Chronic lymphocytic leukemia (CLL) is the most common adult leukemia in the Western world. Pathogenic mechanisms involve multiple external events (such as microenvironmental and antigenic stimuli) and internal events (genetic and epigenetic alterations) that are associated with the transformation, progression and evolution of CLL. CLL is characterized by an accumulation of mature B cells in peripheral blood, bone marrow and lymphoid tissues. Extracellular stimuli play an important role in the development and maintenance of neoplastic cells. B-CLL cells proliferate and activate pathogenic signaling pathways in anatomical structures known as proliferation centers, which are usually more conspicuous in involved lymph nodes.1 Its clinical course is quite heterogeneous, whereby some patients progress rapidly and have short survival, whereas others have a more stable clinical course that may not need treatment for years.
Several clinical and biological prognostic factors for CLL have been identified, such as the Rai and Binet clinical staging systems, specific cytogenetic alterations, mutational status of immunoglobulin (IgHv) genes, TP53 mutations and the expression levels of CD38 and ZAP70. More recently, massive sequencing data have revealed new genetic alterations, some of which have a significant impact on the clinical course. Of these, mutations in the NOTCH1 and SF3B1 genes, which are associated with an adverse clinical outcome, are emerging as the most frequent mutations in CLL.2
The NOTCH1 gene codes for a trans-membrane receptor that regulates critical cellular functions, such as proliferation, apoptosis and differentiation, and is associated with tumorigenesis. NOTCH1 signaling is initiated when a ligand from the Jagged or Delta families binds the receptor and induces a cascade of proteolytic processes that results in the release and nuclear translocation of the NOTCH1 intracellular domain (NCID). This, in conjunction with the transcription factor CBF1/RBP-Jk, leads to the activation or repression of its target genes.3,4
Although a considerable number of studies have addressed the consequences of NOTCH1 and SF3B1 mutations in CLL,2 few have so far analyzed the frequency and biological impact of these mutations in lymph nodes (LN) affected by CLL (lnCLL). There is a growing interest in therapeutic targeting of NOTCH1 activation signaling in tumors with agonists such as γ-secretase inhibitors.5 It has also been proposed that mutation-independent NOTCH1 -activation contributes to tumor cell growth and survival.6,7
Therefore, we have analyzed the frequency of NOTCH1 and SF3B1 mutations and the functional status of the NOTCH1 pathway through the expression analysis of NOTCH1-induced targets in a series of samples of lnCLL.
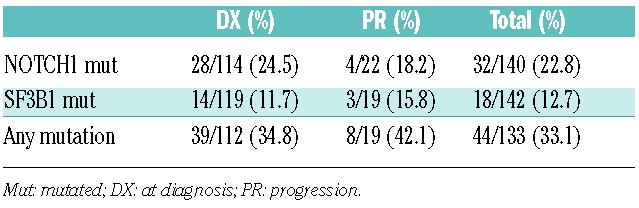
The first step was the analysis of NOTCH1 and SF3B1 mutations in the LN infiltrated with CLL. The series included 155 LN samples from 147 patients, who were biopsied at diagnosis or during the course of the disease (Online Supplementary Appendix). Patients’ characteristics are summarized in Table 1. NOTCH1 and SF3B1 mutations were successfully analyzed in 140 and 142 samples by qPCR, respectively; 32 of 140 (22.8%) were positive for the p.P2515fs*4 NOTCH1 mutation and 14 of 142 (9.8%) for the p.K700E SF3B1 mutation. As this SF3B1 mutation constitutes only around 50%–60% of all SF3B1 mutations identified in CLL, we also studied exons 14 and 16 in which most of the other mutations reside. Although capillary sequencing is a less sensitive method than qPCR, we were still able to identify four additional SF3B1 mutations in exon 14 and none in exon 16. Therefore, 18 of 142 (12.7%) SF3B1 gene mutations were detected. Three out of 132 samples showed a mutation in both genes (Table 2). When comparing the rate of mutations in both genes in samples taken at the time of diagnosis or progression, no significant differences were found (Table 2). Samples at diagnosis and during progression were available for 8 patients. NOTCH1 status did not change in any of the patients (2 mutated and 6 non-mutated), while one acquired an SF3B1 mutation during progression.
Table 1.
Summary of the main clinical and biological characteristics of patients with samples at diagnosis.
