Abstract
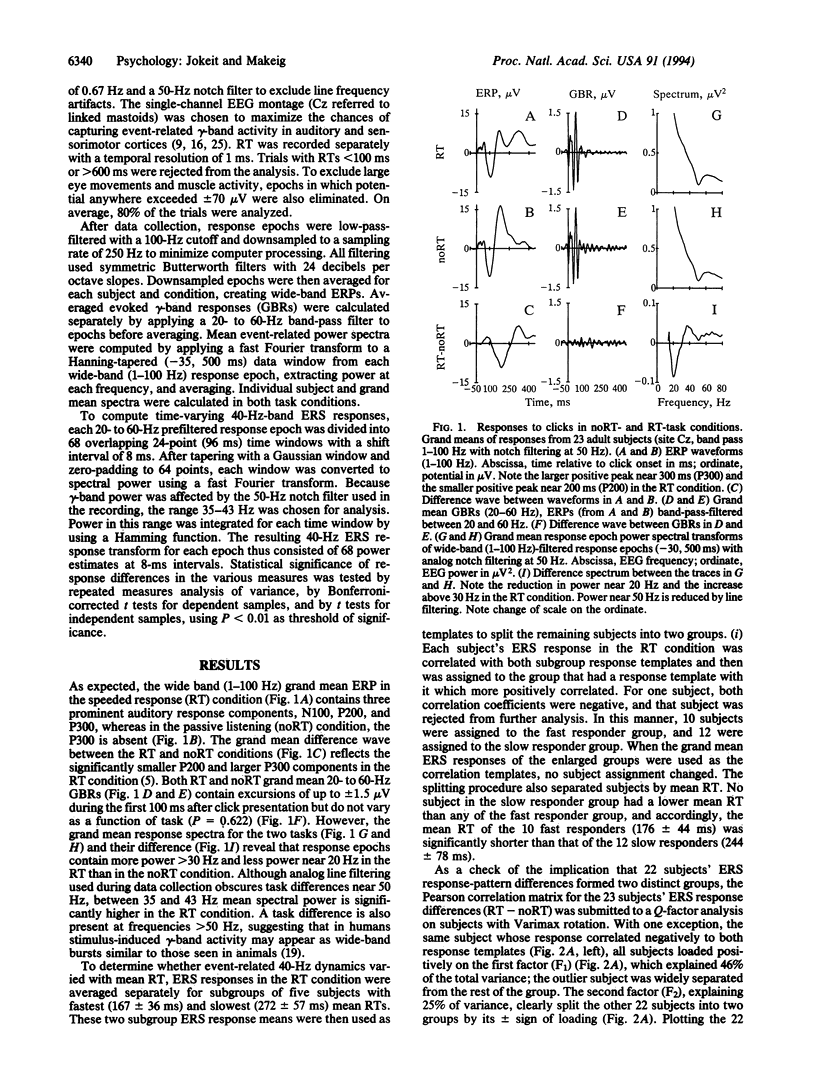
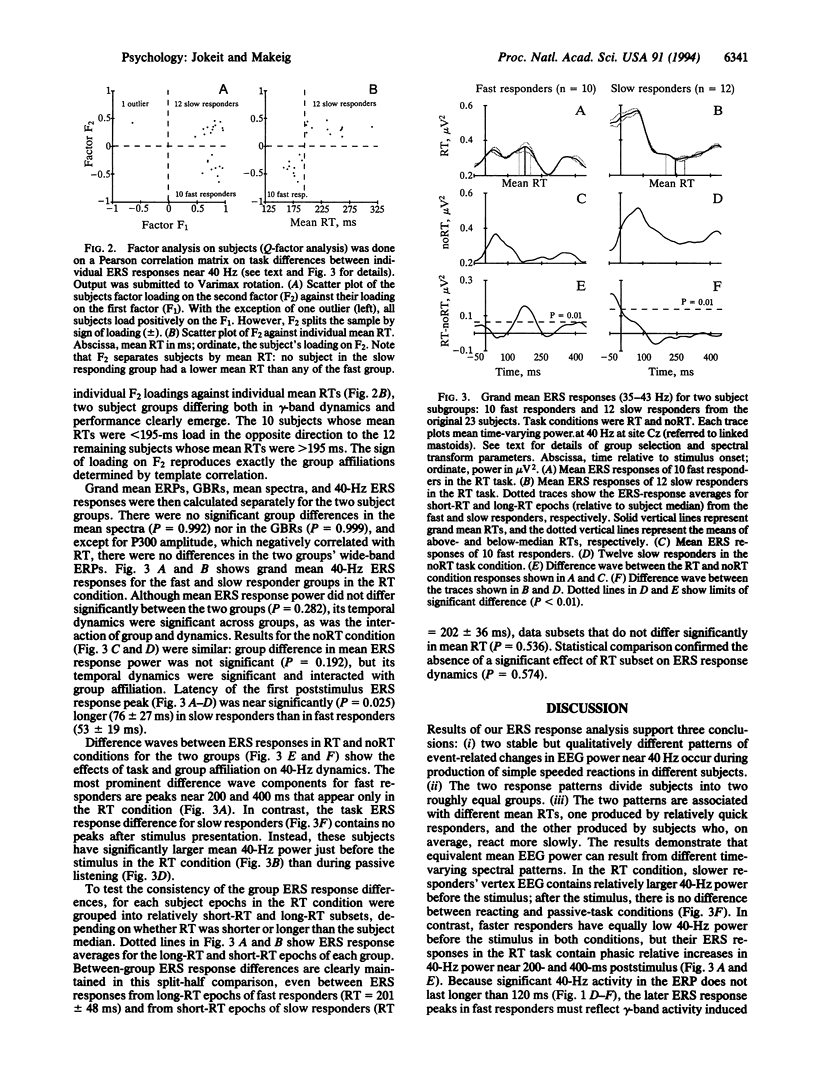
Fast- and slow-reacting subjects exhibit different patterns of gamma-band electroencephalogram (EEG) activity when responding as quickly as possible to auditory stimuli. This result appears to confirm long-standing speculations of Wundt that fast- and slow-reacting subjects produce speeded reactions in different ways and demonstrates that analysis of event-related changes in the amplitude of EEG activity recorded from the human scalp can reveal information about event-related brain processes unavailable using event-related potential measures. Time-varying spectral power in a selected (35- to 43-Hz) gamma frequency band was averaged across trials in two experimental conditions: passive listening and speeded reacting to binaural clicks, forming 40-Hz event-related spectral responses. Factor analysis of between-subject event-related spectral response differences split subjects into two near-equal groups composed of faster- and slower-reacting subjects. In faster-reacting subjects, 40-Hz power peaked near 200 ms and 400 ms poststimulus in the react condition, whereas in slower-reacting subjects, 40-Hz power just before stimulus delivery was larger in the react condition. These group differences were preserved in separate averages of relatively long and short reaction-time epochs for each group. gamma-band (20-60 Hz)-filtered event-related potential response averages did not differ between the two groups or conditions. Because of this and because gamma-band power in the auditory event-related potential is small compared with the EEG, the observed event-related spectral response features must represent gamma-band EEG activity reliably induced by, but not phase-locked to, experimental stimuli or events.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Başar-Eroglu C., Başar E. A compound P300-40 Hz response of the cat hippocampus. Int J Neurosci. 1991 Oct;60(3-4):227–237. doi: 10.3109/00207459109167035. [DOI] [PubMed] [Google Scholar]
- Başar E., Gönder A., Ungan P. Important relation between EEG and brain evoked potentials. I. Resonance phenomena in subdural structures of the cat brain. Biol Cybern. 1976 Dec 15;25(1):27–40. doi: 10.1007/BF00337046. [DOI] [PubMed] [Google Scholar]
- Belyavin A., Wright N. A. Changes in electrical activity of the brain with vigilance. Electroencephalogr Clin Neurophysiol. 1987 Feb;66(2):137–144. doi: 10.1016/0013-4694(87)90183-0. [DOI] [PubMed] [Google Scholar]
- Bouyer J. J., Montaron M. F., Vahnée J. M., Albert M. P., Rougeul A. Anatomical localization of cortical beta rhythms in cat. Neuroscience. 1987 Sep;22(3):863–869. doi: 10.1016/0306-4522(87)92965-4. [DOI] [PubMed] [Google Scholar]
- Campbell M. J., Lewis D. A., Foote S. L., Morrison J. H. Distribution of choline acetyltransferase-, serotonin-, dopamine-beta-hydroxylase-, tyrosine hydroxylase-immunoreactive fibers in monkey primary auditory cortex. J Comp Neurol. 1987 Jul 8;261(2):209–220. doi: 10.1002/cne.902610204. [DOI] [PubMed] [Google Scholar]
