Abstract
Hypothesis
Our objective was to evaluate whether the use of midline venous catheters in place of central line venous catheters, when appropriate, decreased the overall incidence of central line–associated bacteremia in a ventilator unit.
Methods
The time interval between February 2012 and February 2013 was divided into 2 periods. Group A was the first half of the year, before the introduction of midline catheters, and group B was the second half of the year, 6 months after their introduction. Central line–associated bloodstream infection (CLABSI) was calculated using the equation: (total number of CLABSI/total number of catheter days) × 1000. The Z test was used for proportions between independent groups to compare the significance in the difference in CLABSI between groups A and B.
Results
There was a significant decrease in the total number of catheter days on the ventilator unit in group A from 2408 catheter days in 1 year (August 1, 2011, to July 31, 2012) before the introduction of midline catheters to 1521 catheter days in group B in the following year (November 1, 2012, to October 31, 2013; P < 0.05 for both groups).
Conclusions
Midline catheters in place of central lines decrease the rate of CLABSI in a ventilator unit. In addition, no bloodstream infections were associated with midline catheters.
Key Words: sepsis, infection, bacteremia, catheter
The Centers for Disease Control and Prevention has put in place guidelines to reduce the incidence of central line–associated bloodstream infection (CLABSI). These guidelines brought about by numerous studies for years have helped bring down the number of CLABSI in intensive care units (ICUs). However, the increasing use of central lines outside ICUs has increased the incidence of CLABSI in patients outside the ICU setting.1,2 Studies have found an incidence of 2.79 to 4.79 bloodstream infections (BSIs) per 1000 catheter days for peripherally inserted central catheters (PICCs) and 1.0 to 3.2 BSIs per 1000 catheter days for noncuffed central venous catheters (CVCs).3,4 Health care–associated infections such as CLABSI result in 12% to 25% mortality and an average cost of approximately $25,000 to $32,000 per episode with an increased length of ICU and hospital stay of 2.4 and 7.5 days, respectively.5–7
Catheter dwell time is an important factor for CLABSI. Prolonged dwell time has been shown to increase CLABSI rates rapidly after 9 catheter days.6,8,9 Removing catheters promptly when not required or not inserting them when other means are available helps reduce total catheter days and dwell time. A home infusion care study comparing the complications in different types of catheters found the rate of BSI to be highest with tunneled and untunneled CVCs compared with midline catheters.10
There are other complications of central lines, including inadvertent arterial puncture (3%), hemothorax, or pneumothorax (1%–2%).11 These complications are associated with poorer patient outcomes, causing an increase in the risk for morbidity and mortality, prolongation of hospital stay, and increased cost.2 Studies have shown that the cost of insertion-related pneumothorax is approximately $71,000.5 Central lines and PICC lines require x-ray confirmation of tip placement, leading to additional costs and exposing the patient to unnecessary radiation.
Midline catheters are peripheral intravenous catheters inserted with or without ultrasound guidance and placed in deeper larger peripheral veins. They are generally longer than peripheral venous catheters between 3 and 8 in long and have a dwell time of up to 4 weeks. They can be used in home therapy as well as inpatient management. They are placed in proximal veins such as the brachial, basilic, or cephalic, with the tip distal to the axillary vein. These catheters are termed midlines because they are longer than peripheral intravenous catheters, which are 1 to 3 in long but shorter than PICC, which extend into the vena cava. Several studies have demonstrated that central line use can be decreased through the use of midline catheters.12–14 The use of CVCs was decreased by 80% to 85% in an emergency department setting over the span of 5 years using midlines instead for patients with difficult venous access.13
Midline catheters have a much lower incidence of BSI compared with CVCs. The BSI rate of midlines in various studies has been reported between 0% and 0.9%.4,10,15–17
It stands to reason that the introduction and regular use of these midlines when warranted may reduce the overall incidence of central line–associated bacteremia and its sequelae in certain hospital environments. Our study compares the number of CLABSI per 1000 catheter days in the ventilator unit in the periods 1 year before and after the active introduction of midline catheters and the CLABSI rates during these periods.
