Abstract
Due to its unique physicochemical properties and remarkable antimicrobial activity, nanosilver (nAg) is increasingly being used in a wide array of fields, including medicine and personal care products. Despite substantial progress being made towards the understanding of the acute toxicity of nAg, large knowledge gaps still exist on the assessment of its chronic toxicity to humans. Chronic effects of nAg, typically at low doses (i.e. sublethal doses) should be different from the acute toxicity at high doses (i.e., lethal doses), which is analogous to other environmental pollutants. Although a few review papers have elaborated the findings on nAg-mediated toxicity, most of them only discussed overt toxicity of nAg at high-level exposure and failed to evaluate the chronic and cumulative effects of nAg at sublethal doses. Therefore, it is necessary to more stringently scrutinize the sublethal toxicity of nAg under environmentally relevant conditions. Herein, we recapitulated recent findings on the sublethal effects of nAg toxicity performed by our groups and others. We then discussed the molecular mechanisms by which nAg exerts its toxicity under low concentrations and compared that with nAg-induced cell death.
Keywords: nanosilver, cell death, sublethal toxicity, molecular mechanisms
1. Introduction
With the rapid development of nanotechnology, a variety of engineered nanomaterials, including carbon nanotubes, gold nanoparticles, silver nanoparticles and quantum dots, have been extensively explored for applications in biology and medicine.1–4 Among these engineered nanomaterials, nanosilver (nAg) is the most commonly used inorganic metal-based particle. nAg, also referred to as silver nanoparticles, is a cluster of silver atoms that are at least one dimensional with a size ranging from 1 to 100 nm.5 Compared to its bulk counterpart, silver nanoparticles exhibit distinct physical, chemical and optical properties, as well as excellent antimicrobial activities, and they are widely used in a myriad of real life applications such as catalysis, electronics, food packaging materials, textiles, cosmetics and room sprays.5–8 In fact, more than 400 nAg-related products (over 30% of total nanoproducts) have been in the market around the world.9, 10 One of the extensive applications for nAg is in biomedicine such as wound dressing, coating of implantable devices, medical imaging and drug delivery.6, 11 Specifically, nAg has been added as a desirable component in a number of clinical products, including Actioat™, Silverline®, SilverSorb® and ON-Q SilverSoaker™.4 With the increasing usage of nAg in consumer and medical products, it is crucial to comprehensively understand its potential toxicity and the mechanism of toxicity under environmentally relevant conditions to promote its safe use in diagnostics and therapeutics.
Exposure dosages are critical for the toxic assessment of environmental pollutants, and nanomaterials are not different.12, 13 It should be noted that there are differences between nanoparticle and heavy metal pollutants in ionic form, although both of them induce toxicity in a dose-dependent manner. Their differences are mainly in the cellular uptake pathways and intracellular behaviors through which nanoparticles and metals pose toxicity to cells and organisms. Compared to metal ions, metal-based nanoparticles are easier to pass through biological barriers and cell membranes.14 In addition, metal nanoparticles have a large surface that has the potential to adsorb toxic substances and biological molecules on their surface, resulting in an increased reactivity, availability and toxicity.15 It has long been known that the chronic toxicity caused by environmental pollutants could be very different from their acute toxicity, as listed in Table 1. High-dose exposure to inorganic and organic pollutants such as arsenic, cadmium and methylmercury in occupational settings or by accident usually causes acute and often fatal effects, ranging from gastrointestinal distress or respiratory distress to death.16–18 In contrast, long-term exposure to these pollutants from contaminated food and drinking water can induce chronic organ injuries, diseases and cancer in adults,19, 20 as well as lead to the development of toxicity in the fetus even when the mother shows no poisoning symptoms.18, 21 Ambient particulate matter (PM) is another example. Long-term cumulative exposure to PM can cause cardiovascular diseases, respiratory diseases, and cancer in susceptible humans and animals, compared with air pollution, which leads to respiratory distress and death.22–25 Moreover, the signaling pathways that are activated at low doses are distinct from that activated at high doses when cellular and organismic defense systems are overwhelmed.12 Therefore, the health risk from exposure to a low dose of environmental pollutants, which is more relevant to real-world situations, should be particularly considered.
Table 1.
Toxicity induced by the representative environmental pollutants at the acute high dose and the chronic low dose.
| Pollutants | Adverse effects/toxicity |
Ref. | |
|---|---|---|---|
| Acute, high-dose | Chronic, low-dose | ||
| Arsenic (As) | Gastrointestinal distress and death | Dermal lesions, cardiovascular disease, liver disease, neuropathy, and carcinogenicity |
16, 19 |
| Cadmium (Cd) | Respiratory distress, hepatotoxicity and death |
Kidney tubular damage, bone disease (e.g. itai-itai disease) and carcinogenicity |
17, 20 |
| Methylmercury (MeHg) | Death | Nervous system diseases (e.g. Minamata disease), reproductive toxicity and developmental neurotoxicity |
18, 21 |
| Fine particulate matter (PM2.5, aerodynamic Diameter ≤ 2.5 µm) |
Cardiovascular disease, respiratory disease and death |
Higher risk for cardiovascular disease, respiratory disease, lung cancer and mortality |
22–25 |
The most common routes of nAg exposure to humans include inhalation, skin contact, food ingestion and medication.11, 26, 27 After translocation into the circulatory system, nAg could be transported to various internal organs, causing adverse effects in susceptible organs.5, 28 Clinical research and animal studies reported that the adverse effect of nAg is systemic neurotoxicity and death after acute exposure, and argyria, local inflammation, and organ damages after chronic exposure.5, 27, 29, 30 At the cellular level, nAg can cause overt cytotoxicity at high concentrations, including abnormalities of cellular morphology, enhancement of membrane permeability, significant decline of cell growth and even cell death.31–33 If the exposure levels are relatively low, no cell death can be observed; however, nAg can disturb the normal functions of cells at the molecular level.34–36 These preliminary studies suggest that low-dose nAg has the potential to pose chronic health hazards that warrant attention in future studies.
To date, most studies on silver focused on the acute effects or overt toxicity of nAg at lethal doses without considerable attention being paid to sublethal doses. In the current review, we recapitulate the potential detrimental effects of nAg under lethal and sublethal exposure conditions and highlight the molecular mechanisms responsible for nAg-induced sublethal cellular responses. It should be noted that the description of low doses in this review represents not necessarily the physiologically relevant doses, but the exposure levels, in which nAg induces adverse effects on animals and cells without lethal effects.
2. What are the sublethal effects of nAg?
It is well known that the toxicity induced by nAg is a dose-dependent process.26 nAg added into consumer products and coatings will slowly, mainly through laundering, release from its substrate material into domestic wastewater. It has been reported that nearly one-third nAg-containing products in the market in 2007 had the potential to disperse silver or silver nanoparticles into the environment.37 Although the exact concentration of nAg exposed to human has not been reported to date, it should be of relatively low levels that can be deduced from silver exposure levels under environmental and everyday settings. The probability for human daily exposure to very low levels of silver is mainly from food and drinking water, and less from air.5, 26 The average concentration of silver is 0.2–2 µg L−1 in surface water of rivers and lakes, 200–300 µg kg−1 in soils, and less than 1 × 10−6 µg L−1 in air.38 Given the extremely low background concentrations of silver in the environment, the addition of nAg due to human activities, even a small mass, will result in significant environmental risks.37 Moreover, silver in the form of nanoparticles exhibits an enhanced capability to penetrate protection barriers and tissues, and thus gain access to cells and biological molecules in the body, which results in acute or chronic effects such as organ injuries.
2.1. Effects of nAg on the environment
It has been suggested that the leached nAg will first pass through sewage treatment plants, in which the majority of nAg is precipitated in the sludge and the minority left in the effluent will reach the aquatic environment.39 If the sludge from sewage plants is used in soil as a fertilizer or deposited onto a landfill, nAg will deposit into the soil.40 The disposal of nAg into water and soil will raise risks to aquatic and terrestrial organisms. The potential risk of nAg lies in their bioaccumulative ability and the unique properties of nanoparticles. Moreover, nAg dispersed into the natural environment will undergo further transformation, such as agglomerating, dissolving ionic Ag, reacting with other toxic substances, and/or binding to natural organic matter. For example, humic acid can reduce the aggregation of nAg and enhance its mobility in natural water. Depending on solar light, nAg can be regenerated through the organic matter conducted reduction of Ag ions. These transformations can largely affect the bioavailability and toxicity of nAg.
