Summary
Background
We aimed to compare overall survival after standard-dose versus high-dose conformal radiotherapy with concurrent chemotherapy and the addition of cetuximab to concurrent chemoradiation for patients with inoperable stage III non-small-cell lung cancer.
Methods
In this open-label randomised, two-by-two factorial phase 3 study in 185 institutions in the USA and Canada, we enrolled patients (aged ≥ 18 years) with unresectable stage III non-small-cell lung cancer, a Zubrod performance status of 0–1, adequate pulmonary function, and no evidence of supraclavicular or contralateral hilar adenopathy. We randomly assigned (1:1:1:1) patients to receive either 60 Gy (standard dose), 74 Gy (high dose), 60 Gy plus cetuximab, or 74 Gy plus cetuximab. All patients also received concurrent chemotherapy with 45 mg/m2 paclitaxel and carboplatin once a week (AUC 2); 2 weeks after chemoradiation, two cycles of consolidation chemotherapy separated by 3 weeks were given consisting of paclitaxel (200 mg/m2) and carboplatin (AUC 6). Randomisation was done with permuted block randomisation methods, stratified by radiotherapy technique, Zubrod performance status, use of PET during staging, and histology; treatment group assignments were not masked. Radiation dose was prescribed to the planning target volume and was given in 2 Gy daily fractions with either intensity-modulated radiation therapy or three-dimensional conformal radiation therapy. The use of four-dimensional CT and image-guided radiation therapy were encouraged but not necessary. For patients assigned to receive cetuximab, 400 mg/m2 cetuximab was given on day 1 followed by weekly doses of 250 mg/m2, and was continued through consolidation therapy. The primary endpoint was overall survival. All analyses were done by modified intention-to-treat. The study is registered with ClinicalTrials.gov, number NCT00533949.
Findings
Between Nov 27, 2007, and Nov 22, 2011, 166 patients were randomly assigned to receive standard-dose chemoradiotherapy, 121 to high-dose chemoradiotherapy, 147 to standard-dose chemoradiotherapy and cetuximab, and 110 to high-dose chemoradiotherapy and cetuximab. Median follow-up for the radiotherapy comparison was 22·9 months (IQR 27·5–33·3). Median overall survival was 28·7 months (95% CI 24·1–36·9) for patients who received standard-dose radiotherapy and 20·3 months (17·7–25·0) for those who received high-dose radiotherapy (hazard ratio [HR] 1·38, 95% CI 1·09–1·76; p=0·004). Median follow-up for the cetuximab comparison was 21·3 months (IQR 23·5–29·8). Median overall survival in patients who received cetuximab was 25·0 months (95% CI 20·2–30·5) compared with 24·0 months (19·8–28·6) in those who did not (HR 1·07, 95% CI 0·84–1·35; p=0·29). Both the radiation-dose and cetuximab results crossed protocol-specified futility boundaries. We recorded no statistical differences in grade 3 or worse toxic effects between radiotherapy groups. By contrast, the use of cetuximab was associated with a higher rate of grade 3 or worse toxic effects (205 [86%] of 237 vs 160 [70%] of 228 patients; p<0·0001). There were more treatment-related deaths in the high-dose chemoradiotherapy and cetuximab groups (radiotherapy comparison: eight vs three patients; cetuximab comparison: ten vs five patients). There were no differences in severe pulmonary events between treatment groups. Severe oesophagitis was more common in patients who received high-dose chemoradiotherapy than in those who received standard-dose treatment (43 [21%] of 207 patients vs 16 [7%] of 217 patients; p<0·0001).
Interpretation
74 Gy radiation given in 2 Gy fractions with concurrent chemotherapy was not better than 60 Gy plus concurrent chemotherapy for patients with stage III non-small-cell lung cancer, and might be potentially harmful. Addition of cetuximab to concurrent chemoradiation and consolidation treatment provided no benefit in overall survival for these patients.
Funding
National Cancer Institute and Bristol-Myers Squibb.
Introduction
The commonly accepted radiation therapy dose (60–63 Gy in 1·8–2·0 Gy fraction sizes) for patients with stage III non-small-cell lung cancer was established by the Radiation Therapy Oncology Group (RTOG) 7301 trial and has remained unchanged for more than 30 years.1 With the idea that increasing radiation dose would improve both local-regional control and overall survival, the RTOG and other investigators did separate prospective phase 1 and 2 trials to establish the safety and efficacy of increasing the total radiation dose in the setting of concurrent chemotherapy while reducing irradiated volumes by use of image guidance and either three-dimensional conformal or intensity-modulated radiation therapy for locally advanced non-small-cell lung cancer.2–7 Findings from these trials were similar, showing that a maximum tumour dose of 74 Gy given with concurrent weekly paclitaxel and carboplatin was safe and resulted in a median overall survival of roughly 24 months3–6 versus a median overall survival of around 17·1 months in patients given a 60 Gy dose in RTOG 9410.8
Our trial (RTOG 0617) was designed to establish whether a 74 Gy dose was better than a 60 Gy dose and whether adding cetuximab to concurrent chemoradiation would confer an overall survival benefit. Cetuximab is a chimerised antibody of the immunoglobulin G1 subclass that blocks binding of EGF and TGF α to EGFR.9 The use of cetuximab in this setting was tested in RTOG 0324, a phase 2 study combining chemoradiation with cetuximab in patients with unresectable stage III non-small-cell lung cancer.10 The trial enrolled 93 patients, showed a median survival of 22·7 months, and 24-month overall survival of 49·3%. On the basis of these encouraging data, we investigated cetuximab in this trial.
Methods
Study design and participants
In this randomised phase 3 study, we recruited patients aged 18 years and older with stage IIIA/IIIB non-small cell-lung cancer from 185 institutions in the USA and Canada. Eligibility criteria included having stage IIIA or IIIB non-small-cell lung cancer, no previous invasive cancer during the previous 3 years, Zubrod performance status score of 0–1, less than 10% weight loss (in the month before study entry), and pulmonary function (before or after bronchodilation) of 1·2 L per s or higher. Tumour histology was classified as squamous cell, adenocarcinoma, large-cell carcinoma, or non-small-cell lung cancer not otherwise specified. Specific mutational analyses were not necessary for trial entry. Patients with contralateral hilar or supraclavicular adenopathy or Pancoast tumours were excluded because of the risk of lung or brachial plexus toxic effects. Minimum pleural effusions were allowed if they were transudative and cytologically negative by thoracentesis. CT of the lung and upper abdomen and brain MRI with contrast was needed within 6 weeks of registration. Laboratory investigation requirements included the following: absolute neutrophil count of 1800 cells per μL or higher, platelets 100 000 cells per μL or higher, haemoglobin 10 g/dL or higher, normal serum creatinine and bilirubin, and aspartate amino transferase and alanine aminotransferase concentrations 2·5 times or lower the upper institutional normal limit.
The institutional review board of each participating institution approved the study protocol. All patients were required to read and sign an institutional review board approved informed consent document.
Randomisation and masking
We randomly assigned (1:1:1:1) eligible patients to one of four treatment groups: 60 Gy versus 74 Gy with concurrent and consolidation chemotherapy, with or without cetuximab. Treatment groups were assigned with the permuted block randomisation scheme described by Zelen11 and stratified by radiotherapy technique (three dimensional conformal radiotherapy vs intensity-modulated radiation therapy), Zubrod performance status at the time of enrollment (0 vs 1), use of PET during tumour staging (no vs yes), and histology (squamous vs non-squamous). Allocation sequences were generated algorithmically at the RTOG statistics and data management centre, and access to these sequences by participating centres and statistics and data management was prohibited. Participating centres enrolled patients initially via the RTOG website, and then beginning on June 2, 2011, via the National Cancer Institute’s Oncology Patient Enrollment Network (OPEN) enrolment system, which remotely accessed the allocation sequence as necessary through a secure connection. Treatment group assignments, once allocated, were not masked.
Procedures
Radiation therapy was given 5 days per week (ie, Monday to Friday with the weekend off) in 2 Gy fractions daily by use of 6–18 MV x-rays. Use of image-guided radiation therapy was encouraged. Both three-dimensional conformal and intensity-modulated radiation therapy were allowed. Compliance with normal tissue dose constraints was encouraged but not neccessary (appendix p 1). Radiation doses were prescribed to the planning target volume. Motion management was required, and internal target volumes, clinical target volumes, and planning target volumes depended on which motion management method was used. Use of PET or CT and four-dimensional CT for radiation therapy planning was encouraged. Elective nodal irradiation was not permitted. The gross tumour volume was defined as the primary tumour and any regionally involved nodes on CT (>1 cm on short axis) or pretreatment PET scan (standardised uptake value >3). The internal target volume was defined as the envelope that encompasses the gross tumour volume plus ventilatory motion. Clinical target volume margins were 0·5–1·0 cm beyond the internal target volume. Planning target volume margins were 0·5–1·5 cm beyond the clinical target volume, depending on the use of four-dimensional CT for planning and image-guided radiation therapy for delivery. The appendix shows institutional credentialing protocol compliance definitions for both radiation therapy and chemotherapy (appendix p 2). Radiation therapy plans were reviewed centrally and scored for both target delineation and dose and normal tissue delineation and dose on submitted plans. Per-protocol planning target volume coverage was achieved when more than 99% of the planning target volume received 93% or more of the prescribed dose and when minimum margin values for both clinical target volume and planning target volume were achieved.
