminished inhibition of the response to repeated stimuli (Adler et al., 1982) (Figure 2A), and animal models of this phenomenon point to a defect in hippocampal inhibition. Recent studies provide evidence both that nicotinic signaling partially underlies these schizophrenia-related inhibitory defects and that nicotinic drugs have possible therapeutic roles.
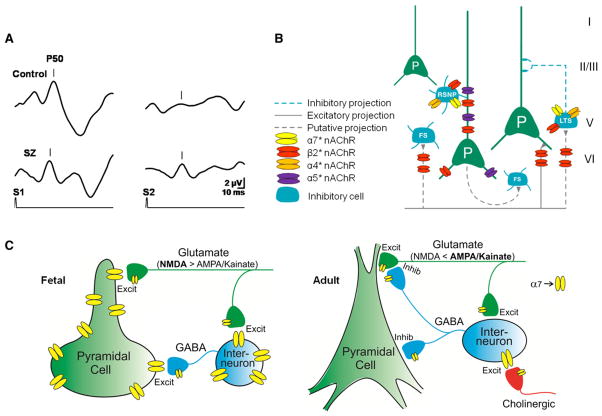
Figure 2. Aspects of nAChR Subtypes on Circuit Function.
(A) Sensory inhibition deficits in schizophrenia. Cerebral evoked P50 potentials to repeated sounds (S1, S2) are inhibited in a normal (control, upper trace) but not in a schizophrenia patient (SZ, bottom trace).
(B) Differential localization of nAChRs subtypes on neurons in the prefrontal cortex. Green cells are excitatory pyramidal neurons (P) and blue cells are inhibitory interneurons. FS, fast-spiking interneurons; LTS, low threshold spiking; RSNP, regular spiking nonpyramidal neuron. Adapted with permission from Poorthuis et al. (2009).
(C) Development of α7nAChRs in hippocampus. In the fetal brain before cholinergic innervation occurs (left), α7 nAChRs are somatodendritic and presynaptic on both GABAergic and glutamatergic neurons. In adults (right), α7 nAChR expression is generally reduced. Receptors are still expressed on GABAergic and glutamatergic presynaptic terminals, but only GABAergic neurons express somatodendritic α7 nAChRs (figure courtesy of William Proctor).
Cerebral α7 nAChRs in Cortex and Thalamus
The hippocampus responds to repeated stimuli with rapid habituation, which is dependent upon cholinergic input from the medial septal nucleus, an input that is driven by the brainstem reticular formation. α7 nAChRs on inhibitory interneurons throughout the hippocampus and presynaptic α7 nAChRs on mossy fiber terminals in the dentate gyrus participate in the control of sensory response in the hippocampus (Gray et al., 1996; Alkondon et al., 1999). Nicotinic activation of inhibitory interneurons increases their activity and activates nitric oxide synthetase. The neurons release additional GABA, activating presynaptic GABAB receptors on the excitatory inputs to pyramidal neurons, which diminish the release of glutamate onto the pyramidal neurons (Figure 2). The result is diminished pyramidal neuron response to repeated sensory stimuli. Thus, the brainstem can regulate hippocampal response in the presence of high sensory input. Although α7 nAChRs have both presynaptic and postsynaptic expression (Frazier et al., 1998), their postsynaptic expression in humans is especially marked on inhibitory neurons of the hippocampus (Alkondon et al., 2000). Rodents have similar expression in the hippocampus, but primates have much more expression in the interneurons of the nucleus reticularis thalamis; the selective advantage of this higher expression may be greater inhibitory control of sensory input to the cerebral cortex.
Three lines of evidence support the possibility that the failure of sensory inhibition in schizophrenia results from decreased expression of α7 nAChRs. First, postmortem studies of the hippocampus and thalamus show diminished labeling of putative inhibitory neurons by α-bungarotoxin, an antagonist of α7 nAChRs (Court et al., 1999). Second, the defect in inhibition is linked to the chromosome 15q14 locus of CHRNA7, the gene for the α7 nAChR subunit. Polymorphisms in the α7 5′ promoter and in a nearby partial duplication of the gene, FAM7A, are associated with both schizophrenia and the defect in inhibition (Leonard et al., 2002). It should, however, be noted that many genes have been associated with schizophrenia and there is no definitive model of its genetic transmission. Yet some of the other genes identified, such as NRG1, are involved in the assembly of α7 nAChRs, further supporting a potential link between α7 nAChRs and schizophrenia (Mathew et al., 2007). Third, persons with schizophrenia have the greatest rate and intensity of cigarette smoking of any identifiable subgroup in the population. Over 80% smoke, most of them multiple packs per day. Per cigarette they extract more nicotine than other smokers with comparable cigarette consumption by inhaling more deeply and holding the smoke in their lungs. Cigarette smoking transiently improves their sensory inhibition. While it is not yet possible to know precisely how well α7 nAChRs are activated by smoked nicotine, one can reasonably hypothesize that the patients’ higher dose of nicotine activates α7 nAChRs (Adler et al., 1993; Papke and Thinschmidt, 1998; Royal College of Physicians, 2007). Inhibition of the evoked response to auditory stimuli is significantly increased after patients smoke, an effect that is blocked by antagonists of α7 nAChRs in animal models (Luntz-Leybman et al., 1992). In schizophrenics, long-term cellular and molecular sequelae of this heavy exposure to nicotine may transcend chaperone-dependent upregulation (see next section) and also arise from the high Ca2+ permeability of nAChRs, especially of α7 nAChRs (Brunzell et al., 2003). Ca2+ activated signal transduction pathways reshape synaptic transmission and neural circuits, in some cases leading to gene activation (Kauer and Malenka, 2007).
If part of the genetic risk for schizophrenia involves variants in genes involved in formation of α7 nAChRs, then that risk has developmental significance as well. Schizophrenia generally appears in early adulthood, but long before the eruption of hallucinations and delusions, there is neurocognitive and psychophysiological evidence for abnormalities in children with schizophrenic parents (which increase their risk of the illness). Such is the case with sensory inhibitory deficits. These are apparent at birth in some neonates with a parent who has schizophrenia (Hunter et al., 2010). Mothers who smoke during pregnancy are also likely to have a neonate with a sensory inhibitory deficit. Chronic exposure to nicotine would be expected to desensitize α7 nAChRs and thus lead to their dysfunction during development. Immature neurons that express α7 nAChRs are more likely to be injured by neonatal nicotine, whereas the expression of heteromeric α4β2* nAChRs by more mature neurons may contribute to increased survival (Huang et al., 2007).
Like other nicotinic receptors, α7 nAChRs are thus potential targets for new therapeutic interventions for neural diseases such as schizophrenia. Several clinical trials involving schizophrenics have utilized more specific agonists for α7 nAChRs. 3-(2,4 dimethoxy)-benzylidene-anabaseine, derived from an alkaloid produced by nemertine worms, is a partial agonist at α7 nAChRs. It improves sensory inhibition in schizophrenics and also moderately improves their neuropsychological deficits in attention (Olincy et al., 2006). Clinical ratings of their negative symptoms, particularly anhedonia (absence of a sense of pleasure) and alogia (poverty of content in their speech), also improve during treatment. The atypical antipsychotic clozapine uniquely reduces smoking in schizophrenia, possibly because it releases acetylcholine in the hippocampus, activating α7 nAChRs (George et al., 1995). These clinical observations indicate that the patients’ cognitive deficits are more amenable to treatment than many previously believed and their heavy cigarette smoking suggests that prescribed neurobiological treatment does not yet adequately address the brain pathophysiology of schizophrenia.