Silver particles at the nanoscale exert robust inhibitory effects on the growth for a wide spectrum of microorganisms. Numerous studies concerning its strong antimicrobial activity showed that nAg could induce bacterial death by damaging membrane structure, increasing reactive oxygen species (ROS) levels and enhancing Ag ion release.41–43 Thus, the biocidal silver dispersed into the environment may inhibit the growth of beneficial bacteria in sewage systems and disrupt the normal function of key soil microbial communities.6, 40 Especially, the contamination of nAg in water and soil will further raise the possibility of silver resistance in bacteria, a growing concern around the world.6, 7 Several studies showed that low levels of silver released from wound dressing are more likely to promote resistance than that at high levels, especially if the silver level is sublethal.44–46 It has been suggested that nAg under sublethal concentrations may promote bacterial survival rates, although relevant studies are still limited. In the recent study by Xiu and colleagues, E. coli cells were exposed to a panel with nAg of different sizes and coatings for 6 h. They found that the survival rates were improved in the presence of lower concentrations of nAg (2–7 µg mL−1) with 6%-21% higher viability compared to the untreated control group, indicating an apparent hormetic effect.47
Moreover, given that silver is the second most toxic metal for aquatic organisms next to mercury, nAg dispersed into the aquatic systems may affect the development of fish embryos at even lower concentrations.37 For example, in a study by Lee and colleagues, single nAg particles were observed in zebrafish embryos, including brain, heart, gill arches and tail, resulting in developmental malformations and morphological deformities.48 The concentrations used in this study (4–71 ng L−1) were realistic in terms of what is expected for contaminated waters. Although mortality was observed in a dose-dependent manner, the toxicity of nAg for the development of zebrafish occurs likely at lower concentrations.
Together, it is reasonable to conclude that nAg can be released from commercial products into the environment, where nAg is likely to disrupt bacterial ecosystems and interfere with the development of fish embryos under sublethal concentrations.
2.2. Effects of nAg on human health and animals
Because of the effective antimicrobial activity, nAg has been extensively applied in biomedicine, which poses a substantially increased risk to human health. For example, the accidental inhalation of nAg may occur during the usage of nasal drops for rhinitis treatment. The dermal absorption of nAg from the wound dressing may take place after local treatment.27 Moreover, due to the enhanced permeation and preferential optical property, nAg has emerged as an effective regime for disease therapeutics and can be used to deliver therapeutic compounds or image tumor cells.49–51 However, the accumulation of nanoparticles in the target tissues is always a small fraction of the total injected dose, e.g. 1%–10% for tumor, whereas majority of the administered particles end up in liver and spleen and ultimately remain in the body.52 The internal crystal structure, designed for strong plasmonic resonance properties, may increase the catalytic effect of nanoparticles and thus enhance their adverse effects.53 In addition, nAg may have the Trojan horse effect after cellular uptake, in which the particles continue to release silver ions in cells that can bind to proteins overwhelming the antioxidant defense, leading to toxicity.54–56
Clinical reports concerning the side effects of nAg are relatively rare, despite the rapid development of nAg in biomedical applications. Chronic toxicity of nAg is accompanied with skin discolouration (argyria), which is a local or systemic effect observed in individuals that are occupationally exposed to nAg, such as people who ingested a nAg suspension, or patients that have been treated with nAg-containing burn dressings.27 Fewer cases or studies showed neurological toxicity of nAg on humans. One clinical case has reported that a 71-year-old man developed myoclonic status epilepticus and coma and death after 5.5 months, resulting from daily ingestion of colloidal silver (a nAg suspension in a liquid) for 4 months.29 A localized nerve lesion was observed in a patient after a five-year usage of silver impregnated bone cement in a revisional Christiansen total hip arthroplasty because of a large amount of silver released from the implanted cement.57 The higher level of released silver led to a severe local tissue disruption and even necrosis.58 The applications of nAg-coated implantable devices and increased release rate of silver suggests that a low concentration of nAg has potential to incur nerve and tissue damage.6
In the literature, many studies have described in vivo toxic effects of nAg using various animal models.5, 28 It has been demonstrated that nAg can accumulate in various organs, such as liver, spleen, kidney, heart, lung, olfactory bulb, brain and testes, based on ultrastructural analysis, histological staining and Ag content measurement.5, 13, 27, 59 The form of silver in these organs is difficult to determine because the quantification methods, such as ICP-MS, only provide total Ag element concentrations; therefore, they should include both nanoparticles and Ag ions.5 After deposition in the body, short-term or acute effects of nAg have been reported at relatively high concentrations of its exposure (acute exposure). Death has been observed in rats after oral ingestion of very high-dose colloidal silver (1680 mg kg−1) for four days.5 Moreover, Schmaehl and Steinhoff administered rats with an intravenous injection of LD50 amounts of colloidal silver (67 mg kg−1) and observed brown discoloration in the liver, spleen and kidney, lung edema, and death of rats.30 At lower concentrations (chronic exposure), nAg has been reported to cause different toxic effects without a significant sign of morbidity. Local inflammation and individual organ damage, such as lung abnormalities and liver damage, may occur in administrated animals, upon the absorption of nAg in lung, skin, or gastrointestinal tract.59–63 For example, pulmonary inflammation and mild fibrosis were observed in mice after an inhaled administration of nAg by oropharyngeal aspiration at lower doses (0.1, 0.5 and 1.0 mg kg−1). The dosage used was environmentally relevant because it was extrapolated from the real life nAg concentrations in the air in a manufacturing facility (range of 5–289 ng L−1 nAg in the injection room).13, 64
It has been found in preliminary studies that nAg more easily crosses the biological barriers in animals, including the blood-brain barrier (BBB) and the placental barrier. For example, inhaled nAg could gain access to the brain through olfactory nerves and/or the BBB, resulting in the alterations of gene expression in the central nervous system of mice.5, 12, 65 Tang and colleagues demonstrated that nAg could pass through the BBB mainly by transcytosis of capillary endothelial cells and induce astrocyte swelling and neuronal degeneration after subcutaneous administration in rats.66, 67 In recent studies by our group and other groups, the presence of silver in murine fetuses and rat pups suggested the translocation from maternal blood to embryonic blood via the placental barrier, although the form of sliver (nanoparticle or Ag ions) was unclear.68, 69 Our group further demonstrated that nAg accumulated in embryos could induce pronounced developmental retardation on embryonic day 14.5 (E14.5) following intraperitoneal injection in mice before pregnancy.36 Under the current exposure doses (22 and 108 µg kg−1), the gross toxicity, organ injuries and hematological changes were not observed in the parental mice, except for histological abnormalities in the liver. These studies indicate that low-dose nAg may also elicit chronic toxicity to brains and embryonic development.
In addition to the general toxicity as well as tissue and organ damage in animals, the adverse effects of nAg on various cells and the corresponding mechanisms have been investigated, which will be discussed in more detail in the next section. Briefly, at high concentrations, nAg can cause overt cytotoxicity via ROS production. In contrast, the low exposure levels of nAg tend to interrupt biological processing and signaling and to disturb the normal functions of organelles at a cellular level, which is not associated with viability reduction or cell death.
The damage of tissues and dysfunction of cells indicate that, similar to environmental pollutants described above, the chronic toxicity of nAg at low levels is different from its lethal effects at high doses. Although the doses used in previous studies may be comparatively higher, it is difficult to extrapolate these toxic effects to human health risks at the current stage; low-dose nAg, which is more relevant to daily life, appears to be toxic without causing cell death. Given the realistic exposure scenarios for humans using nAg-related products, it is necessary to consider the sublethal toxicity of nAg at low doses. In future studies, lower doses of nAg exposure such as the no observable adverse effect level (NOAEL) and especially the environmental relevant exposure levels should be conducted.
3. Mechanisms of nAg-induced cytotoxicity
Herein, we present an overview of the major molecular mechanisms whereby nAg can cause damage to cells and thus organisms. These mechanisms are organized around the cellular responses upon nAg under lethal and sublethal concentrations, as presented in Table 2. In order to highlight sublethal effects of low-dose nAg, we compare its effects to the cytotoxic mechanisms underlying lethal nAg-induced apoptosis and necrosis under high-dose exposure. The major physicochemical properties, including size, shape and surface coating largely contribute, at various extents, to nAg-conducted biological effects. The mechanisms of nAg toxicity can be attributed to released Ag ions and/or nanoparticles deposited inside the cells and detailed mechanisms mostly include reactive oxygen species (ROS) generation, disruption of energy metabolism and gene transcription.28, 41, 70, 71 The damage of membrane integrity induced by extracellular nAg is presumably an additional mechanism responsible for cell death.53
Table 2.
