Abstract
E1-deleted adenovirus (FG Ad) transducing vectors are limited for use in vivo by their induction of strong innate and adaptive inflammatory responses. We have examined the contribution of the transgene cassette, particularly the foreign promoter driving transgene expression, in the induction of innate inflammation using a mouse ear model in which swelling is measured as a sensitive surrogate marker of the total innate inflammatory response. The commonly used cytomegalovirus major immediate early (CMV) promoter led to high-level swelling that was independent of transgene expression, while the Rous sarcoma virus and human ubiquitin C promoters led to intermediate levels of swelling and the Ad E1A promoter or no promoter led to equally low levels of swelling. Significant swelling was induced by a virus in which the E1A promoter directed pIX expression, supporting the possibility that activation of expression of Ad genes retained in the vector plays an important role in the inflammatory response. Taken together, our findings support the idea that strong foreign promoters likely play the limiting role in the induction of innate and adaptive immune responses that limit the duration of transgene expression after transduction by FG Ad vectors.
Keywords: Adenovirus, Vector, CMV promoter, Innate inflammation
Introduction
Replication-defective first-generation adenovirus (FG Ad) vectors, typically derived from Ad5, have the E1 region replaced by a foreign gene, most often under the control of the cytomegalovirus major immediate early (CMV) promoter. These vectors have promise for gene therapy, but are limited by their induction of strong adaptive (Choi et al., 1993; Smith et al., 1993) and innate (Muruve et al., 1999) immune responses.
In contrast to the high level of inflammation induced by replication-defective Ad vectors, wild-type Ad5 frequently causes asymptomatic infections in people with normal immune responses (Strauss, 1984; Lichtenstein and Wold, 2004). The relatively modest pathogenicity of the wild-type virus in immune-competent individuals raises the question of why replication-defective Ad5 vectors are so inflammatory. The three most obvious explanations, aside from the very large vector doses used in gene therapy, are that 1) deleted viral genes play an important role in inhibiting inflammation, 2) introduced foreign sequences play an inflammatory role in the context of the virus, and 3) use of Ad vectors in tissues not normally infected by Ad5 leads to enhanced inflammatory responses.
Deletion of the Ad E1 genes significantly increases the inflamma-tory response provoked by the virus lacking foreign sequences, as assayed using swelling induced after subdermal injection of viruses into BALB/c mouse ears as a sensitive and readily measured surrogate marker of the total inflammatory response (Schaack et al., 2004). In the context of no introduced foreign sequence, E1A, E1B, and E3 play important roles in inhibiting virally-induced innate inflammation, albeit in a complicated manner (Schaack et al., 2004; Schaack, 2005). The contributions of other Ad gene products and introduced foreign sequences to ear swelling have not been addressed.
In this study, we have examined the role of the transgene cassette, particularly the foreign promoter introduced to direct expression of the transgene, in the induction of inflammation. We demonstrate that the presence of the CMV promoter leads to induction of swelling in a manner independent of transgene expression, regardless of whether the foreign proteins LacZ (E. coli β-galactosidase) or green fluorescent protein (GFP), the “self” protein murine adipophilin (ADPH), or no foreign protein is encoded by the virus. We also demonstrate that the presence of other promoters leads to swelling that correlates well with the strength of the promoter.
Results
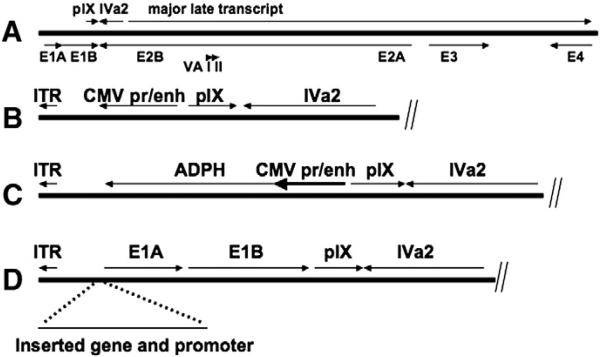
The genotypes of viruses used in this study are presented in Table 1 and the left end structure of the viruses is schematically presented in Fig. 1.
Table 1.
Viruses used in this study are deleted for the pTP gene and the E1A 289R coding sequence and are wild-type for other genes not indicated.
| Virus | Promoter | Orientation | Poly A | Encoding | E1A 243R | E1B | E3 |
|---|---|---|---|---|---|---|---|
| A AdE1Apr | E1A | right | – | – | – | – | + |
| B AdE1Apr pA | E1A | right | + | – | – | – | + |
| C AdΔE1 | – | – | – | – | – | + | |
| D AdCMVR pA | CMV | right | + | – | – | – | + |
| E AdCMVR-LacZ | CMV | right | + | LacZ | – | – | + |
| F AdCMVR | CMV | right | – | pIX | – | – | + |
| G AdCMVL | CMV | left | + | – | – | – | + |
| H AdCMVL-GFP | CMV | left | + | GFP | – | – | + |
| I AdCMVL-ADPH | CMV | left | + | ADPH | – | – | + |
| J AdCMVL-MetVal | CMV | left | + | MetVal | – | – | + |
| K AdRSVL | RSV | left | + | – | – | – | + |
| L AdUbCL | UbC | left | + | – | – | – | + |
| M AdCMVR-LacZ E1A | CMV | right | + | LacZ* | + | – | + |
| N AdCMVL-GFP E1AE1B | CMV | left | + | GFP* | + | + | + |
| O AdCMVL-ADPH E3 | CMV | left | + | ADPH | – | – | + |
| P AdCMVL-ADPH | CMV | left | + | ADPH | – | – | – |
| Q AdApTP E1A 12S | E1A, E1B | right | + | E1A243R, E1B | + | + | + |
The orientation of the promoter is indicated by “R” (rightward, or normal E1A, orientation) or “L” (leftward orientation). The presence or absence of sequences is indicated by “+” and “–”, respectively.
The transgene cassettes are inserted between the packing sequence and the E1A promoter.
Fig. 1.
Schematic of the Ad chromosome. (A) The entire Ad5 chromosome is presented schematically. (B–D) The left end of the viral vector chromosomes are presented schematically. (B) The CMV promoter was inserted in the leftward orientation in the absence of a coding sequence. The RSV and UbC promoters were similarly introduced. (C) The structure of the CMV promoter directing ADPH expression is shown. (D) For viruses in which a gene was inserted and the E1A 243R coding sequence or E1A 243 coding sequence and E1B gene are retained, the insertion site is immediately downstream of the packaging sequence. The entire E1A promoter and enhancer (Hearing and Shenk, 1983) was used to direct E1A 243 expression, so the packaging sequence is reiterated.
