Abstract
The hippocampus and medial temporal lobes (MTL) support the successful formation of new memories without succumbing to interference from related, older memories. Computational models and animal findings have implicated the dentate gyrus (DG), CA3, CA1, and entorhinal cortex (EC) in the disambiguation and encoding of well-established, episodic events that share common elements. However, it is unknown if these hippocampal subfields and MTL (entorhinal, perirhinal, parahippocampal) cortices also contribute during working memory when overlapping stimuli that share related features are rapidly encoded and subsequently maintained over a brief temporal delay. We hypothesized that activity in CA3/DG hippocampal subfields would be greater for the rapid encoding of stimuli with overlapping features than for the rapid encoding of stimuli with distinct features. In addition, we predicted that CA1 and EC, regions that are associated with creating long-term episodic representations, would show greater sustained activity across both encoding and delay periods for representations of stimuli with overlapping features than for those with distinct features. We used high-resolution fMRI during a delayed matching-to-sample (DMS) task using face pairs that either shared (overlapping condition, OL) or did not share (non-overlapping condition, NOL) common elements. We contrasted the OL condition with the NOL condition separately at sample (encoding) and during a brief delay (maintenance). At sample, we observed activity localized to CA3/DG, the subiculum, and CA1. At delay, we observed activity localized to the subiculum and CA1 and activity within the entorhinal, perirhinal, and parahippocampal cortices. Our findings are consistent with our hypotheses and suggest that CA3/DG, CA1 and the subiculum support the disambiguation and encoding of overlapping representations while CA1, subiculum and entorhinal cortex maintain these overlapping representations during working memory.
Keywords: high-resolution fMRI, dentate gyrus, CA3, CA1, delayed match-to-sample
INTRODUCTION
The hippocampus is critically involved in disambiguation, the separation of episodic elements that share common representations. At the subfield level, computational models have proposed a critical role for the CA3 and dentate gyrus (DG) hippocampal subfields in minimizing interference between events that share overlapping elements (O’Reilly and McClelland, 1994; McClelland et al., 1995; Levy, 1996; Hasselmo and Wyble, 1997; Sohal and Hasselmo, 1998; Hasselmo and Eichenbaum, 2005). Empirical evidence from single neuron recordings and lesion studies (Frank et al., 2000; Wood et al., 2000; Agster et al., 2002; Lee et al., 2006; Lipton et al., 2007; Lee and Solivan, 2008; Lee and Solivan, 2010; Ginther et al., 2011) as well as recent high-resolution fMRI work (Bakker et al., 2008; Lacy et al., 2011) supports a role for these structures, along with CA1 and the entorhinal cortex (EC), in disambiguation in an episodic context.
Evidence also suggests that the DG and CA1 are critical in a working memory (WM) setting when overlapping representations are encoded. For example, lesions to the DG in rats significantly impair WM (Lee and Kesner, 2003; Gilbert and Kesner, 2006). In particular, rats with DG lesions show deficits on tasks that require distinguishing between highly similar spatial representations (Gilbert et al., 2001). Additionally, single neuron recordings show increased CA1 activity as rats navigate common locations in overlapping navigation routes (Frank et al., 2000; Wood et al., 2000; Bahar et al., 2011; Ginther et al., 2011), and CA1 has also been implicated in maintaining information across a brief temporal delay (Furusawa et al., 2006; Hunsaker et al., 2006; Manns et al., 2007; Kesner et al., 2010). These results suggest that both DG and CA1 may be engaged when encoding overlapping representations during WM, while CA1 may be recruited for maintaining representations over a brief working memory delay.
Empirical evidence also suggests that in addition to CA1, the EC may be recruited for maintaining overlapping representations across a brief working memory delay. Converging findings from single unit recordings (Suzuki et al., 1997; Young et al., 1997) and persistent spiking activity in slice preparations (Klink and Alonso, 1997; Fransén et al., 2002) have demonstrated sustained EC activity at the cellular level during brief delay periods. Additionally, human neuroimaging studies have shown that hippocampal and EC activity during the delay portion of WM tasks is associated with long-term retention of the information presented (Schon et al., 2004; Ben-Yakov and Dudai, 2011). Theoretical models have proposed that the EC may aid in binding related episodes that overlap in time, or that share the same temporal context (Hasselmo and Eichenbaum, 2005; Hasselmo, 2007; Hasselmo et al., 2007; Hasselmo, 2009) suggesting that the EC may play a crucial role in maintaining representations that distinguish overlapping events. In support of this role, increased neuronal firing within the entorhinal cortex (EC) has also been observed when overlapping spatial routes are disambiguated (Lipton et al., 2007). As such, the EC may be recruited to maintain distinct representations of overlapping events across a delay, regardless of the nature of the overlap.
Our goal was to use high-resolution fMRI to examine the contributions of hippocampal subfields and extrahippocampal MTL cortices to the encoding and maintenance of overlapping representations during working memory. We predicted greater transient activity in CA3/DG and CA1 during encoding of stimuli with overlapping features than during encoding of distinct stimuli. In addition, we predicted greater sustained recruitment of the EC and CA1 during a brief working memory delay period when overlapping stimuli are maintained than when distinct stimuli are maintained. To test these hypotheses, we used a delayed matching-to-sample (DMS) task using face stimulus pairs with either overlapping or non-overlapping features. To evaluate activation related to separating overlapping face pairs and to maintaining distinct representations of these overlapping face pairs, we contrasted the overlapping conditions (same identity, different facial expressions) with the non-overlapping conditions (different identities, different facial expressions) during the sample and delay periods.
MATERIALS AND METHODS
Subjects
Seventeen healthy individuals from the Boston University community (6 male, 11 female, ages 19–31) with no history of neurological or psychiatric illness participated in the study after giving informed consent in accordance with the Human Research Committee of the Massachusetts General Hospital and the Charles River Campus Institutional Review Board of Boston University. One subject was eliminated because of poor behavioral task performance. Vision was either normal or corrected to normal. Analysis of the fMRI data was performed on the remaining 16 subjects (5 male, 11 female, mean age = 21.1 ± 3.6 years).