Detrimental effects induced by nAg on different cells.
| Characteristics of nAg | Cell types | Doses (cell viability) |
ROS levels |
Major conclusions | Ref. |
|---|---|---|---|---|---|
| 6–20 nm, starch coating | Human lung fibroblast IMR-90 and human glioblastoma cell line U251 |
25–400 µg mL−1 | Increased | Intracellular nAg, uptake mainly through macropinocytosis and clathrin-dependent endocytosis, induced stress responses, chromosomal abnormalities and proliferation inhibition |
75 |
| 43.9 nm, sphere, citrate coating |
Murine macrophage line RAW 264.7 |
30–100 µg mL−1 (from less than 40% to 90%) |
Increased | Uptake and localization of nAg in cells resulted in oxidative stress and cell apoptosis |
74 |
| 15, 100 nm, no coating | Rat liver cell line BRL 3A |
5–50 µg mL−1 (from less than 20% to 70%) |
Increased | Increased LDH leakage and dysfunction of mitochondria, along with ROS generation, contributed to cellular morphological modifications |
32 |
| 15, 30 and 55 nm, no coating | Rat alveolar macrophages |
10–75 µg mL−1 (from less than 20% to 80%) |
Increased | Oxidative stress induced mitochondrial function reduction, membrane leakage increase and inflammatory response in a size-dependent manner |
31 |
| 1–100 nm, no coating | Mouse fibroblast NIH 3T3 |
50–100 µg mL−1 (less than 50%) |
Increased | ROS generation and JNK activation triggered mitochondrial-dependent apoptosis |
91 |
| ≤ 100 nm, no coating | Human Chang liver cells and Chinese hamster lung fibroblasts |
3–4 µg mL−1 (50%) |
Not reported |
Endoplasmic reticulum stress signaling mediated cellular apoptosis |
92 |
| 26.2 ± 7.6 nm, sphere, citrate coating |
Mouse fibroblast NIH 3T3 |
2–30 µg mL−1 (from less than 50% to 95%) |
Increased | ROS generation resulted in cellular morphological abnormalities, autophagy dysfunction and cell death |
90 |
| 5, 28 and 100 nm, sphere, PVP coating |
Human blood monocytes |
0.3–1.25 µg mL−1 (from less than 40% to 80%) |
Increased | Enhanced hydrogen peroxide caused mitochondrial membrane superoxide, resulting in inflammasome formation and cytokine IL-1β release |
87 |
| 65 nm, sphere, citrate coating | Human umbilical vein endothelial cells |
1–2 µg mL−1 (from less than 10% to 90%) |
Increased | Oxidative damage led to in cell dysfunction and apoptosis following NF κB activation |
88 |
| 25 nm, polysaccharide coating versus no coating |
Mouse embryonic stem cells and mouse embryonic fibroblasts |
50 µg mL−1 (less than 60%) |
Not reported | Coated nAg elicited more severe DNA damage and apotosis than uncoated |
89 |
| 6–20 nm, sphere, starch coating |
Human lung fibroblast cell line IMR-90 and human glioblastoma cell line U251 |
25–400 µg mL−1 (from less than 20% to 90%) |
Increased | Disruption of the mitochondrial respiratory chain resulted in ROS production and ATP synthesis reduction, leading to DNA damage and cell cycle arrest |
70 |
| 5–10 nm, no coating | Human Jurkat T cells | 0.05–0.2 µg mL−1 (70–90%) |
Increased | Oxidative damage led to DNA strand breaks, cell cycle arrest and apoptosis |
96 |
| 15.9 ± 7.6 nm, sphere, citrate coating |
Human lung epithelial cell line A549 |
12.1 µg/ml (80%) |
Increased | Up-regulated expression of stress response-related genes caused cell cycle arrest |
103 |
| 10, 20, and 40 nm, sphere, PVP coating; 45 × 10 nm, plate, PVP coating; 60 nm × 20 µm, wires, PVP coating |
Rainbow trout gill fish cell line RT-W1 |
0.39–25 µg mL−1 | Increased | High surface reactivity are responsible for contributing to shape-dependent effects of cell membrane lysis |
53 |
| 57.7 ± 6.9 nm, sphere, PEG coating |
Murine macrophage cell line J774A.1 |
1.2–8.7 µg mL−1 (100%) |
Not reported |
Scavenger receptor-mediated phagocytosis and clathrin- and actin-dependent endocytosis coexisted in cellular uptake of nAg |
73 |
| 10, 40 and 75 nm, citrate coating; 10 nm, PVP coating; 50 nm, no coating |
Bronchial epithelial cell line BEAS-2B |
5–50 µg mL−1 (from less than 20% to 100%) |
Not detected |
Cellular uptake, intracellular localization and Ag release were responsible for size-dependent cytotoxicity |
56 |
| 10, 25 and 40 nm, sphere, PVP coating; 45 × 10 nm, plate, PVP coating |
Human embryonic kidney cell line EK293T, human cervical cancer cell line HeLa, human prostate cancer cell line PC3, human hepatic carcinoma cell line HepG2, and human renal carcinoma cell line A498 |
2–8 µg mL−1 (100%) |
Not detected |
Changes of energy metabolism-related gene expression and protein levels resulted in cellular energy reprogramming in a size- and shape-dependent manner |
35 |
| 7–10 nm, no coating | Human hepatic carcinoma cell line HepG2 |
0.1–3 µg mL−1 (from less than 50% to 160%) |
Not detected |
A number of genes related to cell proliferation and cell cycle, not stress response, were up-regulated under a nontoxic dose |
34 |
| 10, 25, 40 and 110 nm, sphere, PVP coating; 45 × 10 nm, plate, PVP coating |
Mouse erythroleukemia cells |
1–8 µg mL−1 (100%) |
Not detected |
Reciprocal interaction between nAg with RNA polymerase resulted in a robust inhibition on overall RNA transcription |
36 |
ROS, reactive oxygen species; PVP, polyvinylpyrrolidone; PEG, polyethylene glycols; LDH, lactate dehydrogenase; JNK, c-Jun N-terminal kinases
3.1. Physicochemical properties: important factors influencing cellular uptake
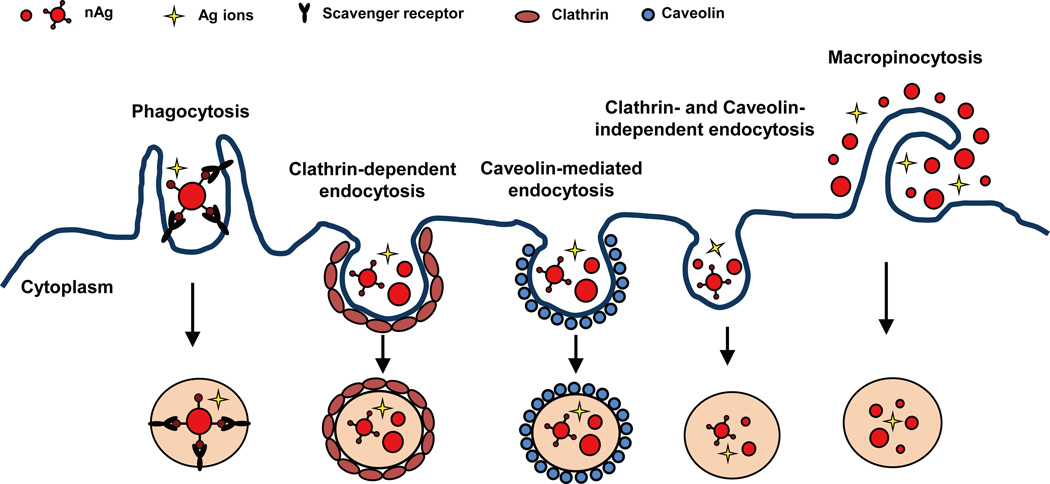
Cellular uptake and subcellular distribution of nAg in cells form the basis for its biological and toxic effects.72 For non-phagocytic eukaryotic cells, nAg can be taken up via various endocytic pathways, including (i) clathrin-dependent endocytosis, (ii) caveolin- mediated endocytosis, (iii) clathrin- and caveolin-independent endocytosis, and (iv) macropinocytosis, as depicted in Figure 1. For phagocytes, including macrophages and monocytes, phagocytosis is the main mechanism responsible for the cellular uptake of nAg.73, 74 The different internalization pathways can be elucidated by treatments with selective pharmacological inhibitors, dominant negative mutants, as well as gene knockdowns. Moreover, the process of nAg uptake involves a combination of different endocytic routes. For example, both clathrin-dependent endocytosis and macropinocytosis coexisted for the uptake of nAg by human mesenchymal stem cells, as demonstrated by the decreased uptake following treatment with chlorpromazine (a clathrin inhibitor) and wortmannin (a macropinocytosis inhibitor). On the other hand, Wang and colleagues demonstrated that nAg bound to scavenger receptor on the J774A.1 cell surface and was subsequently phagocytosed by clathrin- and actin-dependent endocytosis with different inhibitors, including polyinosinic acid, chlorpromazine, nystatin and cytochalasin.73 After entering cells, nAg mainly localizes in endocytic vesicles, which ultimately merge with lysosomes, in which nAg can escape into cytosol and target subcellular structures.70, 75, 76 In our recent study with mouse erythroid progenitor cells, we verified that higher percentage of Ag mass (75%) was in the cytoplasmic fraction than in the nucleus (25%).36
Figure 1.
Pathways for cellular uptake of nAg. nAg can be internalized through different endocytosis routes.