α7 nAChRs and the Development of Inhibitory Neuronal Circuitry
Like many genes expressed in the brain, the expression of α7 nAChRs is maximal during development. α7 nAChRs first appear on neuroblasts as soon as they differentiate from the neuroepithelium, and the peak expression occurs just after birth in rodents (Adams, 2003). In the third trimester, the expression of α7nAChRs in the hippocampus is greater than three times the level in adults. The postsynaptic expression, confined to inter-neurons in adults, is prominent on fetal pyramidal neurons as well (Figure 2C). One important role for α7 nAChRs, in conjunction with α3-containing nAChRs, is the induction of the KCC2 chloride transporter in pyramidal neurons (Liu et al., 2006). This transporter lowers the internal Cl− concentration of the neuron and changes GABA from a depolarizing to a hyperpolarizing or inhibitory neurotransmitter. A specific role of α7 nAChRs was demonstrated by failure of the induction of KCC2 by treatment with α7 nAChR antagonists and in α7 KO mice (Zhang and Berg, 2007). At the time of birth, α7 nAChRs are involved in the transformation of glutamate neurotransmission from primarily NMDA-type receptors to kainate-aspartate receptors. α7 nAChRs remain embedded in the glutamate receptor-containing postsynaptic density.
Cholinergic innervation of the hippocampus occurs near the time of birth; therefore, the endogenous ligand for fetal α7 nAChRs cannot be synaptically released acetylcholine (Derrington and Borroni, 1990). A possible candidate is choline, which, in addition to its other development roles, activates α7 nAChRs at levels several fold higher than acetylcholine. Choline levels in human neonatal cord blood (~35 μM) are three times higher than those in adult blood (Zeisel et al., 1980). These levels are sufficient to selectively downregulate α7 nAChRs on hippocampal neurons in tissue culture, perhaps reflecting a chronic low level of receptor stimulation (Alkondon et al., 1997; Uteshev et al., 2003). Brief choline treatment during gestation is associated with increased excitability and dendritic development in hippocampal pyramidal neurons (Li et al., 2004).
Choline is an essential dietary nutrient. Normally humans have adequate choline, but during pregnancy many women are thought to be deficient because the fetus makes large demands for use in the synthesis of cell membranes (Meck and Williams, 2003). In addition to poor maternal diet, choline deficiency for the fetus can occur because of maternal stress, which leads the mother to sequester choline in her own liver. Variants in the gene for phosphatidylethanolamine methyl transferase, which synthesizes phosphatidylcholine and thus provides a source of choline, are also associated with choline deficiency and with schizophrenia. Experiments in animal models suggest that choline supplementation during gestation and early postnatal development may produce a reversal of sensory inhibitory deficits that lasts through adulthood (Li et al., 2004). Clinical trials are currently in progress.
In addition to genetic risk, exposure to nicotine, and dietary deficiency, maternal infection is a risk factor for schizophrenia (Patterson, 2007). In some cases the infectious agent enters the fetus, but in most cases, like influenza, it remains in the mother’s respiratory tract. It is the deleterious effect of her cytokine response to the infection on the placenta that appears to be pathogenic. α7 nAChRs are involved in the macrophage and placental cytokine response, which may be an additional role for genetic variants in these receptors in the pathogenesis of schizophrenia (Wang et al., 2003).
In short, schizophrenia remains a challenging and mysterious disease. Yet the perinatal development of α7 nAChRs, the role of the endogenous agonist choline on α7 nAChRs, and the consequences for maturation of inhibitory circuits provide both a partial pathophysiological role and a promising avenue for therapy of schizophrenia.
Effects of Chronic Nicotine: Role of Upregulation
“It’s easy to quit smoking,” Mark Twain reportedly said. “I’ve done it a hundred times.” Nicotine dependence may be the most complex of the addictions, perhaps both because HS nAChRs occur in so many brain areas and because unlike acute opioid administration, nicotine allows a user to remain active and productive.
Maintained or repeated intake of nicotine occurs during tobacco smoking or chewing and during the use of snus, lozenges, gums, or patches. The peak and maintained nicotine concentrations during such intake are lower than those presumably associated with schizophrenics’ smoking, and they primarily activate HS nAChRs (Matta et al., 2007; Royal College of Physicians, 2007). In contrast to nicotine addiction, and somewhat surprisingly, such chronic exposure to nicotine produces inadvertent therapeutic effects in at least two other conditions, Parkinson’s disease and a specific form of epilepsy.
This section discusses the status of the unifying hypothesis that these three effects of chronic nicotine exposure are explained by a common molecular and cellular phenomenon. In brief, the interaction between chronic nicotine and HS nAChRs, especially α4β2, appears to cause selective upregulation of these nAChRs via posttranslational mechanisms.
A Pathological Effect: Brain Mechanisms of Nicotine Dependence
Nicotine-dependent people value the effects produced by the smoking-induced nicotine bolus that activates and then desensitizes nAChRs; but longer-term exposure is essential for nicotine dependence (Markou, 2008; Kalivas, 2009; Koob and Volkow, 2010). The meaning of “longer term” depends on one’s definition of nicotine dependence, a lively topic in itself (DSM-V Nicotine Workgroup, 2010; DiFranza et al., 2000; Difranza, 2010); the time required may be as brief as several days.
Some people use tobacco repeatedly because it provides a feeling of well-being, which probably begins when nicotine reaches midbrain nAChRs (Matta et al., 2007; Royal College of Physicians, 2007). Nicotine both activates and desensitizes nAChRs in midbrain dopaminergic neurons (Brodie, 1991; Pidoplichko et al., 1997), and the pleasurable effects associated with nicotine intake occur in large part via the mesolimbic dopaminergic reward system (Corrigall et al., 1992; Koob and Volkow, 2010). Recent studies also show important contributions from insular cortex (Naqvi et al., 2007). The nAChR-rich medial habenula may actually participate in aversive effects of nicotine (Fowler et al., 2011), which apparently underlie moderate smokers’ (but not schizophrenics’) habit of carefully titrating the nicotine dose generated by each cigarette.
In addition to alterations in reward, many nicotine-dependent people display improved declarative memory for several minutes to one hour after smoking (Myers et al., 2008). Because smokers gradually learn to exploit this effect, it is called “cognitive sensitization.” However, it is not known whether the nicotine-enhanced cognitive performance exceeds the level that would occur if the person had never begun to smoke, or after remaining abstinent for one year (the usual criterion for successful smoking cessation) (Levin et al., 2006). Cognitive sensitization probably involves fore-brain-dependent processes (Xu et al., 2005; Davis and Gould, 2009; Kenny, 2011). In rodents and humans, the hippocampus is importantly implicated in cognitive sensitization, and α4β2* nAChRs play key roles (Levin et al., 2006; Davis and Gould, 2009). Chronic or acute nicotine enhances LTP in several regions of hippocampus, especially dentate gyrus (Nashmi et al., 2007; Tang and Dani, 2009; Pentonet al., 2011). The effects may proceed via HS receptors on both the axons of the perforant path and the intrinsic GABAergic interneurons (Gahring and Rogers, 2008).
Other nicotine-dependent people find that nicotine helps them to cope with stressors; the soldier dangling a cigarette after battle is an enduring image (Brandt, 2007). Relapse in response to environmental or contextual stimuli such as stress—even after months of abstinence—constitutes a major challenge in smoking cessation. Stress- and cue-induced reinstatement of nicotine administration is studied far less frequently than analogous phenomena for cocaine and opioids. The VTA-nucleus accum-bens system does play a role. Several additional candidate brain areas receive dopaminergic and other monoaminergic nerve terminals, and these terminals all presumably express HS nAChRs. For instance, dopamine increases in the extended amygdala during stress, fear, and nicotine withdrawal (Inglis and Moghaddam, 1999; Pape, 2005; Grace et al., 2007; Gallagher et al., 2008; Koob, 2009; Marcinkiewcz et al., 2009). We do not know whether either nAChR upregulation, or its sequelae, can account for stress- or cue-induced relapse in nicotine dependence.
nAChR Upregulation: The “Selectivity Hypothesis” and Its Functional Implications
Which molecular and cellular mechanisms could account for the widespread actions of chronic nicotine on neuronal circuit properties? This puzzle does not yet have a complete answer, but it is clear that chronic nicotine increases the number of nAChRs themselves (Marks et al., 1983; Schwartz and Kellar, 1983). In an emerging hypothesis, this “upregulation” is both necessary and sufficient for the initial stages of nicotine exposure—minutes, hours, days, and weeks. Remarkably, the upregulation shows selectivity at every level thus far examined.