- Eckhorn R., Bauer R., Jordan W., Brosch M., Kruse W., Munk M., Reitboeck H. J. Coherent oscillations: a mechanism of feature linking in the visual cortex? Multiple electrode and correlation analyses in the cat. Biol Cybern. 1988;60(2):121–130. doi: 10.1007/BF00202899. [DOI] [PubMed] [Google Scholar]
- Engel A. K., König P., Singer W. Direct physiological evidence for scene segmentation by temporal coding. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9136–9140. doi: 10.1073/pnas.88.20.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman W. J., Skarda C. A. Spatial EEG patterns, non-linear dynamics and perception: the neo-Sherringtonian view. Brain Res. 1985 Dec;357(3):147–175. doi: 10.1016/0165-0173(85)90022-0. [DOI] [PubMed] [Google Scholar]
- Freeman W. J., van Dijk B. W. Spatial patterns of visual cortical fast EEG during conditioned reflex in a rhesus monkey. Brain Res. 1987 Oct 6;422(2):267–276. doi: 10.1016/0006-8993(87)90933-4. [DOI] [PubMed] [Google Scholar]
- Galambos R., Makeig S., Talmachoff P. J. A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin D. S., Aminoff M. J., Shefrin S. L. Organization of sensory discrimination and response selection in choice and nonchoice conditions: a study using cerebral evoked potentials in normal humans. J Neurophysiol. 1990 Oct;64(4):1270–1281. doi: 10.1152/jn.1990.64.4.1270. [DOI] [PubMed] [Google Scholar]
- Gray C. M., Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk J. C., Keifer J., Barto A. G. Distributed motor commands in the limb premotor network. Trends Neurosci. 1993 Jan;16(1):27–33. doi: 10.1016/0166-2236(93)90049-r. [DOI] [PubMed] [Google Scholar]
- Krieger D., Dillbeck M. High frequency scalp potentials evoked by a reaction time task. Electroencephalogr Clin Neurophysiol. 1987 Sep;67(3):222–230. doi: 10.1016/0013-4694(87)90020-4. [DOI] [PubMed] [Google Scholar]
- Kristeva-Feige R., Feige B., Makeig S., Ross B., Elbert T. Oscillatory brain activity during a motor task. Neuroreport. 1993 Sep 30;4(12):1291–1294. doi: 10.1097/00001756-199309150-00001. [DOI] [PubMed] [Google Scholar]
- Kristofferson A. B. Quantal and deterministic timing in human duration discrimination. Ann N Y Acad Sci. 1984;423:3–15. doi: 10.1111/j.1749-6632.1984.tb23413.x. [DOI] [PubMed] [Google Scholar]
- Llinás R., Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madler C., Pöppel E. Auditory evoked potentials indicate the loss of neuronal oscillations during general anaesthesia. Naturwissenschaften. 1987 Jan;74(1):42–43. doi: 10.1007/BF00367044. [DOI] [PubMed] [Google Scholar]
- Makeig S. Auditory event-related dynamics of the EEG spectrum and effects of exposure to tones. Electroencephalogr Clin Neurophysiol. 1993 Apr;86(4):283–293. doi: 10.1016/0013-4694(93)90110-h. [DOI] [PubMed] [Google Scholar]