Due to their small size, large surface area, and surface reactivity, engineered nanoparticles have the ability to bind to receptors and proteins on cell membranes.77 Interactions between nanoparticles and proteins, which will form a protein layer on the nanoparticle surface called protein corona, are operated by a number of forces, specific or nonspecific such as hydrogen bonds and interactions such as van der Waals, electrostatic, charge, steric and solvation forces.78, 79 Surface ligands of nanoparticles also play an important role in determining the interactions between particles and receptors.78 Furthermore, nanoparticles in the biological system are likely to adsorb proteins onto their surfaces, causing alterations of particle properties and biological activity.79 In addition, the adsorbed proteins may undergo structural changes, function and avidity alterations, further affecting particles’ interactions with receptors and other proteins.80 With regard to nAg, one of the most commonly used engineered nanoparticles is its interactions with receptors and proteins may be related to the biophysicochemical forces, as described above. Additionally, the higher affinity of silver to thiol groups also contributes to the binding of nAg with sulfur-containing proteins on the membrane.41 Therefore, the intrinsic properties of nAg significantly impact its interactions with cells and determine its efficiency of cellular uptake and intracellular behavior.52 It has been demonstrated that nAg with a smaller size has a higher cellular uptake compared to a larger sized nAg.81 Moreover, the shape of nanoparticles plays an important role in deciding the efficiency of cellular uptake, including phagocytosis by macrophages.82 Spherical nAg has higher cellular uptake compared to plate-like nAg as demonstrated by a study with a fish gill epithelial cell line.53 Another study found that uncoated nAg had higher cellular uptake (10 pg Ag/cell) than PVP and citrate coated nAg (less than 4 pg Ag/cell) in a normal bronchial epithelial cell line, indicating that surface coating also affects the cellular uptake of nAg.56 Additionally, physical and chemical transformation of nAg in biological settings, such as particle agglomeration and Ag ion dissolution, may considerably alter its cellular uptake and cytotoxicity.48, 56, 83
3.2. ROS generation and oxidative stress: the major basis for nAg-induced cytotoxicity
Numerous studies have suggested that the generation of ROS and resultant oxidative stress were the major mechanism responsible for nAg-induced lethal toxicity.27, 28, 41 This is supported by various in vitro studies in which nAg exposure is at high concentrations with a significant decrease of cell viability. Deposition of nAg in the cytosol can disrupt mitochondrial function by inducing mechanical injury and blocking the electron transport in the mitochondrial respiratory chain, resulting in an increased ROS production. 70 The Fenton-like effects in an acidic environment may be another reason for the generation of hydroxyl radicals.84, 85 An additional mechanism is that Ag can replace ferrous ions from proteins, and subsequently induce Fenton reactions to generate ROS.86 Moreover, Ag can bind to glutathione (GSH, a key antioxidant), depleting GSH and increasing cellular vulnerability to ROS.31, 32 Once ROS production exceeds the level that antioxidants can neutralize, the inflammatory response and mitochondria-related cell death will follow.87, 88
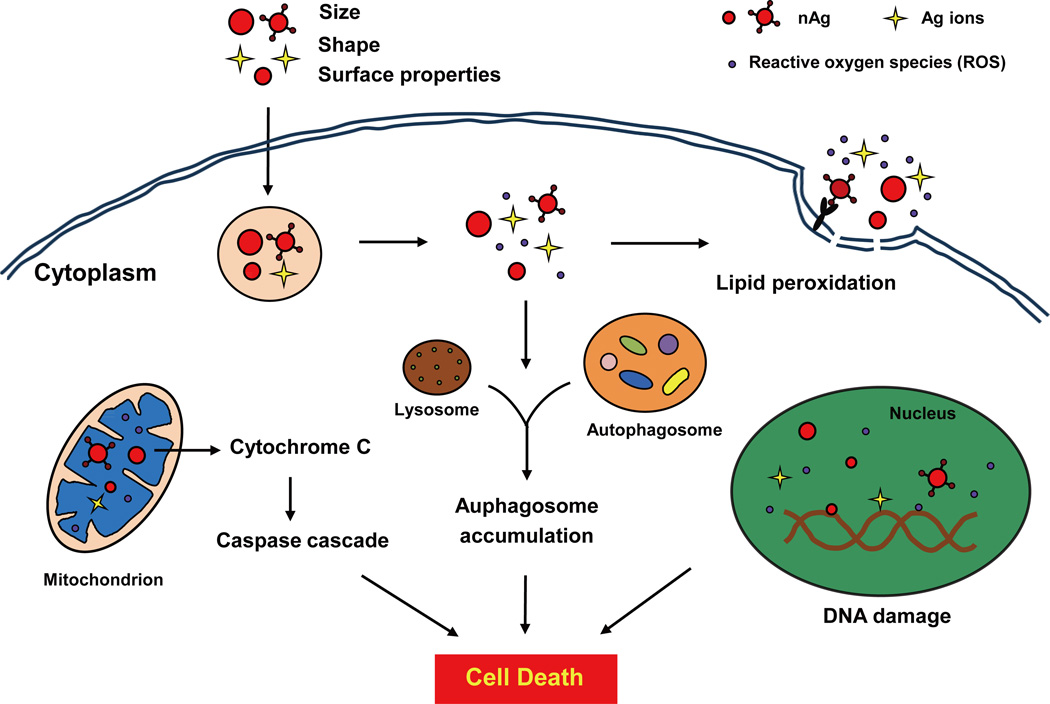
nAg-induced excess ROS can eventually lead to the cell death through a variety of mechanisms, including (i) lipid peroxidation and increased membrane permeability, (ii) activation of signaling cascades involving the mitochondrial pathway, (iii) abnormalities of the autophagy flux, (iv) DNA damage and cell growth arrest, as illustrated in Figure 2. These processes seem to depend on the size and coating of the nanoparticles. A previous study by Carlson and colleagues demonstrated that 15 nm nAg exhibited a stronger cytotoxicity to rat alveolar macrophages with more than 85% decline of cell viability, whereas 55 nm nAg decreased cell viability by only about 15% at the same concentration (50 µg mL−1).31 Another study reported that the surface chemistry of nAg greatly affects its effects on DNA stability: polysaccharide coated nAg elicited more severe DNA damage and apoptosis than uncoated nAg.89
Figure 2.
Schematic overview of the cell death pathways induced by nAg. At exposure levels impacting cell viability, nAg induces excessive ROS generation that could result in lipid peroxidation on membranes, DNA damage in the nucleus, activation of a mitochondria-dependent apoptosis pathway, and disruption of autophagy flux, which ultimately leads to cell death.
nAg treatment causes different types of cell death such as apoptosis, necrosis and/or autophagy.41, 90 It has been revealed that increased ROS could activate Jun N-terminal kinase (JNK) and p53, along with proapoptotic Bax translocation and mitochondrial cytochrome C (initiator of caspase activation) release, triggering a mitochondria-dependent pathway of apoptosis in mouse fibroblasts upon nAg exposure.91 In the same study, researchers found that higher concentration and longer-time exposure of nAg lead to cell necrosis. ROS can also promote apoptosis through other cellular organelles. For example, nAg could cause apoptotic cell death through inducing endoplasmic reticulum (ER) stress.92 Moreover, recent studies indicated that autophagy, a survival mechanism deployed by cells to remove long-lived proteins and damaged organelles, might be a general cellular response to oxidative stress.93, 94 With nAg treatment, excessive ROS led to the disruption of the autophagy flux, resulting in either apoptosis or autophagic cell death in mouse embryonic fibroblasts.90
It is known that ROS is the major source of spontaneous damage to DNA. The highly reactive hydroxyl radical (OH·) can cause DNA damage to generate 8-hydroxyguanine (8-OHdG), leading to a decrease in the stability of repetitive sequences and single- and double-strand breaks.70, 95 DNA damage will cause cell cycle arrest, allowing sufficient time for DNA repair.70, 96 Once the damage is too extensive beyond repair, cells will undergo programmed cell death (apoptosis).89, 97 A study by Eom and Choi demonstrated that increased ROS levels could activate p38 mitogen-activated protein kinase (MAPK) coupled to elevate the levels of oxidative stress, resulting in DNA strand breaks, cell cycle arrest at the G2/M phase and cell viability decline in nAg-treated T cells.96
3.3. Molecular basis of nAg induced sublethal effects
The generation of ROS and oxidative stress appears to play a primary role in nAg-induced lethal effects; however, they are not the only pathway by which nAg exert cytotoxicity. For instance, Gliga and colleagues demonstrated that nAg showed profound cytotoxicity in human lung cells independent of intracellular ROS production.56 Our recent studies also revealed that nAg-mediated inhibition of oxidative phosphorylation and interruption of gene transcription are irrespective of ROS generation.35, 36 In the following subsection, we will show the molecular basis for nAg-mediated detrimental effects at concentrations where excessive ROS are not detected and no significant decline of cell viability are observed.