Plaque size is dependent on foreign promoter introduced into the Ad chromosome
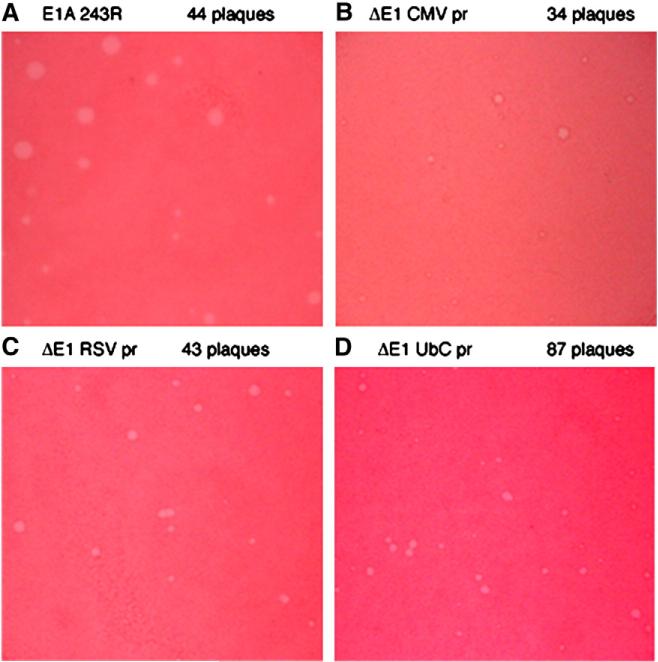
Purified viruses containing strong foreign promoters with no introduced genes and AdΔpTP E1A 12S (virus Q; the genotypes of all viruses tested are presented in Table 1), which does not induce and does inhibit ear swelling (Schaack et al., 2004), were used to infect HEK 293-pTP cells to assay plaque formation (Fig. 2). In HEK 293 cells, the CMV promoter is considerably stronger than the RSV promoter, which is considerably stronger than the E1A promoter (Schaack et al., 2001). The RSV and UbC promoters are expected to be of similar strength.
Fig. 2.
Plaque sizes vary as a function of the promoter introduced into the virus. HEK 293 plates were infected in triplicate for plaque assays. After incubation for 8 days, plates were stained with neutral red and plaques counted. The viruses used for plaquing and the numbers of plaques counted on the entire plate are listed above each panel (A–D). A representative field from each plate is shown. The estimated efficiencies of plaque counting, relative to 300ΔpTP 12S, were CMV 11%; RSV 37%; UbC 49%.
As expected, a range of plaque sizes was seen for each of the viruses. However, plaques formed by AdΔpTP E1A 12S (virus Q; average plaque area in arbitrary units 100±68 (standard deviation)) were generally considerably larger than those formed by Ad-CMV (virus G; average area 8.5±11 units; Fig. 2A and B). Plaques formed by Ad-Ubp (virus L; average area 12±11 units) were similar in size to those formed by Ad-CMV and Ad-RSV plaques (virus K; average area 38±25 units; Fig. 2C and D), were intermediate in size between those induced by AdΔpTP E1A 12S and Ad-CMV. The differences in size between plaques formed by Ad-Ubp and Ad-CMV were not statistically significant while differences in all other pairwise comparisons were highly significant. Because plaque sizes roughly correlate inversely with the strength of the promoter introduced into the virus (compare Fig. 2 with data below) and larger plaques are more efficiently counted, plaque titers underestimate the infectivity of viruses containing strong promoters. For that reason, care was taken to grow and purify viruses using as similar conditions as possible, and particle numbers were used to normalize amounts of virus used.
E1-deleted Ad induces modest ear swelling
Understanding the contribution of Ad vector components to the induction of innate inflammatory processes requires determination of the background induced by E1-deleted Ads devoid of foreign sequences. We have used a mouse ear model in which innate inflammation induced by Ads is monitored using swelling, a sensitive surrogate marker that correlates well with inflammation (Moorhead et al., 1999; Schaack et al., 2004).
We previously showed that deletion of the E1A and E1B genes leads to a modest level of ear swelling (Schaack et al., 2004). To test the effect of the E1A promoter on the innate inflammatory response to Ad, we constructed a virus deleted for the E1A coding sequence as well as the E1B gene. A poly A site was introduced immediately downstream of the E1A promoter. This virus (virus B) induced a modest level of ear swelling (Fig. 3), statistically identical to swelling induced by a virus deleted for the entire E1 region (virus C). These data raise the possibility that relatively weak promoters have little effect on the induction of innate inflammatory responses by Ad vectors.
Fig. 3.
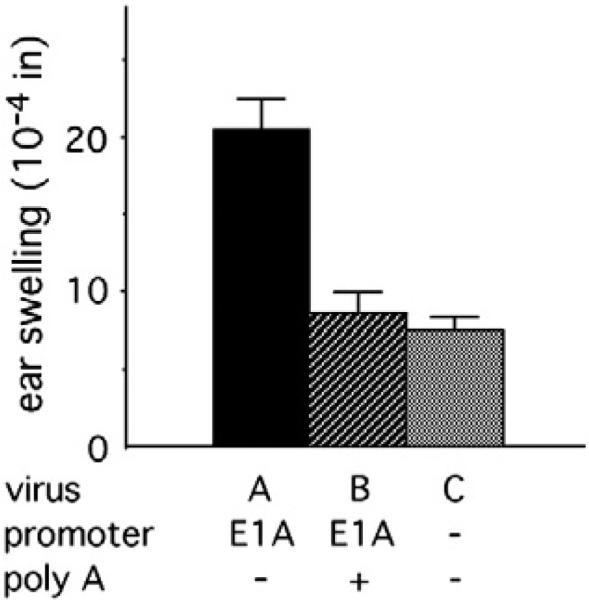
E1A promoter effects on ear swelling. Viruses A–C are deleted for E1A and E1B as well as pTP. The data presented for virus A include previously published (Schaack et al., 2004) as well as new data. Virus B is identical to virus A except that the SV40 poly A site was inserted immediately downstream of the E1A promoter. In this and succeeding figures, results are presented for swelling induced 24 h after subdermal injection of BALB/c mouse ears, with background due to injection of buffer alone subtracted. The numbers of ears analyzed were: virus A, 16; virus B, 8; virus C, 11.