- Makeig S., Inlow M. Lapses in alertness: coherence of fluctuations in performance and EEG spectrum. Electroencephalogr Clin Neurophysiol. 1993 Jan;86(1):23–35. doi: 10.1016/0013-4694(93)90064-3. [DOI] [PubMed] [Google Scholar]
- Metherate R., Cox C. L., Ashe J. H. Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992 Dec;12(12):4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy V. N., Fetz E. E. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz T. A., Goodin D. S., Aminoff M. J. Neural processing in a three-choice reaction-time task: a study using cerebral evoked-potentials and single-trial analysis in normal humans. J Neurophysiol. 1993 May;69(5):1499–1512. doi: 10.1152/jn.1993.69.5.1499. [DOI] [PubMed] [Google Scholar]
- Pantev C., Makeig S., Hoke M., Galambos R., Hampson S., Gallen C. Human auditory evoked gamma-band magnetic fields. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8996–9000. doi: 10.1073/pnas.88.20.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G. Graphical display and statistical evaluation of event-related desynchronization (ERD). Electroencephalogr Clin Neurophysiol. 1977 Nov;43(5):757–760. doi: 10.1016/0013-4694(77)90092-x. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G., Neuper C. Simultaneous EEG 10 Hz desynchronization and 40 Hz synchronization during finger movements. Neuroreport. 1992 Dec;3(12):1057–1060. doi: 10.1097/00001756-199212000-00006. [DOI] [PubMed] [Google Scholar]
- Picton T. W., Hillyard S. A., Krausz H. I., Galambos R. Human auditory evoked potentials. I. Evaluation of components. Electroencephalogr Clin Neurophysiol. 1974 Feb;36(2):179–190. doi: 10.1016/0013-4694(74)90155-2. [DOI] [PubMed] [Google Scholar]
- Pirch J. H., Corbus M. J., Rigdon G. C., Lyness W. H. Generation of cortical event-related slow potentials in the rat involves nucleus basalis cholinergic innervation. Electroencephalogr Clin Neurophysiol. 1986 May;63(5):464–475. doi: 10.1016/0013-4694(86)90128-8. [DOI] [PubMed] [Google Scholar]
- Scherg M., Volk S. A. Frequency specificity of simultaneously recorded early and middle latency auditory evoked potentials. Electroencephalogr Clin Neurophysiol. 1983 Nov;56(5):443–452. doi: 10.1016/0013-4694(83)90227-4. [DOI] [PubMed] [Google Scholar]
- Schiller P. H., Sandell J. H., Maunsell J. H. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol. 1987 Apr;57(4):1033–1049. doi: 10.1152/jn.1987.57.4.1033. [DOI] [PubMed] [Google Scholar]
- Singer W. Synchronization of cortical activity and its putative role in information processing and learning. Annu Rev Physiol. 1993;55:349–374. doi: 10.1146/annurev.ph.55.030193.002025. [DOI] [PubMed] [Google Scholar]
- Steriade M., Dossi R. C., Paré D., Oakson G. Fast oscillations (20-40 Hz) in thalamocortical systems and their potentiation by mesopontine cholinergic nuclei in the cat. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4396–4400. doi: 10.1073/pnas.88.10.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]