3.3.1. Disruption of energy homeostasis
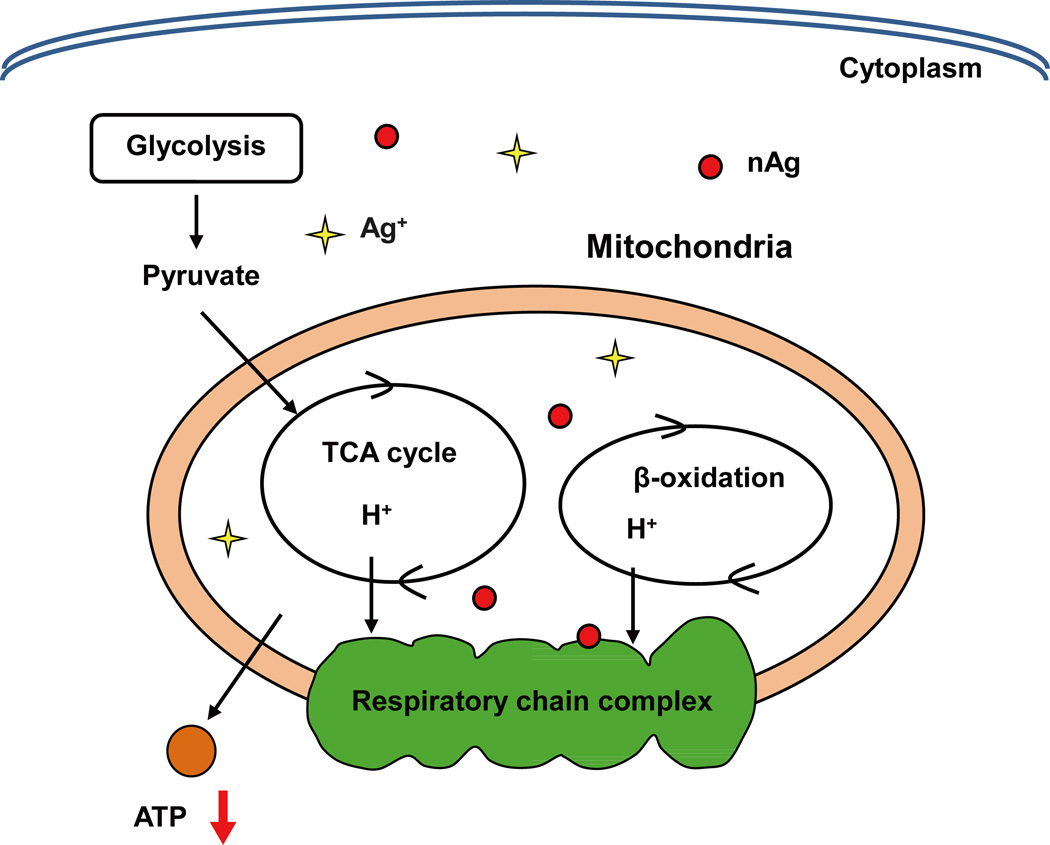
Mitochondria represent the most important organelle in-charge of energy production and maintaining energy homeostasis in mammalian cells.98 It seems that nAg at lethal doses tends to disturb cellular redox homeostasis, induce excess ROS production, and then increase mitochondrial membrane permeability, which uncouples the oxidative phosphorylation from the respiratory chain.32, 99, 100 Given that ATP is essential for numerous cellular processes, including proteins and DNA damage repair, the interruption of ATP synthesis will disturb DNA repair, leading to a cell cycle arrest and apoptosis.70, 101 In contrast, nAg at sublethal doses could interfere with cellular energy balance, including the activity of respiratory chain complexes in mitochondria, and/or alter energy metabolism-related gene expression and protein levels, without ROS generation or cell death.35, 102 Our own recent study demonstrated that sublethal nAg could reduce ATP synthesis and resulted in energy metabolism reprogramming, as depicted in Figure 3.35 We treated a panel of human tumor and nontumor cells with nAg at low concentrations (2–8 µg mL−1) and found that intracellular nAg could reduce the concentration of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α, a central regulator of mitochondrial energy transduction) and pyruvate dehydrogenase (PDH, a key enzyme for oxidative phosphorylation initiation). These nAg-induced alternations at crucial protein levels forced cellular energy homeostasis to switch from oxidative phosphorylation-based aerobic metabolism to anaerobic glycolysis to satisfy basal energy demand for cell survival. In this study, spherical nAg with smaller size presented greater alterations on cellular energy metabolism compared to larger size nanospheres and nanoplates. Moreover, non-tumor HEK293T cells showed higher susceptibility to switch on glycolysis than tumor cells for a greater respiration rate in non-tumor cells.35
Figure 3.
nAg induces reprograming of energy metabolism at sublethal doses. The schematic diagram reveals that nAg could inhibit a key regulator of cellular energy metabolism, PGC-α expression as well as many energy metabolism-related proteins. These changes result in a decrease in oxidative phosphorylation and compensatory increase of glycolysis. This is a reproduction from our research group with modifications.35
3.3.2. Alteration of proliferation-related genes
Recent studies indicated that both lethal and sublethal nAg could alter gene expression profiles once exposed to mammalian cells. Coupled with increased ROS and decreased cell viability, nAg tends to up-regulate gene expression levels related to stress response but down-regulate gene expression levels related to cell cycle execution.103 Inversely, it appears that nAg is more inclined to increase the expression levels of proliferation-related genes in exposed cells at concentrations without reducing cell viability. In a study by Kawata and colleagues, a number of up-regulated genes (122 of total 236) were involved in cell proliferation and cell cycle progression, such as M phase, microtubule-based process, DNA biosynthesis and intracellular transport, upon 1 µg mL−1 nAg with 100% cell viability.34 Oxidative stress-related genes were not induced at a similarly low concentration of nAg. The elevated genes implicated in the cell cycle resulted in an increase in the cell number > 20% upon the addition of nAg at doses of less than 0.5 µg mL−1, indicating that sublethal nAg potentially promotes cell proliferation.
3.3.3. Inhibition of RNA transcription
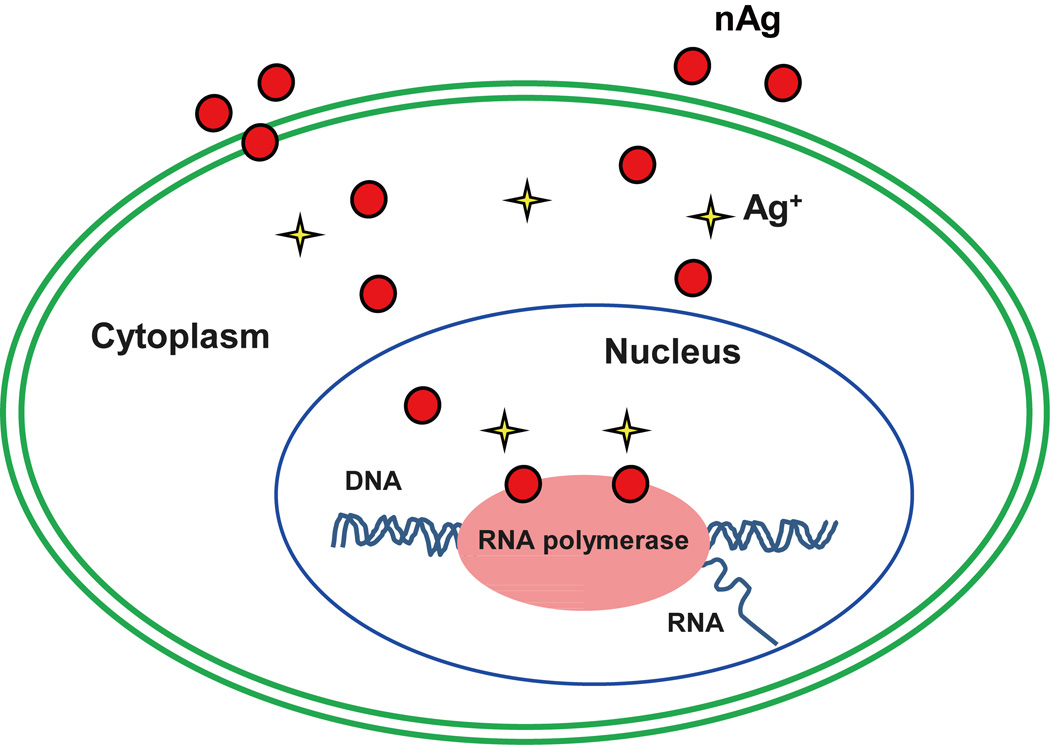
Nanoparticles can impair the cellular transcription machinery and interrupt genetic integrity, which is different from ROS-induced DNA point mutations or/and single- or double-strand breaks.104 Although lethal nAg can induce DNA damage via ROS production, our current study demonstrated that low concentrations of nAg could interrupt the transcription machinery and reduce overall RNA synthesis through directly binding to RNA polymerase, as illustrated in Figure 4.36 Mouse erythroid progenitor (MEL) cells were exposed to PVP-coated nAg at concentrations (1–8 µg mL−1) without provoking ROS and without a decline in cell viability. After exposure for 48 h, cellular total RNAs, including 18S rRNA and 28S rRNA, were substantially inhibited, as characterized by a large reduction in the intensity of nascent RNAs through autoradiography. The acellular pull-down assay of nAg with RNA polymerase showed that nAg could precipitate RNA polymerase. Furthermore, when we used an anti-RNA polymerase II antibody to precipitate RNA polymerase and an ICP-MS assay to detect Ag content in the immunoprecipitates, Ag was present only in the co-immunoprecipitated complexes extracted from nAg-treated cells in a dose-dependent manner. These results proved a direct reciprocal interaction between nAg and RNA polymerase, which interrupted the transcription machinery and led to a robust inhibition on overall RNA transcription. The interactions of nanoparticles with polymerase, similar to that of nanoparticles with proteins on the membrane, may be involved in various physicochemical forces and sulfur-containing amino acids, which needs further investigation in future studies.