Chemotherapy consisted of weekly paclitaxel (45 mg/m2 per week) and carboplatin (area under the curve [AUC] 2 per week) during radiation therapy. 2 weeks after chemoradiation, two cycles of consolidation chemotherapy separated by 3 weeks were given consisting of paclitaxel (200 mg/m2) and carboplatin (AUC 6). Paclitaxel was given for 3 h 30 min after diphenhydramine (25–50 mg), followed by an H2 blocker, and dexamethasone (oral or intravenous administration allowed). Carboplatin was given for 30 min with standard antiemetics after paclitaxel.
Patients in the cetuximab groups received the agent during both concurrent and consolidative phases. Cetuximab was given at 400 mg/m2 intravenously on day 1, with concurrent chemoradiation starting on day 8. Weekly cetuximab dosing was 250 mg/m2, given before chemotherapy and radiation therapy that day. Consolidation cetuximab (250 mg/m2 per week) was given weekly during consolidation.
We did follow-up assessments every 3 months for the first year, every 4 months for year 2, every 6 months for years 3–5, then every year. Routine follow-up assessments included assessment of vital signs, Zubrod performance status, and any adverse events. CT scans were done every 6 months for the first 2 years, and then once a year. Pulmonary functioning was assessed at 6 months and then 1 year after completion of treatment. All data were collected by the enrolling site and then reported to RTOG via standard case report forms. All adverse events were graded with the CTCAE version 3.0 criteria; response was assessed per the RECIST criteria.12
Pathological biomarker analysis was based on the FLEX trial,13 which suggested that the use of cetuximab in patients with EGFR expressing non-small-cell lung cancer conferred a survival benefit. We assessed the association between EGFR status in tumour samples that were available and the effect of cetuximab on patient outcomes. EGFR status established centrally was reported as H scores on the basis of EGFR immunohistochemical staining, with a score of 200 or more defined as a positive score.11
Outcomes
The coprimary objectives of this trial were to compare the overall survival of patients given 74 Gy with those given 60 Gy conformal radiation therapy with concurrent chemotherapy and to compare the overall survival of patients given cetuximab with those not given cetuximab. There were several secondary objectives including a comparison of progression-free survival and local regional tumour control, comparison of toxic effects between 74 Gy versus 60 Gy, and between cetuximab versus without cetuximab, to assess patient-reported quality of life in each group of the trial (Movsas et al, unpublished data), and to explore biological markers that might predict clinical outcome (ie, EGFR expression by use of H score).
Statistical analysis
The trial was a two-by-two factorial design, with radiation therapy dose as one treatment factor and cetuximab as the other. A log-rank test for each factor at one-sided α of 0·0125 (α of 0·0250 for both factors to account for multiple comparisons) would yield statistical power of 80% to detect an improvement in median overall survival from 17·1 months to 24 months after 339 deaths were reported from a sample of 500 patients. Three interim analyses with early stopping criteria with Haybittle14 and Peto15 boundaries for efficacy and Freidlin and Korn16 methods for futility were planned after 85, 170, and 225 events, and overseen by the independent RTOG data monitoring committee.
Results are reported on a modified intent-to-treat basis with all patients included in the assigned group, irrespective of treatment received, but excluding those patients who were found not to meet the pre-defined eligibility criteria. Endpoints of overall survival, progression-free survival, local failure, and distant metastasis were measured from the date of randomisation. We estimated overall survival and progression-free survival with the Kaplan-Meier method,17 compared with the log-rank test,18 and modelled with the Cox proportional hazards method.19 We used the cumulative incidence method20 to estimate local failure and distant metastasis rates, which were compared with the Gray’s test,21 and that were modelled with the Fine-Gray method.22 We compared categorical data with χ2 test statistics; continuous data were compared with t tests or Wilcoxon rank sum tests as appropriate. All analyses were done with SAS version 9.2 except for Fine-Gray modelling, which was done with R (version 2.11.1). The appendix shows additional methods (appendix p 8).
At the first interim analysis in June, 2011, the monitoring committee established that the trial had crossed the futility boundary with respect to high-dose radiation. The high-dose radiation groups were then closed, and the trial continued accruing patients to the 60 Gy with and without cetuximab groups. At the third interim analysis in June, 2013, it was likewise established that a futility boundary with respect to cetuximab had been crossed.
The study is registered with ClinicalTrials.gov, number NCT00533949.
Role of the funding source
The trial design, data collection, analysis, interpretation of the data, and writing of the report was the responsibility of the authors. The NCI approved the trial design, monitored trial progress, and received the two interim futility analyses of both the radiotherapy and cetuximab endpoints. Bristol-Myers Squibb agreed to the initial trial design and received the data reports for both the radiotherapy and cetuximab endpoints. RTOG statisticians (RP and CH) had access to the raw data. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Between Nov 27, 2007, and Nov 22, 2011, the trial accrued 544 patients from 185 institutions (hospital and outpatient centres; median two per institution, range 1–18), 464 while the radiotherapy dose randomisation was still in effect, and 514 while the cetuximab randomisation was in effect. The radiotherapy randomisation was closed early because of futility, but the cetuximab randomisation met targeted accrual goals. This report includes all data reported as of Oct 24, 2013; median follow-up was 21·2 months (IQR 10·5–30·3). Figure 1 shows the trial profile. After exclusions, 495 patients were available for analysis (424 for the radiation therapy endpoint and 465 for the cetuximab endpoint). Table 1 shows patient characteristics. The median age was 64 years (IQR 57–70), and most patients were white men. Treatment technique was equally distributed between three-dimensional conformal radiation therapy and intensity-modulated radiation therapy. 449 (91%) patients underwent diagnostic PET staging.
Figure 1. Trial profile.
The trial was initially opened only to the radiation dose groups. After the initial 30 patients were enrolled, the cetuximab groups were opened to accrual. After the 464th patient was enrolled, the 74 Gy and 74 Gy plus cetuximab groups were closed to accrual because the futility boundary relating to the radiation therapy endpoint had been crossed. We then continued to enrol to the 60 Gy and 60 Gy plus cetuximab groups. Thus the numbers of patients enrolled to the radiation therapy and cetuximab endpoints differ according to these three enrolment periods. CBC=complete blood count.
Table 1.
Patient characteristics
| 60 Gy (n=217) | 74 Gy (n=207) | Cetuximab (n=237) | No cetuximab (n=228) | |
|---|---|---|---|---|
| Age | ||||
|
| ||||
| Median (range) | 64 (38–83) | 64 (41–83) | 64 (38–83) | 64 (37–82) |
|
| ||||
| Sex | ||||
|
| ||||
| Men | 128 (59%) | 121 (58%) | 130 (55%) | 147 (64%) |
| Women | 89 (41%) | 86 (42%) | 107 (45%) | 81 (36%) |
|
| ||||
| Race | ||||
|
| ||||
| American Indian/Alaskan native | 1 (<1%) | 1 (<1%) | 1 (<1%) | 1 (<1%) |
| Asian | 5 (2%) | 5 (2%) | 7 (3%) | 6 (3%) |
| Black/African American | 20 (9%) | 23 (11%) | 32 (14%) | 17 (7%) |
| Native Hawaiian/Other Pacific Islander | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| White | 189 (87%) | 177 (86%) | 194 (82%) | 202 (89%) |
| Unknown | 1 (<1%) | 1 (<1%) | 3 (1%) | 2 (<1%) |
|
| ||||
| Ethnic origin | ||||
|
| ||||
| Hispanic or Latino | 8 (4%) | 5 (2%) | 4 (2%) | 10 (4%) |
| Not Hispanic or Latino | 199 (92%) | 194 (94%) | 222 (94%) | 211 (93%) |
| Unknown | 10 (4%) | 8 (5%) | 11 (5%) | 7 (3%) |
|
| ||||
| Zubrod performance status | ||||
|
| ||||
| 0 | 129 (59%) | 121 (58%) | 138 (58%) | 126 (55%) |
| 1 | 88 (41%) | 86 (42%) | 99 (42%) | 102 (45%) |
|
| ||||
| Smoking history | ||||
|
| ||||
| Non-smoker (<100 cigarettes in lifetime) | 15 (7%) | 14 (7%) | 19 (8%) | 8 (4%) |
| Former light smoker (≤10 pack years and quit ≥1 year ago) | 19 (9%) | 16 (8%) | 23 (10%) | 17 (7%) |
| Former heavy smoker (>10 pack years) | 75 (35%) | 65 (31%) | 70 (30%) | 87 (38%) |
| Current smoker (quit <1 year ago or currently smoke) | 93 (43%) | 105 (51%) | 111 (47%) | 102 (45%) |
| Unknown | 15 (7%) | 7 (3%) | 14 (6%) | 14 (6%) |
|
| ||||
| Radiotherapy technique | ||||
|
| ||||
| Three-dimensional conformal radiation therapy | 118 (54%) | 109 (53%) | 123 (52%) | 108 (47%) |
| Intensity-modulated radiation therapy | 99 (46%) | 98 (47%) | 114 (48%) | 120 (53%) |
|
| ||||
| PET staging | ||||
|
| ||||
| No | 19 (9%) | 23 (11%) | 22 (9%) | 21 (9%) |
| Yes | 198 (91%) | 184 (89%) | 215 (91%) | 207 (91%) |
|
| ||||
| Histology | ||||
|
| ||||
| Squamous cell carcinoma | 92 (42%) | 97 (47%) | 103 (43%) | 104 (46%) |
| Adenocarcinoma | 85 (39%) | 71 (34%) | 99 (42%) | 86 (38%) |
| Large cell undifferentiated | 5 (2%) | 6 (3%) | 5 (2%) | 8 (4%) |
| Non-small-cell lung cancer not otherwise specified | 35 (16%) | 33 (16%) | 30 (13%) | 30 (13%) |
|
| ||||
| AJCC stage | ||||
|
| ||||
| IIIA/N2 + undetectable NSCLC primary | 144 (66%) | 131 (63%) | 154 (65%) | 150 (66%) |
| IIIB/N3 + undetectable NSCLC primary | 73 (34%) | 76 (37%) | 83 (35%) | 78 (34%) |
|
| ||||
| EGFR H score | ||||
|
| ||||
| H-score available | 102 (47%) | 94 (45%) | 101 (41%) | 102 (45%) |
| H-score unavailable | 115 (53%) | 113 (55%) | 136 (57%) | 126 (55%) |
| <200 | 52 (51%) | 47 (50%) | 48 (48%) | 55 (54%) |
| ≥200 | 50 (49%) | 47 (50%) | 53 (52%) | 47 (46%) |
AJCC=American Joint Committee on Cancer.