Expression of pIX induces ear swelling
Our primary hypothesis underlying this study was that strong promoters contained within Ad vectors are primarily responsible for the strong innate inflammatory response induced by the vector. In particular, expression of the pIX gene, which is immediately adjacent to the transgene cassette, was expected to be activated by the enhancer present within the promoter-containing DNA fragment introduced into the vector.
To test the effect of pIX expression on ear swelling, the E1A promoter was introduced immediately upstream of the pIX gene in the absence of an intervening poly A site. This virus (virus A, Table 1) induced a significantly greater innate inflammatory response than did the viruses deleted for all of E1 or containing the E1A promoter followed by a poly A site (Fig. 3; P<0.0002 for both pairwise comparisons), in agreement with the results of Nakai et al. (2007).
The CMV promoter induces high-level ear swelling
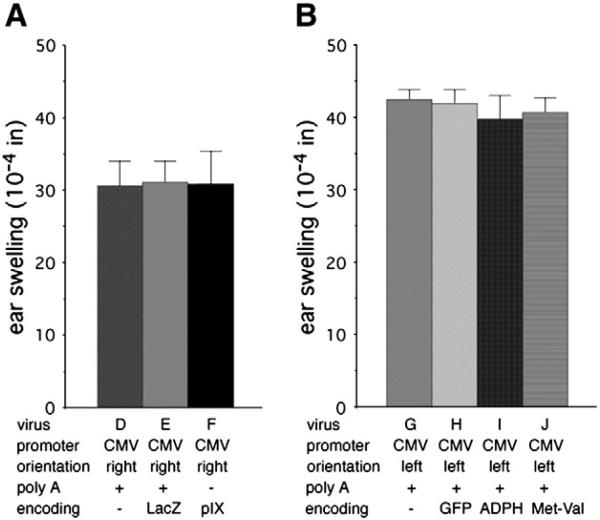
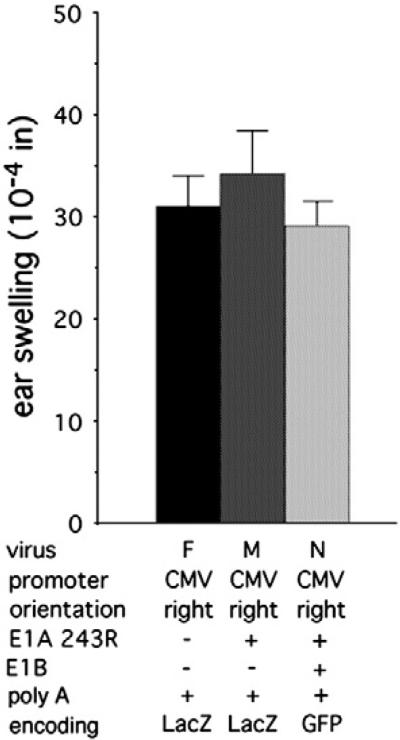
Adaptive immune responses to LacZ encoded by Ad vectors play an important role in limiting the efficacy of the vectors (Yang et al., 1996; Chen et al., 1997). However, innate inflammatory responses induced by Ad vectors that contain the CMV promoter are apparent within 24 h after injection (Moorhead et al., 1999; Muruve et al., 1999; Schaack et al., 2004), prior to the development of an adaptive immune response. We hypothesized that the CMV promoter, and not transgene expression, is the major contributor to the induction of early innate responses, specifically through CMV enhancer-dependent activation of expression of Ad genes retained in the vector. To test this hypothesis, we compared the inflammatory effects of viruses with the E1 region replaced by the CMV promoter directing expression of no transgene (virus D), LacZ (virus E), or pIX expression (virus F) (Fig. 4A). The presence of the CMV promoter alone led to induction of swelling that was statistically the same as that induced by the virus encoding LacZ (P>0.9; Fig. 4B), suggesting that the CMV promoter accounts for the majority of transgene cassette-dependent ear swelling. Interestingly, the virus containing the CMV promoter immediately adjacent to the pIX gene and lacking a poly A site between the CMV promoter and the pIX promoter induced swelling that was not different from that induced by the virus encoding LacZ or the virus containing the CMV promoter with a poly A site between the CMV promoter and the pIX gene.
Fig. 4.
Coding region has no apparent effect but CMV promoter orientation has a significant effect on induction of swelling. (A) Viruses D, E, and F contain the CMV promoter in the rightward orientation. The numbers of ears analyzed were: virus D, 18; virus E, 12; virus F, 6. (B) Viruses G-J contain the CMV promoter in the leftward (anti-E1A) orientation. The numbers of ears analyzed were: virus F, 9; virus G, 6; virus H, 18; virus I, 12.
The CMV promoter induces swelling in an orientation-dependent manner
E1-deleted viruses were constructed with the CMV promoter in the leftward (anti-E1A) orientation directing expression of no introduced cDNA (virus G) or of cDNAs encoding GFP (virus H) or the “self” gene murine ADPH (virus I). In the absence of a cDNA, downstream AUG codons that could be present in an mRNA are in poor context for translation initiation (Kozak, 1987) and little or no polypeptide synthesis should occur. To control for unintended polypeptide synthesis, the strong translation initiation site from pEGFP-N1 with codons for Met and Val followed by a termination codon was introduced behind the CMV promoter (virus J). Translation initiation at the introduced AUG should inhibit initiation at downstream sites (Liu et al., 1984). Met–Val, rather than Met alone, was encoded because the +4 position (position 1 of codon 2) contributes to translation initiation efficiency (Kozak, 1987). A literature search uncovered no evidence for an inflammatory effect of the dipeptide Met–Val.
All of the viruses carrying the CMV promoter in the leftward orientation induced swelling that was statistically equivalent (Fig. 4B; P>0.5 for all pairwise comparisons). The effect of promoter orientation was determined by comparison of swelling induced by viruses with rightward-facing (Fig. 3A) and leftward-facing (Fig. 4B) CMV promoters. The virus with the leftward-facing CMV promoter directing ADPH expression yielded differences that were slightly above statistical significance for viruses with the CMV promoter in the rightward orientation (P<0.06–0.1 for the three comparisons) while all other pairwise comparisons of viruses carrying leftward oriented CMV promoters with the viruses carrying rightward oriented CMV promoters were statistically significant (P<0.02–0.048). Comparison of the combined results demonstrates that significantly greater swelling is induced when the CMV promoter is in the leftward than rightward orientation (P=0.011).
