Abstract
In recent decades, cilia have moved from relative obscurity to a position of importance for understanding multiple complex human diseases. Now termed the ciliopathies, these diseases inflict devastating effects on millions of people worldwide. In this review, written primarily for teachers and students who may not yet be aware of the recent exciting developments in this field, we provide a general overview of our current understanding of cilia and human disease. We start with an introduction to cilia structure and assembly and indicate where they are found in the human body. We then discuss the clinical features of selected ciliopathies, with an emphasis on primary ciliary dyskinesia, polycystic kidney disease, and retinal degeneration. The history of ciliopathy research involves a fascinating interplay between basic and clinical sciences, highlighted in a timeline. Finally, we summarize the relative strengths of individual model organisms for ciliopathy research; many of these are suitable for classroom use.
Keywords: cilia, flagella, ciliopathies, cystic kidney disease, blindness
In the summer of 1674, the Dutch scientist Antoni van Leeuwenhoek looked through a homemade microscope at a sample of rain water and revolutionized the human view of the world. His report of animalcules, little animals, with “divers incredibly thin little feet or little legs” was the first description of the single-celled protozoa and the cilia that many of them use for locomotion (see box 1; van Leeuwenhoek 1677). Almost exactly 300 years later, the observations of Afzelius (1976) led to another paradigm shift, when he linked defects in the machinery required for the movement of human cilia to Kartagener's syndrome, a disease characterized by chronic sinus and respiratory infections; male infertility; and, amazingly, the misplacement of the heart and other organs. Other cilia, such as primary cilia, lack motility but also are crucial to human health. Although primary cilia were discovered on mammalian cells in the 1890s, and most cells in the human body assemble a primary cilium, until 2000, they were widely considered to be vestigial evolutionary relics with no important function (Bloodgood 2009). In that year, defective primary cilia in the kidney were linked to the development of polycystic kidney disease (PKD; Pazour et al. 2000). These studies led to the realization that primary cilia function as cellular antennae that receive signals from the environment and transmit them to the cell body to control many important cellular functions.
Box 1. From Leeuwenhoek to Leber's congenital amaurosis: Little legs at the heart of ciliopathies.
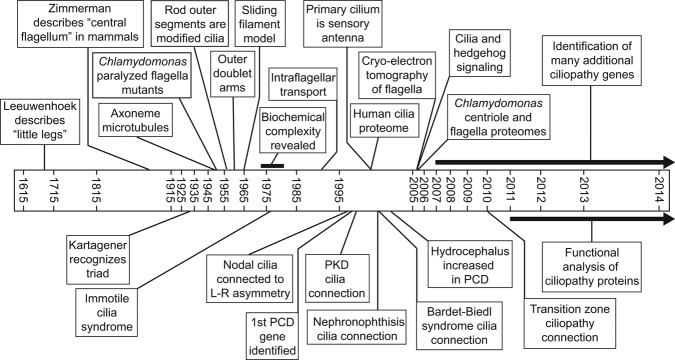
The history of discoveries leading to our current molecular understanding of ciliopathies has been closely tied to the development of new technologies. In the 1670s, Antoni van Leeuwenhoek used a new type of microscope that he designed and built to see for the first time the “little legs” that we now know are the cilia that many single-celled protozoa use for locomotion (van Leeuwenhoek 1677). In 1933, Manes Kartagener recognized the clinical triad of bronchiectasis, chronic sinusitis, and situs inversus as a distinct congenital syndrome (Kartagener 1933). In 1952, the first “paralyzed” flagellar mutants were described in Chlamydomonas (Lewin 1952). Also in the early 1950s, electron microscopy, newly applied to biological specimens, revealed that the flagellar axoneme contains nine peripheral filaments, later shown to be doublet microtubules (Manton 1952). Soon thereafter, De Robertis (1956) and Porter (1957) observed these same filaments in the sensory outer segments of rod cells in the retina, establishing that the outer segments are modified cilia and that motile cilia, photoreceptor outer segments, and basal bodies all belong to the same family of organelles. In 1959, Bjorn Afzelius, using an improved electron microscopy (EM) fixation technique, discovered arms extending from the doublet microtubules (Afzelius 1959). During the 1960s, further EM and biochemical analyses by Peter Satir and Ian Gibbons led to the development of the sliding filament model of ciliary motility, in which the arms contain an ATPase (adenylpyrophosphatase) termed dynein that uses the energy from ATP (adenosine triphosphate) hydrolysis to slide adjacent doublet microtubules relative to each other (Gibbons and Rowe 1965, Satir 1965, and see Satir et al. 2014 in this issue). In the 1970s, newly developed gel electrophoresis techniques were applied to isolated C. reinhardtii flagella, revealing an unexpected compositional complexity (Witman et al. 1972, Piperno et al. 1977). In the mid-1970s, Afzelius discovered that the arms were missing from the sperm flagella of four infertile men, three of whom had Kartagener's syndrome, thus establishing the first link between cilia and disease (Afzelius 1976); Afzelius termed the disorder immotile cilia syndrome, later renamed primary ciliary dyskinesia (PCD). A mechanistic explanation of the establishment of left–right asymmetry came two decades later, when it was shown for the first time that cilia in the mouse embryonic node are motile (Nonaka et al. 1998). In the early 1990s, advances in light microscopy enabled the discovery of intraflagellar transport (Kozminski et al. 1993). In the late 1990s, studies of dynein assembly in C. reinhardtii led to the identification of the first PCD genes in humans (Pennarun et al. 1999). After 2000, studies of human PCD patients and mice mutants linked defective motility of ependymal cilia to the accumulation of fluid in the brain, known as hydrocephalus (Ibanez-Tallon et al. 2004).
Over 200 years after van Leeuwenhoek observed motile cilia, Zimmerman described the “central flagellum” of mammalian cells (for a review, see Bloodgood 2009), establishing the distinction between motile cilia and primary cilia in mammals. However, it would be over 100 years before it was recognized that these primary cilia were anything other than an evolutionary relic: In 2000, studies of intraflagellar transport in C. reinhardtii and a mouse mutant linked primary cilia to polycystic kidney disease (PKD) and showed that the primary cilium functions as a cellular antenna (Pazour et al. 2000, 2003). This discovery was a precursor to a flood of new connections between multiple human diseases and cilia malfunction. These included nephronophthisis, blindness, and syndromic diseases such as Bardet–Biedl syndrome (Fliegauf et al. 2007). Advancements in mass spectrometry led to the first ciliary proteome—specifically, that of the human cilium—in 2002 (Ostrowski et al. 2002); 2005 saw the publication of both the flagellar and basal body proteomes from C. reinhardtii, revealing that many proteins associated with human diseases are located in one or the other of these structures (Keller et al. 2005, Pazour et al. 2005). Also in 2005, it was discovered that the hedgehog signaling pathway is mediated through cilia (Huangfu and Anderson 2005). That year also saw the first of many publications in which the detailed three-dimensional structure of the ciliary axoneme was determined by recently developed cryoelectron tomographic methods (Nicastro et al. 2005; also see Satir et al. 2014 in this issue). In the same decade, advances in DNA sequencing led to the discovery of an increasing number of specific genetic mutations underlying various ciliopathies. Beginning in 2010, the study of disease proteins located in the transition zones of C. reinhardtii, Caenorhabditis elegans, and the mouse established that these proteins and the transition zone control entry of membrane proteins into the cilium (Craige et al. 2010, Garcia-Gonzalo et al. 2011, Williams et al. 2011); more recent work has explored the physical properties of this barrier.