Protocol compliance reviews were done for both radiation therapy and for systemic treatment (appendix pp 3–7). Rates of protocol non-compliance with radiation therapy were greater in the high-dose group than in the standard-dose group (54 [26%] of 207 patients vs 37 [17%] of 217 patients; p=0·02), as were treatment delays. Contouring scores were poorer in the high-dose group than in the low-dose group for the planning target volume, heart, and brachial plexus. 13 patients did not receive any radiation therapy. Most of the remaining patients received full dose with no interruptions in radiation therapy. There were no differences in receipt of radiation therapy in the cetuximab comparison. We recorded no difference in chemotherapy delivery between groups. Concurrent chemotherapy delivery was per-protocol in 192 (88%) of 217 patients in the 60 Gy group and 175 (85%) of 207 patients in the 74 Gy group, with corresponding acceptable variation in 14 (6%) of 217 and 12 (6%) of 207 patients. Consolidation chemotherapy delivery did not differ between groups. However, fewer patients completed this treatment: per-protocol consolidation chemotherapy was completed by 151 (70%) of 217 patients in the 60 Gy group and 133 (64%) of 207 patients in the 74 Gy group, with acceptable variations noted of 11 (5%) of 217 and 11 (5%) of 207 patients. Concurrent chemotherapy delivery was per protocol in 198 (84%) of 237 patients in the cetuximab group and 203 (89%) of 228 patients in the no cetuximab group, with corresponding acceptable variation in 19 (8%) of 237 and 10 (4%) of 228 patients. Consolidation chemotherapy was completed by 159 (67%) of 237 patients in the cetuximab group and 153 (67%) of 228 patients in the no cetuximab group, with acceptable variations noted of 9 (4%) of 237 and 15 (7%) of 208 patients.
Cetuximab compliance in patients assigned to cetuximab was not different between the standard-dose and high-dose groups (131 [96%] of 137 patients in the standard-dose group vs 90 (90%) of 100 patients in the high-dose group) during the concurrent phase or in the consolidation phase (107 [78%] of 137 vs 73 [74%] of 99 patients).
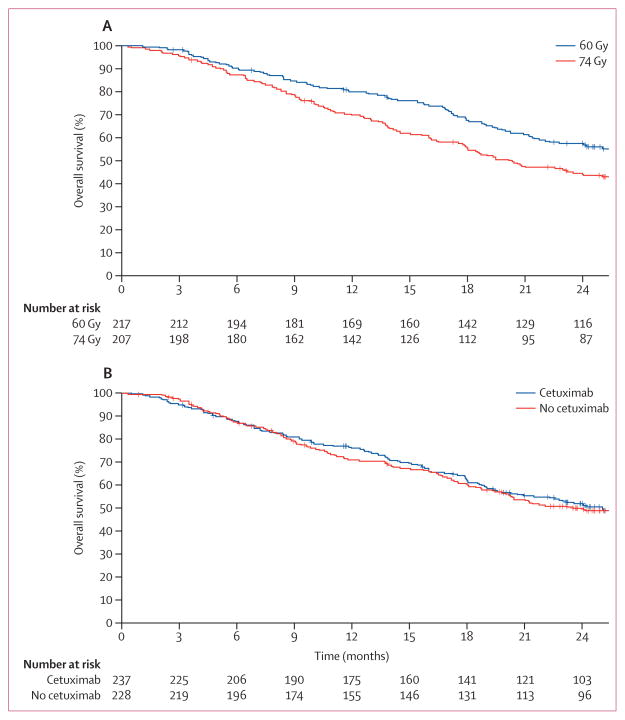
Median follow-up for the 419 analysable patients randomly assigned to the radiation therapy dose question was 22·9 months (IQR 27·5–33·3). Table 2 shows overall survival, progression-free survival, and failure rates. Median overall survival for the standard-dose group was 28·7 months (95% CI 24·1–36·9) and that for the high-dose group was 20·3 months (95% CI 17·7–25·0; one-sided p=0·996 for superiority of 74 Gy; two-sided p=0·008). The hazard ratio for radiation dose (74 Gy vs 60 Gy) on overall survival was 1·38 (95% CI 1·09–1·76; figure 2). 116 (58%) of 217 patients were alive at 2 years in the standard-dose group compared with 87 (45%) of 207 patients in the high-dose group. The primary cause of death was lung cancer (96 [76%] of 127 patients in the 60 Gy group and 105 [75%] of 140 patients in the 74 Gy group).
Table 2.
Primary and secondary outcomes
| 60 Gy (n=217)* | 74 Gy (n=207) | Cetuximab (n=237)* | No cetuximab (n=228) | |
|---|---|---|---|---|
| Overall survival | ||||
|
| ||||
| Dead | 127 | 140 | 140 | 139 |
| 1 year | 80·0% (73·9–84·7) | 69·8% (63·1–75·6) | 76·2% (70·2–81·1) | 71·1% (64·7–76·6) |
| 2 year | 57·6% (50·6–63·9) | 44·6% (37·7–51·3) | 52·3% (45·6–58·5) | 50·1% (43·3–56·6) |
| Median (months) | 28·7 (24·1–36·9) | 20·3 (17·7–25·0) | 25·0 (20·2–30·5) | 24·0 (19·8–28·6) |
| HR | 1·38 (1·09–1·76) | ·· | 1·07 (0·84–1·35) | ·· |
| p value (log-rank, one-sided) | 0·004 | ·· | 0·29 | ·· |
|
| ||||
| Progression-free survival | ||||
|
| ||||
| Fail | 164 | 164 | 181 | 168 |
| 1 year | 49·2% (42·3–55·6) | 41·2% (34·4–47·8) | 44·3% (37·8–50·5) | 46·3% (39·6–52·7) |
| 2 year | 29·1% (23·1–35·3) | 21·4% (16·1–27·3) | 24·2% (18·8–29·9) | 27·5% (21·7–33·6) |
| Median (months) | 11·8 (10·2–14·3) | 9·8 (8·8–11·6) | 10·8 (9·8–12·3) | 10·7 (9·3–13·2) |
| HR | 1·19 (0·95–1·47) | ·· | 0·99 (0·80–1·22) | ·· |
| p value (log-rank, two-sided) | 0·12 | ·· | 0·89 | ·· |
|
| ||||
| Local failure | ||||
|
| ||||
| Fail | 77 | 86 | 95 | 77 |
| 1 year | 16·3% (11·4–21·3) | 24·8% (18·9–30·7) | 22·2% (16·8–27·5) | 17·6% (12·6–22·7) |
| 2 year | 30·7% (24·5–36·9) | 38·6% (31·9–45·3) | 38·2% (31·9–44·5) | 30·7% (24·6–36·9) |
| HR | 1·26 (0·93–1·71) | ·· | 0·82 (0·61–1·11) | ·· |
| p value (Gray, two-sided) | 0·13 | ·· | 0·20 | ·· |
|
| ||||
| Distant metastasis | ||||
|
| ||||
| Fail | 106 | 107 | 124 | 98 |
| 1 year | 32·2% (25·9–38·5) | 35·1% (28·5–41·6) | 35·0% (28·9–41·2) | 29·8% (23·8–35·9) |
| 2 year | 46·6% (39·9–53·4) | 51·0% (44·1–57·9) | 52·6% (46·0–59·1) | 42·0% (35·4–48·6) |
| HR | 1·10 (0·84–1·43) | ·· | 0·80 (0·61–1·04) | ·· |
| p value (Gray, two-sided) | 0·48 | ·· | 0·09 | ·· |
Data are % (95% CI), median (95% CI), or HR (95% CI).