Behavioral Procedures
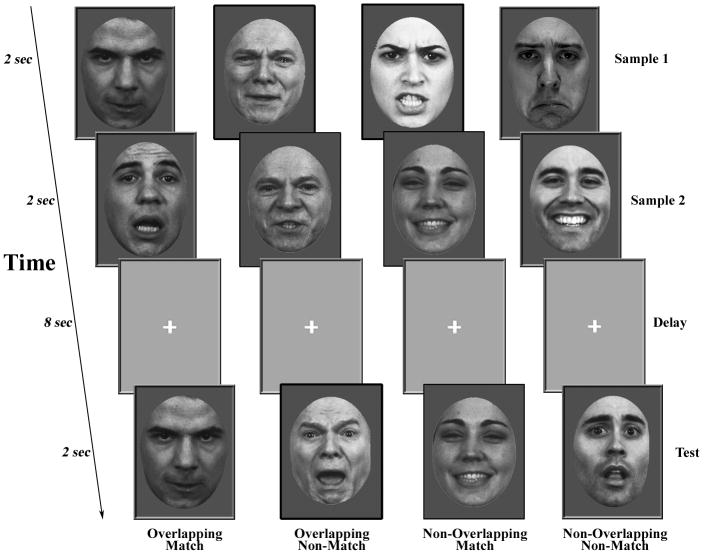
Subjects performed a DMS task using familiarized face stimuli selected from the University of Pennsylvania database of facial expressions (Gur et al., 2002) and other freely available online databases (Ekman and Friesen, 1976; Lyons et al., 1998). The stimulus set consisted of gray scale images of young, non-famous faces of men and women. In total, there were 120 different face identities. Faces varied in range of expression including happy, sad, angry, and fearful. The stimuli were cropped to 350 x 467 pixels at 28.35 pixels/cm resolution (12.35 cm x 16.47 cm), and the central facial features were isolated by cropping out the peripheral features (e.g. hair, clothes; see Fig. 1).
Fig. 1.
One day prior to scanning, subjects were familiarized with the neutral expressions of all 120 face identities. During three different cycles, the subjects made subjective judgments (male/female, young/old, and attractive/non-attractive) while viewing the stimuli. These decision requirements were designed to engage the subject in a deeper level of encoding and to minimize any reliance on familiarity-based recognition decisions.
The following day, the DMS task was performed during 5 functional scans (Fig. 1). Each trial consisted of two faces presented sequentially for 2 seconds each (Sample), followed by a gray background image with a black fixation cross presented for an 8-second delay-period (Delay), followed by a single face presented for 2 seconds (Test). A variable length (8, 10, or 12 s) inter-trial interval (ITI) separated each trial. Specifically, the task consisted of two conditions: overlapping (OL) and non-overlapping (NOL). The conditions differed only in the type of faces presented during the Sample phase. The face pairs for the OL condition consisted of 2 different expressions from a single individual, and those for the NOL condition consisted of 2 different expressions from 2 different individuals. During the Test period, subjects selected one of three possible button-press responses: 1 indicated that the Test face matched both the identity and the expression of the first Sample face, 2 indicated that the Test face matched both the identity and expression of the second Sample face, 3 indicated that the Test face matched the identity of one of the two Sample faces, but did not match the emotional expression (non-match). For both OL and NOL trials, non-match trials contained stimuli that were the same identity as one of the two Sample faces, but with a different expression. Overlapping/Non-overlapping conditions, match/non-match trials, and facial expressions were counterbalanced across 5 fMRI runs. Subjects performed 16 trials per run for a total of 80 trials (40 OL and 40 NOL). For all tasks, stimuli were presented and responses were recorded using E-Prime 2 (Psychology Software Tools, Inc., Pittsburgh, PA).
fMRI data acquisition
Imaging data were acquired on a 3.0 Tesla Siemens MAGNETOM Trio™Tim® System scanner (Siemens AG, Medical Solutions, Erlangen, Germany) with a 12-channel Tim® Matrix head coil at the Athinoula A. Martinos Center for Biomedical Imaging (Massachusetts General Hospital, Charlestown, MA). Two high-resolution T1-weighted magnetization prepared rapid acquisition gradient echo (MP-RAGE) structural scans were acquired using generalized autocalibrating partially parallel acquisitions (GRAPPA) (TR = 2.530 s, TE = 3.44–3.48 ms, flip angle = 7°, number of slices = 176, field of view = 256 mm, resolution = 1 x 1 x 1 mm3). A total of 960 functional volumes were acquired for each participant using T2*-sensitive gradient echo echo-planar imaging (EPI) blood-oxygen-level-dependent (BOLD) scans (TR = 2 s, TE = 34 ms, flip angle = 90°, 22 interleaved slices, field of view (FoV) = 96 mm, matrix size = 64 x 64, resolution = 1.5 x 1.5 x 1.5 mm3, no interslice skip). We obtained a single T1-EPI scan for each subject (TR = 18.280 s; TE = 52 ms; flip angle = 90°, field of view = 192 mm; matrix size = 128 x 128 mm2; in-plane resolution = 1.5 mm2, slice thickness = 1.5 mm, interslice skip = 0.3 mm; 90 interleaved slices) using the GRAPPA method. All EPI image volumes were acquired using slices that were oriented approximately parallel to the long axis of the hippocampus, allowing inclusion of all hippocampal subfields (CA3/DG, CA1, subiculum), including the hippocampal tail, and MTL subregions (perirhinal, entorhinal, parahippocampal cortices, amygdala) in the axial plane.
Preprocessing of fMRI Data
Data were preprocessed using the SPM8 software package (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology, London, UK). First, we averaged together the two high-resolution T1 structural scans to increase the visual quality of the MTL for manual tracings. The BOLD images were then reoriented such that the origin was 8 mm ventral to the anterior commissure (AC). The images were then corrected for differences in slice timing, realigned to the first image collected within a series, and unwarped to correct for image distortions due to susceptibility-by-movement interactions. In addition, the averaged MP-RAGE scans and the BOLD scans were coregistered to the T1-EPI scan, and the MP-RAGE scans were segmented into gray and white matter images. The segmentation step also produced a bias-corrected MP-RAGE scan using the default tissue probability maps as priors. Because regions of interest (ROIs) included anatomically defined hippocampal subfields, the standard normalization and spatial smoothing procedures were omitted. Instead, ROI-based regional cross-participant alignment (ROI-AL) procedures were applied (Stark and Okado, 2003). The protocol used for ROI definition and anatomical tracings and the ROI-AL procedures are described in the following paragraphs.