Figure 4.
A schematic indicating that nAg binds RNA polymerase and inhibits RNA transcription in erythroid progenitor cells, leading to overall repression of RNA synthesis. This is a reproduction from our research group with modifications.36
nAg-induced inhibition on RNA transcription in erythroid cells in vivo was confirmed using fetal liver at E14.5, an optimal model for embryonic definitive erythropoiesis, following intraperitoneal administration of 22 µg kg−1 nAg in parental mice for 4 weeks.36 The microarray analysis showed that 264 genes of a total of 301 differentially expressed genes were repressed in fetal liver cells of embryos from nAg-treated mice. A large array of these down-regulated genes was associated with the regulation of erythropoiesis in erythroid cells. The significant inhibition of nAg on vital genes and overall RNA transcription, different from ROS-induced placental damage, resulted in anemia and developmental retardation in mouse embryos.
3.4. Contribution of Ag ion toxicity and particle effects
Previous studies have demonstrated that nAg has the potential to release Ag ions in biological settings.83, 105 The release rate is not dependent on nAg’s exposure levels, and the release of Ag ions from nAg can happen at both lethal and sublethal concentrations. Ag ions are generally considered the main mechanism of nAg-induced toxicity. Ag ions have the ability to bind to sulfur-containing proteins in the plasma membrane and inside cells, resulting in the disruption of membrane integrity, dysfunctions of proteins and formation of ROS.41, 106 Interaction with DNA base pairs and induction of DNA damage are also mechanistic responses in cells exposed to Ag ions.41 The role of Ag ions is further supported by findings showing that no toxicity was observed when Ag ions were complexed by their ligands.
However, nAg-induced cytotoxicity is also reported to depend on Ag nanoparticles.35, 36, 56 These studies suggested that the nanoparticle form of Ag could provide particle specific effects associated with impacting toxicity in addition to Ag dissolution. In other words, both nano-sized particles of Ag and ionic Ag could be involved in nAg-mediated detrimental effects. It has been recently considered that nAg acts as a Trojan horse (a vehicle or carrier) to transport ions/particles into cells, promoting the penetrability and bioavailability of particles and Ag ions in cells and organisms.54–56 Although whether and to what extent nAg exists in the form of nanoparticles is still ambiguous, recent studies by our group and the other groups demonstrated that more than 80% of Ag presented in the form of nanoparticles via the cloud point extraction (CPE) method, indicating that particles might be the main form of nAg inside cells.36, 107 On the other hand, nAg particles could be more active during their interactions with biological molecules.5, 11 The smaller nanoparticles have larger surface area, more binding sites, and thus higher Ag ion release and ROS production, leading to more harmful effects than the bulk particles. Furthermore, due to higher surface reactivity, Ag nanoplates were more toxic than Ag nanospheres and Ag nanowires to fish gill epithelial cells, despite their lower rates of dissolution and bioavailability.53 Additionally, surface coating can reduce dissolution rates of nAg and/or bind to Ag ions after dissolution, consequently decreasing the toxicity.105
In summary, the physicochemical properties of nAg affect its cellular availability, interaction activity, Ag ion release and thus its consequent cytotoxicity, indicating similarities in the response of cells to lethal and sublethal doses of nAg. However, there are still pronounced differences. The direct indication of lethal cytotoxicity of nAg is cell death, which masks other predominant mechanisms underlying nAg-induced biological effects. Increased ROS generation and oxidative stress are the major routes through which high-dose nAg induces overt toxic effects. Under sublethal doses, nAg interferes with biomolecules in a more subtle manner with minimal ROS production and little impact on cell viability. As discussed above, sublethal nAg can disturb cellular energy homeostasis not through ROS-mediated depolarization of mitochondrial membrane potential, but mainly through indirect alternations of energy supply-related mRNA and protein levels. Moreover, sublethal doses of nAg can interfere with more biological processes, including cell proliferation, RNA transcription and erythropoiesis and be devoid of significant stress responses. The similarities (i.e. common mechanisms) and differences (i.e. novel mechanisms) between lethal toxicity and sublethal toxicity of nAg are summarized in Table 3.
Table 3.
Similarities and differences in the response of cells to nAg at lethal and sublethal concentrations.
| Mechanisms | Similarity | Difference |
|---|---|---|
Lethal effects
|
|
Lethal effects
|
4. Conclusions and Perspectives
In this review, we emphasize the importance of sublethal effects of nAg at low exposure levels to better assess health hazards of nAg under environmentally relevant conditions. Analogous to a variety of environmental pollutants, nAg exhibits chronic impairments (i.e., sublethal effects) that are more relevant to environmental conditions but have long been under-researched. Based on in vitro studies, nAg can perturb cellular pathways at the molecular level under sublethal doses without eliciting overt cytotoxicity. Enhancement of aerobic glycolysis, induction of cell proliferation-related genes, and inhibition of RNA polymerase-mediated transcription by nAg suggest a distinct effect of nAg at sublethal doses.
Although the sublethal doses are possibly still higher than actual human exposure levels that are still unclear to date, we can claim that studying the chronic effects of nAg at low (sublethal) doses is a necessary step in the right direction. However, there have been very limited investigations related to sublethal effects from exposure to nAg. To assess these effects of nAg, we need to perform dose-response studies and determine the sublethal doses for cell models through different methods such as morphological observation, cell counting, MTT and Alarmar Blue assays. Then, we should survey its subcellular localization and effects on cellular functions and search for appropriate indicators of sublethal toxicity such as alterations of gene transcription and biochemical markers specific to cell functions. Thereafter, animal exposure studies should be carried out to validate the in vitro findings. Moreover, due to transformation of nAg in biological media, researchers should use various nAg with different physicochemical properties in order to verify how the alternation of basic properties impact its bioavailability, cellular uptake and subcellular localization and subsequent biological effects. The improved understanding of nAg’s sublethal effects will assist in elucidating their potential adverse effects on human health.
Supplementary Material
Impact Statement.
Analogous to the health impacts induced by environmental pollutants, the chronic toxicity of nAg at low doses is possibly different from the acute toxicity at high doses. Previous findings indicate that sublethal nAg exposure could also cause significant adverse effects in cells and animals. Therefore, to evaluate the potential hazards of nAg, it is desirable to examine not only acute lethal effects but also chronic sublethal effects. Further understanding of nAg-induced detrimental effects at low concentrations will be helpful in investigating the health effects of nAg under environmentally relevant conditions.
Acknowledgments
This work was supported by a grant under the national “973” program (grant number: 2014CB932000), grants from the National Natural Science Foundation of China (grant numbers: 21407172, 21377159, 21177151, 21207152, 21425731), the Strategic Priority Research Program of the Chinese Academy of Sciences (grant No. XDB14000000), and the International Postdoctoral Exchange Fellowship Program (the approval document number: No.57 Document of OCPC, 2013). TX was supported by National Institute of Environmental Health Sciences, U19ES019528, R01 ES016746, and the National Science Foundation and the Environmental Protection Agency under Cooperative Agreement Number DBI 0830117 and 1266377.
Footnotes
Competing interests
No potential conflicts of interest were disclosed from the authors.