The discovery that all cilia may have crucial functions for human health has led to an explosion of interest in these fascinating organelles. The term ciliopathy has been coined to refer to the group of diseases now recognized to be caused by defects in ciliary assembly or function (Badano et al. 2006). As patients with defects in ciliary motility share common symptoms, so too do patients with defects in nonmotile cilia often exhibit overlap in their symptoms. These ciliopathies are characterized to a greater or lesser degree by cystic kidneys, retinal degeneration and blindness, extra digits and other skeletal abnormalities, fluid accumulation in the brain, cognitive deficits, and obesity. A great deal of effort has been expended over the last decade to identify genes mutated in human ciliopathies and to understand the underlying functional connections between the encoded proteins. Excellent reviews of these advances have been written for professionals in the field (Drummond 2012, Fliegauf et al. 2007); here, we target teachers and students who may not be aware of the recent exciting developments in cilia biology.
What are cilia, and where are they found?
Cilia are hair-like structures that extend from the cell body into the fluid surrounding the cell. They are found on many types of single-celled eukaryotes, in which they are adapted for moving the cells through their surrounding fluid, for food uptake, and for sensing the environment. Many plants have sperm cells that swim using flagella (in this review, we will use the terms cilia and flagella interchangeably, because they are nearly identical in their basic structure and composition). In animals, cilia are found on multiple cell types throughout the body and are crucial for normal development and homeostasis.
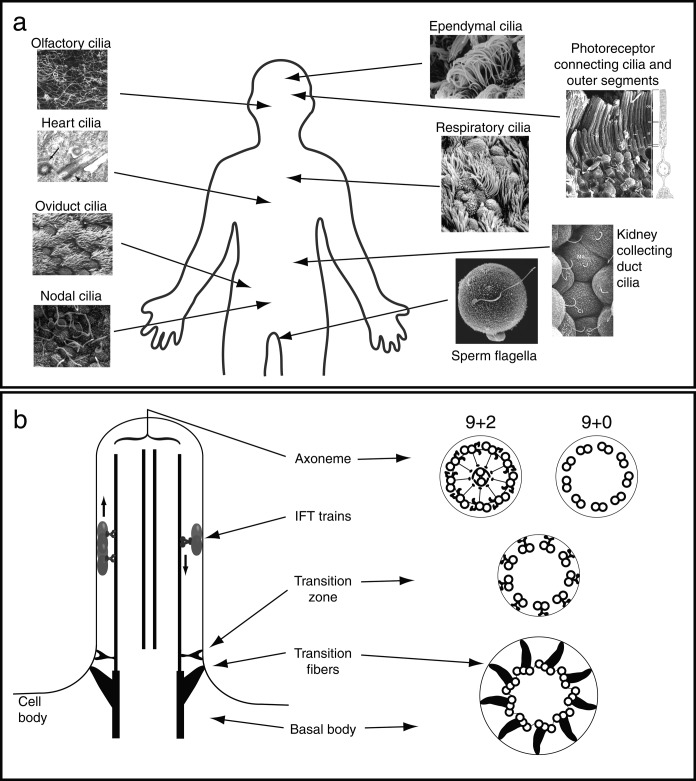
In humans, cilia are found on almost every cell in the body, and they can be broadly categorized into motile and nonmotile cilia (figure 1a); individual cells appear to assemble either motile or nonmotile cilia but not both. Motile cilia include ependymal cilia in the brain that are responsible for circulating cerebrospinal fluid, and respiratory cilia that move mucus and inhaled particulate matter up and out of the lungs. Following ejaculation, millions of motile sperm use their flagella to swim through the uterus and up into the oviducts. As the sperm cells move through the oviduct, they encounter a flow oriented against their direction of movement generated by oviduct cilia sweeping the ovum from the ovary toward the uterus. Cilia in the node of the embryo are crucial for the correct placement of organs relative to the left–right body axis during development (Nonaka et al. 1998, Yoshiba et al. 2012). Among the nonmotile cilia, the kidney collecting duct and tubule cilia stand out as crucially important. These cilia detect fluid flow within the ducts and tubules and help kidney cells maintain proper patterns of cell division (Pazour et al. 2002a, Fliegauf et al. 2007). The rod and cone photoreceptor cells in the retina detect light using membrane-associated molecules that are concentrated in structures known as the outer segments, which are modified cilia (Wheway et al. 2013). The perception of our environment is further facilitated by olfactory cilia, the membranes of which are filled with odorant receptor proteins that detect chemicals in our surroundings and send signals to the brain to be processed into our sense of smell (Jenkins et al. 2009). Of central importance to mammalian homeostasis is the establishment of the proper heart anatomy during embryonic development; cilia on the cells of the developing heart are crucial for heart morphogenesis (Willaredt et al. 2012). Figure 1a is not exhaustive; additional cell types crucial for human health are ciliated. The wide distribution of cilia throughout the body explains why multiple organ systems are affected in the syndromic ciliary diseases discussed below.
Figure 1.
What are cilia and where are they found? (a) Selected locations of motile and nonmotile cilia in the human body. Micrographs: Olfactory, oviduct, photoreceptor, and kidney cilia reprinted with permission from Kessel and Kardon (1979); heart cilia reprinted with permission from Willaredt and colleagues (2012); nodal cilia reprinted with permission from Follit and colleagues (2014); ependymal cilia reprinted with permission from O'Callaghan and colleagues (1999); respiratory cilia, reprinted with permission from Rosenbaum and Witman (2002); sperm on oocyte, micrograph: George B. Witman. (b) Ciliary structure. On the left is a longitudinal representation of the cilium. On the right are cross sections at different levels, including the axoneme, transition zone, and basal body. Abbreviation: IFT, intraflagellar transport.
The cilium includes the cylindrical protein core known as the axoneme, surrounded by a specialized extension of the plasma membrane (figure 1b). The ciliary matrix, akin to the cell cytosol, is the soluble portion of the cilium bounded by the ciliary membrane and not attached to the axoneme. In nonmotile cilia, a 9+0 axoneme of nine doublet microtubules is assembled from polymers of the alpha and beta tubulin proteins. In motile cilia, this basic structure is augmented to generate a 9+2 axoneme, which has two central singlet microtubules and accessory substructures that generate and regulate motility (see Satir et al. 2014, in this issue). These include the inner and outer dynein arms, which generate the force needed for ciliary movement; the radial spokes and central microtubule projections, which work together to regulate dynein arm activity; and a dynein regulatory complex that relays mechanical signals from the radial spokes to the dynein arms.