ROI-based cross-participant alignment
We used the ROI-AL method (Stark and Okado, 2003; Bakker et al., 2008) to accomplish cross-participant alignment. This method optimizes regional-alignment and allows more precise localization within anatomically defined regions within the MTL and hippocampus (Miller et al., 2005; Yassa and Stark, 2009). To perform this cross-participant alignment, we first manually delineated each participant’s anatomically defined region-of-interest (ROI) using techniques adapted for the visualization and analysis of MTL structures and described in more detail below (Insausti et al., 1998; Pruessner et al., 2000; Zeineh et al., 2000; Pruessner et al., 2002; Preston et al., 2010). To create the high-resolution ROI-model, we selected a single participant to serve as the initial model for the transformation calculations for all other participants. Each participant’s set of ROIs were aligned to the model by creating a displacement field, and the resulting displacement field was then applied to each participant’s structural and statistical data.
ROI definition
ROIs included the hippocampal subfields (CA1, dentate gyrus/CA3, subiculum), amygdala, entorhinal cortex, perirhinal cortex, and parahippocampal cortex. ROIs were manually traced on the bias-corrected, averaged MP-RAGE scans in native space (single subject) by one researcher (RN) using the ITK-SNAP software package (Yushkevich et al., 2006) and previously published guidelines (Insausti et al., 1998). Boundaries for the hippocampus included the fimbria, the inferior horn of the lateral ventricle, the uncus, and the quadrigeminal cistern. The subfields of the hippocampus, the subiculum, CA1, and CA3/DG, were defined bilaterally using methods described in a previous study (Kirwan and Stark, 2007; Kirwan et al., 2007) and using the Duvernoy atlas (Duvernoy et al., 2005). Because the border between CA3 and dentate gyrus could not be anatomically distinguished, the two structures were combined to create a CA3/DG subfield. Briefly, eight coronal slices that were detailed in the Duvernoy atlas were delineated on the corresponding slices. The manual segmentation continued in both anterior and posterior directions to create a smooth transition between slices. The perirhinal cortex was delineated using landmarks including the gyrus of Schwalbe and the lateral edge of the collateral sulcus. The entorhinal cortex was defined by landmarks including the disappearance of the hippocampal uncus, and the infero-medial bank of the collateral sulcus. The parahippocampal cortex was defined using borders including the splenium of the corpus callosum and the perirhinal cortex.
Data analysis
Data were statistically analyzed using the SPM8 software package. All single-subject statistical analyses were performed in native space before cross-participant alignment with ROI-AL. BOLD activity during the DMS task was assessed with multiple regression with near orthogonal regressors, allowing for simultaneous and independent analysis of hemodynamic changes during time-locked task components (Sample, then Delay, then Test). We created 12 regressors to account for the slow onset of the BOLD signal relative to the stimulus presentation. The regressors modeled the 5 components of the task (Sample1, Sample2, Delay, Test Match, Test Non-match, and ITI) for each of the two conditions (overlapping, OL; and non-overlapping, NOL). Given that participants made only very few errors (average number of errors across participants: OL: 4.31 errors, NOL: 2.37 errors), our analysis included correct and incorrect trials. Regressors were constructed by using positive stick functions convolved with a Gamma hemodynamic response function (HRF) (Boynton et al., 1996) in MATLAB 7.5 (The Mathworks, Inc., Natick, MA) (Fig. 2). Additionally, the delay regressor was separated into 4 quarter sized stick functions spread across the 4 TRs (8 seconds) of the delay period to account for the sustained time-course and expected weaker signal during this phase of the task (Schluppeck et al., 2006; LoPresti et al., 2008). The 5 functional runs were concatenated in time and treated as a single time series. Additional regressors were included in the model to account for run number. Linear contrasts were created to compare OL and NOL conditions at the Sample, Delay, and Test components. Contrasts of the Sample component consisted of a combination of both Sample 1 and Sample 2 regressors because of collinearity between the Sample 1 and Sample 2 regressors. Statistical parametric t-maps (SPM[T]) were generated for each contrast and participant. These contrast images were calculated at the single-subject level in native space before ROI-based cross-participant alignment. The pre-processed BOLD images were spatially smoothed with a full-width-half-maximum of 3 mm to account for variations in individual subjects’ functional anatomy. Group-analyses were performed after ROI-based cross-participant alignment of the contrast images. Group analyses were performed on each component of the task by entering the contrast images from each subject into a second-level random-effects one-sample t-test treating subjects as a random factor. A cluster extent threshold was enforced to correct for multiple comparisons. Specifically, an individual voxel statistical threshold of p < 0.03 was enforced with a minimum cluster extent threshold of 20 voxels to correct for multiple comparisons to p < 0.03. The cluster extent threshold value was derived from a Monte Carlo simulation with 10,000 iterations using AFNI’s AlphaSim program (Ward, 2000). Localization of activity (OL > NOL) to a particular region was determined by calculating the percent overlap between the functionally defined areas and the manual segmentations. Additionally, for visualization of our parameter estimates, we averaged the extracted values across participants using SPM.
Fig. 2.