References
- 1.Chen Z, Tabakman SM, Goodwin AP, Kattah MG, Daranciang D, Wang XR, Zhang GY, Li XL, Liu Z, Utz PJ, Jiang KL, Fan SS, Dai HJ. Nature Biotechnology. 2008;26:1285–1292. doi: 10.1038/nbt.1501. [DOI] [PubMed] [Google Scholar]
- 2.Qian X, Peng XH, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S. Nat Biotechnol. 2008;26:83–90. doi: 10.1038/nbt1377. [DOI] [PubMed] [Google Scholar]
- 3.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaloupka K, Malam Y, Seifalian AM. Trends in Biotechnology. 2010;28:580–588. doi: 10.1016/j.tibtech.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, Roszek B, Bisschops J, Gosens I, Van de Meent D, Dekkers S, De Jong WH, Van Zijverden M, Sips AJAM, Geertsma RE. Nanotoxicology. 2009;3:U109–U178. [Google Scholar]
- 6.Faunce T, Watal A. Nanomedicine. 2010;5:617–632. doi: 10.2217/nnm.10.33. [DOI] [PubMed] [Google Scholar]
- 7.Seltenrich N. Environ Health Persp. 2013;121:A220–A225. doi: 10.1289/ehp.121-a220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schluesener JK, Schluesener HJ. Arch Toxicol. 2013;87:569–576. doi: 10.1007/s00204-012-1007-z. [DOI] [PubMed] [Google Scholar]
- 9. http://www.nanotechproject.org/inventories/consumer/analysis_draft/ [Google Scholar]
- 10.Glover RD, Miller JM, Hutchison JE. ACS Nano. 2011;5:8950–8957. doi: 10.1021/nn2031319. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Schluesener HJ. Toxicol Lett. 2008;176:1–12. doi: 10.1016/j.toxlet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Oberdorster G, Oberdorster E, Oberdorster J. Environ Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Ji ZX, Chang CH, Zhang HY, Wang MY, Liao YP, Lin SJ, Meng H, Li RB, Sun BB, Winkle LV, Pinkerton KE, Zink JI, Xia T, Nel AE. Small. 2014;10:385–398. doi: 10.1002/smll.201301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limbach LK, Wick P, Manser P, Grass RN, Bruinink A, Stark WJ. Environ Sci Technol. 2007;41:4158–4163. doi: 10.1021/es062629t. [DOI] [PubMed] [Google Scholar]
- 15.Li YF, Chen C. Small. 2011;7:2965–2980. doi: 10.1002/smll.201101059. [DOI] [PubMed] [Google Scholar]
- 16.Hughes MF, Beck BD, Chen Y, Lewis AS, Thomas DJ. Toxicol Sci. 2011;123:305–332. doi: 10.1093/toxsci/kfr184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thevenod F, Lee WK. Metal ions in life sciences. 2013;11:415–490. doi: 10.1007/978-94-007-5179-8_14. [DOI] [PubMed] [Google Scholar]
- 18.Hong YS, Kim YM, Lee KE. Journal of preventive medicine and public health = Yebang Uihakhoe chi. 2012;45:353–363. doi: 10.3961/jpmph.2012.45.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benbrahim-Tallaa L, Waalkes MP. Environ Health Perspect. 2008;116:158–164. doi: 10.1289/ehp.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarup L, Akesson A. Toxicol Appl Pharm. 2009;238:201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Grandjean P, Satoh H, Murata K, Eto K. Environ Health Perspect. 2010;118:1137–1145. doi: 10.1289/ehp.0901757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruckerl R, Schneider A, Breitner S, Cyrys J, Peters A. Inhal Toxicol. 2011;23:555–592. doi: 10.3109/08958378.2011.593587. [DOI] [PubMed] [Google Scholar]
- 23.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD, American Heart Association Council on E, Prevention CotKiCD, Council on Nutrition Metabolism PA. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 24.Weichenthal SA, Godri-Pollitt K, Villeneuve PJ. Environmental health : a global access science source. 2013;12:40. doi: 10.1186/1476-069X-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia YL, Stone D, Wang WT, Schrlau J, Tao S, Simonich SLM. Environ Health Persp. 2011;119:815–820. doi: 10.1289/ehp.1003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckhardt S, Brunetto PS, Gagnon J, Priebe M, Giese B, Fromm KM. Chem Rev. 2013;113:4708–4754. doi: 10.1021/cr300288v. [DOI] [PubMed] [Google Scholar]
- 27.Johnston HJ, Hutchison G, Christensen FM, Peters S, Hankin S, Stone V. Critical reviews in toxicology. 2010;40:328–346. doi: 10.3109/10408440903453074. [DOI] [PubMed] [Google Scholar]
- 28.Ahamed M, Alsalhi MS, Siddiqui MK. Clin Chim Acta. 2010;411:1841–1848. doi: 10.1016/j.cca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Mirsattari SM, Hammond RR, Sharpe MD, Leung FY, Young GB. Neurology. 2004;62:1408–1410. doi: 10.1212/01.wnl.0000120671.73335.ec. [DOI] [PubMed] [Google Scholar]
- 30.Schmaehl D, Steinhoff D. Zeitschrift fur Krebsforschung. 1960;63:586–591. [PubMed] [Google Scholar]
- 31.Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL, Schlager JJ. J Phys Chem B. 2008;112:13608–13619. doi: 10.1021/jp712087m. [DOI] [PubMed] [Google Scholar]
- 32.Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. Toxicol In Vitro. 2005;19:975–983. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 33.Braydich-Stolle L, Hussain S, Schlager JJ, Hofmann MC. Toxicol Sci. 2005;88:412–419. doi: 10.1093/toxsci/kfi256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawata K, Osawa M, Okabe S. Environmental Science & Technology. 2009;43:6046–6051. doi: 10.1021/es900754q. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Wang Z, Xu M, Wang X, Liu R, Liu Q, Zhang Z, Xia T, Zhao J, Jiang G, Xu Y, Liu S. ACS Nano. 2014;8:5813–5825. doi: 10.1021/nn500719m. [DOI] [PubMed] [Google Scholar]
- 36.Wang Z, Liu SJ, Ma J, Qu GB, Wang XY, Yu SJ, He JY, Liu JF, Xia T, Jiang GB. ACS Nano. 2013;7:4171–4186. doi: 10.1021/nn400594s. [DOI] [PubMed] [Google Scholar]
- 37.Luoma SN. Washington, DC:Project on Emerging Nanotechnologies. 2008 Woodrow Wilson International Center for Scholars. [Google Scholar]
- 38.Statement AfTSaDRPH, Services edUSDoHaH. Public Health Service, Atlanta, GA1990 [Google Scholar]
- 39.Gottschalk F, Sonderer T, Scholz RW, Nowack B. Environ Sci Technol. 2009;43:9216–9222. doi: 10.1021/es9015553. [DOI] [PubMed] [Google Scholar]
- 40.Colman BP, Arnaout CL, Anciaux S, Gunsch CK, Hochella MF, Jr, Kim B, Lowry GV, McGill BM, Reinsch BC, Richardson CJ, Unrine JM, Wright JP, Yin L, Bernhardt ES. PLoS One. 2013;8:e57189. doi: 10.1371/journal.pone.0057189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volker C, Oetken M, Oehlmann J. Rev Environ Contam Toxicol. 2013;223:81–106. doi: 10.1007/978-1-4614-5577-6_4. [DOI] [PubMed] [Google Scholar]
- 42.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ. Nanotechnology. 2005;16:2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 43.Li WR, Xie XB, Shi QS, Zeng HY, Ou-Yang YS, Chen YB. Appl Microbiol Biot. 2010;85:1115–1122. doi: 10.1007/s00253-009-2159-5. [DOI] [PubMed] [Google Scholar]
- 44.Landsdown AB, Williams A. Journal of wound care. 2007;16:15–19. doi: 10.12968/jowc.2007.16.1.26983. [DOI] [PubMed] [Google Scholar]
- 45.Chopra I. The Journal of antimicrobial chemotherapy. 2007;59:587–590. doi: 10.1093/jac/dkm006. [DOI] [PubMed] [Google Scholar]
- 46.Brett DW. Ostomy/wound management. 2006;52:34–41. [PubMed] [Google Scholar]
- 47.Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJ. Nano Lett. 2012;12:4271–4275. doi: 10.1021/nl301934w. [DOI] [PubMed] [Google Scholar]
- 48.Lee KJ, Nallathamby PD, Browning LM, Osgood CJ, Xu XH. ACS Nano. 2007;1:133–143. doi: 10.1021/nn700048y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Love SA, Maurer-Jones MA, Thompson JW, Lin YS, Haynes CL. Annual review of analytical chemistry. 2012;5:181–205. doi: 10.1146/annurev-anchem-062011-143134. [DOI] [PubMed] [Google Scholar]
- 50.Skirtach AG, Antipov AA, Shchukin DG, Sukhorukov GB. Langmuir. 2004;20:6988–6992. doi: 10.1021/la048873k. [DOI] [PubMed] [Google Scholar]
- 51.Skirtach AG, Munoz Javier A, Kreft O, Kohler K, Piera Alberola A, Mohwald H, Parak WJ, Sukhorukov GB. Angew Chem Int Ed Engl. 2006;45:4612–4617. doi: 10.1002/anie.200504599. [DOI] [PubMed] [Google Scholar]
- 52.Albanese A, Tang PS, Chan WC. Annual review of biomedical engineering. 2012;14:1–16. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 53.George S, Lin SJ, Jo ZX, Thomas CR, Li LJ, Mecklenburg M, Meng H, Wang X, Zhang HY, Xia T, Hohman JN, Lin S, Zink JI, Weiss PS, Nel AE. Acs Nano. 2012;6:3745–3759. doi: 10.1021/nn204671v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park EJ, Yi J, Kim Y, Choi K, Park K. Toxicol In Vitro. 2010;24:872–878. doi: 10.1016/j.tiv.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Jiang X, Miclaus T, Wang L, Foldbjerg R, Sutherland DS, Autrup H, Chen C, Beer C. Nanotoxicology. 2014 doi: 10.3109/17435390.2014.907457. [DOI] [PubMed] [Google Scholar]