MATERIALS AND METHODS
This was a retrospective cohort study approved by our institutional review board as a quality improvement research. The records of catheter days 1 year before the introduction of midlines (group A: August 1, 2011, to July 31, 2012) and catheter days of 1 year after the regular use of midlines (group B: November 1, 2012, to October 31, 2013) in the ventilator unit were collected (Table 1). During the period from August 2012 to November 2012, internal medicine residents and nursing staff underwent training on the use and insertion of midline catheters and their care. This dedicated team was responsible for replacing CVCs by midlines as per our new practices. We have both antibiotic-coated and noncoated CVCs in our department. We did not randomize or document which patients had antibiotic-coated catheters. The midlines that we used were POWERWAND (Access Scientific, San Diego, Calif).
Table 1.
Before Midline Catheter, Patient Days: Number of Days the Patient was in the Ventilator Unit; Catheter Days: Number of Days the Patient had a Central Venous Catheter and After Midline Catheter, Patient Days: Number of Days the Patient was in the Ventilator Unit; Catheter Days: Number of Days the Patient had a Midline Catheter
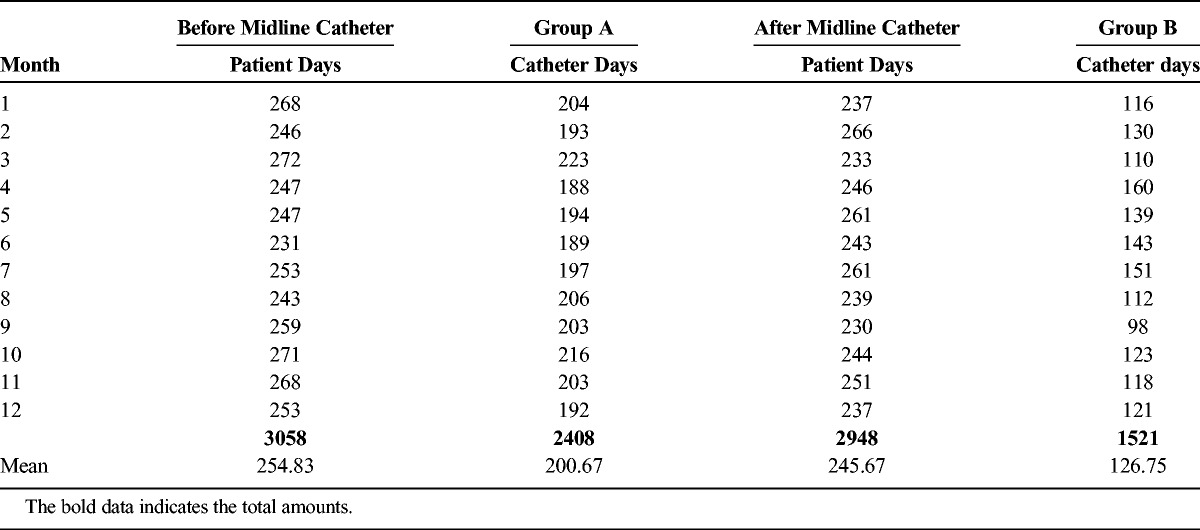
We chose this unit because it has a constant denominator of the number and type of patients. Most of the patients in this unit are ventilator dependent and being treated for problems such as health care–associated pneumonia, urinary tract infections, wound and pressure ulcer infections, or other long-term conditions requiring acute care that cannot be administered at a nursing home facility. The patients here have longer length of stay compared with other units and generally have difficult venous access. This unit also has the largest number of reported catheter days outside the ICU. The most common reason for CVC use in this unit is for difficult and long-term intravenous access. Indications of CVCs in these patients included antibiotic therapy, infusion therapy, diagnostic procedures, transfusions, and blood draws. None of these patients required vasopressors in this unit. We do not have a policy of administering vasopressors through midlines.
The Department of Infection Control of the hospital reports all the CLABSI per 1000 catheter days in the hospital, categorized by location and unit. These records for the ventilator unit were collected. The number of CLABSI during this period along with the type of bacteria and the date of culture was obtained from the microbiology department. We compared the CLABSI rates between group A and group B to see whether it decreased through the use of midlines in comparison with central lines.
We trained a team of residents for a period of 3 months in the insertion of ultrasound-guided midline catheters, which would replace CVCs by midline catheters whenever possible. The same antiseptic precautions used in CVC catheter placements under ultrasound guidance were applied to the placement of midlines. The policy for replacing the central lines included the following guidelines:
Any patient with a CVC in a femoral vein had their line removed and replaced by a midline catheter.
All patients who were not on an ionotropic agent or total parenteral nutrition had their CVC replaced by midlines.