At the level of whole brain, chronic nicotine causes selective upregulation of nAChRs among major brain regions. Upregulation occurs in cortex, midbrain, and hypothalamus, but not in thalamus or cerebellum (Pauly et al., 1991; Marks et al., 1992; Nguyen et al., 2003; Nashmi et al., 2007; Doura et al., 2008). In several brain regions, chronic nicotine administration produces ~50% upregulation of HS nAChRs after just two days. Continued administration then produces additional increases over one to several weeks (Marks et al., 1991; Pietilä et al., 1998).
Within individual brain regions, there is selective upregulation among cell types. In the midbrain, both DA neurons (in substantia nigra pars compacta and ventral tegmental area [VTA]) and GABAergic neurons (in substantia nigra pars reticulata and VTA) express high levels of α4β2* nAChRs on their somata, but only GABAergic neurons display somatic upregulation (Nashmi et al., 2007; Xiao et al., 2009). Another example of cell-selective upregulation occurs in the projection from medial entorhinal cortex to dentate gyrus. In the medial perforant path, which mainly arises from layer II stellate cells, chronic nicotine upregulates α4β2* nAChRs. However in the temporoammonic pathway, which mainly arises from layer III pyramidal neurons, α4β2* nAChRs are present but are not upregulated (Nashmi et al., 2007).
Chronic nicotine also produces selective upregulation between somatodendritic versus axon terminal regions of individual neurons. In midbrain, chronic nicotine treatment elicits a general increase in α4β2* nAChRs in GABAergic neurons, but only in axon terminals of DA neurons. Such “tiers of selectivity” in mesostriatal and mesolimbic upregulation have the power to explain two components of nicotine dependence: tolerance to some rewarding effects of nicotine and sensitization to others (Nashmi et al., 2007; Lester et al., 2009).
Nicotine also interacts with specific nAChR subtypes, and nicotine-induced upregulation is governed in part by these interactions at agonist binding interfaces (Figure 1). Further work is needed to understand how chronic nicotine differentially upregulates some but not all HS nAChRs. Part of the cell selectivity in upregulation presumably arises because each neuronal type expresses a distinct repertoire of subunits. GABAergic neurons in DA brain regions express mostly α4β2 nAChRs along with a few α4α5β2 nAChRs (McClure-Begley et al., 2009), whereas DA neurons express at least three α4-containing nAChRs (α4α6β2β3, α4α5β2, and a few α4β2) (Salminen et al., 2004; Gotti et al., 2007). Although α6β3* nAChRs are, like α4β2 nAChRs, highly sensitive to nicotine, several studies demonstrate that chronic nicotine treatment elicits either no change or a decrease in α6β3* nAChRs in mouse brain (McCallum et al., 2006a, 2006b; Mugnaini et al., 2006). Nicotine may also subvert the coordinated regulation in place by other control mechanisms, such as lynx proteins, through the preferential up-regulation of one subtype resulting in imbalance in nicotinic receptor signaling. It is too early, however, to conclude that lynx proteins influence the effects of chronic nicotine exposure.
Emerging clues to selectivity at the subunit level, especially in the context of nicotine dependence, concern the α5 subunit. Figure 1 shows that this subunit never participates at the agonist binding interface between α and β subunit but occupies a fifth or “auxiliary” position. In rodent brain, most α5* nAChRs are thought to be (α4)2(β2)2α5 pentamers (Gotti et al., 2006; Albuquerque et al., 2009). In all known animals, the α5, α3, β4 genes form a cluster. Indeed, (α3)2(β4)2α5 pentamers are widespread in the peripheral nervous system, in the medial habenula, and in some nonneuronal cell types. [We do not emphasize (α3)2(β4)2α5 nAChRs or α3β4 nAChRs, because such nAChRs have relatively low nicotine sensitivity and relatively low susceptibility to upregulation.]
Single-nucleotide polymorphisms found in the human α5, α3, β4 gene cluster are associated with nicotine dependence and its age-dependent onset; number of cigarettes smoked per day and “pleasurable buzz” elicited by smoking; alcoholism, sensitivity to the depressant effects of alcohol, and age of alcohol initiation; cocaine dependence; opioid dependence; lung cancer; and cognitive flexibility (Erlich et al., 2010; Hansen et al., 2010; Improgo et al., 2010; Saccone et al., 2010; Zhang et al., 2010). A major “risk allele” is in a noncoding region of α5 and is associated with decreased expression of α5 subunit mRNA (Wang et al., 2009). A second “risk allele” occurs in the coding region, within the M3–M4 loop, and also produces decreased function of (α4)2(β2)2α5 nAChRs (Wang et al., 2009; Kuryatov et al., 2011). Furthermore in experiments using chronic nicotine exposure in rats, (α4)2(β2)2α5 nAChRs are not upregulated, but (presumptive) (α4)2(β2)3 nAChRs in the same brain region are (Mao et al., 2008). Summarizing the available data, the “risk alleles” may decrease the fraction of (α4)2(β2)2α5, increasing that of α4β2 nAChRs. Because α4β2 nAChRs are the most susceptible to nicotine-induced upregulation, the data again seem consistent with the idea that selective upregulation of α4β2 nAChRs underlies nicotine dependence. The potential power of α4β2 upregulation to explain the initial events of nicotine dependence thus derives from its selectivity, displayed at every level of organization: regional, neuronal, cellular, and stoichiometric.
Selective upregulation would directly result in modified neuronal excitability and neuronal interactions. As noted above, in the context of nicotine dependence, selective upregulation presently has been studied in detail only in midbrain and in the perforant path. Thus it remains an audacious hypothesis that the initial stages of nicotine dependence can be explained solely by “selective upregulation,” with no additional mechanisms of regulation, adaptation, neuroadaptation, homeostasis, or plasticity.
Despite the selectivity described above, upregulation also displays an important generality. The upregulation of α4β2* nAChRs by chronic nicotine treatment has been replicated many times in numerous systems—transfected cell lines, neurons in culture, brain slices, and smokers’ brains (Albuquerque et al., 2009; Fu et al., 2009; Lester et al., 2009; Srinivasan et al., 2011). Upregulation is not accompanied by an increase in nAChR subunit mRNA (Marks et al., 1992; Huang et al., 2007). Instead, the membrane-permeant nicotine molecule appears to act intracellularly, as a selective pharmacological chaperone of acetylcholine receptor and stoichiometry (SePhaChARNS) (Kuryatov et al., 2005; Sallette et al., 2005; Lester et al., 2009). SePhaChARNS arises in part from the thermodynamics of pharmacological chaperoning: ligand binding, especially at subunit interfaces, stabilizes AChRs during assembly and maturation, and this stabilization is most pronounced for the highest-affinity nAChR subunit compositions (especially α4β2*), stoichiometries, and functional states of nAChRs.
Upregulation Magnifies Both Activation and Desensitization
Another general aspect of upregulation is its applicability to two functional states induced by nicotine at nAChRs—activation and desensitization (Figure 3). Smoked nicotine acts differently from ACh in three ways (Lester et al., 2009). (1) Acetylcholinesterase does not hydrolyze nicotine; therefore, nicotine remains near nAChRs thousands of times longer than ACh. (2) Nicotine efficiently permeates membranes; therefore, it accumulates within cells (Putney and Borzelleca, 1971; Lester et al., 2009). (3) Nicotine activates α4β2 nAChRs ~400-fold more effectively than it activates muscle-type nAChRs, because of cation-π and H-bond interactions at the agonist binding site (Xiu et al., 2009). These factors lead nicotine to activate and desensitize the basal and nicotine-upregulated nAChRs for prolonged periods (minutes to hours). Therefore, desensitization influences actions of exogenous nicotine more than of endogenous ACh.