Not surprising for a superstructure of such complexity, the cilium is compositionally complex. Proteomic analysis of the Chlamydomonas reinhardtii flagellum has revealed that it contains over 600 different proteins, most of which are associated with the axoneme (Pazour et al. 2005). Each of the major substructures of the axoneme is, itself, a large, multisubunit complex; for example, the outer dynein arm contains at least 16 different proteins, and the radial spokes contain over 20 different proteins. Even so, most axonemal proteins have not yet been identified with a specific substructure, so many important discoveries are waiting to be made.
The outer doublet microtubules are continuous with the triplet microtubules of the basal body, a cylindrical structure derived from mitotic centrioles. Also associated with basal bodies are accessory structures, such as the transition fibers, which anchor the basal body to the cell surface (figure 1b). Between the basal body and the axoneme proper is the transition zone, a region that is a major focus of ciliopathy research. Connected to the nine peripheral doublets in this region are Y-shaped structures, visible through electron microscopy (EM) and depicted in the diagram on the right side of figure 1b, that connect the transition zone membrane to the underlying microtubules. Several studies have now linked proteins normally found in the transition zone to a function in regulating ciliary protein composition. This has led to the model that the transition zone is a kind of gate or sieve that excludes nonciliary proteins from the cilium while allowing the entry of appropriately targeted ciliary proteins (Craige et al. 2010, Garcia-Gonzalo et al. 2011, Williams et al. 2011).
Despite the fact that cilia are composed of many hundreds of proteins, these proteins are not synthesized within the cilia. Therefore, new proteins incorporated into the growing or steady-state axoneme must be transported into cilia from their sites of synthesis in the cell body; many of these proteins are known to be incorporated into the axoneme at the ciliary tip (Rosenbaum and Witman 2002). The question of how cells deliver axonemal proteins from the cell body to the ciliary tip was answered in part in 1993 with the description of intraflagellar transport (IFT), a bidirectional movement of large particles along the doublet microtubules just beneath the flagellar membrane. IFT is evolutionarily highly conserved and is required for the assembly of almost all cilia, including both primary and motile cilia, in every organism in which it has been studied. Visible through EM, IFT particles appear to be linear arrays of subunits (Kozminski et al. 1993, Pigino et al. 2009); such arrays are now termed IFT trains. The locomotives for anterograde trains—the trains moving toward the tip—are members of the kinesin family of microtubule motors; retrograde trains are carried back to the cell body by another microtubule motor, a specialized cytoplasmic dynein. Both anterograde and retrograde IFT trains carry cargo, including both axonemal and membrane-associated proteins. In further analogy to freight trains, some IFT trains include specialized cars that are adapted for carrying specific types of cargo. One example of a specialized cargo adapter with particular significance for human health is the BBSome; genes encoding many of its proteins are mutated in the human disease Bardet–Biedl syndrome (BBS; Nachury et al. 2007, Lechtreck et al. 2009).
Although the specifics of IFT function have been somewhat elusive, some details have emerged over the last 15 years. IFT trains are composed of two separable protein complexes known as IFT-A and IFT-B, which, together, contain at least 20 different proteins, known as IFT-particle proteins. Because most IFT-B mutants fail to assemble cilia and many IFT-A mutants have ciliary bulges containing material that would normally be transported out of the cilia by retrograde trains, it has been proposed that IFT-B is more important for anterograde transport and that IFT-A is more important for retrograde transport (Rosenbaum and Witman 2002, Taschner et al. 2012). In addition, it is now abundantly clear that IFT has important cargo transport functions other than transporting axonemal precursors for ciliary assembly. In fact, mammalian extracellular signaling pathways, including hedgehog and possibly canonical and noncanonical Wnt pathways, depend on IFT for their function (Goetz and Anderson 2010, Lancaster et al. 2011). These pathways play crucial roles during embryonic development, which explains why many ciliopathies caused by defects in IFT are congenital developmental disorders rather than degenerative diseases. In addition, for these pathways to function properly, the signaling receptors must be included or excluded from the ciliary membrane at specific times and under precise sets of conditions. Therefore, the regulation of ciliary membrane composition, which occurs at the basal body–transition zone region, is of special importance for human health.
Diseases caused by defects in cilia
To date, all diseases caused by defective cilia are due to mutations in the nuclear genome; as a result, all are inherited and many are manifested in the embryo or newborn. Below we discuss some of the most well characterized ciliopathies.
Primary ciliary dyskinesia
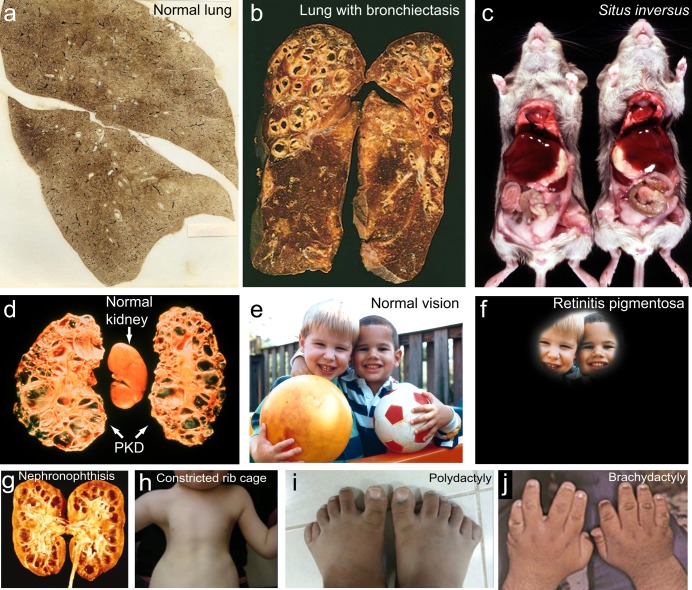
Because cilia are so widespread in the human body, defects in cilia frequently cause syndromes—that is, collections of symptoms in which multiple tissues are affected by a single underlying cause. The first of these to be identified was immotile cilia syndrome or primary ciliary dyskinesia (PCD; Afzelius 1976, Knowles et al. 2013), which is caused by defects in motile cilia and should not be confused with disorders caused by defects in primary cilia, which are discussed below. Patients with PCD have chronic bronchitis and sinusitis because their cilia fail to clear mucus and inhaled bacteria out of their airways. Because of their recurrent bacterial infections, they also develop bronchiectasis, an irreversible dilation of the bronchi that leads to severely impaired respiratory function (figure 2a, 2b). The male patients are infertile, because of impaired motility of the sperm flagellum. About half of the patients have situs inversus due to impaired motility of the nodal cilia that initiate left–right asymmetry in the early embryo. Situs inversus is a condition in which the positions of the heart and other internal organs are reversed during development (figure 2c). Normal nodal cilia (figure 1a) generate a leftward flow of fluid over the node, and this flow initiates a signaling cascade that leads to normal left–right asymmetry (Nonaka et al. 1998, Yoshiba et al. 2012). When this flow is missing or abnormal, it is a matter of chance whether this signaling cascade is initiated on the left or right side; therefore, half of PCD patients have normal left–right asymmetry, and half have situs inversus. Finally, patients with PCD have a greatly increased incidence of hydrocephalus, which is an abnormal accumulation of cerebrospinal fluid in the ventricles of the brain that leads to an enlargement of the head; this is due to defective motility of the ependymal cilia (figure 1a) that help circulate the cerebrospinal fluid (Ibanez-Tallon et al. 2004).