RESULTS
Behavioral Performance
Behavioral responses and reaction times were recorded and analyzed. A repeated-measures ANOVA (overlapping/non-overlapping by match/nonmatch) was performed for both accuracy and reaction time. As expected, participants performed significantly better on NOL trials than on OL trials (Mean ± Standard Error, OL: 89.2% ± 1.20; NOL: 94.0% ± 4.9: F(1,15)= 4.62, p < 0.05) and on non-match trials compared to match trials (Non-Match: 94.8% ± 1.9; Match: 88.4% ± 2.3, F(1,15)= 16.09; p < 0.05). Reaction time performance showed no significant differences between OL and NOL trials (OL: 1091.22 ms ± 138.61; NOL: 1058.30 ms ± 141.80, F(1,15)= 2.98; p = NS) and between match and non-match trials (Non-match: 1053.74 ms ± 36.7; Match: 1095.76 ms ± 34.9, F(1,15)= 3.06; p = NS). There were no significant interactions for both reaction time (F(1,15)=2.46, NS) and accuracy (F(1,15)=1.46, NS).
fMRI results
Encoding OL representations
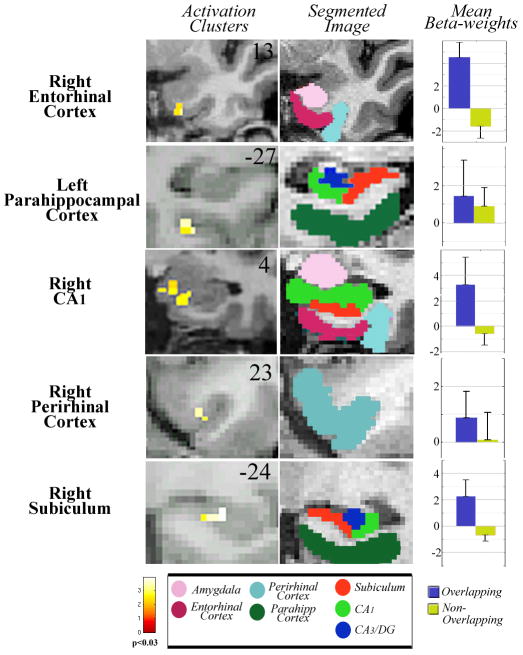
Analysis during encoding of OL face pairs compared to NOL face pairs (OL Sample > NOL Sample) demonstrated significant activation in the left CA1 (T=3.39; Z=2.88; p<0.03Corr) and right hippocampal CA1, CA3/DG, and subiculum (Fig. 2; T=3.89; Z=3.19; p<0.03Corr). We also contrasted NOL trials with OL trials (NOL, Sample > OL, Sample) during encoding at p<0.03 and the contrast yielded no significant clusters of activity.
Maintaining OL representations
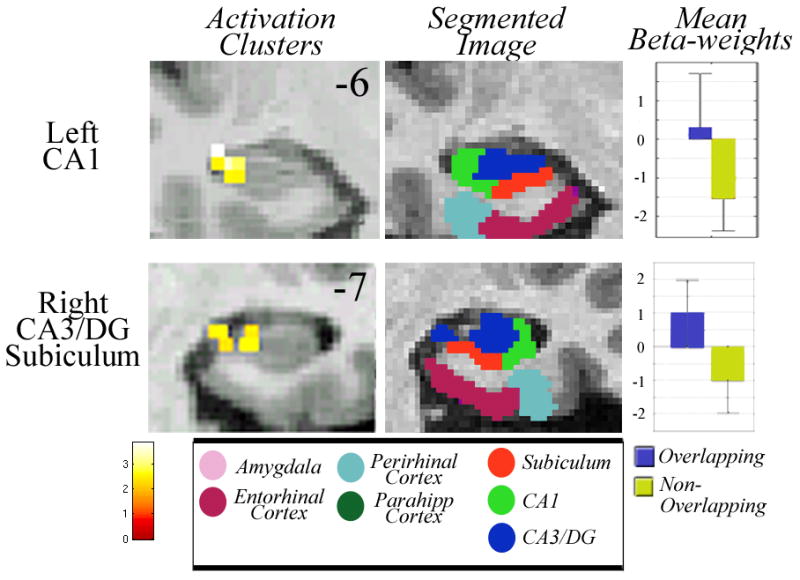
When subjects were required to maintain face pairs with overlapping features compared to those with non-overlapping features during the delay period (OL Delay > NOL Delay) there was significant activation in right entorhinal cortex (Fig. 3; T=2.92; Z=2.56; p<0.03Corr), right perirhinal cortex (T=3.20; Z=2.75; p<0.03Corr), left parahippocampal cortex (T=4.01; Z=3.26; p<0.03Corr), right subiculum (T=3.91; Z=3.20; p<0.03Corr), and right CA1 (T=2.86; Z=2.52; p<0.03Corr). A contrast of NOL trials with OL trials during the delay period (NOL Delay > OL Delay) at p<0.03 showed no significant clusters of activity.
Fig. 3.
DISCUSSION
In the current study, we used high-resolution fMRI to examine the contributions of hippocampal subfields and extrahippocampal MTL cortices in encoding and maintaining overlapping stimuli during working memory. We used photographs of faces as stimuli and defined overlapping faces pairs as two faces of the same identity, but different expression and non-overlapping face pairs as two faces of different identity and different expression. We found activation within the CA3/DG, CA1, and subiculum subfields while encoding overlapping face pairs compared to encoding non-overlapping face pairs. During the delay period, CA1 and subiculum were recruited along with EC, PrC, and PHC to actively maintain the previously presented overlapping stimuli. Together, our results identify hippocampal subfield and specific MTL cortical mechanisms related to disambiguation during working memory.
Our current results are consistent with computational models (Rolls, 1990; Shapiro and Hetherington, 1993; O’Reilly and McClelland, 1994; Rolls, 1996) and electrophysiological and gene expression data (Guzowski et al., 2004; Leutgeb et al., 2004; Gilbert and Kesner, 2006; Leutgeb et al., 2007; Satvat et al., 2011) that implicate the DG and CA3 in minimizing interference between conflicting or overlapping input patterns. Anatomically, these two regions are suited to help resolve interference between overlapping pairs through broadly divergent connections from EC to DG, sparse connections that link the DG to the CA3 (Amaral et al., 1990), and through extensive collateral connections within CA3 (Amaral and Witter, 1989). Rodents with DG lesions show deficits in WM discrimination of highly similar contexts (Gilbert et al., 2001; Gilbert and Kesner, 2003; Hunsaker and Kesner, 2008). Similarly, high-resolution fMRI studies have shown increased BOLD activity within CA3/DG region during presentation of objects that were highly similar to previously seen objects (Bakker et al., 2008; Lacy et al., 2011). This previous work established a role for the DG and CA3 in disambiguation of overlapping episodic events (i.e., long-term memory). Our findings extend the role of the CA3/DG region in minimizing interference between overlapping input patterns to working memory.