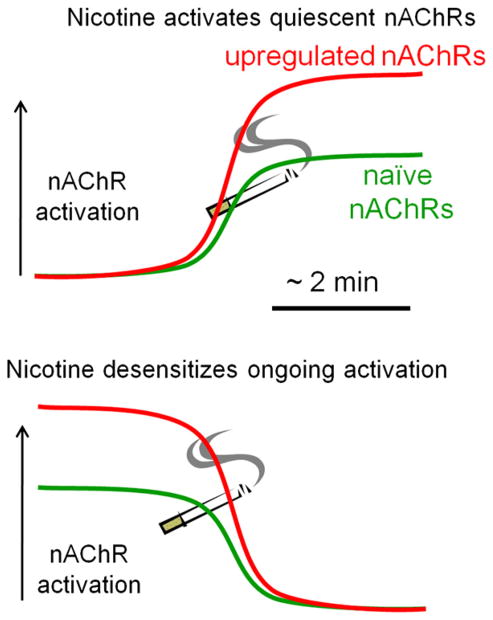
Figure 3. A Graphical View that Upregulation of nAChRs Can Amplify Both the Effects of nAChR Activation and the Effects of Desensitization.
The vertical black arrow represents the level of nAChR activation at a synapse, and the x axis represents the time course of activation and/or desensitization. The cigarette represents an acute exposure to nicotine, in the context of either nicotine-naive nAChRs (green) or nicotine-upregulated receptors (red). (Top) Exposure to nicotine produces stronger activation at upregulated receptors than at naive nAChRs, because upregulated nAChRs are both more numerous and more sensitive.
(Bottom) A synapse where ongoing endogenous ACh mediates stronger nAChR activation than at a naive synapse. Desensitization then produces a correspondingly larger decrement of activity. The most common example of such a desensitizing response to nicotine occurs at the presynaptic terminals of nigrostriatal dopaminergic neurons (Xiao et al., 2009).
In summary, upregulation due to chronic nicotine can magnify either activation or desensitization by acute nicotine. While it has been debated whether the acute effects of nicotine arise from activation or from desensitization, in the contemporary view (Figure 3) (Picciotto et al., 2008) both are thought to occur at appropriate neurons and synapses.
An Inadvertent Therapeutic Effect: Parkinson’s Disease Neuroprotection
At first glance, nicotine addiction and Parkinson’s disease seem related only by the participation of neighboring dopaminergic neuron populations: the former involves dopamine release from VTA neurons, and the latter involves degeneration in the substantia nigra pars compacta. In fact, more than 50 studies document an inverse correlation between a person’s history of tobacco use and his/her risk of Parkinson’s disease (Ritz et al., 2007). The effect is remarkably large—roughly a factor of two—when one considers that it derives from retrospective epidemiological studies (Hernán et al., 2002). Some Parkinson’s disease cases (~10%) are directly linked to genetic mutations. However, when all genetic factors are eliminated by studying monozygotic twins who are discordant for both tobacco use and Parkinson’s disease, tobacco smoking and chewing still decrease the risk of Parkinson’s disease (Tanner et al., 2002; Wirdefeldt et al., 2005). Could selective upregulation contribute to the apparent neuroprotective effects? We discuss three possible mechanisms.
One mechanism may be via regulation of nAChR-containing circuits (Nashmi et al., 2007; Xiao et al., 2009). While chronic nicotine does not change the abundance or function of α4* nAChRs in the somata of substantia nigra pars compacta dopaminergic neurons, it does suppress baseline firing rates of these DA neurons. In mice exposed to chronic nicotine, GABA neurons in substantia nigra pars reticulata have increased baseline firing rates, both in brain slices and in anesthetized animals. These contrasting effects on GABA and DA neurons are due to upregulated α4* nAChR responses in GABA neurons, at both somata and synaptic terminals. Thus chronic nicotine could regularize the firing rates of substantia nigra DA neurons, preventing them from experiencing bursts that could lead to excitotoxic Ca2+ influx.
Another neuroprotective mechanism may occur at nerve terminals in the striatum. Chronic nicotine upregulates α4* nAChRs in dopaminergic presynaptic terminals, apparently leading to increased resting dopamine release from those terminals. This effect produces a basal decrease in the level of glutamate release from corticostriatal neurons (Xiao et al., 2009). The process may counteract the increased effectiveness of corticostriatal glutamatergic inputs during degeneration of the DA system.
A third neuroprotective mechanism may operate entirely within DA neurons. The chaperoning of nAChRs by nicotine enhances the export of α4β2 nAChRs from the endoplasmic reticulum (ER), and this leads to a general increase in ER exit sites (Srinivasan et al., 2011). This aspect of SePhaChARNS eventually leads to plasma membrane upregulation. We hypothesize that, in addition, this process lowers the demands on the general proteostatic machinery in the ER, thereby altering ER stress, which is frequently invoked as a toxic mechanism in Parkinson’s disease.
A Second Inadvertent Therapeutic Effect: ADNFLE
Autosomal-dominant nocturnal frontal lobe epilepsy (ADNFLE) is caused by missense mutations in either the α4 or the β2 subunit. Several strains of knock-in mice bearing these mutations have seizure phenotypes related to ADNFLE (Klaassen et al., 2006; Teper et al., 2007; Xu et al., 2010), but α4 KO and β2 KO mice display no seizure phenotypes, implying that ADNFLE has a subtle, as yet unexplained pathophysiology. ADNFLE patients who use a nicotine patch or tobacco have fewer seizures (Willoughby et al., 2003; Brodtkorb and Picard, 2006). Recent data suggest that ADNFLE mutations bias nAChR composition away from the (α4)2(β2)3 stoichiometry, which is then re-established by nicotine exposure (Son et al., 2009). Thus, changes in α4β2* nAChR stoichiometry, subunit composition, and sorting could contribute both to the etiology of ADNFLE and to the inadvertent therapeutic effects of nicotine. This highly penetrant monogenic disease could eventually provide important clues to the pathophysiology and therapy of complex polygenic diseases such as Parkinson’s disease and nicotine dependence.
Thus, chaperoning of nascent nAChRs by smoking-relevant concentrations of nicotine represents a form of nicotine-nAChR interaction that is not directly associated with ion flux through active nAChRs. Chaperoning may provide a partial explanation for the pathological process of nicotine addiction and also for the inadvertent therapeutic effects of tobacco use in Parkinson’s disease and ADNFLE. Some effects of chaperoning may actually occur at the level of nAChR stabilization in the endoplasmic reticulum, and others arise from the consequent upregulation at the plasma membrane.
Conclusions
The Introduction posed the problem of explaining how manipulations of nicotinic synapses, which have been considered all-or-none machines, can produce the graded modulation of neuronal circuits and behaviors. Here we summarize the four (admittedly partial) explanations. First, recent evidence supports the graded “volume transmission” hypothesis (Ren et al., 2011). Second, the prototoxin lynx can function, probably both intracellularly and extracellularly, to direct the localization and activity of nAChRs. Absence of lynx has the profound modulatory effect of lengthening the critical period for ocular dominance plasticity. Third, α7 nAChRs can be activated in extrasynaptic regions by ambient concentrations of choline, with possible consequences for neuronal development as well as for circuit function during schizophrenia. Finally, the pharmacokinetics and stability of nicotine allow it to influence nAChRs in environments not reached by acetylcholine itself—extracellularly on somata, and intracellularly in the ER, where nicotine functions as a pharmacological chaperone to upregulate certain HS receptors. Furthermore, nicotine’s persistence leads to desensitization of nAChRs.
For more than four centuries, nicotinic systems have unfortunately played a role in drug abuse, but we have reviewed ways in which nicotinic systems can also be manipulated to provide help for neural illnesses such as Parkinson’s disease, cognitive decline, epilepsy, and schizophrenia. Nicotinic systems will continue to serve as touchstones for advances in neuroscience.
Acknowledgments
We thank William Proctor and Susan Moriguchi for help with Figure 2 and T.K. Hensch, T.N. Wiesel, and R.L. Parker for helpful discussions. We received support from AG-33954, DA-11729, MH-86386, NS-11756, and the California Tobacco-Related Disease Research Program (17RT-0127, 19KT-0032). J.M.M. is founder and shareholder of Ophidion, Inc. She has applied for U.S. patents 10322359 and 20080221013, on the use of lynx for therapeutic purposes. R.F. has received U.S. patent 10322359 on the use of alpha7 nAChR sequence variants in schizophrenia diagnosis. H.A.L. has received U.S. patent 6753456 on mice with hypersenitive alpha4 nicotinic receptors.