Figure 2.
Representative ciliopathy phenotypes. (a) Normal human lungs. Photograph: National Institute for Occupational Safety, Centers for Disease Control. (b) Lungs with the dilated bronchioles characteristic of bronchiectasis. Photograph: Matthew M. Fitz, Loyola University Chicago, Stritch School of Medicine. (c) On the left is a normal mouse, and on the right is a mouse with situs inversus, in which there is a reversal of the asymmetric placement of internal organs. Photograph: Noah's Arkive Database, Department of Pathology, University of Georgia College of Veterinary Medicine (http://dlab.vet.uga.edu/NA). (d) polycystic kidney disease (PKD). Photograph: Vicente E. Torres, Mayo Clinic. (e) Normal vision and (f) what the world might look like with the loss of vision, which progresses from the periphery inward, in retinitis pigmentosa. Images: National Eye Institute, National Institutes of Health, reference nos: EDS01 and EDS07. (g) Nephronophthisis, another form of cystic kidney disease in which the cysts form at the corticomedulary junction and are associated with little or no kidney enlargement. Source: Reprinted with permission from Hildebrandt and Zhou (2007). (h) Constricted rib cage characteristic of asphyxiating thoracic dystrophies. Source: Reprinted with permission from Huber and Cormier-Daire (2012). (i) Polydactyly—extra digits. Source: Reprinted with permission from Aldahmesh and colleagues (2014). (j) Brachydactyly—shortened digits. Source: Reprinted with permission from Forsythe and Beales (2013).
As more and more proteins that generate motility in the cilium have been identified, this has facilitated the discovery of the genes that cause PCD when they are mutated. Currently, approximately 20 PCD genes are known (Knowles et al. 2013). Most of these genes encode proteins of the outer dynein arms, the radial spokes, the dynein regulatory complex, and the central microtubule projections, as well as proteins necessary for preassembly of the dynein arms, which occurs in the cytoplasm. However, all together, these known PCD genes account for only about half of all PCD cases, so it is likely that there are many more PCD genes yet to be identified. In general, mutations specifically affecting ciliary motility and causing PCD lead to a different set of symptoms than those affecting nonmotile cilia, which are discussed below.
Polycystic kidney disease
Polycystic kidney disease, or PKD, is the most common life-threatening inherited disease in humans, and it affects 12.5 million people worldwide. The disease comes in two major forms: autosomal dominant and autosomal recessive. Autosomal dominant PKD (ADPKD) affects mostly adults and has an incidence of at least 1 in 1000. Autosomal recessive PKD (ARPKD) occurs in neonates and children; it affects up to 1 in 6000 live births, and in 75% of these cases, death occurs within a few days (Grantham 1998). Both types of the disease result from excessive proliferation of the epithelial cells lining the ducts and tubules of the kidney, so that the lumens of the ducts become closed off as cysts, which then expand, leading to a massive enlargement of the kidneys, which can become as big as an American football (figure 2d). Ultimately, this leads to end-stage renal failure.
The first hint of a connection between PKD and cilia came from the finding that homologues of the human proteins polycystin-1 and polycystin-2, which, together, account for most cases of ADPKD, are localized to the sensory cilia involved in the mating behavior of the nematode Caenorhabditis elegans (Barr and Sternberg 1999). Shortly thereafter, sequencing of IFT-particle proteins in Chlamydomonas revealed that one of these proteins, IFT88, was a homologue of a mouse protein of then-unknown function defective in the murine model for ARPKD. Genetic analysis showed that IFT88 was essential for flagellar formation in Chlamydomonas; EM examination of the mutant mouse kidney then showed that the mouse homologue was similarly necessary for the assembly of the primary cilia in the kidney's collecting ducts and tubules (Pazour et al. 2000). Therefore, the underlying defect in the mouse model for ARPKD was a failure to form kidney primary cilia because of a malfunction in IFT. However, at that time, the function of the primary cilium was not known, and why an inability to form these cilia in the kidney would lead to ARPKD was not clear. A key insight was gained from the finding that polycystin-2 at the surface of mouse and human kidney cells was specifically localized to the primary cilium (Pazour et al. 2002a). Because polycystin-1 and polycystin-2 interact to form a receptor-channel complex that acts at an early step in a signaling pathway that controls kidney epithelial cell differentiation and proliferation, it was concluded that the primary cilium was functioning as a sensory antenna, displaying these two PKD proteins to the environment and relaying signals from them to the cell body. Mutation of either of these two ciliary proteins, or an inability to assemble the cilium as a whole, as in the mouse model, results in the defective ciliary signaling that leads to PKD. Therefore, PKD is a disease of the cilium. Although this proposal was initially met with considerable resistance from some investigators in the PKD field, most proteins associated with PKD have now been localized to primary cilia or their basal bodies, and the ciliary theory of PKD is now well established (Fliegauf et al. 2007).
Blindness
Blindness due to retinitis pigmentosa (photoreceptor cell degeneration) is also frequently associated with defects in IFT, including defects in IFT-particle proteins and the BBSome, an IFT-cargo adaptor (figure 2e, 2f; Wheway et al. 2013). The rod and cone cells of the retina have an inner segment, in which proteins are synthesized, and an outer segment, which contains the membranous disks on which the opsins responsible for photoreception are located (figure 1a). The entire outer segment is a modified cilium. The only connection between it and the inner segment is via a 9+0 structure known as the connecting cilium, which is a greatly elongated transition zone. Therefore, all of the billions of copies of proteins (such as opsin) that are necessary to build and maintain the outer segment must pass through the connecting cilium (Crouse et al. 2014). Many of these proteins are likely to be dependent on IFT for their movement into the outer segment. Studies in the 1990s showed that the anterograde IFT motor, kinesin-II, was located in the vertebrate connecting cilium and that knockout of this motor in the mouse retina resulted in outer segment abnormalities and retinal degeneration (Marszalek et al. 2000). More definitive evidence for the involvement of IFT came shortly thereafter, when it was shown that IFT-particle proteins also localized to the connecting cilium and that the same IFT88 mutation that causes PKD in mice (see above) also caused abnormal outer segment development and the death of photoreceptor cells by apoptosis (Pazour et al. 2002b). Subsequent studies have documented similar results for mice lacking other IFT-particle proteins. Therefore, it seems likely that photoreceptor cells are exquisitely sensitive to perturbations in the transport of proteins to the outer segment and respond by initiating apoptosis to commit cell suicide, thereby ensuring the elimination of defective cells from the retina.