Reference level for the hazard ratios presented.
Figure 2. Kaplan-Meier overall survival curves for radiation dose (A) and the use of cetuximab (B).
(A) One-sided log-rank p=0·0042. (B) one-sided log-rank, p=0·2938.
Planning target volumes were similar in the two groups (mean 494·8 cm3 [SD 287·3] for standard-dose group vs 509·9 cm3 [274·7] for high-dose group). The percentages of the planning target volume covered by either 95% of the prescription dose (median 99·8% [IQR 98·9–100·0] in the standard-dose group vs 99·3% [97·1–99·9] in the high-dose group) or 100% of the prescription dose (median 95·3% [IQR 92·8–97·0] in the standard-dose group vs 94·3% [77·8–96·3] in the high-dose group) were significantly higher in the standard-dose group (p<0·0001). Mean lung dose was significantly higher in the high-dose group (mean 16·5 Gy vs 18·9 Gy; p<0·0001). We noted no difference in lung V5 distribution (mean 57·7% [SD 16·2] in the standard-dose group vs 58·0% [15·0] in the high-dose group), but lung V20 was higher in the high-dose group (mean 28·7% [7·9] vs 30·9% [7·8]; p=0·0012). The mean and maximum margin between planning target volume and clinical target volume were similar in both groups, but the minimum margin was smaller in the high-dose group (mean 4·5 mm [2·9] in the standard-dose group vs 3·9 mm [3·0] in the high-dose group; p=0·0047). Oesophageal doses were significantly higher in the high-dose group. Heart dose was also significantly higher in the high-dose group. The appendix shows a complete summary of dosimetric variables (appendix p 9–10).
On univariate analysis, increasing values of gross tumour volume, planning target volume, lung V5, heart V5, and heart V30 were associated with increased risk of death. On multivariate analyses, factors predicting overall survival were radiation dose (60 Gy), maximum oesophagitis grade, planning target volume, and heart V5 and V30. Notably, neither radiotherapy compliance nor technique (three-dimensional conformal radiation therapy or intensity-modulated radiation therapy) were significant in these analyses. The appendix shows the multivariate analyses for both the radiation therapy and cetuximab endpoints (appendix pp 10–11).
To assess whether radiation therapy quality compromised overall survival or local failure within the trial, we did separate analyses limiting the dataset to cases compliant on two measures—physician radiation therapy review and 95% of the dose covering 90% or more of the planning target volume. Significant overall survival benefits were maintained for the 60 Gy group (appendix p 12). Although this unplanned subset analysis excludes patients who did not complete the radiation therapy course, it strongly suggests that radiation therapy compliance was not the cause for the poorer performance of the high-dose group.
Additionally, we assessed the interaction of radiation therapy dose and cetuximab use to establish whether the use of cetuximab affected the dose results. We recorded no interactive effect (pinteraction=0·3984; appendix p 13).
Median follow-up for the 465 analysable patients randomly assigned to the cetuximab question was 21·3 months (IQR 23·5–29·8). Table 2 shows survival and failure endpoints. Cetuximab showed no evidence of benefit in terms of overall survival (25·0 months [95% CI 20·2–30·5] with cetuximab vs 24·0 months [19·8–28·6] without; one-sided p=0·29 for superiority; two-sided p=0·58; HR 0·94 [95% CI 0·74–1·19]; table 2). 103 (52%) of 237 patients who received cetuximab and 96 (50%) of 228 patients who did not were alive at 2 years. The primary cause of death in both cohorts was lung cancer (107 [76%] of 140 patients in the cetuximab group and 103 [74%] of 139 patients in the no-cetuximab group). There was no difference in survival with the use of cetuximab based on histological subsets (data not shown).
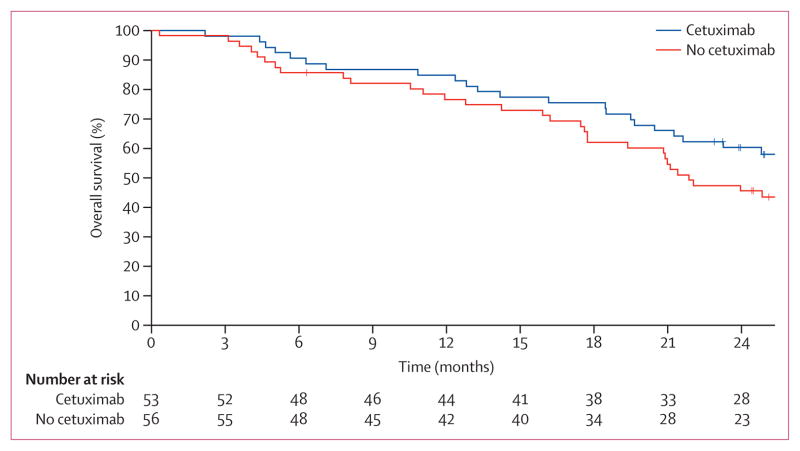
We did a separate planned retrospective analysis of the association of EGFR expression and outcome with cetuximab by use of prospectively obtained specimen. There were 203 patients (101 given cetuximab and 102 not given cetuximab) with usable samples (table 1). An EGFR H-score less than 200 (low EGFR expression) was noted more commonly in patients with non-squamous histology whereas an EGFR H score of 200 or more (EGFR-overexpression) was more common in those with squamous histology (p=0·0003). We noted no differences in outcomes between H-score groups. Cetuximab might have had a detrimental effect on patients with an H score of less than 200 and a benefit in the group with an H score of 200 or more: in patients with EGFR H score lower than 200, median overall survival for those who received cetuximab was 19·5 months (95% CI 15·6–27·8) versus 29·6 months (18·0–57·3) for those who did not receive cetuximab (p=0·056, statistical power=0·36). By contrast, in patients with an H score of 200 or higher, median overall survival for the cetuximab group was 42·0 months (95% CI 20·6–not reached) versus 21·2 months (17·2–29·2) for the no-cetuximab group (HR 1·72, 95% CI 1·04–2·84; two-sided log-rank p=0·032, statistical power=0·4; figure 3).
Figure 3. Kaplan-Meier overall survival curves for patients with high EGFR expression (H score ≥200).
Two-sided log-rank, p=0·0325.
We noted no difference in severe (grade ≥3) toxic effects between the radiation therapy dose groups (165 [76%] of 217 patients in the standard-dose group and 163 [79%] of 207 in the high-dose group); by contrast, 205 (86%) of 237 patients in the cetuximab group had severe toxic effects versus 160 (70%) of 228 in the no-cetuximab group (p<0·0001; appendix p 14–26). Notably, eight treatment-related deaths arose in the high-dose group versus three in the standard-dose group, and ten patients in the cetuximab group died because of treatment-related deaths versus five deaths in the no-cetuximab group (appendix p 27). The overall number of patients with grade 3 or worse pulmonary events was 44 (20%) of 217 in the standard-dose group versus 39 (19%) of 207 in the high-dose group (p=0·71). Rates of grade 3 or higher radiation pneumonitis were similar between groups, arising in 15 (7%) of 217 patients in the standard-dose and nine (4%) of 207 patients in the high-dose group (p=0·25). As expected, grade 3 or worse radiation oesophagitis was more common in the high-dose group (43 [21%] of 207 vs 16 [7%] of 217; p<0·0001). Table 3 shows the most frequently reported adverse events and table 4 shows them by category.
Table 3.