References
- Adams CE. Comparison of α7 nicotinic acetylcholine receptor development in the hippocampal formation of C3H and DBA/2 mice. Brain Res Dev Brain Res. 2003;143:137–149. doi: 10.1016/s0165-3806(03)00106-8. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX. Neuronal nicotinic acetylcholine receptor activation modulates γ-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther. 1997;283:1396–1411. [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Nicotinic receptor activation in human cerebral cortical interneurons: A mechanism for inhibition and disinhibition of neuronal networks. J Neurosci. 2000;20:66–75. doi: 10.1523/JNEUROSCI.20-01-00066.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, Grando SA. The nicotinic receptor antagonists abolish pathobiologic effects of tobacco-derived nitrosamines on BEP2D cells. J Cancer Res Clin Oncol. 2006;132:653–663. doi: 10.1007/s00432-006-0113-9. [DOI] [PubMed] [Google Scholar]
- Arredondo J, Chernyavsky AI, Grando SA. SLURP-1 and -2 in normal, immortalized and malignant oral keratinocytes. Life Sci. 2007;80:2243–2247. doi: 10.1016/j.lfs.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer S, Stürzebecher AS, Jüttner R, Santos-Torres J, Hanack C, Frahm S, Liehl B, Ibañez-Tallon I. Silencing neurotransmission with membrane-tethered toxins. Nat Methods. 2010;7:229–236. doi: 10.1038/nmeth.1425. [DOI] [PubMed] [Google Scholar]
- Aznavour N, Watkins KC, Descarries L. Postnatal development of the cholinergic innervation in the dorsal hippocampus of rat: Quantitative light and electron microscopic immunocytochemical study. J Comp Neurol. 2005;486:61–75. doi: 10.1002/cne.20501. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: From molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Singer W. Modulation of visual cortical plasticity by acetylcholine and noradrenaline. Nature. 1986;320:172–176. doi: 10.1038/320172a0. [DOI] [PubMed] [Google Scholar]
- Bernard C. Action du curare et de la nicotine sur le système nerveux et sur le système musculaire. C R Soc Biol. 1851:2. [Google Scholar]
- Bertrand D, Picard F, Le Hellard S, Weiland S, Favre I, Phillips H, Bertrand S, Berkovic SF, Malafosse A, Mulley J. How mutations in the nAChRs can cause ADNFLE epilepsy. Epilepsia. 2002;43(Suppl 5):112–122. doi: 10.1046/j.1528-1157.43.s.5.16.x. [DOI] [PubMed] [Google Scholar]
- Brandt A. The Cigarette Century. New York: Basic Books; 2007. [Google Scholar]
- Brodie M. Low concentrations of nicotine increase the firing rate of neurons of the rat ventral tegmental area in vitro. In: Thurau FAK, editor. Advances in Pharmacological Sciences. Basel: Birkhäuser Verlag; 1991. pp. 373–377. [Google Scholar]
- Brodtkorb E, Picard F. Tobacco habits modulate autosomal dominant nocturnal frontal lobe epilepsy. Epilepsy Behav. 2006;9:515–520. doi: 10.1016/j.yebeh.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Russell DS, Picciotto MR. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem. 2003;84:1431–1441. doi: 10.1046/j.1471-4159.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- Cheng SB, Amici SA, Ren XQ, McKay SB, Treuil MW, Lindstrom JM, Rao J, Anand R. Presynaptic targeting of α4β 2 nicotinic acetylcholine receptors is regulated by neurexin-1β. J Biol Chem. 2009;284:23251–23259. doi: 10.1074/jbc.M109.017384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimienti F, Hogg RC, Plantard L, Lehmann C, Brakch N, Fischer J, Huber M, Bertrand D, Hohl D. Identification of SLURP-1 as an epidermal neuromodulator explains the clinical phenotype of Mal de Meleda. Hum Mol Genet. 2003;12:3017–3024. doi: 10.1093/hmg/ddg320. [DOI] [PubMed] [Google Scholar]
- Chou JH, Bargmann CI, Sengupta P. The Caenorhabditis elegans odr-2 gene encodes a novel Ly-6-related protein required for olfaction. Genetics. 2001;157:211–224. doi: 10.1093/genetics/157.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Couey JJ, Meredith RM, Spijker S, Poorthuis RB, Smit AB, Brussaard AB, Mansvelder HD. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54:73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, Kerwin R, Perry R, Perry E. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: α-bungarotoxin and nicotine binding in the thalamus. J Neurochem. 1999;73:1590–1597. doi: 10.1046/j.1471-4159.1999.0731590.x. [DOI] [PubMed] [Google Scholar]
- Cragg SJ. Meaningful silences: How dopamine listens to the ACh pause. Trends Neurosci. 2006;29:125–131. doi: 10.1016/j.tins.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001;31:349–352. doi: 10.1016/s0896-6273(01)00379-8. [DOI] [PubMed] [Google Scholar]
- Darvas M, Morsch M, Racz I, Ahmadi S, Swandulla D, Zimmer A. Modulation of the Ca2+ conductance of nicotinic acetylcholine receptors by Lypd6. Eur Neuropsychopharmacol. 2009;19:670–681. doi: 10.1016/j.euroneuro.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Hippocampal nAChRs mediate nicotine withdrawal-related learning deficits. Eur Neuropsychopharmacol. 2009;19:551–561. doi: 10.1016/j.euroneuro.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, Chen L. Crystal structure of the extracellular domain of nAChR α1 bound to α-bungarotoxin at 1.94 A resolution. Nat Neurosci. 2007;10:953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- Derrington EA, Borroni E. The developmental expression of the cholinergic-specific antigen Chol-1 in the central and peripheral nervous system of the rat. Brain Res Dev Brain Res. 1990;52:131–140. doi: 10.1016/0165-3806(90)90228-q. [DOI] [PubMed] [Google Scholar]
- Dessaud E, Salaün D, Gayet O, Chabbert M, deLapeyrière O. Identification of lynx2, a novel member of the ly-6/neurotoxin superfamily, expressed in neuronal subpopulations during mouse development. Mol Cell Neurosci. 2006;31:232–242. doi: 10.1016/j.mcn.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol. 1997;53:603–625. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Difranza JR. Thwarting science by protecting the received wisdom on tobacco addiction from the scientific method. Harm Reduct J. 2010;7:26. doi: 10.1186/1477-7517-7-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Rigotti NA, McNeill AD, Ockene JK, Savageau JA, St Cyr D, Coleman M. Initial symptoms of nicotine dependence in adolescents. Tob Control. 2000;9:313–319. doi: 10.1136/tc.9.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney AA, Aoki C, Hawken MJ. Gain modulation by nicotine in macaque v1. Neuron. 2007;56:701–713. doi: 10.1016/j.neuron.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, Perry DC. Adult and peria-dolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney SR, Miwa JM, Bupp S, Heintz N, McIntosh JM, Bencherif M, et al. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity α 6 nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DSM-V Nicotine Workgroup. Nicotine-use disorder. 2010 http://www.dsm5.org/ProposedRevisions/Pages/proposedrevision.aspx?rid=459.