Inflammation induced by other promoters introduced into the Ad chromosome
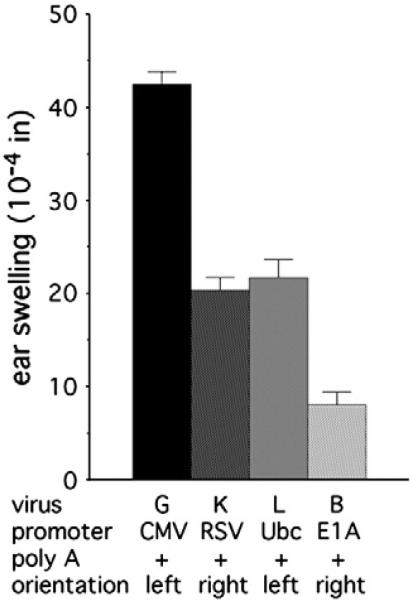
To test the effect of the relative strength of the introduced promoter on the induction of swelling by Ad vectors, promoters of varying strengths followed immediately by the SV40 poly A site were inserted in place of the E1 region. Viruses containing the relatively weak Ad E1A promoter in its normal, rightward, orientation (virus A) or the strong RSV LTR (virus K) or human ubiquitin C (Ub; virus L) promoters, both in the leftward orientation, induced swelling that, in comparison with the CMV promoter (Fig. 5), correlated well with the approximate strengths of the introduced promoters (Schaack et al., 2001). The virus carrying the CMV promoter induced significantly increased swelling relative to each of the other promoters. Swelling induced in the presence of the UbC and RSV promoters was not statistically different (P>0.5), while the viruses carrying the UbC or RSV promoters both induced significantly greater swelling than the virus carrying the E1A promoter with a poly A site (virus A) (P<0.0002 for both).
Fig. 5.
Dependence of swelling induced as a function of promoter. Viruses that are isogenic except for the promoter introduced in place of the E1 region were tested for induction of swelling. Results for virus F are from Fig. 4 and for virus B are from Fig. 3. The numbers of ears analyzed were: virus G, 9; virus K, 8; virus L, 8; virus B, 8.
E1 genes have little effect on ear swelling induced by a virus carrying the CMV promoter
E1A 243R inhibits type 1 interferon responses (Reich et al., 1988), transcription of the IL-6 gene (Janaswami et al., 1992), and IL-6-dependent transcriptional activation (Takeda et al., 1994). There are likely additional roles of E1A in inhibiting innate immune responses provoked by the virus. To test the effects of E1A 243R on Ad vector-induced inflammation, swelling induced by viruses encoding LacZ under the control of the CMV promoter with (virus M) or without (virus E) co-expression of E1A 243R protein under its own promoter was compared. E1A 243R expression had no effect on swelling (PN0.9; Fig. 6).
Fig. 6.
E1 proteins do not inhibit ear swelling induced by Ad vectors containing the CMV promoter. The virus encoding LacZ in the rightward orientation was modified to encode E1A 243R and the virus encoding GFP in the rightward orientation was modified to encode E1A 243R and the E1B proteins. The poly A site for all clones is immediately downstream of the transgene coding sequence. Results for virus E are from Fig. 2A and for virus G are from Fig. 3. The numbers of ears analyzed were: virus E, 12; virus L, 6; virus G, 6; virus M, 14.
E1A 243R acts in concert with the E1B proteins to inhibit swelling (Schaack et al., 2004). To test whether E1B proteins in concert with E1A 243R inhibit CMV promoter-dependent swelling, we constructed a virus encoding GFP under the control of the CMV promoter immediately upstream of the E1 region containing the wild-type E1B gene and the modified E1A gene encoding E1A 243R only (virus N). Swelling was partially reduced relative to viruses containing the CMV promoter in the leftward orientation and lacking the E1 genes. However, plaque assays carried out with the stock demonstrated that approximately 20% of the virus had lost GFP expression. PCR analysis demonstrated that the CMV–GFP cassette had been deleted from the chromosomes of these viruses, apparently through repeated sequences of the cis-acting packaging sequence flanking the CMV– GFP cassette (the viral cis-acting packaging sequence overlaps the E1A promoter and was therefore present immediately in front of the E1A coding sequence as well as immediately in front of the E1A 243R gene). Given that viruses deleted for the CMV–GFP cassette should induce no swelling, it is likely that the high-level swelling that occurred was caused by the vectors that maintained the CMV–GFP cassette, and that the presence of the E1 genes has little or no effect on ear swelling induced by the vector encoding GFP under the control of the CMV promoter.
The role of the E3 region in inhibiting innate inflammation induced by the CMV promoter
The E3 gene encodes a variety of proteins that act to inhibit innate and adaptive immune responses (Horwitz, 2004; Lichtenstein et al., 2004; Windheim, et al. 2004). To test the role of E3 in inhibiting CMV promoter-dependent innate inflammation, viruses with the E1 region replaced by the CMV promoter in the leftward orientation driving expression of murine ADHP and either wild-type (virus O) or deleted (virus P) for the E3 gene were tested (Fig. 7). The presence of the E3 gene had no effect on swelling induced (PN0.5).
Fig. 7.
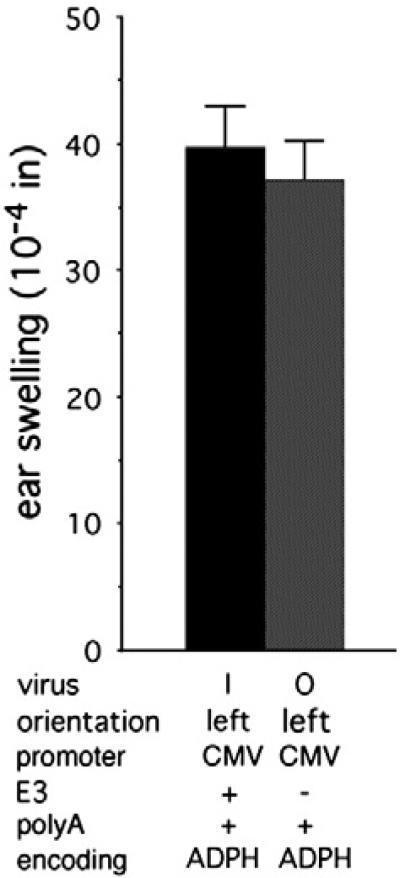
E3 does not inhibit swelling induced by a virus encoding ADPH under the control of the CMV promoter. E1-deleted viruses encoding ADPH under the control of the CMV promoter that are isogenic except for the presence or absence of E3 were tested for induction of swelling. Results for virus H are from Fig. 3. The numbers of ears analyzed were: virus H, 18; virus N, 12.