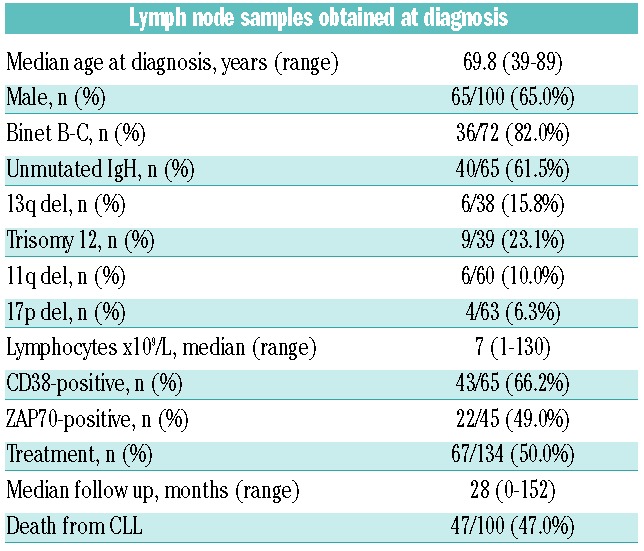
Table 2.
Summary of samples with mutations in NOTCH1 and/or SF3B1.
We found that, although the percentage of cases with mutated SF3B1 at the time of diagnosis (11.7%) was similar to that of other series analyzed in peripheral blood (PB) lymphocytes,2 the NOTCH1 mutation rate we found in lnCLL (24.5% at diagnosis) seems to be higher than in other studies performed in PB samples, in which the percentages of mutations range from 8% to 31%8,9 depending on the series and its characteristics. The lowest values (<15%) were obtained from the analysis of samples taken at diagnosis and the highest were found in patients transformed to Richter syndrome8 or who showed refractoriness to treatment.10 It is also higher than the frequency found in a different series of CLL PB taken at the time of diagnosis analyzed in our laboratory using the same method in which we found 12% of cases with p.P2515fs*4 NOTCH1 mutation (data not shown).
We only analyzed the p.P2515fs*4 mutation, which accounts for around 80% of all NOTCH1 mutations reported in CLL (66%–98%)11 and, therefore, the real percentage of mutated cases in our lnCLL might be even higher. This could be due to the greater aggressiveness of lnCLL and/or to the high sensitivity of the qPCR technique used for mutation detection.
We next analyzed the association of NOTCH1 and SF3B1 mutations in samples taken at diagnosis with patient, clinical, biological and cytogenetic characteristics. We found no significant associations between the presence of NOTCH1 or SF3B1 mutations and any biological and clinical characteristics, or with overall survival and time to treatment (Online Supplementary Table S1).
The expression of NOTCH1 itself together with NOTCH1 targets, including NFATc1, p52, p50, C-MYC, MUM1, XBP1s, LEF and HES1,3,4,12–14 and Ki67, were analyzed by immunohistochemistry in the 155 CLL paraffin samples and in 6 samples of reactive lymphoid tissue included in the tissue micro arrays (TMA) (Online Supplementary Appendix).
Lymphocytes in reactive LN exhibited mainly cytoplasmic expression of NOTCH1, NFAT, p52 and p50. MUM1 expression was restricted to plasma cells and scattered cells within the germinal centers, XBP1 only recognized plasma cells, while MYC, LEF1 and HES1 expression were negative in normal B cells.
NOTCH1 nuclear accumulation was analyzed with a rabbit-mAb recognizing the NICD1 epitope,15 a surrogate of NOTCH1 activation, and was detected in both 23 of 29 (79.3%) of NOTCH1-mutated cases and in 82 of 91 (90%) of NOTCH1-wild-type cases. This means that in a large proportion of samples (105 of 120, 87.5%), NOTCH1 could be detected in the nuclear compartment of the tumoral B cells (mainly in the proliferation centers) (Online Supplementary Table S2), indicating that the WNT-NOCTH1 pathway is frequently activated in lnCLL, irrespective of the presence of the NOTCH1 mutation, as has been previously reported by others.15
The NOTCH1 mutation was associated with nuclear expression of NFAT (χ2=8.081, P=0.005), NF-κB p52 subunit (χ2=5.841, P=0.016) and CMYC (χ2=4.077, P=0.043), which was selectively expressed in the proliferation center B cells (Figure 1 and Online Supplementary Table S2). The intensity and selectiveness of the expression of C-MYC by neoplastic B cells within proliferation centers were unexpected, being similar to those found in tumoral cells in Burkitt lymphoma samples, although they were restricted to the proliferation centers in our study (Figure 1). Other NOTCH1 downstream targets (such as HES1, LEF1 and XBP1) were also diffusely expressed by neoplastic B cells, and were detected in mutated and non-mutated samples, further supporting the hypothesis that the WNT-NOCTH1 pathway is frequently activated in lnCLL, irrespective of the presence of the NOTCH1 mutation.