- Erlich PM, Hoffman SN, Rukstalis M, Han JJ, Chu X, Linda Kao WH, Gerhard GS, Stewart WF, Boscarino JA. Nicotinic acetylcholine receptor genes on chromosome 15q25.1 are associated with nicotine and opioid dependence severity. Hum Genet. 2010;128:491–499. doi: 10.1007/s00439-010-0876-6. [DOI] [PubMed] [Google Scholar]
- Exley R, Cragg SJ. Presynaptic nicotinic receptors: A dynamic and diverse cholinergic filter of striatal dopamine neurotransmission. Br J Pharmacol. 2008;153(Suppl 1):S283–S297. doi: 10.1038/sj.bjp.0707510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonck C, Cohen BN, Nashmi R, Whiteaker P, Wagenaar DA, Rodrigues-Pinguet N, Deshpande P, McKinney S, Kwoh S, Munoz J, et al. Novel seizure phenotype and sleep disruptions in knock-in mice with hypersensitive α 4* nicotinic receptors. J Neurosci. 2005;25:11396–11411. doi: 10.1523/JNEUROSCI.3597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011 doi: 10.1038/nature09797. in press Published online January 30, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier CJ, Rollins YD, Breese CR, Leonard S, Freedman R, Dunwiddie TV. Acetylcholine activates an α-bungarotoxin-sensitive nicotinic current in rat hippocampal interneurons, but not pyramidal cells. J Neurosci. 1998;18:1187–1195. doi: 10.1523/JNEUROSCI.18-04-01187.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XW, Lindstrom J, Spindel ER. Nicotine activates and up-regulates nicotinic acetylcholine receptors in bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;41:93–99. doi: 10.1165/rcmb.2008-0352OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahring LC, Rogers SW. Nicotinic acetylcholine receptor expression in the hippocampus of 27 mouse strains reveals novel inhibitory circuitry. Hippocampus. 2008;18:737–749. doi: 10.1002/hipo.20430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher JP, Orozco-Cabal LF, Liu J, Shinnick-Gallagher P. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008;583:215–225. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George TP, Sernyak MJ, Ziedonis DM, Woods SW. Effects of clozapine on smoking in chronic schizophrenic outpatients. J Clin Psychiatry. 1995;56:344–346. [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: Native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Gaimarri A, Zanardi A, Clementi F, Zoli M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem Pharmacol. 2007;74:1102–1111. doi: 10.1016/j.bcp.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning: Implications for addiction. Mol Neurobiol. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, Dani JA. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature. 1996;383:713–716. doi: 10.1038/383713a0. [DOI] [PubMed] [Google Scholar]
- Hansen SB, Taylor P. Galanthamine and non-competitive inhibitor binding to ACh-binding protein: Evidence for a binding site on non-alpha-subunit interfaces of heteromeric neuronal nicotinic receptors. J Mol Biol. 2007;369:895–901. doi: 10.1016/j.jmb.2007.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. EMBO J. 2005;24:3635–3646. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen HM, Xiao Y, Rice T, Bracci PM, Wrensch MR, Sison JD, Chang JS, Smirnov IV, Patoka J, Seldin MF, et al. Fine mapping of chromosome 15q25.1 lung cancer susceptibility in African-Americans. Hum Mol Genet. 2010;19:3652–3661. doi: 10.1093/hmg/ddq268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath CJ, Picciotto MR. Nicotine-induced plasticity during development: Modulation of the cholinergic system and long-term consequences for circuits involved in attention and sensory processing. Neuropharmacology. 2009;56(Suppl 1):254–262. doi: 10.1016/j.neuropharm.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann S, Asouline G, Ballivet G, Boulter M, Connolly J, Deneris E, Evans K, Forrest J, Gardener P, Goldman D, et al. The Neural and Muscle Nicotinic Acetylcholine Receptors. New York: Plenum Press; 1987. [Google Scholar]
- Hernán MA, Takkouche B, Caamaño-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52:276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaër E, Trocmé-Thibierge C, Sarter M. Enhancement of attentional performance by selective stimulation of α4β2(*) nAChRs: Underlying cholinergic mechanisms. Neuro-psychopharmacology. 2010;35:1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska M, Keefe J, Wert D, Tekinay AB, Hulce JJ, Ibañez-Tallon I, Nishi R. Prostate stem cell antigen is an endogenous lynx1-like prototoxin that antagonizes α7-containing nicotinic receptors and prevents programmed cell death of parasympathetic neurons. J Neurosci. 2009;29:14847–14854. doi: 10.1523/JNEUROSCI.2271-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LZ, Abbott LC, Winzer-Serhan UH. Effects of chronic neonatal nicotine exposure on nicotinic acetylcholine receptor binding, cell death and morphology in hippocampus and cerebellum. Neuroscience. 2007;146:1854–1868. doi: 10.1016/j.neuroscience.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Kisley MA, McCarthy L, Freedman R, Ross RG. Diminished cerebral inhibition in neonates associated with risk factors for schizophrenia: Parental psychosis, maternal depression, and nicotine use. Schizophr Bull. 2010 doi: 10.1093/schbul/sbq036. in press Published online April 19, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez-Tallon I, Miwa JM, Wang HL, Adams NC, Crabtree GW, Sine SM, Heintz N. Novel modulation of neuronal nicotinic acetylcholine receptors by association with the endogenous prototoxin lynx1. Neuron. 2002;33:893–903. doi: 10.1016/s0896-6273(02)00632-3. [DOI] [PubMed] [Google Scholar]
- Improgo MR, Scofield MD, Tapper AR, Gardner PD. The nicotinic acetylcholine receptor CHRNA5/A3/B4 gene cluster: Dual role in nicotine addiction and lung cancer. Prog Neurobiol. 2010;92:212–226. doi: 10.1016/j.pneurobio.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Jeanclos EM, Lin L, Treuil MW, Rao J, DeCoster MA, Anand R. The chaperone protein 14-3-3η interacts with the nicotinic acetylcholine receptor α 4 subunit. Evidence for a dynamic role in subunit stabilization. J Biol Chem. 2001;276:28281–28290. doi: 10.1074/jbc.M011549200. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Tobacco dependence, the insular cortex and the hypocretin connection. Pharmacol Biochem Behav. 2011;97:700–707. doi: 10.1016/j.pbb.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen A, Glykys J, Maguire J, Labarca C, Mody I, Boulter J. Seizures and enhanced cortical GABAergic inhibition in two mouse models of human autosomal dominant nocturnal frontal lobe epilepsy. Proc Natl Acad Sci USA. 2006;103:19152–19157. doi: 10.1073/pnas.0608215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsy-chopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J. Nicotine acts as a pharmacological chaperone to up-regulate human α4β2 acetylcholine receptors. Mol Pharmacol. 2005;68:1839–1851. doi: 10.1124/mol.105.012419. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini WH, Lindstrom JM. Acetylcholine receptor (AChR) α5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (α4β2)2α5 AChR function. Mol Pharmacol. 2011;79:119–125. doi: 10.1124/mol.110.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdell SJ, Gee VJ, Harkness PC, Doward AI, Baker ER, Gibb AJ, Millar NS. RIC-3 enhances functional expression of multiple nicotinic acetylcholine receptor subtypes in mammalian cells. Mol Pharmacol. 2005;68:1431–1438. doi: 10.1124/mol.105.017459. [DOI] [PubMed] [Google Scholar]