Discussion
Strong innate and adaptive immune responses are induced by E1-deleted Ad vectors and adaptive immune responses to both viral (Yang et al., 1994) and transgene (Yang et al., 1996) antigens are important in limiting vector efficacy. Helper-dependent Ad vectors induce innate immune responses that, for the inflammatory mediators examined, are similar to those induced by FG Ad vectors early after transduction (Muruve et al., 2004). However, the innate response remains high for FG vectors for a week or more in DBA/2 mice while it declines for hd vectors. The fact that hd Ad vectors effectively transduce tissues in vivo for extended periods of time (Chen et al., 1997; Morsy et al., 1998; Toietta et al., 2005) suggests that transient innate immune responses do not necessarily limit the effectiveness of Ad vectors. However, innate responses prime the adaptive immune responses to vector-encoded proteins, including the transgene product. Therefore, it is important to understand the induction of innate inflammatory responses by Ad vectors, especially widely used FG vectors.
Enhancer effects
The presence of the CMV promoter or other strong promoters in the virus induces substantial ear swelling (Fig. 4) as well as activation of expression of Ad genes retained in the vector (Shapiro et al., 2006 and Schaack et al., accompanying manuscript). Our underlying hypothesis for these effects is that the enhancer contained within the CMV promoter fragment is responsible for these effects.
Enhancers have come to be defined as elements that act in a position- and orientation-independent manner to activate transcription, although this definition is simplistic. The prototypical, and by far best studied, enhancer is that of SV40. The SV40 enhancer displays modest orientation- and significant position-dependent activity (Wasylyk et al., 1984; Zenke et al., 1986). This is important in consideration of the consistency of our results with our primary hypothesis. The CMV promoter in the leftward orientation places the pIX promoter immediately adjacent to the enhancer. In contrast, the rightward orientation places the CMV promoter between the CMV enhancer and the pIX promoter, so it is possible that the reduced innate response induced by pIX results from dilution of enhancer activity by the intervening CMV promoter. Alternatively, the CMV enhancer may exhibit orientation dependence in its activity, a possibility that literature searches did not indicate to have been tested. An additional uncertainty arises from the fact that Ad chromosome is bound by Ad core proteins, at least for a significant time after virus uptake by the cell (Chatterjee et al., 1986; Chen et al., 2007). It is not clear how Ad chromatin affects the function of enhancers compared to the effects of host cell and native viral chromatin, although it is possible that the CMV promoter alters Ad chromatin structure as it does in CMV DNA (Nitzsche et al., 2008).
CMV promoter induction of innate inflammatory processes
It is possible that increased and inappropriate expression of Ad genes is responsible for the increased innate inflammation. The mechanisms by which Ad early gene products contribute to the induction of innate inflammatory processes remain to be determined, but pIX, as suggested by Nakai et al. (2007), and possibly other Ad proteins, could be at least partly responsible. Our evidence suggests that pIX expression explains only part of the innate inflammatory response. Positioning of the E1A promoter to direct pIX expression led to a substantial increase in ear swelling. In contrast, positioning of the CMV promoter to direct pIX expression led to no increase in ear swelling compared to that induced when a poly A site was placed between the CMV promoter and the pIX gene (Fig. 4A), and the level of ear swelling induced is not maximal, as placement of the CMV promoter in the opposite orientation led to a significant increase in swelling (Fig. 4B).
E4-dependent activation of TAp73 expression
Shapiro et al. demonstrated that transduction of BALB/c MEFs with Ad vectors containing the CMV promoter led to increased TAp73 expression in an E4 orf6/7-dependent manner that was independent of transgene expression (Shapiro et al., 2006). TAp73 expression was induced to a greater extent when the CMV promoter was in the leftward than the rightward orientation. When compared with data presented here (Fig. 4), this suggests a possible correlation between TAp73 induction in vitro and activation of innate inflammatory processes in vivo, although it is not clear what mechanism(s) are involved. Induction of TAp73 expression does not correlate completely with the inflammatory potential of the virus. Virus A, containing the E1A promoter but lacking a poly A site before the pIX gene, induced swelling that was similar to that induced by virus J, containing the UbC promoter (compare Figs. 2 and 4). In contrast, a virus containing the UbC promoter directing GFP expression induced TAp73 to a lesser extent than did virus A in MEFs. In addition, the virus containing a poly A site between the pIX gene and the E1A promoter also induced TAp73 expression to a higher level than did the more inflammatory virus containing the UbC promoter (Shapiro et al., 2006).
Finally, the fact that the presence of the CMV promoter led to greater TAp73 expression than did the UbC promoter in MEFs while the presence of the UbC promoter led to greater activation of TAp73 expression than did the CMV promoter in human foreskin fibroblasts suggests that control of expression of Ad genes retained within vectors is complex, with potential variability dependent on host cells transduced.
Potential unintended consequences of gene deletions
pIX is normally expressed only after the onset of viral DNA replication (Crossland and Raskas, 1983). The pIX gene is nested within the 3’ end of the E1B transcription unit and the pIX promoter is normally inhibited prior to Ad DNA replication in part through promoter occlusion dependent on transcription from the E1B promoter (Vales and Darnell, 1989). E1B promoter activity is also regulated, although in a positive manner, by read-through transcription directed by the upstream E1A promoter (Parks and Spector, 1990; Shen and Spector, 2003). The transcription-regulatory mechanisms raise the possibility that pIX expression in cells transduced by E1-deleted Ads could result, at least in part, from deletion of the E1A and E1B promoters as well as from activation by strong foreign enhancers.
The contribution of transgene expression to innate and adaptive inflammatory processes
Our results indicate that induction of innate immune responses by Ad vectors in the first 24 h after transduction exhibits little or no dependence on expression of a transgene or, among the three transgenes that we tested, on the nature of the transgene. (Fig. 4). However, other lines of experimentation have indicated that the transgene product plays an important role in limiting the efficacy of Ad vectors.