As was noted above, the connecting cilium of photoreceptor cells is thought to be a greatly elongated ciliary transition zone, and proteins that localize to the transition zone in other cell types localize to the connecting cilium of photoreceptor cells. Interestingly, defects in these proteins seem to have a high probability of causing blindness. For example, mutations in the transition zone and connecting cilium protein CEP290 cause Leber's congenital amaurosis (amaurosis is Greek for “darkness”), an isolated (i.e., only one organ is affected) early-onset form of retinal degeneration (den Hollander et al. 2006). Inasmuch as the transition zone appears to form a barrier to the entry of nonciliary proteins into the cilium and may also be the site at which ciliary proteins are sorted to gain access to the cilium, it is likely that these functions are particularly important in photoreceptor cells. This may explain why the transition zone is so elongated in these cells and why the cells are so sensitive to defects in the sorting or trafficking of proteins to the outer segment.
In disorders such as Leber's congenital amaurosis, in which degeneration of the photoreceptor cells is progressive whereas other organs are spared, there is a window between diagnosis and the complete loss of the photoreceptors during which the disorder could be amenable to gene therapy. Gene therapy by subretinal injection of a vector expressing a wild-type form of the mutated protein has been successful for a form of Leber's congenital amaurosis caused by defects in a protein specific to retinal pigmented epithelial cells, which are located adjacent to the tips of the photoreceptor outer segments (Maguire et al. 2008). Although significant challenges remain, efforts are now underway to develop the methodology for similar rescue of photoreceptor cells affected by defects in ciliary proteins such as CEP290.
Syndromic developmental disorders
As the group of ciliopathy-associated phenotypes has become more well defined, several additional syndromes have been identified as ciliopathies. These range in severity from disorders that include one or two common ciliopathy phenotypes that are not acutely life threatening to more severe lethal disorders combining multiple different symptoms. On the less severe end of the spectrum, Senior–Løken syndrome primarily involves only retinitis pigmentosa and nephronophthisis, a cystic kidney disease that involves kidney malfunction with little to no kidney enlargement (figure 2g; Hildebrandt and Zhou 2007). A more severely life-threatening syndrome, Jeune asphyxiating thoracic dystrophy (JATD), involves severe constriction of the rib cage due to the development of abnormally short ribs, in some cases leading to death from insufficiency in lung ventilation (figure 2h). The rib cage defect in JATD is accompanied by short limbs and, occasionally, by polydactyly, the development of extra digits (figure 2i; Huber and Cormier-Daire 2012).
Skeletal malformations such as those in JATD are relatively common among ciliopathies and are shared to varying degrees by different syndromes. They also highlight the importance of specific cilia-dependent signaling pathways for the development of the skeleton. As was mentioned above, hedgehog signaling is dependent on IFT in cilia and is required for normal skeletal patterning during development. The receptor protein for this pathway is found in the ciliary membrane, and once hedgehog ligand binds to this receptor, a complex series of events occurs involving the export of the receptor from the cilium, the trafficking of transcriptional regulatory proteins through the cilium and to the nucleus, and their ultimate activation of the downstream genes involved in normal tissue patterning (Goetz and Anderson 2010).
Another disorder with polydactyly as a common feature is BBS. In addition to polydactyly, the hallmarks of BBS are obesity, cystic kidneys, retinal degeneration, brachydactyly (short digits; figure 2j), and small genitalia (Forsythe and Beales 2013). This disorder has been attributed to defects in the BBSome, which functions as a cargo adapter linking IFT particles to certain membrane proteins, including signaling proteins (Lechtreck et al. 2009). Although the exact mechanism of disease development is unclear, at least some of the clinical manifestations of BBS are likely to involve specific signaling defects.
A number of other syndromic ciliopathies are caused by mutations in IFT-associated genes. Mutations in both IFT-A and IFT-B proteins have been associated with skeletal ciliopathies (Huber and Cormier-Daire 2012). However, IFT-A may be particularly closely connected with these disorders because causative mutations for these diseases have now been identified in the genes encoding all known IFT-A proteins. In one example, the gene encoding the IFT-A protein IFT140 was found to be mutated in multiple cases of Mainzer–Saldino syndrome (MSS; Perrault et al. 2012). This disorder is defined by an abnormal cone-shaped appearance of the normally rounded ends of the long bones in the fingers, kidney disease, severe early-onset retinal degeneration, and mild abnormalities of the upper portion of the femur (Perrault et al. 2012). An interesting possible clue about a specific function for the IFT140 protein is that skeletal ciliopathies caused by other IFT-A mutations generally do not include retinal degeneration. Therefore, the retinal problems seen in MSS may indicate a specific function for IFT140 in the assembly and maintenance of the retina (Perrault et al. 2012).
Some syndromic ciliopathies also have distinctive central nervous system (CNS) abnormalities as hallmarks of the diseases. One of the most well-studied examples is Joubert syndrome, which presents with extremely variable multiorgan involvement. Many of these defects, including retinal degeneration, polydactyly, and kidney disease are shared with other ciliopathies. However, the diagnostic feature of Joubert syndrome is the presence of the molar tooth sign—a striking tooth-shaped abnormal growth of tissue visible in evaluations of the brain created through magnetic resonance imaging. The brain abnormalities are accompanied by some level of cognitive disability in essentially all patients (Romani et al. 2013). A more severe ciliopathy exhibiting a characteristic CNS defect is Meckel syndrome (MKS). Ninety percent of MKS cases have an occipital encephalocele, a protrusion of brain tissue and associated protective membranes through a hole in the back of the skull. MKS also includes the prenatal development of enlarged cystic kidneys, enlarged liver, and polydactyly (Salonen and Paavola 1998). The combination of these multiple severe defects prior to birth explains the almost exclusively prenatal and perinatal lethality characteristic of MKS.
Model organisms in ciliopathy research
Pedigree analysis combined with modern DNA-sequencing technologies have led to the identification of a number of new candidate ciliopathy genes in recent years. Research in which immortalized human cells and primary cell culture from ciliopathy patients were used has also been important for answering some human-specific questions and for testing hypotheses generated by work in other organisms. However, for obvious ethical reasons, many types of questions related to ciliopathy mechanisms cannot be answered directly by research on humans. In addition, the growth of tissue culture cells is expensive, and there are technical limitations on what can be accomplished with research on human cells. For instance, classical genetic analysis is not available in cell lines. These cells cannot be mated, and, until very recently, no tools were available to easily make targeted gene knockouts in cell lines. In addition, the biochemical analysis of human cilia has so far been extremely challenging because of the need to grow large numbers of cells and the difficulty of isolating cilia in large quantities and high purity.
Because of the limitations of working with human cell lines, nonhuman model organisms have been crucial in the discovery of ciliopathies and the subsequent explosion of ciliopathy research. These different model organisms each have their own advantages, which are highlighted below. Importantly, some can be grown cheaply and easily and are ideal for use in the teaching laboratory.