Encoding face pairs that share overlapping components may require pattern separation. Theoretical models have suggested that pattern separation allows similar stimuli to be represented with distinct neural patterns (Treves and Rolls, 1994; O’Reilly and Rudy, 2001; Norman and O’Reilly, 2003). According to these models, DG can perform pattern separation by enhancing differences between similar spatial or temporal inputs through orthogonalization of the neural representations. This idea is supported by findings from both animal (Rolls and Kesner, 2006; Leutgeb et al., 2007; McHugh et al., 2007) and human studies (Bakker et al., 2008). Although previous models do not specifically articulate the role of the hippocampal subfields in pattern separation during WM, our results suggest that pattern separation may contribute to encode and separate overlapping, similar stimuli.
While CA3/DG, subiculum and CA1 were engaged during encoding, the EC, together with the CA1 and subiculum, contributed when overlapping stimuli were maintained across a brief delay. A number of findings support the role of EC in maintaining information across a brief delay. For example, depolarizing current injections have produced persistent firing within the entorhinal cortex (Klink and Alonso, 1997; Egorov et al., 2002) and slice recording studies (Tahvildari et al., 2007; Yoshida et al., 2008) along with functional neuroimaging investigations (Schon et al., 2004; Brickman et al., 2011) show EC activity during a brief delay period. Delay-period activity (OL > NOL) during WM may be related to long-term memory formation. Neuroimaging studies have shown a strong association between EC activity and delayed cue-recall performance (Fernández et al., 1999) and long-term memory encoding (Ranganath and D’Esposito 2005; Schon 2005; Nichols et al., 2006), and between post-stimulus activity in the hippocampus and subsequent memory (Ben-Yakov and Dudai, 2011). Anatomical connections from the EC to the CA1 and subiculum (Witter and Amaral, 2006) may enable coordinated involvement in long-term memory to support the formation of long-term stable representations (Remondes and Schuman, 2004).
Our findings of hippocampal involvement during encoding and maintaining overlapping stimuli are in line with a large body of whole-brain fMRI studies showing hippocampal support in working memory for novel information (Ranganath and D’Esposito, 2001; Stern et al., 2001; Schon et al., 2004; Hannula and Ranganath, 2008; Schon et al., 2012) as well as complex information (Ranganath et al., 2005; Piekema et al., 2009). Additionally, recent studies show that patients with hippocampal lesions have memory deficits when working memory capacity is exceeded (Jeneson et al., 2010) and during an extended working memory delay (Jeneson et al., 2011). Our results add to this work by suggesting that stimulus similarity also recruits the hippocampus during the working memory delay. While our data fit nicely with this previous body of work, an alternative interpretation is that the hippocampal activity may be related to the presentation of two images of the same face. This alternative seems unlikely given that previous studies have demonstrated that repeated presentations of the same stimuli result in a reduction in activity in the hippocampus in both long-term encoding and working memory studies (Stern et al., 1996; Stern et al., 2001; Kirchhoff et al., 2000; Brozinsky et al., 2005; Ranganath and Blumenfeld, 2005).
Several models theorize that EC and CA1 encode specific events by bridging temporal gaps between common or overlapping elements within these events (Howard et al., 2005; Jensen and Lisman, 2005). Recently, it has been shown that EC and CA1 contribute to process temporally separated events (Suh et al., 2011). Additionally, recording studies in rodents have suggested that CA1 and subiculum work in a complementary manner to encode and maintain information during a working memory task (Deadwyler & Hampson, 2004). Our present work suggests that if a delay occurs following the presentation of stimuli with overlapping features, the EC together with CA1 and subiculum may play a critical role in encoding and maintaining a sustained representation of overlapping information across a temporal lag. Together with previous work, our results suggest a role for the subiculum, the CA1, and the EC in linking separated elements into long-term coherent representations.
During the delay, the PrC and PHC were also recruited when subjects maintained overlapping stimuli compared to non-overlapping stimuli. Recording studies in both rodents and humans show sustained activity within the PrC and PHC during a short delay (Axmacher et al., 2007; Lehky and Tanaka, 2007) while both lesion and recording studies show a role for EC, PrC, and PHC during working memory tasks (Baylis and Rolls, 1987; Otto and Eichenbaum, 1992; Meunier et al., 1993; Suzuki et al., 1993; Meunier et al., 1996; Van Cauter et al., 2008). In particular, evidence from both single-unit recording studies in monkeys (Miyashita and Chang 1988; Naya et al., 2001) and functional imaging studies in humans (Tendolkar et al., 2007; Haskins et al., 2008; Staresina and Davachi, 2008) suggests that the PrC may contribute to encode associations between related items. Furthermore, it has been suggested that the PrC contributes to maintain related items (Hannula and Ranganath, 2008). Our results are consistent with these findings and suggest that the PrC and PHC may also contribute to maintaining associations between face identity and expression during a working memory delay.
SUMMARY AND CONCLUSIONS
Our current study yielded two main findings. First, our finding that the encoding of overlapping face pairs recruited CA3/DG, CA1, and subiculum subfields provides evidence that regions involved in minimizing interference during episodic encoding also play a role during working memory when overlapping stimuli need to be disambiguated. In addition, CA1 and subiculum along with extrahippocampal cortices including EC showed greater activation for overlapping than for non-overlapping stimuli while maintaining these overlapping representations during a brief delay, a finding consistent with the roles of these regions in forming long-term representations and processing temporally separated events.