Most frequently reported adverse events by term, definitely, probably, or possibly related to protocol treatment
| 60 Gy (acute n=151; late n=131)
|
74 Gy (acute n=107; late n=93)
|
60 Gy plus cetuximab (acute n=137; late n=112) |
74 Gy plus cetuximab (acute n=100; late n=86) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
|
|
Acute adverse events
| ||||||||||||||||||||
| Acne | 7 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 47 (34%) | 49 (36%) | 12 (9%) | 0 (0%) | 0 (0%) | 20 (20%) | 39 (39%) | 9 (9%) | 0 (0%) | 0 (0%) |
| Alanine amino-transferase increased | 13 (9%) | 0 (0%) | 1 (<1%) | 0 (0%) | 0 (0%) | 7 (7%) | 2 (2%) | 1 (<1%) | 1 (<1%) | 0 (0%) | 10 (7%) | 0 (0%) | 1 (<1%) | 0 (0%) | 0 (0%) | 12 (12%) | 3 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Alopecia | 27 (18%) | 18 (12%) | 0 (0%) | 0 (0%) | 0 (0%) | 16 (15%) | 16 (15%) | 0 (0%) | 0 (0%) | 0 (0%) | 19 (14%) | 9 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 21 (21%) | 10 (10%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Anorexia | 16 (11%) | 15 (10%) | 7 (5%) | 0 (0%) | 0 (0%) | 13 (12%) | 12 (11%) | 6 (6%) | 0 (0%) | 0 (0%) | 23 (17%) | 19 (14%) | 7 (5%) | 1 (<1%) | 0 (0%) | 17 (17%) | 20 (20%) | 10 (10%) | 0 (0%) | 0 (0%) |
| Constipation | 30 (20%) | 6 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 14 (13%) | 5 (5%) | 1 (<1%) | 0 (0%) | 0 (0%) | 30 (22%) | 8 (6%) | 2 (1%) | 0 (0%) | 0 (0%) | 18 (18%) | 11 (11%) | 2 (2%) | 0 (0%) | 0 (0%) |
| Cough | 30 (20%) | 17 (11%) | 5 (3%) | 0 (0%) | 0 (0%) | 26 (24%) | 7 (7%) | 5 (5%) | 0 (0%) | 0 (0%) | 31 (23%) | 8 (6%) | 2 (1%) | 0 (0%) | 0 (0%) | 19 (19%) | 11 (11%) | 5 (5%) | 0 (0%) | 0 (0%) |
| Dehydration | 6 (4%) | 5 (3%) | 12 (8%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 7 (7%) | 0 (0%) | 0 (0%) | 2 (1%) | 19 (14%) | 16 (12%) | 2 (1%) | 0 (0%) | 0 (0%) | 8 (8%) | 15 (15%) | 1 (1%) | 0 (0%) |
| Dermatitis radiation | 32 (21%) | 9 (6%) | 1 (<1%) | 0 (0%) | 0 (0%) | 21 (20%) | 12 (11%) | 2 (2%) | 0 (0%) | 0 (0%) | 25 (18%) | 16 (12%) | 2 (1%) | 0 (0%) | 0 (0%) | 26 (26%) | 10 (10%) | 3 (3%) | 0 (0%) | 0 (0%) |
| Diarrhea | 19 (13%) | 2 (1%) | 4 (3%) | 0 (0%) | 0 (0%) | 10 (9%) | 1 (<1%) | 1 (<1%) | 0 (0%) | 0 (0%) | 26 (19%) | 6 (4%) | 3 (2%) | 0 (0%) | 0 (0%) | 12 (12%) | 8 (8%) | 8 (8%) | 0 (0%) | 0 (0%) |
| Dry skin | 13 (9%) | 4 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (7%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 34 (25%) | 16 (12%) | 2 (1%) | 0 (0%) | 0 (0%) | 27 (27%) | 11 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dyspepsia | 12 (8%) | 6 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (7%) | 4 (4%) | 1 (<1%) | 0 (0%) | 0 (0%) | 19 (14%) | 9 (7%) | 1 (<1%) | 0 (0%) | 0 (0%) | 13 (13%) | 7 (7%) | 1 (1%) | 0 (0%) | 0 (0%) |
| Dysphagia | 31 (21%) | 34 (23%) | 4 (3%) | 0 (0%) | 0 (0%) | 13 (12%) | 24 (22%) | 11 (10%) | 0 (0%) | 0 (0%) | 35 (26%) | 27 (20%) | 5 (4%) | 0 (0%) | 0 (0%) | 22 (22%) | 19 (19%) | 13 (13%) | 1 (1%) | 0 (0%) |
| Dyspnoea | 31 (21%) | 14 (9%) | 13 (9%) | 2 (1%) | 0 (0%) | 24 (22%) | 14 (13%) | 4 (4%) | 0 (0%) | 0 (0%) | 17 (12%) | 17 (12%) | 7 (5%) | 2 (1%) | 1 (<1%) | 17 (17%) | 11 (11%) | 8 (8%) | 0 (0%) | 0 (0%) |
| Oesophagitis | 23 (15%) | 36 (24%) | 11 (7%) | 0 (0%) | 0 (0%) | 12 (11%) | 30 (28%) | 16 (15%) | 0 (0%) | 0 (0%) | 10 (7%) | 41 (30%) | 8 (6%) | 1 (<1%) | 0 (0%) | 10 (10%) | 25 (25%) | 19 (19%) | 0 (0%) | 0 (0%) |
| Fatigue | 49 (32%) | 44 (29%) | 13 (9%) | 1 (<1%) | 0 (0%) | 30 (28%) | 38 (36%) | 7 (7%) | 1 (<1%) | 0 (0%) | 26 (19%) | 52 (38%) | 16 (12%) | 1 (<1%) | 0 (0%) | 26 (26%) | 32 (32%) | 20 (20%) | 0 (0%) | 0 (0%) |
| Fever | 6 (4%) | 3 (2%) | 2 (1%) | 1 (<1%) | 0 (0%) | 12 (11%) | 0 (0%) | 1 (<1%) | 1 (<1%) | 0 (0%) | 20 (15%) | 3 (2%) | 4 (3%) | 0 (0%) | 0 (0%) | 17 (17%) | 4 (4%) | 2 (2%) | 0 (0%) | 0 (0%) |
| Headache | 9 (6%) | 2 (1%) | 1 (<1%) | 0 (0%) | 0 (0%) | 4 (4%) | 2 (2%) | 1 (<1%) | 0 (0%) | 0 (0%) | 11 (8%) | 5 (4%) | 1 (<1%) | 0 (0%) | 0 (0%) | 8 (8%) | 6 (6%) | 1 (1%) | 0 (0%) | 0 (0%) |
| Haemoglobin decreased | 46 (30%) | 31 (21%) | 10 (7%) | 2 (1%) | 0 (0%) | 23 (21%) | 32 (30%) | 6 (6%) | 2 (2%) | 0 (0%) | 30 (22%) | 28 (20%) | 13 (9%) | 0 (0%) | 0 (0%) | 21 (21%) | 24 (24%) | 5 (5%) | 1 (1%) | 0 (0%) |
| Hyperglycemia | 11 (7%) | 10 (7%) | 7 (5%) | 0 (0%) | 0 (0%) | 5 (5%) | 5 (5%) | 2 (2%) | 1 (<1%) | 0 (0%) | 12 (9%) | 5 (4%) | 5 (4%) | 0 (0%) | 0 (0%) | 7 (7%) | 7 (7%) | 1 (1%) | 0 (0%) | 0 (0%) |
| Hypo-albuminaemia | 11 (7%) | 11 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (6%) | 9 (8%) | 1 (<1%) | 0 (0%) | 0 (0%) | 15 (11%) | 14 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 12 (12%) | 13 (13%) | 2 (2%) | 0 (0%) | 0 (0%) |
| Hypocalcaemia | 16 (11%) | 6 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (7%) | 6 (6%) | 0 (0%) | 1 (<1%) | 0 (0%) | 19 (14%) | 7 (5%) | 0 (0%) | 1 (<1%) | 0 (0%) | 10 (10%) | 4 (4%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Hypokalaemia | 12 (8%) | 0 (0%) | 4 (3%) | 1 (<1%) | 0 (0%) | 8 (7%) | 0 (0%) | 6 (6%) | 0 (0%) | 0 (0%) | 27 (20%) | 0 (0%) | 8 (6%) | 1 (<1%) | 0 (0%) | 14 (14%) | 0 (0%) | 9 (9%) | 1 (1%) | 0 (0%) |
| Hypo-magnesaemia | 14 (9%) | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) | 11 (10%) | 1 (<1%) | 0 (0%) | 1 (<1%) | 0 (0%) | 38 (28%) | 20 (15%) | 7 (5%) | 2 (1%) | 0 (0%) | 33 (33%) | 12 (12%) | 5 (5%) | 0 (0%) | 0 (0%) |
| Hyponatraemia | 20 (13%) | 0 (0%) | 5 (3%) | 1 (1%) | 0 (0%) | 14 (13%) | 0 (0%) | 8 (7%) | 0 (0%) | 0 (0%) | 22 (16%) | 0 (0%) | 8 (6%) | 0 (0%) | 0 (0%) | 18 (18%) | 1 (1%) | 5 (5%) | 0 (0%) | 0 (0%) |