Introduction of Ad vectors encoding LacZ into mice transgenic for LacZ led to increased time of vector-mediated LacZ expression and blunted adaptive immune responses to the vector compared to transduction of mice in which LacZ was a foreign protein (Yang et al., 1996; Chen et al., 1997). This evidence led to the conclusion that the transgene product plays the most important role in limiting E1-deleted Ad vector effectiveness. However, AAV vectors permit prolonged LacZ expression (Clemens et al., 1996; Kessler et al., 1996; Rolling et al., 1997), indicating that LacZ that does not play the limiting role in cessation of transgene expression in cells transduced by Ad vectors. An alternative explanation, consistent with our model of the effects of the CMV promoter on activation of innate immune responses, is that CMV promoter-dependent activation of Ad vector genes leads to induction of innate immune responses that efficiently prime the adaptive immune response to LacZ, which is expressed at extremely high levels when under the control of the CMV promoter. Thus, while adaptive immunity to LacZ limits vector efficacy, the CMV promoter likely acts at the limiting step in induction of the innate inflammatory response that leads to the adaptive immune response to LacZ.
In a second experimental approach, the use of tissue-specific promoters led to increased time of transgene expression with reduced adaptive immune response directed against the vector relative to the strong and broadly active PGK (Pastore et al., 1999) and CMV (Ding et al., 2002) promoters. This finding led to the suggestion that expression of the transgene in cells, particularly dendritic cells and macrophages (Zhang et al., 2001), contributes to the adaptive immune response to vector-transduced cells and limits the efficacy of the vector. Our results suggest an additional mechanism of importance in the function of vectors that carry tissue-specific promoters. Tissue-specific promoters are generally much weaker than the CMV promoter/enhancer and are likely to induce lesser expression of Ad genes retained in the vector in both the targeted cells and in cells that efficiently prime adaptive immune responses. Reduced expression of Ad genes likely leads to reduced innate immune responses and thus to reduced adjuvant activity as well as delayed and reduced adaptive immune responses to both the vector and the transgene product. Data generated by Nakai et al. (2007) support these possibilities. When the CMV promoter was used, expression of the transgene was short lived. In contrast, when the broadly active EF1α promoter was used, transgene expression was prolonged. However, the EF1α promoter directed transgene expression at a level of approximately 10% of that directed by the CMV promoter. These data support the argument that it is the strength of the promoter, including the enhancer, that acts at the limiting step of transgene expression.
Plaque sizes and viruses that induce high levels of swelling
The size of plaques formed by the different viruses (Fig. 2) inversely correlates with the amount of swelling induced after ear injection. Smaller plaque size indicates that the virus grows more slowly in complementing HEK 293 cells and this correlates with greater induction of inflammation in vivo. It is possible that the mechanisms that lead to increased inflammation in the presence of strong promoters are the same as, or at least overlap, the mechanisms that lead to reduced growth rate in vitro. Inappropriate level or timing of expression of Ad genes is likely to be responsible for both effects.
Small, more difficult to count, plaques produced by the more inflammatory viruses lead to underestimation of plaque titers in a manner correlating with the level of inflammation induced by the virus. Thus, plaque titers do not necessarily offer an appropriate method of normalizing the amount of the virus. However, the use of plaque titers would not have affected the general results obtained or the conclusions drawn from these studies: the more inflammatory the virus, the larger the amount of the virus that would have been used, leading to even greater differences in ear swelling.
Potential consequences for the design of Ad vectors
Host innate and adaptive immune responses that limit the efficacy of Ad vectors have been studied in detail. However, the mechanisms by which Ad vector components, particularly the genes retained within the vector, induce inflammation remain poorly understood, and this lack of understanding has impeded attempts to design improved simple Ad gene therapy vectors. The demonstration that strong foreign promoters, and especially the widely used CMV promoter, are responsible for the majority of the strong innate response induced by Ad vectors offers a promising avenue for understanding the mechanisms by which innate inflammation is induced and for the design of improved Ad vectors (see accompanying manuscript).
Materials and methods
Cell lines
HEK293 cells (Graham et al., 1977) and HEK293-pTP cells (Schaack et al., 1995; Schaack et al., unpublished), which constitutively express Ad pTP, were grown in DMEM containing high glucose supplemented with 10% bovine calf serum.
Construction and growth of viruses
Viruses (Table 1) were constructed using the method of He et al. (1998) as previously modified (Orlicky and Schaack, 2001). The plasmids pShuttleE1A (Schaack et al., 2001), pShuttle, pShuttleCMV, and pAdTrack (He et al. 1998) were described previously. New shuttle plasmids containing the CMV, RSV, and Ub promoters in the leftward (anti-E1A) orientation followed by the SV40 poly A signal (a generous gift of David Orlicky) were constructed. The resultant plasmids are pShuttleCMV rev, pShuttleRSV rev, and pShuttle-Ub rev, respectively. The plasmid pShuttleE1A pA was constructed by insertion of the SV40 poly A site immediately downstream of the E1A promoter. The murine ADPH cDNA was introduced into pShuttleCMV rev to generate pShuttleCMV-ADPH. Sequence including the very strong translation start site from the plasmid pEGFP-N1 (Clontech) and encoding Met–Val followed by a nonsense codon was synthesized by PCR and inserted into pShuttleCMV rev to generate pShuttleCMV-Met–Val. pShuttleCMV-LacZ/E1A 243R was constructed by introduction of the Ad2 E1A 12S cDNA (encoding the 243R protein) into pShuttleE1A. The CMV promoter followed by poly A site was introduced in the leftward orientation between the E1A enhancer/packaging sequence and the E1A promoter and the LacZ coding sequence followed by the SV40 poly A site was introduced immediately downstream of the CMV promoter. To introduce a CMV–GFP expression cassette into the virus deleted for the E1A 289R and pTP coding sequences, sequence encoding the E1A enhancer, promoter, and E1A 243R coding sequence in normal orientation was introduced into pShuttleCMV–GFP rev. The plasmid was transferred to an E. coli dam− strain and DNA digested with PacI+ClaI to liberate the desired viral left end fragment. DNA prepared from the virus deleted for the E1A 289R and pTP coding sequences was digested with ClaI and the large viral DNA fragment purified by centrifugation on a 10–40% sucrose gradient and ligated with the left end fragment containing the CMV–GFP expression cassette as well as the majority of the E1A 243R coding sequence.
PmeI-linearized shuttle plasmids were used to transform E. coli strain BJ5183 carrying pAdEasy-300Δ or pAdEasy-327ΔpTP (deleted for the E3 region) to generate recombinant plasmids containing the Ad chromosomes. Ad chromosomes were released from plasmids by digestion with PacI and used to transfect HEK293-pTP cells using calcium phosphate coprecipitation (Jordan et al., 1996) to generate viruses. Viruses were plaque purified and tested by PCR, restriction digestion, and, where appropriate, for expression of the transgene to ensure the correct structure and genotype.