A midline catheter replaced any CVC in place longer than a week.
A patient on antibiotic therapy being sent to nursing home for further management received a midline catheter.
Midlines were not kept longer than 28 days.
Midlines were discontinued as soon as no intravenous access was required.
STATISTICS
The total number of catheter days was compared with the rate of CLABSI in the 2 groups. Catheter days were calculated as the number of central line catheters on the unit every day. Adding the total number of catheters on the unit per day and adding this daily number for the length of time of the study help calculate catheter days.
Central line–associated BSI was reported as rate per 1000 catheter days and can be calculated as follows: (total number of CLABSI/total number of catheter days) × 1000.
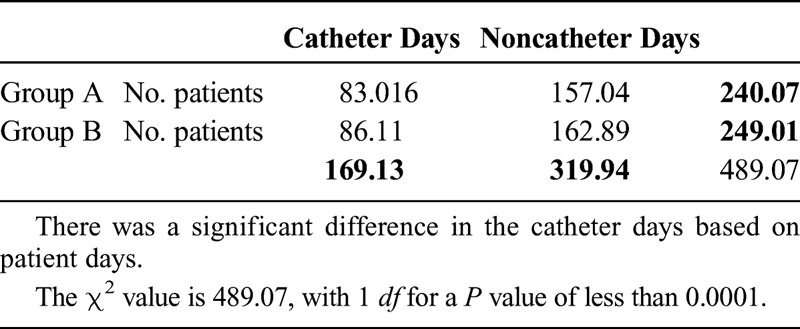
We used a χ2 test to compare the number of catheter days per patient days in the 2 groups as well as to compare infections based on the number of catheter days in each group (Table 2).
Table 2.
Comparing the Number of Catheter Days per Patient Days in the 2 Groups, as well as Comparing Infections Based on the Number of Catheter Days in Each Group
RESULTS
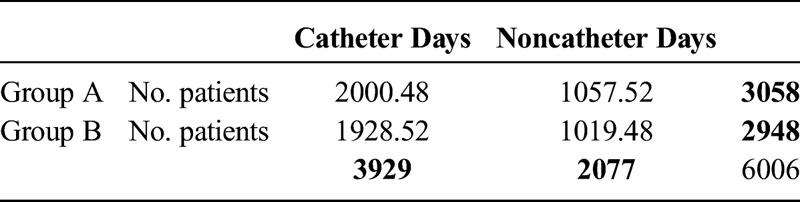
There was a significant decrease in the total number of catheter days on the ventilator unit in group A from 2408 catheter days in the 1 year (August 1, 2011, to July 31, 2012) before the introduction of midline catheters to 1521 catheter days in group B in the following year (November 1, 2012, to October 31, 2013; P < 0.05 in both groups). The total number of CLABSI infections in these periods was also significantly decreased from 8 to 0 (Table 3). This calculates to 3.32 CLABSI per 1000 catheter days and 0 CLABSI per 1000 catheter days, respectively (add χ2). The number of inpatient days during these periods was 3058 and 2948 days (Table 4 and 5).
Table 3.
Continuation of the χ2 Test
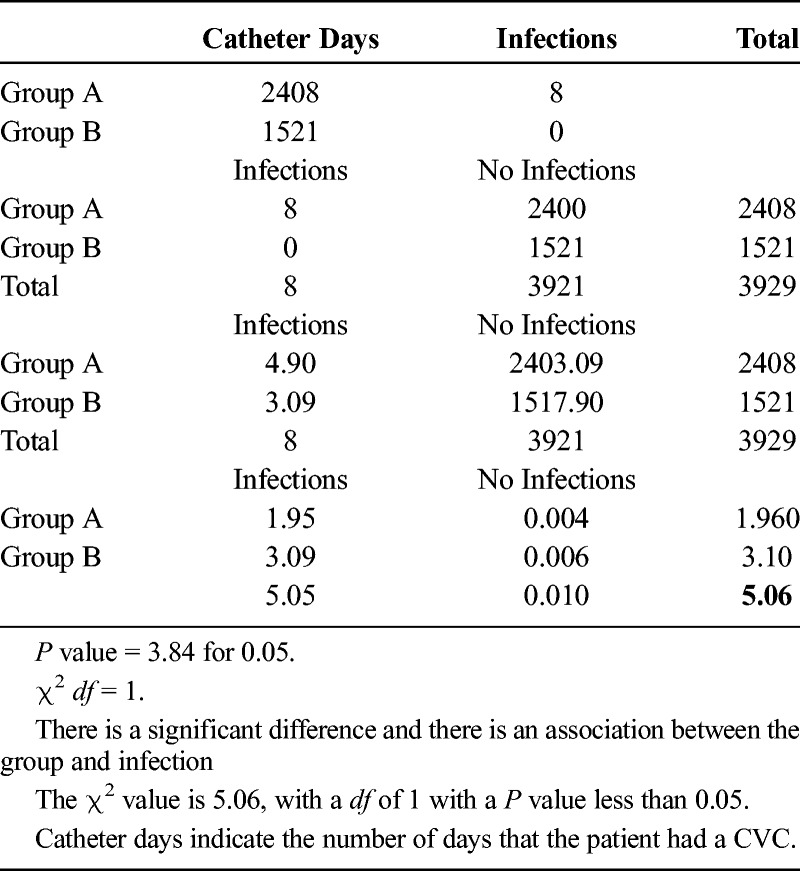
Table 4.