Acknowledgments
Our work was supported in part by CELEST, a National Science Foundation Science of Learning Center (NSF SMA-0835976 and NSF SBE-0354378). Scanning was carried out at the the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41RR14075, a P41 Regional Resource supported by the Biomedical Technology Program of the National Center for Research Resources (NCRR), National Institutes of Health. This work also involved the use of instrumentation supported by the NCRR Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program, specifically, grant number S10RR021110. We would also like to thank Dr. Michael Hasselmo for helpful comments on this manuscript, Meaghan Young for her technical assistance in early processing the fMRI data, Thackery Brown for assisting in fMRI data collection, Dr. Thomas Benner for helping develop the fMRI acquisition protocol, and Dr. Brock Kirwan for helping provide guidelines for accurate anatomical tracings.
References
- Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. Journal of Neuroscience. 2002;22:5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Progress in brain research. 1990;83:1–11. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernández G, Cohen MX, Elger CE, Fell J. Sustained neural activity patterns during working memory in the human medial temporal lobe. Journal of Neuroscience. 2007;27:7807–7816. doi: 10.1523/JNEUROSCI.0962-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar AS, Shirvalkar PR, Shapiro ML. Memory-guided learning: CA1 and CA3 neuronal ensembles differentially encode the commonalities and differences between situations. Journal of Neuroscience. 2011;31:12270–12281. doi: 10.1523/JNEUROSCI.1671-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CEL. Pattern Separation in the Human Hippocampal CA3 and Dentate Gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis GC, Rolls ET. Responses of neurons in the inferior temporal cortex in short term and serial recognition memory tasks. Experimental brain research. 1987;65:614–622. doi: 10.1007/BF00235984. [DOI] [PubMed] [Google Scholar]
- Ben-Yakov A, Dudai Y. Constructing realistic engrams: poststimulus activity of hippocampus and dorsal striatum predicts subsequent episodic memory. Journal of Neuroscience. 2011;31:9032–9042. doi: 10.1523/JNEUROSCI.0702-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozinsky CJ, Yonelinas AP, Kroll NE, Ranganath C. Lag-sensitive repetition suppression effects in the anterior parahippocampal gyrus. Hippocampus. 2005;15:557–61. doi: 10.1002/hipo.20087. [DOI] [PubMed] [Google Scholar]
- Brickman AMA, Stern YY, Small SAS. Hippocampal subregions differentially associate with standardized memory tests. Hippocampus. 2011;21:923–928. doi: 10.1002/hipo.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deadwyler SA, Hampson RE. Differential but complementary mnemonic functions of the hippocampus and subiculum. Neuron. 2004;13:465–76. doi: 10.1016/s0896-6273(04)00195-3. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM, Cattin F, Naidich TP, Raybaud C, Risold PY, Salvolini U, Scarabino U. Vascularization and Serial Sections with MRI. Springer; 2005. The Human Hippocampus: Functional Anatomy. [Google Scholar]
- Egorov AV, Hamam BN, Fransén E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Fernández G, Brewer JB, Zhao Z, Glover GH, Gabrieli JD. Level of sustained entorhinal activity at study correlates with subsequent cued-recall performance: a functional magnetic resonance imaging study with high acquisition rate. Hippocampus. 1999;9:35–44. doi: 10.1002/(SICI)1098-1063(1999)9:1<35::AID-HIPO4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson M. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron. 2000;27:169–178. doi: 10.1016/s0896-6273(00)00018-0. [DOI] [PubMed] [Google Scholar]
- Fransén E, Alonso AA, Hasselmo ME. Simulations of the role of the muscarinic-activated calcium-sensitive nonspecific cation current INCM in entorhinal neuronal activity during delayed matching tasks. Journal of Neuroscience. 2002;22:1081–1097. doi: 10.1523/JNEUROSCI.22-03-01081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa AA, Hori E, Umeno K, Tabuchi E, Ono T, Nishijo H. Unambiguous representation of overlapping serial events in the rat hippocampal formation. Neuroscience. 2006;137:685–698. doi: 10.1016/j.neuroscience.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP. The role of the dorsal CA3 hippocampal subregion in spatial working memory and pattern separation. Behavioural Brain Research. 2006;169:142–149. doi: 10.1016/j.bbr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: A double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- Ginther MR, Walsh DF, Ramus SJ. Hippocampal Neurons Encode Different Episodes in an Overlapping Sequence of Odors Task. Journal of Neuroscience. 2011;31:2706–2711. doi: 10.1523/JNEUROSCI.3413-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RER, McGrath CC, Chan RMR, Schroeder LL, Turner TT, Turetsky BIB, Kohler CC, Alsop DD, Maldjian JJ, Ragland JDJ, Gur RCR. An fMRI study of facial emotion processing in patients with schizophrenia. American Journal of Psychiatry. 2002;159:1992–1999. doi: 10.1176/appi.ajp.159.12.1992. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Knierim JJ, Moser EI. Ensemble dynamics of hippocampal regions CA3 and CA1. Neuron. 2004;44:581–584. doi: 10.1016/j.neuron.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Ranganath C. Medial Temporal Lobe Activity Predicts Successful Relational Memory Binding. Journal of Neuroscience. 2008;28:116–124. doi: 10.1523/JNEUROSCI.3086-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins AL, Yonelinas AP, Quamme JR, Ranganath C. Perirhinal cortex supports encoding and familiarity-based recognition of novel associations. Neuron. 2008;59:554–560. doi: 10.1016/j.neuron.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Arc length coding by interference of theta frequency oscillations may underlie context-dependent hippocampal unit data and episodic memory function. Learning and Memory. 2007;14:782–794. doi: 10.1101/lm.686607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. A model of episodic memory: mental time travel along encoded trajectories using grid cells. Neurobiology of Learning and Memory. 2009;92:559–573. doi: 10.1016/j.nlm.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Networks. 2005;18:1172–1190. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Giocomo LM, Zilli EA. Grid cell firing may arise from interference of theta frequency membrane potential oscillations in single neurons. Hippocampus. 2007;17:1252–1271. doi: 10.1002/hipo.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo MEM, Wyble BPB. Free recall and recognition in a network model of the hippocampus: simulating effects of scopolamine on human memory function. Behavioural Brain Research. 1997;89:1–34. doi: 10.1016/s0166-4328(97)00048-x. [DOI] [PubMed] [Google Scholar]