| Leukopenia | 16 (11%) | 22 (15%) | 37 (25%) | 5 (3%) | 0 (0%) | 9 (8%) | 19 (18%) | 28 (26%) | 5 (5%) | 0 (0%) | 10 (7%) | 19 (14%) | 27 (20%) | 15 (11%) | 0 (0%) | 3 (3%) | 14 (14%) | 29 (29%) | 8 (8%) | 0 (0%) |
| Lymphopenia | 1 (<1%) | 9 (6%) | 20 (13%) | 12 (8%) | 0 (0%) | 0 (0%) | 4 (4%) | 15 (14%) | 6 (6%) | 0 (0%) | 0 (0%) | 4 (3%) | 18 (13%) | 11 (8%) | 0 (0%) | 2 (2%) | 2 (2%) | 12 (12%) | 2 (2%) | 0 (0%) |
| Oral mucositis | 11 (7%) | 2 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (7%) | 1 (<1%) | 1 (<1%) | 0 (0%) | 0 (0%) | 16 (12%) | 10 (7%) | 1 (<%) | 0 (0%) | 0 (0%) | 11 (11%) | 6 (6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Nausea | 47 (31%) | 12 (8%) | 8 (5%) | 0 (0%) | 0 (0%) | 35 (33%) | 7 (7%) | 3 (3%) | 0 (0%) | 0 (0%) | 33 (24%) | 21 (15%) | 6 (4%) | 0 (0%) | 0 (0%) | 26 (26%) | 13 (13%) | 9 (9%) | 0 (0%) | 0 (0%) |
| Neutrophil count decreased | 7 (5%) | 18 (12%) | 20 (13%) | 16 (11%) | 0 (0%) | 8 (7%) | 14 (13%) | 14 (13%) | 14 (13%) | 0 (0%) | 6 (4%) | 13 (9%) | 26 (19%) | 30 (22%) | 0 (0%) | 2 (2%) | 11 (11%) | 24 (24%) | 22 (22%) | 0 (0%) |
| Peripheral sensory neuropathy | 33 (22%) | 12 (8%) | 2 (1%) | 0 (0%) | 0 (0%) | 26 (24%) | 9 (8%) | 3 (3%) | 0 (0%) | 0 (0%) | 25 (18%) | 17 (12%) | 5 (4%) | 0 (0%) | 0 (0%) | 23 (23%) | 8 (8%) | 4 (4%) | 0 (0%) | 0 (0%) |
| Platelet count decreased | 38 (25%) | 9 (6%) | 8 (5%) | 2 (1%) | 0 (0%) | 24 (22%) | 12 (11%) | 4 (4%) | 4 (4%) | 0 (0%) | 27 (20%) | 11 (8%) | 8 (6%) | 3 (2%) | 0 (0%) | 20 (20%) | 8 (8%) | 10 (10%) | 6 (6%) | 0 (0%) |
| Pneumonitis | 2 (1%) | 6 (4%) | 6 (4%) | 1 (<1%) | 0 (0%) | 2 (2%) | 10 (9%) | 0 (0%) | 1 (<1%) | 0 (0%) | 0 (0%) | 7 (5%) | 9 (7%) | 0 (0%) | 1 (<1%) | 1 (1%) | 10 (10%) | 5 (5%) | 1 (1%) | 0 (0%) |
| Pruritus | 18 (12%) | 2 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 11 (10%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 35 (26%) | 11 (8%) | 2 (1%) | 0 (0%) | 0 (0%) | 17 (17%) | 6 (6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Radiation recall reaction (dermatological) | 12 (8%) | 4 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 9 (8%) | 6 (6%) | 4 (4%) | 0 (0%) | 0 (0%) | 14 (10%) | 18 (13%) | 1 (<1%) | 0 (0%) | 0 (0%) | 8 (8%) | 7 (7%) | 4 (4%) | 0 (0%) | 0 (0%) |
| Rash desquamating | 6 (4%) | 3 (2%) | 1 (<1%) | 0 (0%) | 0 (0%) | 8 (7%) | 3 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 19 (14%) | 16 (12%) | 5 (4%) | 0 (0%) | 0 (0%) | 15 (15%) | 13 (13%) | 4 (4%) | 0 (0%) | 0 (0%) |
| Taste alteration | 8 (5%) | 2 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (7%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 15 (11%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (8%) | 5 (5%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Vomiting | 17 (11%) | 7 (5%) | 4 (3%) | 0 (0%) | 0 (0%) | 8 (7%) | 7 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 15 (11%) | 15 (11%) | 3 (2%) | 0 (0%) | 0 (0%) | 7 (7%) | 10 (10%) | 7 (7%) | 0 (0%) | 0 (0%) |
| Weight loss | 32 (21%) | 14 (9%) | 0 (0%) | 0 (0%) | 0 (0%) | 20 (19%) | 19 (18%) | 4 (4%) | 0 (0%) | 0 (0%) | 35 (26%) | 20 (15%) | 3 (2%) | 0 (0%) | 0 (0%) | 21 (21%) | 22 (22%) | 7 (7%) | 0 (0%) | 0 (0%) |
|
| ||||||||||||||||||||
|
Late adverse events
| ||||||||||||||||||||
| Cough | 12 (9%) | 3 (2%) | 1 (<1%) | 0 (0%) | 0 (0%) | 8 (9%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 9 (8%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (6%) | 3 (3%) | 2 (2%) | 0 (0%) | 0 (0%) |
| Dysphagia | 5 (4%) | 2 (2%) | 1 (<1%) | 0 (0%) | 0 (0%) | 2 (2%) | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 6 (5%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (7%) | 3 (3%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dyspnoea | 5 (4%) | 6 (5%) | 5 (4%) | 1 (<1%) | 0 (0%) | 4 (4%) | 2 (2%) | 4 (4%) | 0 (0%) | 0 (0%) | 8 (7%) | 3 (3%) | 2 (2%) | 0 (0%) | 0 (0%) | 3 (3%) | 6 (7%) | 4 (5%) | 0 (0%) | 0 (0%) |
| Fatigue | 8 (6%) | 7 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (3%) | 3 (3%) | 3 (3%) | 0 (0%) | 0 (0%) | 9 (8%) | 0 (0%) | 2 (2%) | 0 (0%) | 0 (0%) | 8 (9%) | 4 (5%) | 1 (1%) | 0 (0%) | 0 (0%) |
| Haemoglobin decreased | 5 (4%) | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 8 (7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) |
| Peripheral sensory neuropathy | 9 (7%) | 5 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (2%) | 4 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (3%) | 4 (5%) | 1 (1%) | 0 (0%) | 0 (0%) |
| Pneumonitis | 5 (4%) | 11 (8%) | 1 (<1%) | 0 (0%) | 1 (<1%) | 2 (2%) | 1 (1%) | 2 (2%) | 0 (0%) | 0 (0%) | 2 (2%) | 2 (2%) | 2 (2%) | 0 (0%) | 0 (0%) | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pulmonary fibrosis | 10 (8%) | 3 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (4%) | 3 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (3%) | 0 (0%) | 1 (<1%) | 0 (0%) | 0 (0%) | 3 (3%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Weight loss | 4 (3%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (3%) | 3 (3%) | 2 (2%) | 0 (0%) | 0 (0%) | 6 (5%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (3%) | 4 (5%) | 1 (1%) | 0 (0%) | 0 (0%) |
Adverse events were graded with CTCAE version 3.0. Acute adverse events are those arising within 90 days of completion of all protocol treatment. Late adverse events are those arising after 90 days of completion of all protocol treatment. Limited to acute adverse events arising in at least 10% of patients and late adverse events arising in at least 5% of patients.
Table 4.