χ2 Test: Observed Frequency
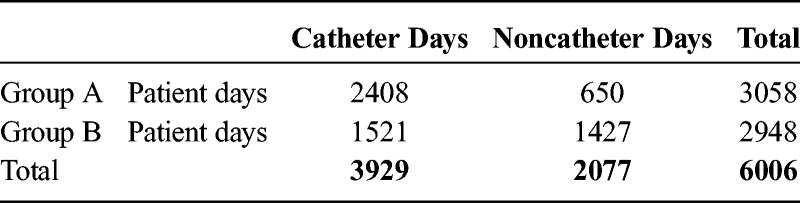
Table 5.
χ2 Test: Expected (Theoretical) Frequency, Asserted by the Null Hypothesis
There were no BSIs associated with midlines in this study.
One patient developed CVC insertion–related pneumothorax.
Two patients with midlines developed phlebitis.
DISCUSSION
The Centers for Disease Control and Prevention has put in place guidelines to reduce the incidence of CLABSI. These guidelines brought about by numerous studies for years have helped bring down the rate of CLABSI.
These measures include the following2:
reducing the number of dwell days by removing catheters as early as possible;
reducing the use of central lines by using other means of venous access;
proper care and technique for CVCs;
maximum sterile barrier precautions during insertion;
use of chlorhexidine for skin disinfection before catheter insertion;
avoidance of the femoral insertion site; and
use of recommended insertion site dressing care practices.
Catheter dwell time is an important factor for CLABSI. Prolonged dwell time has been shown to increase CLABSI rates rapidly after 9 catheter days.6,8,9 Replacing central lines with midlines decreases the dwell time and also the total number of catheter days. It may be argued that the decrease in the use of central lines and thereby reducing the number may be responsible in decreasing CLABSI; however, our denominator is constant for comparison. The use of midlines reduces the use of and the dwell days for central lines, and with this study, we show that it can be a factor in reducing CLABSI as well. Another study showed that CLABSI rates were higher in patients who had central lines for longer than 7 days.18 In our study, by replacing central lines with midlines in patients, we essentially decreased risk factors such as dressing changes, catheter care, and duration of central line use. A more obvious outcome is the decrease in use of central lines itself, causing a drop in the infection rates. The decrease in catheter days in the 2 groups was highly significant. We discontinued most of the central lines after the first week. The fall in infection rate may be attributed to the fewer number of catheters in place for longer than 7 days. Before the introduction of midlines, difficult intravenous access in these chronically ill patients mandated the use of central lines.
There are other complications of central lines, including inadvertent arterial puncture (3%), hemothorax, or pneumothorax (1%-2%).11 Central lines and PICC lines require x-ray confirmation of tip placement, exposing the patient to radiation. There are insertion site infections and vein thrombosis. Tip migration may also be a problem. Midlines have complications as well; our most common problem was the loss or malfunction of the midline including extravasations in 2 patients. We encountered no BSI related to midlines.
Midline catheters have a much lower incidence of BSI compared with CVCs. The BSI rate of midlines in various studies has been reported to be between 0% and 0.9%.4,10,15–17 The combination of better available products and the increasing use of ultrasound guidance for intravenous catheter placement has renewed interest in midline catheters. Some limitations of midline catheters are inability to use for vasopressors, total parenteral nutrition or when large peripheral veins are not available such as in amputates and patients with arteriovenous fistulas.