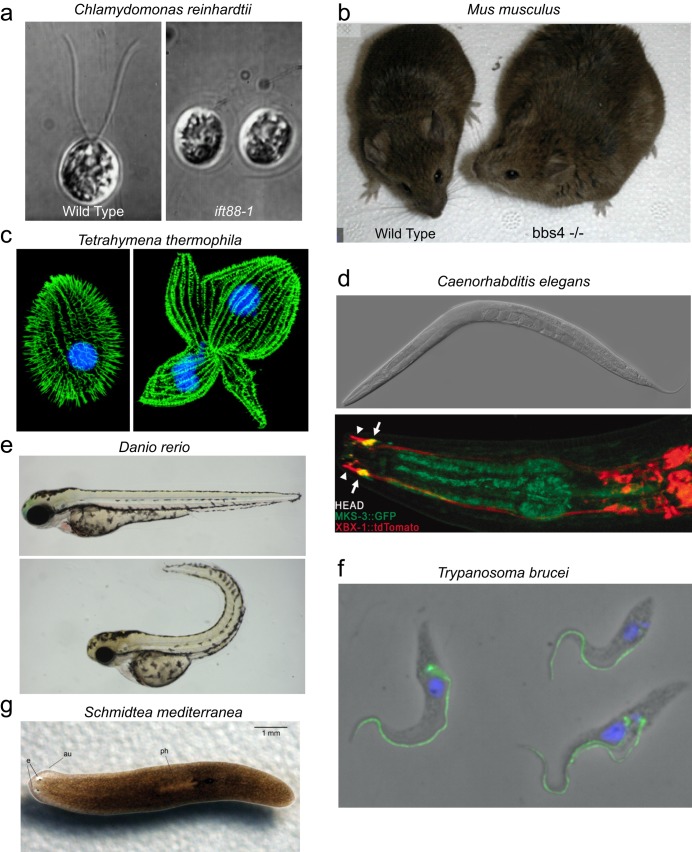
The green alga Chlamydomonas reinhardtii. Perhaps no model organism has been more central to our developing understanding of ciliopathies and basic cilia biology than Chlamydomonas reinhardtii (figure 3a). This single-celled green alga swims using a breaststroke-like motion of its two flagella. It is very easy and inexpensive to grow in large quantities in the laboratory. Because its flagella extend so far away from the cell body, a variety of microscopy techniques can be applied to learn about its flagellar structure and function. C. reinhardtii is a haploid organism with genetics very similar to those of yeast. When DNA-containing selectable markers are introduced into the cell, they integrate randomly throughout the genome, allowing the generation of mutants containing exogenous DNA insertions. Because the flagella of C. reinhardtii are dispensable for survival, insertional mutants have been used to identify mutations in genes encoding a large number of important ciliary proteins. C. reinhardtii also has a distinct biochemical advantage because its flagella can easily be isolated from cell bodies at a very high level of purity. Finally, following flagellar amputation, cells will synchronously regrow new flagella, which allows a detailed analysis of flagellar assembly.
The house mouse, Mus musculus. The common house mouse (figure 3b) is often a useful stand-in for humans in basic and preclinical ciliopathy research. Mice offer the advantage of being a multicellular vertebrate organism that is the most evolutionarily closely related to humans of any of the common model organisms. They have both motile and nonmotile cilia and similar organ and tissue distributions to those found in humans. In addition, there are well-established protocols for generating gene knockout mice. Because human and mouse protein structure and function are highly conserved, mice offer a tractable genetic model system for studying the function of newly identified ciliopathy genes. Mouse models are now available for many ciliopathies, including PCD, ADPKD, ARPKD, hydrocephalus, BBS, Leber's congenital amaurosis, and nephronophthisis (Damerla et al. 2014, Norris and Grimes 2012).
The ciliate Tetrahymena thermophila. The ciliated protozoan Tetrahymena thermophila (figure 3c) offers the biochemical advantages of a single-celled organism because it can be grown cheaply in large quantities and because its hundreds of cilia can be isolated at a high level of purity. However, unlike C. reinhardtii, T. thermophila undergoes frequent homologous recombination. This means that gene knockouts using exogenously introduced DNA sequences are relatively easy in T. thermophila, which gives this organism a genetic toolbox complementary to the one available in C. reinhardtii. Because gene knockouts are brought to expression in the somatic macronucleus in one quick step, even lethal mutations can be studied to determine the mechanism of lethality. This is important because T. thermophila requires cilia for the completion of cytokinesis and for normal feeding, which, under standard growth conditions, leads to eventual cell death in mutants lacking cilia (Brown et al. 1999).
The roundworm Caenorhabditis elegans. Caenorhabditis elegans (figure 3d) is a small multicellular nematode amenable to genetic and molecular techniques. C. elegans lacks motile cilia; however, a subset of its neurons grow nonmotile sensory cilia that are exposed to the external environment. Because these cilia help to control the worm's behavior in response to its environment, researchers can use relatively easy behavioral genetic screens to look for mutants with defects in cilia structure and function. Large mutant collections generated in this way have led to the identification of several important IFT mutants. Well-established methods allow imaging of fluorescently tagged proteins moving in the cilia (Vincensini et al. 2011).
The zebrafish, Danio rerio. The zebrafish (figure 3e) undergoes complex vertebrate development with similarities to those of humans and mice that are relevant to ciliopathy research. These include kidney, eye, and heart development and similar involvement of cilia in the determination of left–right asymmetry. However, zebrafish have the considerable advantage over mice that their embryonic developmental processes are much more visible. Not only are the embryos semi-transparent, but they also develop externally, as opposed to mouse development inside the mother's uterus. Zebrafish are also small and easy to rear and produce much larger numbers of offspring than mice do. Techniques are well developed for knocking down the expression of a single gene, as well as for the generation of mutants. Larvae with defective cilia have a distinctive phenotype (figure 3e, bottom panel) and can be identified a few days after fertilization; large mutant collections have already been developed in which many cilia-related genes are disrupted (Vincensini et al. 2011).
The sleeping sickness parasite, Trypanosoma brucei. As the causative agent of sleeping sickness, a fatal tropical disease, Trypanosoma brucei (figure 3f) has been the subject of intensive molecular and cell biological investigations aimed at vaccine and antiparasite drug development. Many of the tools that have been developed in these studies are applicable to cilia research. T. brucei has a single long flagellum that remains attached to the cell body along most of its length. T. brucei can be maintained in culture in the laboratory and shares some features with both T. thermophila and C. reinhardtii that make it an attractive model. Because of its long flagella, observation of the movement of fluorescently tagged IFT proteins is more feasible than in organisms with shorter cilia. In addition, like T. thermophila cilia development, the preexisting flagellum is not resorbed prior to the initiation of the assembly of the new flagellum. Therefore, flagellar maintenance and assembly can be studied simultaneously in a single cell, something not possible in C. reinhardtii, in which both flagella are resorbed prior to cell division. Like T. thermophila, genes can also be readily replaced by homologous recombination, which makes targeted gene disruption and tagging at the endogenous gene locus possible. Because flagella are required for the survival of T. brucei, stable mutants lacking flagella cannot be generated. However, RNA interference (RNAi) can be used to knock down the expression of proteins required for flagellar assembly and function. Because the double-stranded RNA needed for RNAi can be expressed under the control of an inducible promoter, the knockdown can be turned on and off, which allows the effect of protein loss to be observed in real time (Vincensini et al. 2011).