Most frequently reported adverse events by category, definitely, probably, or possibly related to protocol treatment
| 60 Gy (acute n=151; late n=131)
|
74 Gy (acute n=107; late n=93)
|
60 Gy plus cetuximab (acute n=137; late n=112) |
74 Gy plus cetuximab (acute n=100; late n=86) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Grade 5 |
|
|
Acute adverse events
| ||||||||||||||||||||
| Blood or bone marrow | 12 (8%) | 23 (15%) | 50 (33%) | 28 (19%) | 0 (0%) | 14 (13%) | 23 (21%) | 23 (21%) | 24 (22%) | 0 (0%) | 5 (4%) | 20 (15%) | 36 (26%) | 37 (27%) | 0 (0%) | 4 (4%) | 12 (12%) | 34 (34%) | 27 (27%) | 0 (0%) |
| Cardiac general | 5 (3%) | 4 (3%) | 6 (4%) | 1 (<1%) | 0 (0%) | 0 (0%) | 2 (2%) | 2 (2%) | 0 (0%) | 0 (0%) | 4 (3%) | 11 (8%) | 5 (4%) | 0 (0%) | 0 (0%) | 2 (2%) | 7 (7%) | 3 (3%) | 0 (0%) | 0 (0%) |
| Constitutional symptoms | 55 (36%) | 52 (34%) | 15 (10%) | 2 (1%) | 0 (0%) | 33 (31%) | 41 (38%) | 12 (11%) | 2 (2%) | 0 (0%) | 28 (20%) | 58 (42%) | 23 (17%) | 1 (<1%) | 0 (0%) | 24 (24%) | 38 (38%) | 25 (25%) | 0 (0%) | 0 (0%) |
| Dermatology or skin | 54 (36%) | 36 (24%) | 2 (1%) | 0 (0%) | 0 (0%) | 32 (30%) | 27 (25%) | 4 (4%) | 0 (0%) | 0 (0%) | 35 (26%) | 68 (50%) | 22 (16%) | 0 (0%) | 0 (0%) | 20 (20%) | 52 (52%) | 17 (17%) | 0 (0%) | 0 (0%) |
| Gastro-intestinal | 45 (30%) | 54 (36%) | 29 (19%) | 0 (0%) | 0 (0%) | 21 (20%) | 41 (38%) | 27 (25%) | 0 (0%) | 0 (0%) | 24 (18%) | 63 (46%) | 28 (20%) | 3 (2%) | 0 (0%) | 18 (18%) | 37 (37%) | 37 (37%) | 3 (3%) | 0 (0%) |
| Haemorrhage or bleeding | 13 (9%) | 1 (<1%) | 1 (<1%) | 0 (0%) | 0 (0%) | 8 (7%) | 0 (0%) | 2 (2%) | 0 (0%) | 0 (0%) | 15 (11%) | 0 (0%) | 1 (<1%) | 1 (<1%) | 1 (<1%) | 14 (14%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1%) |
| Infection | 0 (0%) | 13 (9%) | 13 (9%) | 1 (<1%) | 0 (0%) | 1 (<1%) | 9 (8%) | 10 (9%) | 1 (<1%) | 0 (0%) | 1 (<1%) | 8 (6%) | 16 (12%) | 3 (2%) | 1 (<1%) | 0 (0%) | 7 (7%) | 9 (9%) | 2 (2%) | 1 (1%) |
| Metabolic or laboratory | 37 (25%) | 16 (11%) | 14 (9%) | 3 (2%) | 0 (0%) | 21 (20%) | 7 (7%) | 14 (13%) | 4 (4%) | 0 (0%) | 33 (24%) | 23 (17%) | 28 (20%) | 4 (3%) | 0 (0%) | 28 (28%) | 19 (19%) | 18 (18%) | 1 (1%) | 0 (0%) |
| Neurology | 38 (25%) | 22 (15%) | 5 (3%) | 0 (0%) | 0 (0%) | 29 (27%) | 12 (11%) | 4 (4%) | 0 (0%) | 0 (0%) | 23 (17%) | 19 (14%) | 11 (8%) | 1 (<1%) | 0 (0%) | 23 (23%) | 14 (14%) | 5 (5%) | 1 (1%) | 0 (0%) |
| Pain | 25 (17%) | 31 (21%) | 10 (7%) | 0 (0%) | 0 (0%) | 21 (20%) | 23 (21%) | 7 (7%) | 0 (0%) | 0 (0%) | 25 (18%) | 29 (21%) | 10 (7%) | 0 (0%) | 0 (0%) | 18 (18%) | 22 (22%) | 5 (5%) | 0 (0%) | 0 (0%) |
| Pulmonary or upper respiratory | 31 (21%) | 26 (17%) | 20 (13%) | 3 (2%) | 0 (0%) | 32 (30%) | 23 (21%) | 9 (8%) | 1 (<1%) | 1 (<1%) | 29 (21%) | 23 (17%) | 20 (15%) | 3 (2%) | 2 (1%) | 22 (22%) | 18 (18%) | 13 (13%) | 4 (4%) | 0 (0%) |
|
| ||||||||||||||||||||
| Late adverse events | ||||||||||||||||||||
|
| ||||||||||||||||||||
| Blood or bone marrow | 9 (7%) | 5 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (6%) | 3 (3%) | 1 (1%) | 0 (0%) | 0 (0%) | 6 (5%) | 3 (3%) | 1 (<1%) | 0 (0%) | 0 (0%) | 3 (3%) | 1 (1%) | 1 (1%) | 0 (0%) | 0 (0%) |
| Constitutional Symptoms | 10 (8%) | 8 (6%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (6%) | 7 (8%) | 4 (4%) | 0 (0%) | 0 (0%) | 11 (10%) | 2 (2%) | 2 (2%) | 0 (0%) | 0 (0%) | 8 (9%) | 8 (9%) | 2 (2%) | 0 (0%) | 0 (0%) |
| Dermatology/skin | 2 (2%) | 3 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (4%) | 1 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 12 (11%) | 2 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (8%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Gastro-intestinal | 6 (5%) | 5 (4%) | 1 (<1%) | 1 (<1%) | 0 (0%) | 3 (3%) | 6 (6%) | 4 (4%) | 0 (0%) | 1 (1%) | 8 (7%) | 3 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 9 (10%) | 5 (6%) | 2 (2%) | 1 (1%) | 1 (1%) |
| Neurology | 11 (8%) | 5 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (8%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (3%) | 6 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (5%) | 4 (5%) | 1 (1%) | 0 (0%) | 0 (0%) |
| Pain | 7 (5%) | 4 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (3%) | 2 (2%) | 1 (1%) | 0 (0%) | 0 (0%) | 7 (6%) | 1 (<1%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (5%) | 5 (6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Pulmonary or upper respiratory | 18 (14%) | 19 (15%) | 5 (4%) | 1 (<1%) | 1 (<1%) | 13 (14%) | 5 (5%) | 7 (8%) | 0 (0%) | 1 (1%) | 14 (13%) | 5 (4%) | 6 (5%) | 0 (0%) | 0 (0%) | 4 (5%) | 11 (13%) | 6 (7%) | 0 (0%) | 0 (0%) |
Adverse events were graded with CTCAE version 3.0. Acute adverse events are those arising within 90 days of completion of all protocol treatment. Late adverse events are those arising after 90 days of completion of all protocol treatment. Limited to acute adverse events arising in at least 10% of patients and late adverse events arising in at least 5% of patients.
Discussion
We noted two major findings in this study: increasing radiation therapy dose to 74 Gy with 2 Gy per fraction did not improve overall survival and might be potentially harmful, and the addition of concurrent cetuximab, an anti-EGFR antibody, did not improve overall survival. However, use of standard-dose (60 Gy) radiation therapy with concurrent weekly carboplatin and paclitaxel, followed by consolidation chemotherapy with these drugs, resulted in a median overall survival of 28·7 months and 116 (58%) of 217 patients alive at 2 years. These results were better than anticipated and set a new benchmark for patients with inoperable stage III non-small-cell lung cancer given chemoradiation (panel). Notably, this is the first RTOG phase 3 trial that incorporated diagnostic PET for disease staging. Staging PET or PET/CT scans were obtained for most patients in both groups. Thus, stage migration might contribute to the reason the 60 Gy group did better than anticipated.
Findings of trials leading to the development of RTOG 0617 implied a survival benefit from 74 Gy, but this benefit was not realised in this study. In fact, the overall survival interval after 74 Gy was worse than that after 60 Gy (20·3 months vs 28·7 months; one-sided p=0·004). The poorer results with 74 Gy are probably caused by a combination of factors. Treatment-related deaths were more common in the high-dose group than in the low-dose group. Concurrent chemotherapy was more difficult to complete in the high-dose group than in the low-dose group. Radiation therapy planning was more likely to be non-compliant in the high-dose group, and planning target volume coverage by the 95% isodose line was poorer in the high-dose group. Concerns that non-compliance to radiation therapy in the high-dose groups would produce these results led us to analyse overall survival only in those patients with plans compliant with the protocol; nevertheless, overall survival was still better in the standard-dose groups than in the high-dose groups, suggesting that the radiotherapy dose results are attributable to other factors rather than radiation therapy compliance.
Of factors included in multivariate analyses, heart dose might best explain why patients given 74 Gy did worse than patients given the 60 Gy dose. The trial protocol suggested dose-volume guidelines for the heart, but did not need compliance. Thus, when trying to limit normal lung exposure during treatment planning, the heart volume was likely to receive generous doses of radiation therapy in both groups. Multivariate models generated with heart V5 (the percentage of heart volume receiving ≥5 Gy) and V30, on separate multivariate analysis, are both important predictors of patient survival. We chose heart V5 because of its varied distribution across all cases, and heart V30 because it is more meaningful in clinical applications and is not overly informed with the planned dose assignment. Although we were not able to track specific heart toxicity outcomes in this trial, the the findings of heart V5 and heart V30 being predictors of patient death is a major contribution to the specialty of radiation oncology. We noted variability in heart contouring within the submitted plans (appendix p 4). A secondary analysis is planned to analyse heart dose-volume effects on overall survival by use of recontoured heart structures (pericardium, atria, and ventricles). Future lung cancer trials through NRG Oncology (formerly RTOG) will include heart dose-volume limitations. Other issues that might have affected the trial’s outcome include the greater number of deaths in the high-dose groups, the extended duration of radiation therapy to 7·5 weeks, and uncertainty about the true cause of death. Cause of death was reported to RTOG by the local investigator and was probably taken from the death certificate, creating uncertainty about cancer progression or heart-related toxicity events.
Panel: Research in context.