Figure 1.
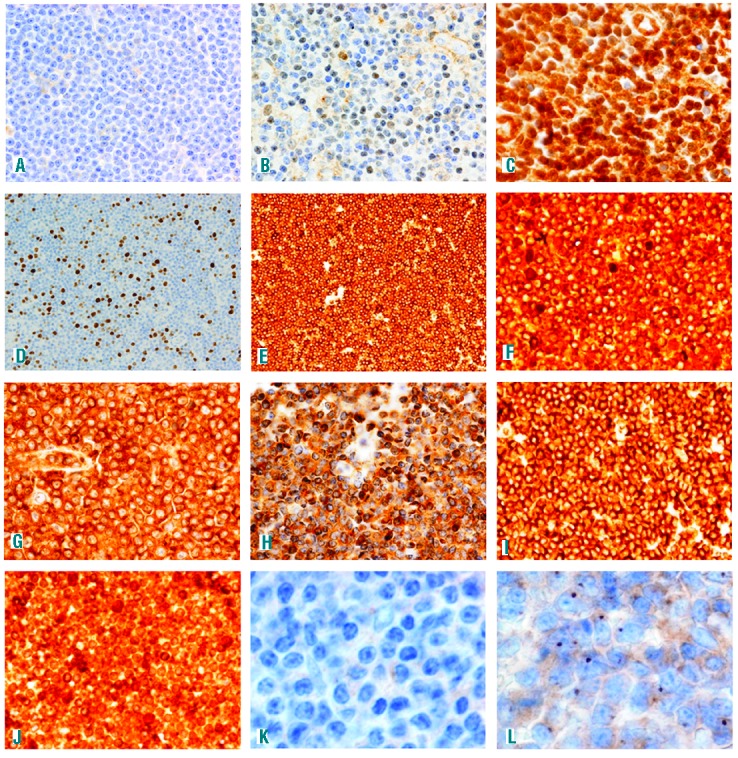
(A) Negative NOTCH1 expression in a wild-type case. (B) Mild NOTCH1 expression in a mutated case. (C) Intense NOTCH1 expression in a wild-type case. (D) NFAT cytoplasmic expression in a negative case. (E) NFAT nuclear expression in a positive case. (F) p50 nuclear expression in a negative case. (G) p50 nuclear expression in a positive case. (H) p52 cytoplasmic expression in a negative case. (I) p52 nuclear expression in a positive case. (J) myc expression in the proliferation center of a wild-type case. (K) JAG-negative stain in a mutated case (L). JAG expression in dots in the Golgi apparatus of a wild-type case.
However, NOTCH1 nuclear expression was not significantly associated with the detection of any of the markers analyzed (Online Supplementary Table S3), their expression being found in samples with or without nuclear NOTCH1 expression.
The NOTCH1 pathway is activated when ligands of the JAG or Delta families bind to their receptors, leading to the release and nuclear translocation of NOTCH1. These ligands are known to be constitutively expressed in B-CLL cells.7 Therefore we decided to analyze JAG1 expression in these samples. We found that 31 of 94 (31.9%) expressed the protein and that its detection was associated with the expression of some NOTCH1 targets such as LEF1 (χ2=7.095, P=0.008), MYC (χ2=5.158, P=0.023) and MUM1 (χ2 =5.369, P=0.021), although not with NOTCH1 expression itself (Online Supplementary Table S4) as NOTCH1 could be activated by mutation independently of JAG1 presence. JAG1 was expressed in the B-CLL cells in the proliferative centers of wild-type and mutated cases in dots in the Golgi apparatus.
These results reflect the complexity of the NOTCH1 pathway in CLL as it could be constitutively activated by different mechanisms including gene mutation, ligand activation or even by the participation of other members such as NOTCH2, as has also been previously reported.7
In conclusion, we have found that the mutational frequency of NOTCH1, but not of SF3B1, is higher in LN with CLL cell involvement than in other series analyzing CLL PB samples. Another major finding of this study is that NOTCH1 pathway is frequently activated in lnCLL, independently of NOTCH1 gene mutational status, thus suggesting the relevance of NOTCH1 pathway in the survival of CLL cells, and the existence of previously reported additional mechanisms of NOTCH1 activation.7 These include the stronger expression of NOTCH1 ligands, which suggests the existence of autocrine/paracrine mechanisms that could lead to NOTCH1 signaling activation in addition to NOTCH1 gene mutation.