- Leonard S, Gault J, Hopkins J, Logel J, Vianzon R, Short M, Drebing C, Berger R, Venn D, Sirota P, et al. Association of promoter variants in the α7 nicotinic acetylcholine receptor subunit gene with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- Lester RA. Activation and desensitization of heteromeric neuronal nicotinic receptors: Implications for non-synaptic transmission. Bioorg Med Chem Lett. 2004;14:1897–1900. doi: 10.1016/j.bmcl.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Lester HA, Xiao C, Srinivasan R, Son CD, Miwa J, Pantoja R, Banghart MR, Dougherty DA, Goate AM, Wang JC. Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery. AAPS J. 2009;11:167–177. doi: 10.1208/s12248-009-9090-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem Pharmacol. 2007;74:1182–1191. doi: 10.1016/j.bcp.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levitin F, Weiss M, Hahn Y, Stern O, Papke RL, Matusik R, Nandana SR, Ziv R, Pichinuk E, Salame S, et al. PATE gene clusters code for multiple, secreted TFP/Ly-6/uPAR proteins that are expressed in reproductive and neuron-rich tissues and possess neuromodulatory activity. J Biol Chem. 2008;283:16928–16939. doi: 10.1074/jbc.M801454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Guo-Ross S, Lewis DV, Turner D, White AM, Wilson WA, Swartzwelder HS. Dietary prenatal choline supplementation alters postnatal hippocampal structure and function. J Neurophysiol. 2004;91:1545–1555. doi: 10.1152/jn.00785.2003. [DOI] [PubMed] [Google Scholar]
- Lin L, Jeanclos EM, Treuil M, Braunewell KH, Gundelfinger ED, Anand R. The calcium sensor protein visinin-like protein-1 modulates the surface expression and agonist sensitivity of the α 4β 2 nicotinic acetylcholine receptor. J Biol Chem. 2002;277:41872–41878. doi: 10.1074/jbc.M206857200. [DOI] [PubMed] [Google Scholar]
- Liu Z, Neff RA, Berg DK. Sequential interplay of nicotinic and GABAergic signaling guides neuronal development. Science. 2006;314:1610–1613. doi: 10.1126/science.1134246. [DOI] [PubMed] [Google Scholar]
- Luntz-Leybman V, Bickford PC, Freedman R. Cholinergic gating of response to auditory stimuli in rat hippocampus. Brain Res. 1992;587:130–136. doi: 10.1016/0006-8993(92)91437-j. [DOI] [PubMed] [Google Scholar]
- Lyukmanova EN, Shenkarev ZO, Shulepko MA, Mineev KS, D’Hoedt D, Kasheverov IE, Filkin SY, Krivolapova AP, Janickova H, Dolezal V, et al. NMR structure and action on nicotinic acetylcholine receptors of water-soluble domain of human lynx1. J Biol Chem. 2011;286:10618–10627. doi: 10.1074/jbc.M110.189100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mao D, Perry DC, Yasuda RP, Wolfe BB, Kellar KJ. The α4β2α5 nicotinic cholinergic receptor in rat brain is resistant to up-regulation by nicotine in vivo. J Neurochem. 2008;104:446–456. doi: 10.1111/j.1471-4159.2007.05011.x. [DOI] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009;34:1743–1752. doi: 10.1038/npp.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A. Review. Neurobiology of nicotine dependence. Philos Trans R Soc Lond B Biol Sci. 2008;363:3159–3168. doi: 10.1098/rstb.2008.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. J Pharmacol Exp Ther. 1983;226:817–825. [PubMed] [Google Scholar]
- Marks MJ, Campbell SM, Romm E, Collins AC. Genotype influences the development of tolerance to nicotine in the mouse. J Pharmacol Exp Ther. 1991;259:392–402. [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SV, Law AJ, Lipska BK, Dávila-García MI, Zamora ED, Mitkus SN, Vakkalanka R, Straub RE, Weinberger DR, Kleinman JE, Hyde TM. α7 nicotinic acetylcholine receptor mRNA expression and binding in postmortem human brain are associated with genetic variation in neuregulin 1. Hum Mol Genet. 2007;16:2921–2932. doi: 10.1093/hmg/ddm253. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, McIntosh JM, Quik M. Differential regulation of mesolimbic α 3/α 6 β 2 and α 4 β 2 nicotinic acetylcholine receptor sites and function after long-term oral nicotine to monkeys. J Pharmacol Exp Ther. 2006a;318:381–388. doi: 10.1124/jpet.106.104414. [DOI] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Bordia T, Fan H, Tyndale RF, Langston JW, McIntosh JM, Quik M. Increases in α4* but not α3*/α6* nicotinic receptor sites and function in the primate striatum following chronic oral nicotine treatment. J Neurochem. 2006b;96:1028–1041. doi: 10.1111/j.1471-4159.2005.03646.x. [DOI] [PubMed] [Google Scholar]
- McClure-Begley TD, King NM, Collins AC, Stitzel JA, Wehner JM, Butt CM. Acetylcholine-stimulated [3H]GABA release from mouse brain synaptosomes is modulated by α4β2 and α4α5β2 nicotinic receptor subtypes. Mol Pharmacol. 2009;75:918–926. doi: 10.1124/mol.108.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meck WH, Williams CL. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neurosci Biobehav Rev. 2003;27:385–399. doi: 10.1016/s0149-7634(03)00069-1. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Ibanez-Tallon I, Crabtree GW, Sánchez R, Sali A, Role LW, Heintz N. lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron. 1999;23:105–114. doi: 10.1016/s0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Stevens TR, King SL, Caldarone BJ, Ibanez-Tallon I, Xiao C, Fitzsimonds RM, Pavlides C, Lester HA, Picciotto MR, Heintz N. The prototoxin lynx1 acts on nicotinic acetylcholine receptors to balance neuronal activity and survival in vivo. Neuron. 2006;51:587–600. doi: 10.1016/j.neuron.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330:1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugnaini M, Garzotti M, Sartori I, Pilla M, Repeto P, Heidbreder CA, Tessari M. Selective down-regulation of [(125)I]Y0-α-conotoxin MII binding in rat mesostriatal dopamine pathway following continuous infusion of nicotine. Neuroscience. 2006;137:565–572. doi: 10.1016/j.neuroscience.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Myers CS, Taylor RC, Moolchan ET, Heishman SJ. Dose-related enhancement of mood and cognition in smokers administered nicotine nasal spray. Neuropsychopharmacology. 2008;33:588–598. doi: 10.1038/sj.npp.1301425. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, et al. Chronic nicotine cell specifically upregulates functional α 4* nicotinic receptors: Basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, Perry DC. Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. J Pharmacol Exp Ther. 2003;307:1090–1097. doi: 10.1124/jpet.103.056408. [DOI] [PubMed] [Google Scholar]
- Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, Allensworth D, Ellis J, Zerbe GO, Leonard S, Stevens KE, et al. Proof-of-concept trial of an α7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–638. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- Pape HC. GABAergic neurons: Gate masters of the amygdala, mastered by dopamine. Neuron. 2005;48:877–879. doi: 10.1016/j.neuron.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Papke RL, Thinschmidt JS. The correction of α7 nicotinic acetylcholine receptor concentration-response relationships in Xenopus oocytes. Neurosci Lett. 1998;256:163–166. doi: 10.1016/s0304-3940(98)00786-1. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Neuroscience. Maternal effects on schizophrenia risk. Science. 2007;318:576–577. doi: 10.1126/science.1150196. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Gross SD, Collins AC. An autoradiographic analysis of cholinergic receptors in mouse brain after chronic nicotine treatment. J Pharmacol Exp Ther. 1991;258:1127–1136. [PubMed] [Google Scholar]
- Penton RE, Quick MW, Lester RA. Short- and long-lasting consequences of in vivo nicotine treatment on hippocampal excitability. J Neurosci. 2011;31:2584–2594. doi: 10.1523/JNEUROSCI.4362-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer CK, Stein V, Keating DJ, Maier H, Rinke I, Rudhard Y, Hentschke M, Rune GM, Jentsch TJ, Hübner CA. NKCC1-dependent GABAergic excitation drives synaptic network maturation during early hippocampal development. J Neurosci. 2009;29:3419–3430. doi: 10.1523/JNEUROSCI.1377-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pietilä K, Lähde T, Attila M, Ahtee L, Nordberg A. Regulation of nicotinic receptors in the brain of mice withdrawn from chronic oral nicotine treatment. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:176–182. doi: 10.1007/pl00005152. [DOI] [PubMed] [Google Scholar]