Viruses were grown and purified by consecutive step and isopycnic CsCl gradient centrifugation as previously described (Schaack et al., 2004). Viral particle titers were determined by OD260, with one OD260=1012 particles/ml. All virus stocks used in this study had less than 1 infectious particle in 1011 particles when assayed on 293 cells.
Analysis of plaque sizes
Digital images taken of plaque plates were converted to TIFF files. Plaque sizes were determined from the TIFF files using the masking function of Slidebook (Intelligent Imaging Innovatiions, Denver CO). Statistical significance was determined using the two-tailed T test.
Injections of mice and analysis of swelling
All procedures involving animals followed the guidelines of, and were approved by, the University of Colorado Institutional Animal Care and Use Committee. Female, 6-week-old BALB/c mice were anesthetized by intraperitoneal injection of avertin prior to all procedures. Virus stocks were diluted with PBS such that the glycerol concentration was 5%. 2×1011 purified particles in 10 μl were injected subdermally into ears. Particle titers were used because viruses differed substantially in the rate of growth of plaques (Fig. 2) and thus in the accuracy with which plaque titers could be determined. Care was taken to prepare and purify virus stocks under identical conditions to ensure that particle titers were representative of viral activities. Ear thickness was measured immediately prior to and every 24 h through 72 h after injection using an engineer's micrometer (Mitutoyo model 7326). Ear measurements are reported in units of 10−4 in. Ears that developed hematomas were excluded. Data represent the average values after subtraction of swelling induced by injection of buffer alone±the standard error of the mean. Swelling induced by injection of ears with buffer alone averaged 4.80±0.237 units. Statistical significance was determined using the two-tailed T test.
Acknowledgments
We thank Ana Ruiz for technical assistance, David Orlicky for advice and the gift of a plasmid encoding the SV40 poly A site, and MK Smith and the Kathryn Holmes laboratory for assistance in capturing plaque images. This work was supported by a grant from the University of Colorado Technology Transfer Office.
The funding agency had no role other than financial support in this study.
Footnotes
All authors declare no actual or potential conflict of interest.
References
- Chatterjee PK, Vayda ME, Flint SJ. Adenoviral protein VII packages intracellular viral DNA throughout the early phase of infection. EMBO J. 1986;5:1633–1644. doi: 10.1002/j.1460-2075.1986.tb04406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Mack LM, Kelly R, Ontell M, Kochanek S, Clemens PR. Persistence in muscle of an adenoviral vector that lacks all viral genes. Proc. Natl Acad. Sci. USA. 1997;94:1645–1650. doi: 10.1073/pnas.94.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Morral N, Engel DA. Transcription releases protein VII from adenovirus chromatin. Virology. 2007;369:411–422. doi: 10.1016/j.virol.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Choi H, Park JY, Coonrod L, Sun J, Kaleko M. Adenovirus mediated expression of therapeutic plasma levels of human factor IX in mice. Nat. Genet. 1993;5:397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- Clemens PR, Kochanek S, Sunada Y, Chan S, Chen HH, Campbell KP, Caskey CT. In vivo muscle gene transfer of full-length dystrophin with an adenoviral vector that lacks all viral genes. Gene Ther. 1996;3:965–972. [PubMed] [Google Scholar]
- Crossland LD, Raskas HJ. Identification of adenovirus genes that require template replication for expression. J. Virol. 1983;46:737–748. doi: 10.1128/jvi.46.3.737-748.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding E, Hu H, Hodges BL, Migone F, Serra D, Xu F, Chen YT, Amalfitano A. Efficacy of gene therapy for a prototypical lysosomal storage disease (GSD-II) is critically dependent on vector dose, transgene promoter, and the tissues targeted for vector transduction. Mol. Ther. 2002;5:436–446. doi: 10.1006/mthe.2002.0563. [DOI] [PubMed] [Google Scholar]
- Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 1977;36:59–72. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc. Natl Acad. Sci. USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing P, Shenk T. The adenovirus type 5 E1A transcriptional control region contains a duplicated enhancer element. Cell. 1983;33:695–703. doi: 10.1016/0092-8674(83)90012-0. [DOI] [PubMed] [Google Scholar]
- Horwitz MS. Function of adenovirus E3 proteins: their interactions with immunoregulatory cell proteins. J. Gene Med. 2004;6(Suppl 1):S172–S183. doi: 10.1002/jgm.495. [DOI] [PubMed] [Google Scholar]
- Janaswami PM, Kalvakolanu DV, Zhang Y, Sen GC. Transcriptional repression of interleukin-6 gene by adenoviral E1A proteins. J. Biol. Chem. 1992;267:24886–24891. [PubMed] [Google Scholar]
- Jordan M, Schallhorn A, Wurm FM. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler PD, Podsakoff GM, Chen X, McQuiston SA, Colosi PC, Matelis LA, Kurtzman GJ, Byrne BJ. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl Acad. Sci. USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein DL, Toth K, Doronin K, Tollefson AE, Wold WS. Functions and mechanisms of action of the adenovirus E3 proteins. Int. Rev. Immunol. 2004;23:75–111. doi: 10.1080/08830180490265556. [DOI] [PubMed] [Google Scholar]
- Lichtenstein DL, Wold WS. Experimental infections of humans with wild-type adenoviruses with replication-competent adenovirus vectors: replication, safety, transmission. Cancer Gene Ther. 2004;10:1–11. doi: 10.1038/sj.cgt.7700765. [DOI] [PubMed] [Google Scholar]
- Liu CC, Simonsen CC, Levinson AD. Initiation of translation at internal AUG codons in mammalian cells. Nature. 1984;309:82–85. doi: 10.1038/309082a0. [DOI] [PubMed] [Google Scholar]