- Howard MW, Fotedar MS, Datey AV, Hasselmo ME. The temporal context model in spatial navigation and relational learning: toward a common explanation of medial temporal lobe function across domains. Psychological Review. 2005;112:75–116. doi: 10.1037/0033-295X.112.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsaker MR, Thorup JA, Welch T, Kesner RP. The role of CA3 and CA1 in the acquisition of an object-trace-place paired-associate task. Behavioral Neuroscience. 2006;120:1252–1256. doi: 10.1037/0735-7044.120.6.1252. [DOI] [PubMed] [Google Scholar]
- Insausti RR, Juottonen KK, Soininen HH, Insausti AMA, Partanen KK, Vainio PP, Laakso MPM, Pitkänen AA. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. American Journal of Neuroradiology. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Mauldin KN, Hopkins RO, Squire LR. The role of the hippocampus in retaining relational information across short delays: the importance of memory load. Learning and Memory. 2011;18:301–305. doi: 10.1101/lm.2010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeneson A, Mauldin KN, Squire LR. Intact Working Memory for Relational Information after Medial Temporal Lobe Damage. Journal of Neuroscience. 2010;30:13624–13629. doi: 10.1523/JNEUROSCI.2895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen O, Lisman JE. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends in Neurosciences. 2005;28:67–72. doi: 10.1016/j.tins.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Hunsaker MR, Ziegler W. The role of the dorsal CA1 and ventral CA1 in memory for the temporal order of a sequence of odors. Neurobiology of Learning and Memory. 2010;93:111–116. doi: 10.1016/j.nlm.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. Journal of Neuroscience. 2000;15:6173–80. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Jones CK, Miller MI, Stark CEL. High-resolution fMRI investigation of the medial temporal lobe. Human Brain Mapping. 2007;28:959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. Overcoming interference: an fMRI investigation of pattern separation in the medial temporal lobe. Learning and Memory. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, Alonso A. Ionic mechanisms of muscarinic depolarization in entorhinal cortex layer II neurons. Journal of Neurophysiology. 1997;77:1829–1843. doi: 10.1152/jn.1997.77.4.1829. [DOI] [PubMed] [Google Scholar]
- Lacy JW, Yassa MA, Stark SM, Muftuler LT, Stark CEL. Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learning and Memory. 2011;18:15–18. doi: 10.1101/lm.1971111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Griffin AL, Zilli EA, Eichenbaum H, Hasselmo ME. Gradual translocation of spatial correlates of neuronal firing in the hippocampus toward prospective reward locations. Neuron. 2006;51:639–650. doi: 10.1016/j.neuron.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behavioral Neuroscience. 2003;117:1044–1053. doi: 10.1037/0735-7044.117.5.1044. [DOI] [PubMed] [Google Scholar]
- Lee I, Solivan F. The roles of the medial prefrontal cortex and hippocampus in a spatial paired-association task. Learning and Memory. 2008;15:357–367. doi: 10.1101/lm.902708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Solivan F. Dentate gyrus is necessary for disambiguating similar object-place representations. Learning & Memory. 2010;17:252–258. doi: 10.1101/lm.1678210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehky SR, Tanaka K. Enhancement of object representations in primate perirhinal cortex during a visual working-memory task. Journal of Neurophysiology. 2007;97:1298–1310. doi: 10.1152/jn.00167.2006. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern Separation in the Dentate Gyrus and CA3 of the Hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser M-B, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305:1295–1298. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- Levy WB. A sequence predicting CA3 is a flexible associator that learns and uses context to solve hippocampal-like tasks. Hippocampus. 1996;6:579–590. doi: 10.1002/(SICI)1098-1063(1996)6:6<579::AID-HIPO3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Lipton PA, White JA, Eichenbaum H. Disambiguation of Overlapping Experiences by Neurons in the Medial Entorhinal Cortex. Journal of Neuroscience. 2007;27:5787–5795. doi: 10.1523/JNEUROSCI.1063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPresti ML, Schon K, Tricarico MD, Swisher JD, Celone KA, Stern CE. Working memory for social cues recruits orbitofrontal cortex and amygdala: a functional magnetic resonance imaging study of delayed matching to sample for emotional expressions. Journal of Neuroscience. 2008;28:3718–3728. doi: 10.1523/JNEUROSCI.0464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons M, Akamatsu S, Kamachi M, Gyoba J. Coding facial expressions with gabor wavelets. Automatic Face and Gesture Recognition, 1998. Proceedings. Third IEEE International Conference on; 1998. pp. 200–205. [Google Scholar]
- Manns JR, Zilli EA, Ong KC, Hasselmo ME, Eichenbaum H. Hippocampal CA1 spiking during encoding and retrieval: Relation to theta phase. Neurobiology of Learning and Memory. 2007;87:9–20. doi: 10.1016/j.nlm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate Gyrus NMDA Receptors Mediate Rapid Pattern Separation in the Hippocampal Network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. Journal of Neuroscience. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M, Hadfield W, Bachevalier J, Murray EA. Effects of rhinal cortex lesions combined with hippocampectomy on visual recognition memory in rhesus monkeys. Journal of Neurophysiology. 1996;75:1190–1205. doi: 10.1152/jn.1996.75.3.1190. [DOI] [PubMed] [Google Scholar]
- Miller MI, Beg MF, Ceritoglu C, Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. PNAS. 2005;102:9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita Y, Chang HS. Neuronal correlate of pictorial short-term memory in the primate temporal cortex. Nature. 1988;331:68–70. doi: 10.1038/331068a0. [DOI] [PubMed] [Google Scholar]
- Naya Y, Yoshida M, Miyashita Y. Backward spreading of memory-retrieval signal in the primate temporal cortex. Science. 2001;291:661–664. doi: 10.1126/science.291.5504.661. [DOI] [PubMed] [Google Scholar]