Systematic review
We did a thorough systematic review of radiation dose escalation and the use of cetuximab in patients with locally advanced non-small-cell lung cancer before we designed this trial. We searched Medline and PubMed between Jan 1, 1980, and Jan 1, 2006, with the search terms “radiation dose”, “radiation dose escalation”, “lung cancer”, “prospective trials”, “cetuximab”, “anti-EGFR”, “systemic therapy”, and “chemotherapy” for publications in English. We identified relevant prospective trials testing radiation dose escalation or the use of anti-EGFR antibody therapy, which formed the basis for this study.
Interpretation
This phase 3 trial was the result of phase 1 and 2 trials that were undertaken and reported from RTOG 0117, CALGB 30105, NCCTG N0628, and institutional studies from the University of North Carolina, NC, USA. These data suggested that a dose of 74 Gy in 2 Gy daily fractions was tolerable and achieved a projected median survival of 24 months in patients with locally advanced non-small-cell lung cancer. However, the results of this work did not translate into a benefit for radiation dose escalation. Our findings show that when 2 Gy daily fractions of radiation therapy are used, 74 Gy is not better and might be worse than 60 Gy; nevertheless, our findings set a new benchmark for median overall survival in patients with locally advanced non-small-cell lung cancer and should be used to design future trials. 60 Gy with 2 Gy daily fractions remains the standard of care in this setting. The use of cetuximab did not result in improvements in overall survival, and strict heart dose constraints will be used in future NRG Oncology trials. NRG Oncology is continuing to pursue radiotherapy dose intensification in clinical trials in progress. RTOG 1106 is using a mid-treatment PET adapted hypofractionated radiation therapy boost to intensify radiation dose to residual tumour volumes during a total duration of 30 fractions (NCT01507428). RTOG 1308 is a phase 3 trial exploiting the potential of protons compared with photons to escalate radiation dose to 70 Gy while applying strict dose volume constraints to adjacent normal tissues (NCT01993810). Both of these trial designs were built on the knowledge gained from RTOG 0617.
Data leading to the incorporation of cetuximab into RTOG 0617 were from a phase 3 study23 of squamous cell cancer of the head and neck and from a single-arm phase 2 study of stage III non-small-cell lung cancer within the RTOG. Bonner and colleagues23 had shown benefits in both locoregional control and overall survival when cetuximab was added to radiation for locally advanced head and neck cancer. Blumenschein and colleagues10 had noted that adding cetuximab to concurrent chemoradiation for patients with non-small-cell lung cancer led to a median overall survival of 22·7 months. However, the use of cetuximab had no meaningful efffect on overall survival in our trial. Patients entered in the trial were not selected on the basis of EGFR status. Thus, most patients in this trial probably did not have EGFR mutations or have amplified EGFR expression. To explore the association between EGFR expression and outcomes with cetuximab, we determined the EGFR H score in the subpopulation of patients with enough pathological material for central review. This was a planned retrospective analysis of prospectively collected pathological specimens. Interpretation of H-score analysis was limited because only about half the patients in the cetuximab analysis had available tissue samples. Nevertheless, the data suggest that patients with an H score of 200 or more might benefit from the addition of cetuximab to chemoradiation but that cetuximab might be detrimental for patients with an H score of less than 200. These data should not change practice, but they are worthy of further exploration in subsequent trials. No additional mutational analyses are planned as part of RTOG 0617 because of limited residual pathological material.
In conclusion, in this trial of patients receiving chemoradiation for stage III non-small cell lung cancer, 74 Gy delivered in 2 Gy daily fractions was not better than 60 Gy and might be potentially harmful. Cetuximab provided no benefit in terms of overall survival. The standard radiation dose with concurrent chemotherapy for patients with inoperable stage III non-small-cell lung cancer should remain 60 Gy.
Supplementary Material
Acknowledgments
This project was supported by grants U10CA21661, U10CA180822, U10 CA37422, U24CA180803 from the National Cancer Institute (NCT00533949) and by Bristol-Myers Squibb. We thank the patients who volunteered to take part in this study; caregivers of the study participants; RTOG Data Management Center for their help in answering thousands of site questions regarding the study and their work to review all submitted case report form data for quality; Julie McIlvaine and Jennifer Presley, RTOG Quality Assurance Department, Philadelphia, PA, USA, for their role in assessing the quality of radiation therapy treatment plans and answering site questions about radiation therapy delivery and the credentialing process; Walter Bosch and William Straube, Image-Guided Therapy QA Center (ITC), Washington University, St Louis, MO, USA, for their efforts providing crucial data from radiation therapy plans submitted to the ITC to the RTOG Statistical Center; and Karma Kerns for her assistance in preparation of this Article for publication.
Footnotes
Contributions
JDB and RP contributed to the literature search, figures, study design, data collection, data analysis, data interpretation, and writing; RK to study design, data analysis, and data interpretation; GM to the literature search, study design, data analysis, data interpretation, and writing; VK, YIG, SN, PI, CR, RBW, CK, JM, JBe, and RG to patient accrual and writing; WCJ and HC contributed to the figures, study design, data analysis, data interpretation, and writing; GB, SS, and JBo to study design, data interpretation, and writing; CH to the literature search, figures, study design, data collection, data analysis, data interpretation, and writing; KF to study design; and AM to data collection, data analysis, data interpretation, and writing.
Declaration of interests
RP reports grants from National Cancer Institute, grants from Bristol-Myers Squibb, during the conduct of the study; GB reports personal fees and non-financial support from Bristol-Myers Squibb, during the conduct of the study, personal fees from Bayer Healthcare, Biothera Pharma, and Novartis Pharmaceuticals outside the submitted work; CH reports grants from Bristol-Myers Squibb (U10CA21661 U10CA37422) and funds for general study support (Bristol-Myers Squibb did not at any time have the right or were they permitted to effect underlying analysis or edit any portion of this manuscript); AM has a consultant or advisory role for Ventana Medical System, receives honoraria from Ventana Medical Systems and Genoptix, and received research funding from Roche and Ventana Medical Systems; HC has a consulting or advisory role for Bayer and EMD Serono, and receives research funding from Celgene (institution); JDB, RK, GM, SS, JBo, KF, VN, YIG, SN, PI, CR, RBW, CK, JM, JBe, RG, and WC declare no competing interests.
References
- 1.Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer. 1980;45:2744–53. doi: 10.1002/1097-0142(19800601)45:11<2744::aid-cncr2820451108>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 2.Bradley JD, Moughan J, Graham MV, et al. A phase I/II radiation dose escalation study with concurrent chemotherapy for patients with inoperable stages I to III non-small-cell lung cancer: phase I results of RTOG 0117. Int J Radiat Oncol Biol Phys. 2010;77:367–72. doi: 10.1016/j.ijrobp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley JD, Bae K, Graham MV, et al. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol. 2010;28:2475–80. doi: 10.1200/JCO.2009.27.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schild SE, McGinnis WL, Graham D, et al. Results of a Phase I trial of concurrent chemotherapy and escalating doses of radiation for unresectable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;65:1106–11. doi: 10.1016/j.ijrobp.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 5.Socinski MA, Blackstock AW, Bogart JA, et al. Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol. 2008;26:2457–63. doi: 10.1200/JCO.2007.14.7371. [DOI] [PubMed] [Google Scholar]
- 6.Stinchcombe TE, Lee CB, Moore DT, et al. Long-term follow-up of a phase I/II trial of dose escalating three-dimensional conformal thoracic radiation therapy with induction and concurrent carboplatin and paclitaxel in unresectable stage IIIA/B non-small cell lung cancer. J Thorac Oncol. 2008;3:1279–85. doi: 10.1097/JTO.0b013e31818b1971. [DOI] [PubMed] [Google Scholar]
- 7.Machtay M, Bae K, Movsas B, et al. Higher biologically effective dose of radiotherapy is associated with improved outcomes for locally advanced non-small cell lung carcinoma treated with chemoradiation: an analysis of the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2012;82:425–34. doi: 10.1016/j.ijrobp.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curran WJ, Jr, Paulus R, Langer CJ, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–60. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJ, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–11. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Blumenschein GR, Jr, Paulus R, Curran WJ, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol. 2011;29:2312–18. doi: 10.1200/JCO.2010.31.7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–75. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Pirker R, Pereira JR, von Pawel J, et al. EGFR expression as a predictor of survival for first-line chemotherapy plus cetuximab in patients with advanced non-small-cell lung cancer: analysis of data from the phase 3 FLEX study. Lancet Oncol. 2012;13:33–42. doi: 10.1016/S1470-2045(11)70318-7. [DOI] [PubMed] [Google Scholar]
- 14.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol. 1971;44:793–97. doi: 10.1259/0007-1285-44-526-793. [DOI] [PubMed] [Google Scholar]
- 15.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976;34:585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freidlin B, Korn EL. A comment on futility monitoring. Control Clin Trials. 2002;23:355–66. doi: 10.1016/s0197-2456(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan E, Meier P. Nonparameteric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 18.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 19.Cox D. Regression models and life tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–229. [Google Scholar]
- 20.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley and Sons; 1980. [Google Scholar]
- 21.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 22.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 23.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.