- Moorhead JW, Clayton GH, Smith RL, Schaack J. An adenovirus vector deleted for the preterminal protein gene elicits reduced inflammation in mouse ears. J. Virol. 1999;73:1046–1053. doi: 10.1128/jvi.73.2.1046-1053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsy MA, Gu M, Motzel S, Zhao J, Lin J, Su Q, Allen H, Franlin L, Parks RJ, Graham FL, Kochanek S, Bett AJ, Caskey CT. An adenoviral vector deleted for all viral coding sequences results in enhanced safety extended expression of a leptin transgene. Proc. Natl Acad. Sci. USA. 1998;95:7866–7871. doi: 10.1073/pnas.95.14.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muruve DA, Barnes MJ, Stillman IE, Libermann TA. Adenoviral gene therapy leads to rapid induction of multiple chemokines acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 1999;10:965–976. doi: 10.1089/10430349950018364. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Cotter MJ, Zaiss AK, White LR, Liu Q, Chan T, Clark SA, Ross PJ, Meulenbroek RA, Maelandsmo GM, Parks RJ. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J. Virol. 2004;78:5966–5972. doi: 10.1128/JVI.78.11.5966-5972.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai M, Komiya K, Murata M, Kimura T, Kanaoka M, Kanegae Y, Saito I. Expression of pIX gene induced by transgene promoter: possible cause of host immune response in first-generation adenoviral vectors. Hum. Gene Ther. 2007;18:925–936. doi: 10.1089/hum.2007.085. [DOI] [PubMed] [Google Scholar]
- Nitzsche A, Paulus C, Nevels M. Temporal dynamics of cytomegalovirus chromatin assembly in productively infected human cells. J. Virol. 2008;82:11167–11180. doi: 10.1128/JVI.01218-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlicky DJ, Schaack J. Adenovirus transduction of 3T3-L1 cells. J. Lipid Res. 2001;42:460–466. [PubMed] [Google Scholar]
- Parks CL, Spector DJ. cis-dominant defect in activation of adenovirus type 5 E1b early RNA synthesis. J. Virol. 1990;64:2780–2787. doi: 10.1128/jvi.64.6.2780-2787.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore L, Morral N, Zhou H, Garcia R, Parks RJ, Kochanek S, Graham FL, Lee B, Beaudet AL. Use of a liver-specific promoter reduces immune response to the transgene in adenoviral vectors. Hum. Gene Ther. 1999;10:1773–1781. doi: 10.1089/10430349950017455. [DOI] [PubMed] [Google Scholar]
- Reich N, Pine R, Levy D, Darnell JE., Jr. Transcription of interferon-stimulated genes is induced by adenovirus particles but is suppressed by E1A gene products. J. Virol. 1988;62:114–119. doi: 10.1128/jvi.62.1.114-119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolling F, Nong Z, Pisvin S, Collen D. Adeno-associated virus-mediated gene transfer into rat carotid arteries. Gene Ther. 1997;4:757–761. doi: 10.1038/sj.gt.3300465. [DOI] [PubMed] [Google Scholar]
- Schaack J. Induction and inhibition of innate inflammatory responses by adenovirus early region proteins. Viral Immunol. 2005;18:79–88. doi: 10.1089/vim.2005.18.79. [DOI] [PubMed] [Google Scholar]
- Schaack J, Allen B, Orlicky DJ, Bennett ML, Maxwell IH, Smith RL. Promoter strength in adenovirus transducing vectors: down-regulation of the adenovirus E1A promoter in 293 cells facilitates vector construction. Virology. 2001;291:101–109. doi: 10.1006/viro.2001.1211. [DOI] [PubMed] [Google Scholar]
- Schaack J, Bennett ML, Colbert JD, Torres AV, Clayton GH, Ornelles D, Moorhead J. E1A and E1B proteins inhibit inflammation induced by adenovirus. Proc. Natl Acad. Sci. USA. 2004;101:3124–3129. doi: 10.1073/pnas.0303709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaack J, Guo X, Ho WY, Karlok M, Chen C, Ornelles D. Adenovirus type 5 precursor terminal protein-expressing 293 and HeLa cell lines. J. Virol. 1995;69:4079–4085. doi: 10.1128/jvi.69.7.4079-4085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GS, VanPeursem C, Ornelles DA, Schaack J, DeGregori J. Adenoviral vectors utilizing the cytomegalovirus promoter induce the expression of p73 via the E4-orf6/7 protein. J. Virol. 2006;80:5349–5360. doi: 10.1128/JVI.02016-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Spector DJ. Local character of readthrough activation in adenovirus type 5 early region 1 transcription control. J. Virol. 2003;77:9266–9277. doi: 10.1128/JVI.77.17.9266-9277.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TA, Mehaffey MG, Kayda DB, Saunders JM, Yei S, Trapnell BC, McClelland A, Kaleko M. Adenovirus mediated expression of therapeutic plasma levels of human factor IX in mice. Nat. Genet. 1993;5:397–402. doi: 10.1038/ng1293-397. [DOI] [PubMed] [Google Scholar]
- Straus SE. Adenovirus infections in humans. In: Ginsberg HS, editor. The Adenoviruses. Plenum; New York: 1984. pp. 451–496. [Google Scholar]
- Takeda T, Nakajima K, Kojima H, Hirano T. E1A repression of IL-6-induced gene activation by blocking the assembly of IL-6 response element binding complexes. J. Immunol. 1994;153:4573–4582. [PubMed] [Google Scholar]
- Toietta G, Mane VP, Norona WS, Finegold MJ, Ng P, McDonagh AF, Beaudet AL, Lee B. Lifelong elimination of hyperbilirubinemia in the Gunn rat with a single injection of helper-dependent adenoviral vector. Proc. Natl Acad. Sci. USA. 2005;102:3930–3935. doi: 10.1073/pnas.0500930102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vales LD, Darnell JEJ. Promoter occlusion prevents transcription of adenovirus polypeptide IX mRNA until after DNA replication. Genes Dev. 1989;3:49–59. doi: 10.1101/gad.3.1.49. [DOI] [PubMed] [Google Scholar]
- Wasylyk B, Wasylyk C, Chambon P. Short and long range activation by the SV40 enhancer. Nucleic Acids Res. 1984;12:5589–5608. doi: 10.1093/nar/12.14.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windheim M, Hilgendorf A, Burgert HG. Immune evasion by adenovirus E3 proteins: exploitation of intracellular trafficking pathways. Curr. Top. Microbiol. Immunol. 2004;273:29–85. doi: 10.1007/978-3-662-05599-1_2. [DOI] [PubMed] [Google Scholar]
- Yang Y, Jooss KU, Su Q, Ertl HC, Wilson JM. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- Yang Y, Nunes FA, Berencsi K, Furth EE, Gönczöl E, Wilson JM. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl Acad. Sci. USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M, Grundström T, Matthes H, Wintzerith M, Schatz C, Wildeman A, Chambon P. Multiple sequence motifs are involved in SV40 enhancer function. EMBO J. 1986;5:387–397. doi: 10.1002/j.1460-2075.1986.tb04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, Tazelaar J, Wilson JM. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells macrophages. Mol. Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]