Acknowledgments
We are indebted to the patients who contributed to this study and to the hospitals who supplied the samples. We acknowledge the Biobanks of the CNIO (RD09/0076/00113), IDIVAL-HUMV (RD09/0076/00076), HU 12 de Octubre (RD09/0076/00118) and HGU Gregorio Marañón for their help in collecting the samples.
Footnotes
Funding: this work was supported by grants from the Ministerio de Economía y Competitividad (MINECO) (SAF2013-47416-R) Instituto de Salud Carlos III (ISCIII)- FEDER – MINECO- AES (CP11/00018, PI10/00621, RD012/0036/0060), and Asociación Española contra el Cancer (AECC). MS-B is supported by a Miguel Servet contract from ISCIII-FEDER (CP11/00018). Salary support to SG is provided by CP11/00018, from ISCIII-FEDER. JG-R is supported by a predoctoral grant from the Fundación Investigación Puerta de Hierro.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Herreros B, Rodriguez-Pinilla SM, Pajares R, et al. Proliferation centers in chronic lymphocytic leukemia: the niche where NF-kappaB activation takes place. Leukemia. 2010;24(4):872–876. [DOI] [PubMed] [Google Scholar]
- 2.Villamor N, Lopez-Guillermo A, Lopez-Otin C, Campo E. Next-generation sequencing in chronic lymphocytic leukemia. Semin Hematol. 2013;50(4):286–295. [DOI] [PubMed] [Google Scholar]
- 3.Vilimas T, Mascarenhas J, Palomero T, et al. Targeting the NF-kappaB signaling pathway in Notch1-induced T-cell leukemia. Nat Med. 2007;13(1):70–77. [DOI] [PubMed] [Google Scholar]
- 4.Guiu J, Shimizu R, D’Altri T, et al. Hes repressors are essential regulators of hematopoietic stem cell development downstream of Notch signaling. J Exp Med. 2012;210(1):71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Guerra M, Xargay-Torrent S, Rosich L, et al. The gamma-secretase inhibitor PF-03084014 combined with fludarabine antagonizes migration, invasion and angiogenesis in NOTCH1-mutated CLL cells. Leukemia. 2015;29(1):96–106. [DOI] [PubMed] [Google Scholar]
- 6.Arruga F, Gizdic B, Serra S, et al. Functional impact of NOTCH1 mutations in chronic lymphocytic leukemia. Leukemia. 2014; 28(5):1060–1070. [DOI] [PubMed] [Google Scholar]
- 7.Rosati E, Sabatini R, Rampino G, et al. Constitutively activated Notch signaling is involved in survival and apoptosis resistance of B-CLL cells. Blood. 2009;113(4):856–865. [DOI] [PubMed] [Google Scholar]
- 8.Fabbri G, Khiabanian H, Holmes AB, et al. Genetic lesions associated with chronic lymphocytic leukemia transformation to Richter syndrome. J Exp Med. 2013;210(11):2273–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi D, Rasi S, Fabbri G, et al. Mutations of NOTCH1 are an independent predictor of survival in chronic lymphocytic leukemia. Blood. 2011;119(2):521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schnaiter A, Paschka P, Rossi M, et al. NOTCH1, SF3B1, and TP53 mutations in fludarabine-refractory CLL patients treated with alemtuzumab: results from the CLL2H trial of the GCLLSG. Blood. 2013;122(7):1266–1270. [DOI] [PubMed] [Google Scholar]
- 11.Jeromin S, Weissmann S, Haferlach C, et al. SF3B1 mutations correlated to cytogenetics and mutations in NOTCH1, FBXW7, MYD88, XPO1 and TP53 in 1160 untreated CLL patients. Leukemia. 2014;28(1):108–117. [DOI] [PubMed] [Google Scholar]
- 12.Mammucari C, Tommasi di Vignano A, et al. Integration of Notch 1 and calcineurin/NFAT signaling pathways in keratinocyte growth and differentiation control. Dev Cell. 2005;8(5):665–676. [DOI] [PubMed] [Google Scholar]
- 13.Palomero T, Lim WK, Odom DT, et al. NOTCH1 directly regulates c-MYC and activates a feed-forward-loop transcriptional network promoting leukemic cell growth. Proc Natl Acad Sci USA. 2006; 103(48):18261–18266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petropoulos K, Arseni N, Schessl C, et al. A novel role for Lef-1, a central transcription mediator of Wnt signaling, in leukemogenesis. J Exp Med. 2008;205(3):515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kluk MJ, Ashworth T, Wang H, et al. Gauging NOTCH1 Activation in Cancer Using Immunohistochemistry. PLoS One. 2013; 8(6):e67306. [DOI] [PMC free article] [PubMed] [Google Scholar]