- Nichols EA, Kao Y-C, Verfaellie M, Gabrieli JDE. Working memory and long-term memory for faces: Evidence from fMRI and global amnesia for involvement of the medial temporal lobes. Hippocampus. 2006;16:604–616. doi: 10.1002/hipo.20190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychological Review. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: principles of cortical and hippocampal function. Psychological Review. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Neuronal activity in the hippocampus during delayed non-match to sample performance in rats: evidence for hippocampal processing in recognition memory. Hippocampus. 1992;2:323–334. doi: 10.1002/hipo.450020310. [DOI] [PubMed] [Google Scholar]
- Piekema C, Kessels RP, Rijpkema M, Fernández G. The hippocampus supports encoding of between-domain associations within working memory. Learning and Memory. 2009;16:231–234. doi: 10.1101/lm.1283109. [DOI] [PubMed] [Google Scholar]
- Preston AR, Bornstein AM, Hutchinson JB, Gaare ME, Glover GH, Wagner AD. High-resolution fMRI of content-sensitive subsequent memory responses in human medial temporal lobe. Journal of cognitive neuroscience. 2010;22:156–173. doi: 10.1162/jocn.2009.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JCJ, Köhler SS, Crane JJ, Pruessner MM, Lord CC, Byrne AA, Kabani NN, Collins DLD, Evans ACA. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cerebral Cortex. 2002;12:1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Pruessner JCJ, Li LML, Serles WW, Pruessner MM, Collins DLD, Kabani NN, Lupien SS, Evans ACA. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cerebral Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 2001;31:865–873. doi: 10.1016/s0896-6273(01)00411-1. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Blumenfeld RS. Doubts about double dissociations between short- and long-term memory. Trends in Cognitive Sciences. 2005;9:374–80. doi: 10.1016/j.tics.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D’Esposito M. Directing the mind’s eye: prefrontal, inferior and medial temporal mechanisms for visual working memory. Current Opinion in Neurobiology. 2005;15:175–182. doi: 10.1016/j.conb.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Cohen MX, Brozinsky CJ. Working memory maintenance contributes to long-term memory formation: neural and behavioral evidence. Journal of Cognitive Neuroscience. 2005;17:994–1010. doi: 10.1162/0898929054475118. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Theoretical and neurophysiological analysis of the functions of the primate hippocampus in memory. Cold Spring Harbor symposia on quantitative biology. 1990;55:995–1006. doi: 10.1101/sqb.1990.055.01.095. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Satvat E, Schmidt B, Argraves M, Marrone DF, Markus EJ. Changes in Task Demands Alter the Pattern of zif268 Expression in the Dentate Gyrus. Journal of Neuroscience. 2011;31:7163–7167. doi: 10.1523/JNEUROSCI.0094-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluppeck D, Curtis CE, Glimcher PW, Heeger DJ. Sustained activity in topographic areas of human posterior parietal cortex during memory-guided saccades. Journal of Neuroscience. 2006;26:5098–5108. doi: 10.1523/JNEUROSCI.5330-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K. Scopolamine Reduces Persistent Activity Related to Long-Term Encoding in the Parahippocampal Gyrus during Delayed Matching in Humans. Journal of Neuroscience. 2005;25:9112–9123. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, LoPresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. Journal of Neuroscience. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Ross RS, Hasselmo ME, Stern CE. Complementary roles of medial temporal lobes and dorsolateral prefrontal cortex for working memory for novel and familiar trial-unique visual stimuli: an fMRI study. The European Journal of Neuroscience. 2012 doi: 10.1111/ejn.12062. [DOI] [PubMed] [Google Scholar]
- Shapiro ML, Hetherington PA. A simple network model simulates hippocampal place fields: parametric analyses and physiological predictions. Behavioral Neuroscience. 1993;107:34–50. doi: 10.1037//0735-7044.107.1.34. [DOI] [PubMed] [Google Scholar]
- Sohal VS, Hasselmo ME. GABA(B) modulation improves sequence disambiguation in computational models of hippocampal region CA3. Hippocampus. 1998;8:171–193. doi: 10.1002/(SICI)1098-1063(1998)8:2<171::AID-HIPO9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. Journal of cognitive neuroscience. 2008;20:1478–1489. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CEL, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. Journal of Neuroscience. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkins S, González RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. PNAS. 1996;93:8660–5. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Sherman SJ, Kirchhoff BA, Hasselmo ME. Medial temporal and prefrontal contributions to working memory tasks with novel and familiar stimuli. Hippocampus. 2001;11:337–346. doi: 10.1002/hipo.1048. [DOI] [PubMed] [Google Scholar]
- Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334:1415–1420. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. Journal of Neurophysiology. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Zola-Morgan S, Squire LR, Amaral DG. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. Journal of Neuroscience. 1993;13:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahvildari B, Fransén E, Alonso AA, Hasselmo ME. Switching between “On” and ‘Off’ states of persistent activity in lateral entorhinal layer III neurons. Hippocampus. 2007;17:257–263. doi: 10.1002/hipo.20270. [DOI] [PubMed] [Google Scholar]
- Tendolkar I, Arnold J, Petersson KM, Weis S, Brockhaus-Dumke Anke, van Eijndhoven P, Buitelaar J, Fernández G. Probing the neural correlates of associative memory formation: a parametrically analyzed event-related functional MRI study. Brain Research. 2007;1142:159–168. doi: 10.1016/j.brainres.2007.01.040. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Van Cauter T, Poucet B, Save E. Delay-dependent involvement of the rat entorhinal cortex in habituation to a novel environment. Neurobiology of Learning and Memory. 2008;90:192–199. doi: 10.1016/j.nlm.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. NeuroImage. 2009;44:319–327. doi: 10.1016/j.neuroimage.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Fransén E, Hasselmo ME. mGluR-dependent persistent firing in entorhinal cortex layer III neurons. European Journal of Neuroscience. 2008;28:1116–1126. doi: 10.1111/j.1460-9568.2008.06409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. Journal of Neuroscience. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Bookheimer SY. Application of Cortical Unfolding Techniques to Functional MRI of the Human Hippocampal Region. NeuroImage. 2000;11:668–683. doi: 10.1006/nimg.2000.0561. [DOI] [PubMed] [Google Scholar]