- 56.Gliga AR, Skoglund S, Wallinder IO, Fadeel B, Karlsson HL. Part Fibre Toxicol. 2014;11:11. doi: 10.1186/1743-8977-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sudmann E, Vik H, Rait M, Todnem K, Andersen KJ, Julsham K, Flesland O, Rungby J. Medical progress through technology. 1994;20:179–184. [PubMed] [Google Scholar]
- 58.Ellender G, Ham KN. British journal of experimental pathology. 1989;70:21–39. [PMC free article] [PubMed] [Google Scholar]
- 59.Samberg ME, Oldenburg SJ, Monteiro-Riviere NA. Environ Health Perspect. 2010;118:407–413. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sung JH, Ji JH, Yoon JU, Kim DS, Song MY, Jeong J, Han BS, Han JH, Chung YH, Kim J, Kim TS, Chang HK, Lee EJ, Lee JH, Yu IJ. Inhal Toxicol. 2008;20:567–574. doi: 10.1080/08958370701874671. [DOI] [PubMed] [Google Scholar]
- 61.Song KS, Sung JH, Ji JH, Lee JH, Lee JS, Ryu HR, Lee JK, Chung YH, Park HM, Shin BS, Chang HK, Kelman B, Yu IJ. Nanotoxicology. 2013;7:169–180. doi: 10.3109/17435390.2011.648223. [DOI] [PubMed] [Google Scholar]
- 62.Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, Choi BS, Lim R, Chang HK, Chung YH, Kwon IH, Jeong J, Han BS, Yu IJ. Inhal Toxicol. 2008;20:575–583. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- 63.Hadrup N, Lam HR. Regul Toxicol Pharmacol. 2014;68:1–7. doi: 10.1016/j.yrtph.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Lee JH, Ahn K, Kim SM, Jeon KS, Lee JS, Yu IJ. J Nanopart Res. 2012:14. [Google Scholar]
- 65.Lee HY, Choi YJ, Jung EJ, Yin HQ, Kwon JT, Kim JE, Im HT, Cho MH, Kim JH, Kim HY, Lee BH. J Nanopart Res. 2010;12:1567–1578. [Google Scholar]
- 66.Tang JL, Xiong L, Wang S, Wang JY, Liu L, Li JA, Wan ZY, Xi TF. Appl Surf Sci. 2008;255:502–504. [Google Scholar]
- 67.Tang J, Xiong L, Wang S, Wang J, Liu L, Li J, Yuan F, Xi T. J Nanosci Nanotechnol. 2009;9:4924–4932. doi: 10.1166/jnn.2009.1269. [DOI] [PubMed] [Google Scholar]
- 68.Lee Y, Choi J, Kim P, Choi K, Kim S, Shon W, Park K. Toxicological research. 2012;28:139–141. doi: 10.5487/TR.2012.28.3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z, Qu GB, Su LN, Wang L, Yang ZZ, Jiang JQ, Liu SJ, Jiang GB. Curr Pharm Design. 2013;19:6691–6697. doi: 10.2174/1381612811319370012. [DOI] [PubMed] [Google Scholar]
- 70.AshaRani PV, Low Kah Mun G, Hande MP, Valiyaveettil S. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 71.Kim S, Ryu DY. J Appl Toxicol. 2013;33:78–89. doi: 10.1002/jat.2792. [DOI] [PubMed] [Google Scholar]
- 72.Xia T, Kovochich M, Liong M, Zink JI, Nel AE. ACS Nano. 2008;2:85–96. doi: 10.1021/nn700256c. [DOI] [PubMed] [Google Scholar]
- 73.Wang H, Wu L, Reinhard BM. ACS Nano. 2012;6:7122–7132. doi: 10.1021/nn302186n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh RP, Ramarao P. Toxicol Lett. 2012;213:249–259. doi: 10.1016/j.toxlet.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 75.Asharani PV, Hande MP, Valiyaveettil S. BMC Cell Biol. 2009;10:65. doi: 10.1186/1471-2121-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Greulich C, Diendorf J, Simon T, Eggeler G, Epple M, Koller M. Acta biomaterialia. 2011;7:347–354. doi: 10.1016/j.actbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 77.Nel A, Xia T, Madler L, Li N. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 78.Nel AE, Madler L, Velegol D, Xia T, Hoek EMV, Somasundaran P, Klaessig F, Castranova V, Thompson M. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 79.Saptarshi SR, Duschl A, Lopata AL. J Nanobiotechnology. 2013;11:26. doi: 10.1186/1477-3155-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brown DM, Dickson C, Duncan P, Al-Attili F, Stone V. Nanotechnology. 2010;21:215104. doi: 10.1088/0957-4484/21/21/215104. [DOI] [PubMed] [Google Scholar]
- 81.Liu W, Wu Y, Wang C, Li HC, Wang T, Liao CY, Cui L, Zhou QF, Yan B, Jiang GB. Nanotoxicology. 2010;4:319–330. doi: 10.3109/17435390.2010.483745. [DOI] [PubMed] [Google Scholar]
- 82.Champion JA, Mitragotri S. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pratsinis A, Hervella P, Leroux JC, Pratsinis SE, Sotiriou GA. Small. 2013;9:2576–2584. doi: 10.1002/smll.201202120. [DOI] [PubMed] [Google Scholar]
- 84.He W, Zhou YT, Wamer WG, Boudreau MD, Yin JJ. Biomaterials. 2012;33:7547–7555. doi: 10.1016/j.biomaterials.2012.06.076. [DOI] [PubMed] [Google Scholar]
- 85.He D, Miller CJ, Waite TD. J Catal. 2014;317:198–205. [Google Scholar]
- 86.Gordon O, Vig Slenters T, Brunetto PS, Villaruz AE, Sturdevant DE, Otto M, Landmann R, Fromm KM. Antimicrobial agents and chemotherapy. 2010;54:4208–4218. doi: 10.1128/AAC.01830-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang EJ, Kim S, Kim JS, Choi IH. Biomaterials. 2012;33:6858–6867. doi: 10.1016/j.biomaterials.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 88.Shi J, Sun X, Lin Y, Zou X, Li Z, Liao Y, Du M, Zhang H. Biomaterials. 2014;35:6657–6666. doi: 10.1016/j.biomaterials.2014.04.093. [DOI] [PubMed] [Google Scholar]
- 89.Ahamed M, Karns M, Goodson M, Rowe J, Hussain SM, Schlager JJ, Hong Y. Toxicol Appl Pharmacol. 2008;233:404–410. doi: 10.1016/j.taap.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 90.Lee YH, Cheng FY, Chiu HW, Tsai JC, Fang CY, Chen CW, Wang YJ. Biomaterials. 2014;35:4706–4715. doi: 10.1016/j.biomaterials.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 91.Hsin YH, Chena CF, Huang S, Shih TS, Lai PS, Chueh PJ. Toxicology Letters. 2008;179:130–139. doi: 10.1016/j.toxlet.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 92.Zhang R, Piao MJ, Kim KC, Kim AD, Choi JY, Choi J, Hyun JW. Int J Biochem Cell B. 2012;44:224–232. doi: 10.1016/j.biocel.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 93.Ryter SW, Choi AM. Curr Pharm Des. 2013;19:2747–2756. doi: 10.2174/1381612811319150010. [DOI] [PubMed] [Google Scholar]
- 94.Kiffin R, Christian C, Knecht E, Cuervo AM. Molecular biology of the cell. 2004;15:4829–4840. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cadet J, Delatour T, Douki T, Gasparutto D, Pouget JP, Ravanat JL, Sauvaigo S. Mutat Res. 1999;424:9–21. doi: 10.1016/s0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 96.Eom HJ, Choi J. Environmental Science & Technology. 2010;44:8337–8342. doi: 10.1021/es1020668. [DOI] [PubMed] [Google Scholar]
- 97.Ishikawa K, Ishii H, Saito T. DNA Cell Biol. 2006;25:406–411. doi: 10.1089/dna.2006.25.406. [DOI] [PubMed] [Google Scholar]
- 98.Friedman JR, Nunnari J. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Teodoro JS, Simoes AM, Duarte FV, Rolo AP, Murdoch RC, Hussain SM, Palmeira CM. Toxicol In Vitro. 2011;25:664–670. doi: 10.1016/j.tiv.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 100.Kim S, Choi JE, Choi J, Chung KH, Park K, Yi J, Ryu DY. Toxicol In Vitro. 2009;23:1076–1084. doi: 10.1016/j.tiv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Wong LY, Recht J, Laurent BC. J Mol Histol. 2006;37:261–269. doi: 10.1007/s10735-006-9047-4. [DOI] [PubMed] [Google Scholar]
- 102.Costa CS, Ronconi JV, Daufenbach JF, Goncalves CL, Rezin GT, Streck EL, Paula MM. Molecular and cellular biochemistry. 2010;342:51–56. doi: 10.1007/s11010-010-0467-9. [DOI] [PubMed] [Google Scholar]
- 103.Foldbjerg R, Irving ES, Hyashi Y, Sutherland DS, Thorsen K, Autrup H, Beer C. Toxicol Sci. 2012;130:145–157. doi: 10.1093/toxsci/kfs225. [DOI] [PubMed] [Google Scholar]
- 104.Singh N, Manshian B, Jenkins GJ, Griffiths SM, Williams PM, Maffeis TG, Wright CJ, Doak SH. Biomaterials. 2009;30:3891–3914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 105.Liu J, Wang Z, Liu FD, Kane AB, Hurt RH. ACS Nano. 2012;6:9887–9899. doi: 10.1021/nn303449n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chernousova S, Epple M. Angew Chem Int Ed Engl. 2013;52:1636–1653. doi: 10.1002/anie.201205923. [DOI] [PubMed] [Google Scholar]
- 107.Yu SJ, Chao JB, Sun J, Yin YG, Liu JF, Jiang GB. Environ Sci Technol. 2013;47:3268–3274. doi: 10.1021/es304346p. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.