CONCLUSIONS
We conclude that the use of midline catheters to replace central lines for difficult intravenous access decreases the rate of CLABSI in a ventilator unit in a community hospital. In addition, no BSIs were associated with midline catheters.
Footnotes
The authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1. Marschall J, Leone C, Jones M, et al. Catheter-associated bloodstream infections in general medical patients outside the intensive care unit: a surveillance study. Infect Control Hosp Epidemiol. 2007; 28: 905– 909. [DOI] [PubMed] [Google Scholar]
- 2. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control. 2011; 39: S1– S34. [DOI] [PubMed] [Google Scholar]
- 3. Chopra V, Flanders SA, Saint S. The problem with peripherally inserted central catheters. JAMA. 2012; 308: 1527– 1528. [DOI] [PubMed] [Google Scholar]
- 4. Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006; 81: 1159– 1171. [DOI] [PubMed] [Google Scholar]
- 5. Kim JS, Holtom P, Vigen C. Reduction of catheter-related bloodstream infections through the use of a central venous line bundle: epidemiologic and economic consequences. Am J Infect Control. 2011; 39: 640– 646. [DOI] [PubMed] [Google Scholar]
- 6. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006; 355: 2725– 2732. [DOI] [PubMed] [Google Scholar]
- 7. Warren DK, Quadir WW, Hollenbeak CS, et al. Attributable cost of catheter-associated bloodstream infections among intensive care patients in a nonteaching hospital. Crit Care Med. 2006; 34: 2084– 2089. [DOI] [PubMed] [Google Scholar]
- 8. McLaws ML, Burrell AR. Zero risk for central line–associated bloodstream infection: are we there yet? Crit Care Med. 2012; 40: 388– 393. [DOI] [PubMed] [Google Scholar]
- 9. O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011; 52: e162– e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moureau N, Poole S, Murdock MA, et al. Central venous catheters in home infusion care: outcomes analysis in 50,470 patients. J Vasc Interv Radiol. 2002; 13: 1009– 1016. [DOI] [PubMed] [Google Scholar]
- 11. Ruesch S, Walder B, Tramer MR. Complications of central venous catheters: internal jugular versus subclavian access—a systematic review. Crit Care Med. 2002; 30: 454– 460. [DOI] [PubMed] [Google Scholar]
- 12. Au AK, Rotte MJ, Grzybowski RJ, et al. Decrease in central venous catheter placement due to use of ultrasound guidance for peripheral intravenous catheters. Am J Emerg Med. 2012; 30: 1950– 1954. [DOI] [PubMed] [Google Scholar]
- 13. Shokoohi H, Boniface K, McCarthy M, et al. Ultrasound-guided peripheral intravenous access program is associated with a marked reduction in central venous catheter use in noncritically ill emergency department patients. Ann Emerg Med. 2013; 61: 198– 203. [DOI] [PubMed] [Google Scholar]
- 14. Schoenfeld E, Shokoohi H, Boniface K. Ultrasound-guided peripheral intravenous access in the emergency department: patient-centered survey. West J Emerg Med. 2011; 12: 475– 477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson NR. Midline catheters: the middle ground of intravenous therapy administration. J Infus Nurs. 2004; 27: 313– 321. [DOI] [PubMed] [Google Scholar]
- 16. Cummings M, Hearse N, McCutcheon H, et al. Improving antibiotic treatment outcomes through the implementation of a midline: piloting a change in practice for cystic fibrosis patients. J Vasc Nurs. 2011; 29: 11– 15. [DOI] [PubMed] [Google Scholar]
- 17. Ramos-Gonzalez E, Moreno-Lorenzo C, Mataran-Penarrocha GA, et al. Comparative study on the effectiveness of myofascial release manual therapy and physical therapy for venous insufficiency in postmenopausal women. Complement Ther Med. 2012; 20: 291– 298. [DOI] [PubMed] [Google Scholar]
- 18. Safdar N, Kluger DM, Maki DG. A review of risk factors for catheter-related bloodstream infection caused by percutaneously inserted, noncuffed central venous catheters: implications for preventive strategies. Medicine (Baltimore). 2002; 81: 466– 479. [DOI] [PubMed] [Google Scholar]