- Poorthuis RB, Goriounova NA, Couey JJ, Mansvelder HD. Nicotinic actions on neuronal networks for cognition: General principles and long-term consequences. Biochem Pharmacol. 2009;78:668–676. doi: 10.1016/j.bcp.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Putney JW, Jr, Borzelleca JF. On the mechanisms of 14C-nicotine distribution in rat submaxillary gland in vitro. J Pharmacol Exp Ther. 1971;178:180–191. [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Ren J, Qin C, Hu F, Tan J, Qiu L, Zhao S, Feng G, Luo M. Habenula “cholinergic” neurons corelease glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. Neuron. 2011;69:445–452. doi: 10.1016/j.neuron.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Ritz B, Ascherio A, Checkoway H, Marder KS, Nelson LM, Rocca WA, Ross GW, Strickland D, Van Den Eeden SK, Gorell J. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64:990–997. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- Royal College of Physicians. A report by the Tobacco Advisory Group of the Royal College of Physicians. London: RCP; 2007. Harm reduction in nicotine addiction: Helping people who can’t quit. [Google Scholar]
- Saccone NL, Schwantes-An TH, Wang JC, Grucza RA, Breslau N, Hatsukami D, Johnson EO, Rice JP, Goate AM, Bierut LJ. Multiple cholinergic nicotinic receptor genes affect nicotine dependence risk in African and European Americans. Genes Brain Behav. 2010;9:741–750. doi: 10.1111/j.1601-183X.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillers-Thiery A, Soudant M, Prado de Carvalho L, Changeux JP, Corringer PJ. Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron. 2005;46:595–607. doi: 10.1016/j.neuron.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, Kellar KJ. Nicotinic cholinergic receptor binding sites in the brain: Regulation in vivo. Science. 1983;220:214–216. doi: 10.1126/science.6828889. [DOI] [PubMed] [Google Scholar]
- Schwarz J, Schwarz SC, Dorigo O, Stützer A, Wegner F, Labarca C, Deshpande P, Gil JS, Berk AJ, Lester HA. Enhanced expression of hypersensitive α4* nAChR in adult mice increases the loss of midbrain dopaminergic neurons. FASEB J. 2006;20:935–946. doi: 10.1096/fj.05-5497com. [DOI] [PubMed] [Google Scholar]
- Sekhon HS, Song P, Jia Y, Lindstrom J, Spindel ER. Expression of lynx1 in developing lung and its modulation by prenatal nicotine exposure. Cell Tissue Res. 2005;320:287–297. doi: 10.1007/s00441-005-1077-9. [DOI] [PubMed] [Google Scholar]
- Son CD, Moss FJ, Cohen BN, Lester HA. Nicotine normalizes intracellular subunit stoichiometry of nicotinic receptors carrying mutations linked to autosomal dominant nocturnal frontal lobe epilepsy. Mol Pharmacol. 2009;75:1137–1148. doi: 10.1124/mol.108.054494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R, Pantoja R, Moss FJ, Mackey EDW, Son CD, Miwa J, Lester HA. Nicotine up-regulates α4β2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol. 2011;137:59–79. doi: 10.1085/jgp.201010532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles JR, Van Helden D, Bartol TM, Jr, Salpeter EE, Salpeter MM. Miniature endplate current rise times less than 100 microseconds from improved dual recordings can be modeled with passive acetylcholine diffusion from a synaptic vesicle. Proc Natl Acad Sci USA. 1996;93:5747–5752. doi: 10.1073/pnas.93.12.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Dani JA. Dopamine enables in vivo synaptic plasticity associated with the addictive drug nicotine. Neuron. 2009;63:673–682. doi: 10.1016/j.neuron.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, Goldman SM, Aston DA, Ottman R, Ellenberg J, Mayeux R, Langston JW. Smoking and Parkinson’s disease in twins. Neurology. 2002;58:581–588. doi: 10.1212/wnl.58.4.581. [DOI] [PubMed] [Google Scholar]
- Tekinay AB, Nong Y, Miwa JM, Lieberam I, Ibanez-Tallon I, Greengard P, Heintz N. A role for LYNX2 in anxiety-related behavior. Proc Natl Acad Sci USA. 2009;106:4477–4482. doi: 10.1073/pnas.0813109106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teper Y, Whyte D, Cahir E, Lester HA, Grady SR, Marks MJ, Cohen BN, Fonck C, McClure-Begley T, McIntosh JM, et al. Nicotine-induced dystonic arousal complex in a mouse line harboring a human auto-somal-dominant nocturnal frontal lobe epilepsy mutation. J Neurosci. 2007;27:10128–10142. doi: 10.1523/JNEUROSCI.3042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsetlin V, Utkin Y, Kasheverov I. Polypeptide and peptide toxins, magnifying lenses for binding sites in nicotinic acetylcholine receptors. Biochem Pharmacol. 2009;78:720–731. doi: 10.1016/j.bcp.2009.05.032. [DOI] [PubMed] [Google Scholar]
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Meyer EM, Papke RL. Regulation of neuronal function by choline and 4OH-GTS-21 through α 7 nicotinic receptors. J Neurophysiol. 2003;89:1797–1806. doi: 10.1152/jn.00943.2002. [DOI] [PubMed] [Google Scholar]
- Venables P. Input dysfunction in schizophrenia. In: Maher B, editor. Experimental Personality Research. Orlando, FL: Academic Press; 1967. pp. 1–64. [PubMed] [Google Scholar]
- Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, Li JH, Wang H, Yang H, Ulloa L, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- Wang JC, Cruchaga C, Saccone NL, Bertelsen S, Liu P, Budde JP, Duan W, Fox L, Grucza RA, Kern J, et al. GELCC collaborators. Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet. 2009;18:3125–3135. doi: 10.1093/hmg/ddp231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathey JC, Nass MM, Lester HA. Numerical reconstruction of the quantal event at nicotinic synapses. Biophys J. 1979;27:145–164. doi: 10.1016/S0006-3495(79)85208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek L, Maas JW, Jr, Muglia LM, Vogt SK, Muglia LJ. Temporal and regional regulation of gene expression by calcium-stimulated adenylyl cyclase activity during fear memory. PLoS ONE. 2010;5:e13385. doi: 10.1371/journal.pone.0013385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby JO, Pope KJ, Eaton V. Nicotine as an antiepileptic agent in ADNFLE: An N-of-one study. Epilepsia. 2003;44:1238–1240. doi: 10.1046/j.1528-1157.2003.58102.x-i1. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K, Gatz M, Pawitan Y, Pedersen NL. Risk and protective factors for Parkinson’s disease: A study in Swedish twins. Ann Neurol. 2005;57:27–33. doi: 10.1002/ana.20307. [DOI] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MN, Joiner WJ, Dean T, Yue Z, Smith CJ, Chen D, Hoshi T, Sehgal A, Koh K. SLEEPLESS, a Ly-6/neurotoxin family member, regulates the levels, localization and activity of Shaker. Nat Neurosci. 2010;13:69–75. doi: 10.1038/nn.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Nashmi R, McKinney S, Cai H, McIntosh JM, Lester HA. Chronic nicotine selectively enhances α4β2* nicotinic acetylcholine receptors in the nigrostriatal dopamine pathway. J Neurosci. 2009;29:12428–12439. doi: 10.1523/JNEUROSCI.2939-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu X, Puskar NL, Shanata JA, Lester HA, Dougherty DA. Nicotine binding to brain receptors requires a strong cation-pi interaction. Nature. 2009;458:534–537. doi: 10.1038/nature07768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mendrek A, Cohen MS, Monterosso J, Rodriguez P, Simon SL, Brody A, Jarvik M, Domier CP, Olmstead R, et al. Brain activity in cigarette smokers performing a working memory task: Effect of smoking abstinence. Biol Psychiatry. 2005;58:143–150. doi: 10.1016/j.biopsych.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Cohen BN, Zhu Y, Dziewczapolski G, Panda S, Lester HA, Heinemann SF, Contractor A. Altered activity-rest patterns in mice with a human autosomal-dominant nocturnal frontal lobe epilepsy mutation in the β2 nicotinic receptor. Mol Psychiatry. 2010 doi: 10.1038/mp.2010.78. in press Published online July 6, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Epstein MF, Wurtman RJ. Elevated choline concentration in neonatal plasma. Life Sci. 1980;26:1827–1831. doi: 10.1016/0024-3205(80)90585-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Berg DK. Reversible inhibition of GABAA receptors by α7-containing nicotinic receptors on the vertebrate postsynaptic neurons. J Physiol. 2007;579:753–763. doi: 10.1113/jphysiol.2006.124578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou FM, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. J Neurosci. 2009;29:4035–4043. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kranzler HR, Poling J, Gelernter J. Variation in the nicotinic acetylcholine receptor gene cluster CHRNA5-CHRNA3-CHRNB4 and its interaction with recent tobacco use influence cognitive flexibility. Neuropsychopharmacology. 2010;35:2211–2224. doi: 10.1038/npp.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]