The flatworm Schmidtea mediterranea. The planarian Schmidtea mediterranea (figure 3g), which is easily and cheaply maintained in the laboratory, is the only multicellular model organism on our list with an external multiciliated epithelium. The ventral surface of the animal is covered with a layer of epithelial cells, each of which grows many cilia, similar to the ciliated respiratory epithelium in humans. The planarian uses the beating of these cilia combined with muscle contraction for locomotion. S. mediterranea also secretes mucus, which adheres to the substrate on which the animal is moving; the cilia presumably gain traction by moving against this mucus highway. Therefore, S. mediterranea is potentially a model system for studying mucus transport by cilia in the airway. Interest in tissue regeneration in S. mediterranea has led to the development of a variety of molecular and cell biological techniques, including a completed genome sequence and facile disruption of gene expression by RNAi (Rompolas et al. 2009).
Figure 3.
Model organisms used in ciliopathy research. (a) The green alga Chlamydomonas reinhardtii with wild type (WT) on left and the ift88–1 mutant on right. Source: Reprinted with permission from Pazour and colleagues (2000). (b) The house mouse, Mus musculus. The WT is on the left, and a bbs4 mutant exhibiting obesity is on the right. Photograph: Val C. Sheffield (www.hhmi.org/research/molecular-genetics-human-obesity-syndrome). (c) The ciliate Tetrahymena thermophila. The WT is on the left, and a kinesin-II mutant, which is unable to complete division, is on the right. Source: Reprinted with permission from Brown and colleagues (1999). (d) The nematode Caenorhabditis elegans. Differential interference contrast microscopy image on top (micrograph: Zeynep F. Altun) and enlargement of head showing fluorescently tagged ciliary (arrowheads) and transition zone (arrows) proteins on bottom. Source: Reprinted with permission from Williams et al. 2010. (e) The zebrafish, Danio rerio. The WT is on top, and a typical ciliary mutant with a curved body axis is on the bottom. Source: Reprinted with permission from Fogelgren and colleagues (2011). (f) The sleeping sickness parasite, Trypanosoma brucei. The nucleus is in blue, and the intraflagellar transport (IFT)-particle protein IFT172 is in green. Source: Reprinted with permission from Absalon and colleagues (2008). (g) The flatworm Schmidtea mediterranea. Source: Reprinted with permission from Rompolas and colleagues (2009).
Conclusions
Although the first years of the twenty-first century have seen an explosion in our understanding of the roles of cilia in human health and disease, there is much more to be learned. For example, cancer and certain neurological diseases, which were not discussed here, also may involve cilia. Moreover, even though most ciliary proteins are likely to cause disease when they are defective, we understand the specific functions of only a tiny subset of these proteins; studies of model organisms will be necessary to elucidate their functions. Recently, there have been hints that some ciliopathies may involve noncoding RNAs; this is another area for future investigation. Just as technical advances have enabled many of the past discoveries related to cilia and flagella, we can anticipate that new and improved techniques will open new avenues for gaining further insight into these immensely important and ever more fascinating cell organelles.
Acknowledgments
The work was supported by National Institutes of Health grant no. GM030626 to GBW and by the Robert W. Booth Endowment at the University of Massachusetts Medical School to GBW. The authors thank all of the individuals who graciously agreed to allow us to include their images.
References cited
- Absalon S, Blisnick T, Kohl L, Toutirais G, Dore G, Julkowska D, Tavenet A, Bastin P. Intraflagellar transport and functional analysis of genes required for flagellum formation in trypanosomes. Molecular Biology of the Cell. 2008;19:929–944. doi: 10.1091/mbc.E07-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzelius BA. Electron microscopy of the sperm tail; results obtained with a new fixative. Journal of Biophysical and Biochemical Cytology. 1959;5:269–278. doi: 10.1083/jcb.5.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–319. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- Aldahmesh MA, et al. IFT27, encoding a small GTPase component of IFT particles, is mutated in a consanguineous family with Bardet–Biedl syndrome. Human Molecular Genetics. 2014;23:3307–3315. doi: 10.1093/hmg/ddu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: An emerging class of human genetic disorders. Annual Review of Genomics and Human Genetics. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Barr MM, Sternberg PW. A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature. 1999;401:386–389. doi: 10.1038/43913. [DOI] [PubMed] [Google Scholar]
- Bloodgood RA. From central to rudimentary to primary: The history of an underappreciated organelle whose time has come. The primary cilium. Methods in Cell Biology. 2009;94:3–52. doi: 10.1016/S0091-679X(08)94001-2. [DOI] [PubMed] [Google Scholar]
- Brown JM, Marsala C, Kosoy R, Gaertig J. Kinesin-II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in Tetrahymena. Molecular Biology of the Cell. 1999;10:3081–3096. doi: 10.1091/mbc.10.10.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. Journal of Cell Biology. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse JA, Lopes VS, Sanagustin JT, Keady BT, Williams DS, Pazour GS. Distinct functions for IFT140 and IFT20 in opsin transport. Cytoskeleton. 2014;71:302–310. doi: 10.1002/cm.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerla RR, Gabriel GC, Li Y, Klena NT, Liu X, Chen Y, Cui C, Pazour GJ, Lo CW. Role of cilia in structural birth defects: Insights from ciliopathy mutant mouse models. Birth Defects Research C. 2014;102:115–125. doi: 10.1002/bdrc.21067. [DOI] [PubMed] [Google Scholar]
- Den Hollander AI, et al. Mutations in the CEP290 (NPHP6) gene are a frequent cause of Leber congenital amaurosis. American Journal of Human Genetics. 2006;79:556–561. doi: 10.1086/507318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond IA. Cilia functions in development. Current Opinion in Cell Biology. 2012;24:24–30. doi: 10.1016/j.ceb.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: Cilia defects and ciliopathies. Nature Reviews Molecular Cell Biology. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- Fogelgren B, Lin SY, Zuo X, Jaffe KM, Park KM, Reichert RJ, Bell PD, Burdine RD, Lipschutz JH. The exocyst protein Sec10 interacts with Polycystin-2 and knockdown causes PKD-phenotypes. PLOS Genetics. 2011;7 doi: 10.1371/journal.pgen.1001361. art. e1001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, San Agustin JT, Jonassen JA, Huang T, Rivera-Perez JA, Tremblay KD, Pazour GJ. Arf4 is required for Mammalian development but dispensable for ciliary assembly. PLOS Genetics. 2014;10 doi: 10.1371/journal.pgen.1004170. art. e1004170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe E, Beales PL. Bardet–Biedl syndrome. European Journal of Human Genetics. 2013;21:8–13. doi: 10.1038/ejhg.2012.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nature Genetics. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons IR, Rowe AJ. Dynein: A protein with adenosine triphosphatase activity from cilia. Science. 1965;149:424–426. doi: 10.1126/science.149.3682.424. [DOI] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: A signalling centre during vertebrate development. Nature Reviews Genetics. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham JJ, Nair V, Winklhofer F. Cystic diseases of the kidney. In: Brenner BM, editor. Brenner and Rector's The Kidney, vol. 2. Saunders; 1998. pp. 1699–1730. [Google Scholar]
- Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. Journal of the American Society for Nephrology. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proceedings of the National Academy of Sciences. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber C, Cormier-Daire V. Ciliary disorder of the skeleton. American Journal of Medical Genetics. 2012;160C:165–174. doi: 10.1002/ajmg.c.31336. [DOI] [PubMed] [Google Scholar]
- Ibanez-Tallon I, Pagenstecher A, Fliegauf M, Olbrich H, Kispert A, Ketelsen UP, North A, Heintz N, Omran H. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Human Molecular Genetics. 2004;13:2133–2141. doi: 10.1093/hmg/ddh219. [DOI] [PubMed] [Google Scholar]
- Jenkins PM, McEwen DP, Martens JR. Olfactory cilia: Linking sensory cilia function and human disease. Chemical Senses. 2009;34:451–464. doi: 10.1093/chemse/bjp020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartagener M. Zur Pathogenese der Bronchiektasien bei Situs viscerum inversus. Beitr Klin Tuberk. 1933;83:13. [PubMed] [Google Scholar]
- Keller LC, Romijn EP, Zamora I, Yates JR, 3rd, Marshall WF. Proteomic analysis of isolated chlamydomonas centrioles reveals orthologs of ciliary-disease genes. Current Biology. 2005;15:1090–1098. doi: 10.1016/j.cub.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Kessel RG, Kardon RH. Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy. Freeman; 1979. [Google Scholar]
- Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia: Recent advances in diagnostics, genetics, and characterization of clinical disease. American Journal of Respiratory and Critical Care Medicine. 2013;188:913–922. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proceedings of the National Academy of Sciences. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Schroth J, Gleeson JG. Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nature Cell Biology. 2011;13:700–707. doi: 10.1038/ncb2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, Pazour GJ, Ikebe M, Witman GB. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. Journal of Cell Biology. 2009;187:1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin RA. Ultraviolet induced mutations in Chlamydomonas moewusii Gerloff. Journal of General Microbiology. 1952;6:233–248. doi: 10.1099/00221287-6-3-4-233. [DOI] [PubMed] [Google Scholar]
- Maguire AM, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. New England Journal of Medicine. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manton I, Clarke B. An electron microscope study of the spermatozoid of sphagnum. Journal of Experimental Botany. 1952;3:265–275. [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Nicastro D, McIntosh JR, Baumeister W. 3D structure of eukaryotic flagella in a quiescent state revealed by cryo-electron tomography. Proceedings of the National Academy of Sciences. 2005;102:15889–15894. doi: 10.1073/pnas.0508274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Norris DP, Grimes DT. Mouse models of ciliopathies: The state of the art. Disease Models and Mechanisms. 2012;5:299–312. doi: 10.1242/dmm.009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C, Sikand K, Rutman A. Respiratory and brain ependymal ciliary function. Pediatric Research. 1999;46:704–707. doi: 10.1203/00006450-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Ostrowski LE, Blackburn K, Radde KM, Moyer MB, Schlatzer DM, Moseley A, Boucher RC. A proteomic analysis of human cilia: identification of novel components. Molecular and Cellular Proteomics. 2002;1:451–465. doi: 10.1074/mcp.m200037-mcp200. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Witman GB. The vertebrate primary cilium is a sensory organelle. Current Opinion in Cell Biology. 2003;15:105–110. doi: 10.1016/s0955-0674(02)00012-1. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. Journal of Cell Biology. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Current Biology. 2002a;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Baker SA, Deane JA, Cole DG, Dickert BL, Rosenbaum JL, Witman GB, Besharse JC. The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. Journal of Cell Biology. 2002b;157:103–113. doi: 10.1083/jcb.200107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennarun G, Escudier E, Chapelin C, Bridoux AM, Cacheux V, Roger G, Clement A, Goossens M, Amselem S, Duriez B. Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. American Journal of Human Genetics. 1999;65:1508–1519. doi: 10.1086/302683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrault I, et al. Mainzer–Saldino syndrome is a ciliopathy caused by IFT140 mutations. American Journal of Human Genetics. 2012;90:864–870. doi: 10.1016/j.ajhg.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Geimer S, Lanzavecchia S, Paccagnini E, Cantele F, Diener DR, Rosenbaum JL, Lupetti P. Electron-tomographic analysis of intraflagellar transport particle trains in situ. Journal of Cell Biology. 2009;187:135–148. doi: 10.1083/jcb.200905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Huang B, Luck DJ. Two-dimensional analysis of flagellar proteins from wild-type and paralyzed mutants of Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences. 1977;74:1600–1604. doi: 10.1073/pnas.74.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani M, Micalizzi A, Valente EM. Joubert syndrome: Congenital cerebellar ataxia with the molar tooth. Lancet Neurology. 2013;12:894–905. doi: 10.1016/S1474-4422(13)70136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompolas P, Patel-King RS, King SM. Schmidtea mediterranea: A model system for analysis of motile cilia. Methods in Cell Biology. 2009;93:81–98. doi: 10.1016/S0091-679X(08)93004-1. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar transport. Nature Reviews Molecular Cell Biology. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Salonen R, Paavola P. Meckel syndrome. Journal of Medical Genetics. 1998;35:497–501. doi: 10.1136/jmg.35.6.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P. Studies on cilia: II. Examination of the distal region of the ciliary shaft and the role of the filaments in motility. Journal of Cell Biology. 1965;26:805–834. doi: 10.1083/jcb.26.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Heuser T, Sale WS. A structural basis for how motile cilia beat. BioScience. 2014;64:1073–1083. doi: 10.1093/biosci/biu180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschner M, Bhogaraju S, Lorentzen E. Architecture and function of IFT complex proteins in ciliogenesis. Differentiation. 2012;83:S12–S22. doi: 10.1016/j.diff.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwenhoek A. Concerning little animals observed in rain-, well-, sea- and snow-water; as also in water wherein pepper had lain infused. Philosophical Transactions of the Royal Society. 1677;12:821–831. [Google Scholar]
- Vincensini L, Blisnick T, Bastin P. 1001 model organisms to study cilia and flagella. Biology of the Cell. 2011;103:109–130. doi: 10.1042/BC20100104. [DOI] [PubMed] [Google Scholar]
- Wheway G, Parry DA, Johnson CA. The role of primary cilia in the development and disease of the retina. Organogenesis. 2013;10:69–85. doi: 10.4161/org.26710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willaredt MA, Gorgas K, Gardner HA, Tucker KL. Multiple essential roles for primary cilia in heart development. Cilia. 2012;1:23. doi: 10.1186/2046-2530-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Masyukova SV, Yoder BK. Normal ciliogenesis requires synergy between the cystic kidney disease genes MKS-3 and NPHP-4. Journal of American Society of Nephrology. 2010;21:782–793. doi: 10.1681/ASN.2009060597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. Journal of Cell Biology. 2011;192:1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman GB, Carlson K, Berliner J, Rosenbaum JL. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. Journal of Cell Biology. 1972;54:507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiba S, et al. Cilia at the node of mouse embryos sense fluid flow for left–right determination via Pkd2. Science. 2012;338:226–231. doi: 10.1126/science.1222538. [DOI] [PMC free article] [PubMed] [Google Scholar]