Abstract
Isolated, surviving sacs of everted small intestine were used to characterize ammonia transport in the golden hamster. Jejunal and ileal sacs incubated aerobically in ammonia-free test solution liberated the same quantity of ammonia as did sacs that were filled and immediately emptied of their contents, indicating no significant evolution of metabolic ammonia.
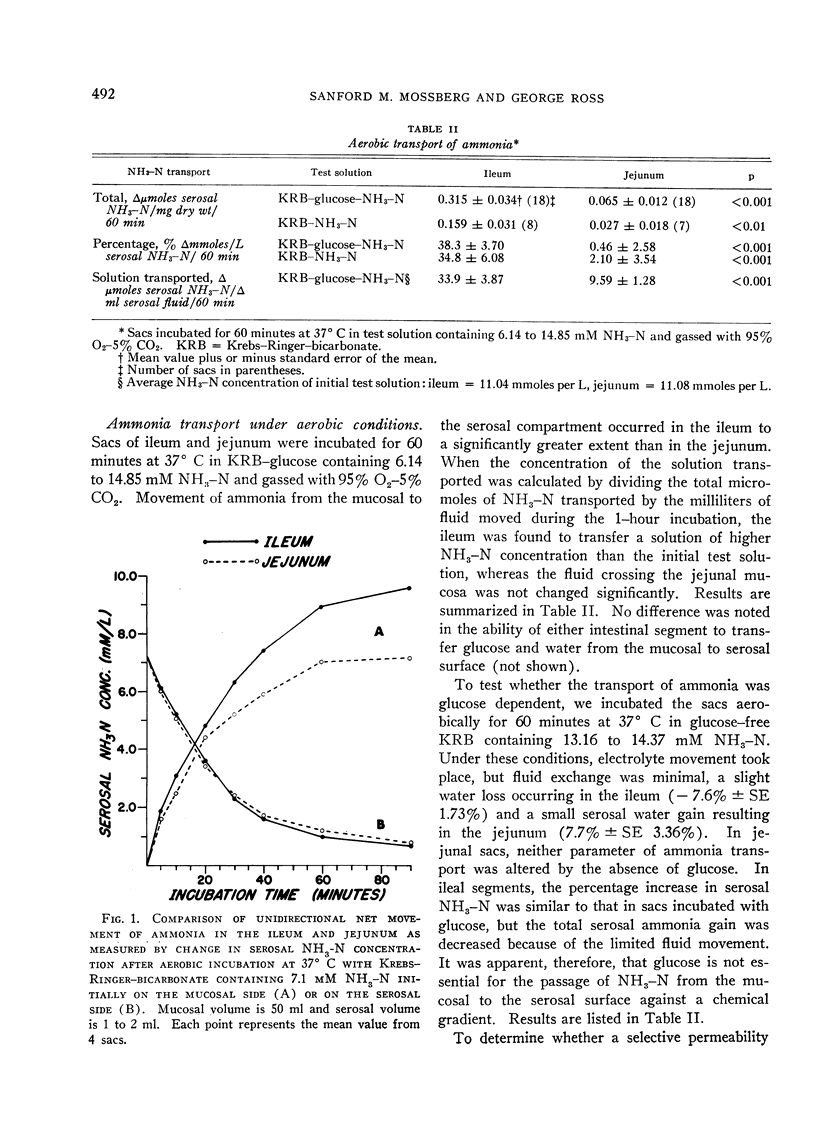
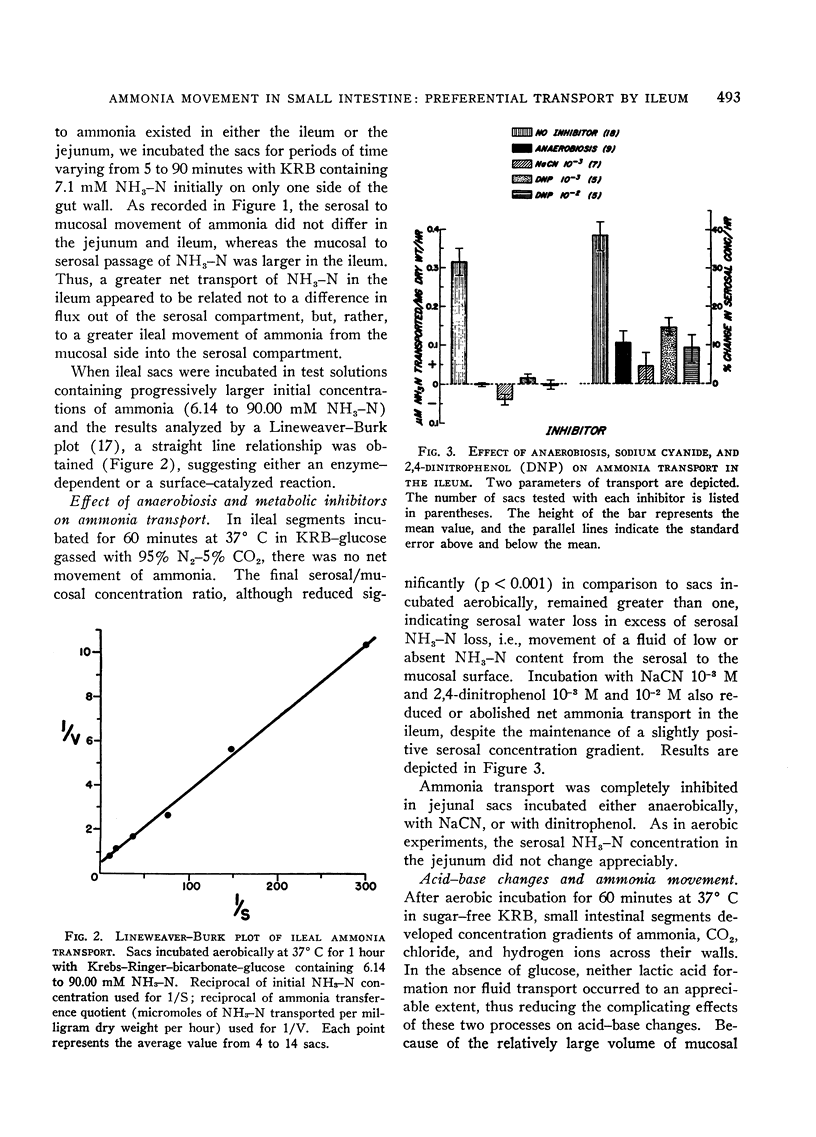
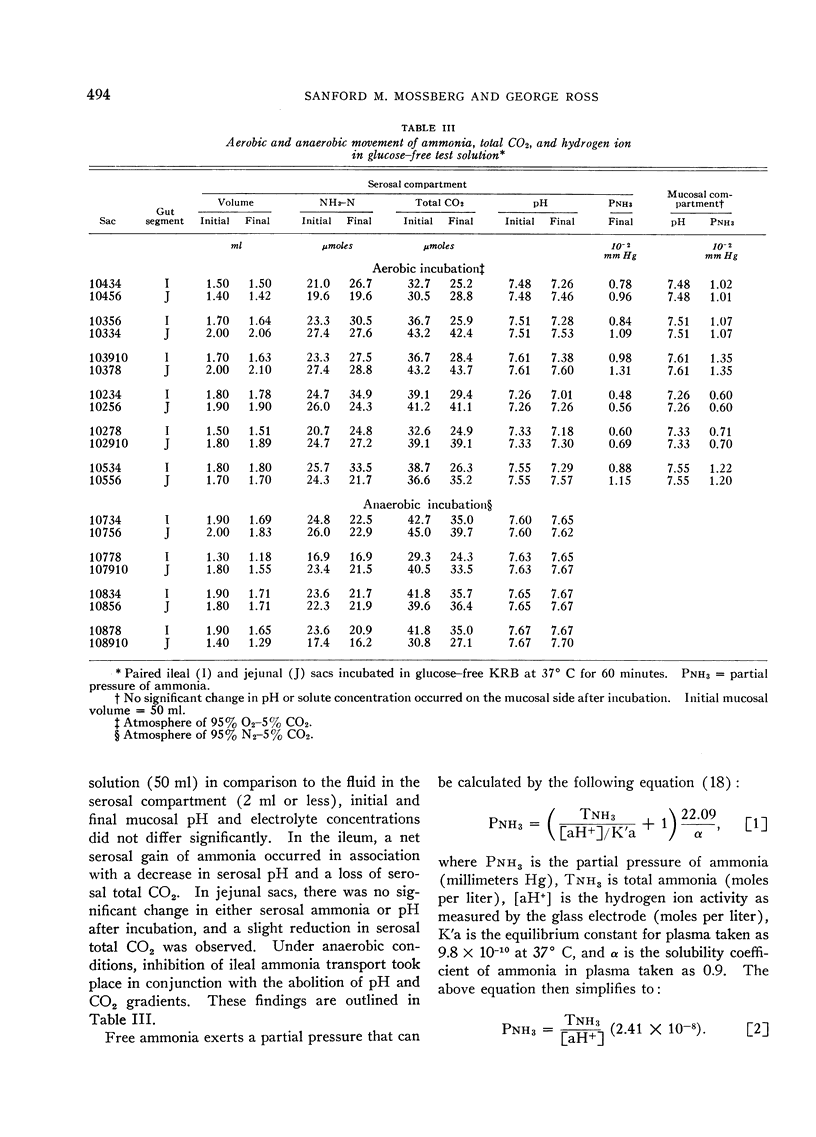
Under aerobic conditions, ileal sacs transferred a solution of high ammonia content from the mucosal surface to the serosal surface against a concentration gradient. This transport was not glucose dependent and exhibited first-order Michaelis-Menten kinetics. Inhibition of absorption occurred with anaerobiosis, 2,4-dinitrophenol, and sodium cyanide. In jejunal segments ammonia was not transported against an adverse chemical gradient. Ileal ammonia absorption was accompanied by bicarbonate secretion and acidification of the serosal solution. Both bicarbonate movement and pH gradients were abolished by inhibitors of ammonia transport. In the jejunum, the absence of ammonia movement occurred in association with minimal bicarbonate secretion and no appreciable change in serosal pH.
Despite the creation of hydrogen ion gradients tending to augment or to retard ammonia absorption by nonionic diffusion, ammonia movement was unaffected, i.e., relative acidification of serosal contents did not augment ammonia absorption, and relative alkalinization of serosal fluid caused no inhibition of ammonia transport. In the absence of bicarbonate ion, ammonia transport did not occur. The significance of these findings is discussed with consideration of both ionic and nonionic mechanisms of ammonia movement. It is suggested that ammonia is absorbed in the ileum by active ionic transport.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BESSMAN S. P., BESSMAN A. N. The cerebral and peripheral uptake of ammonia in liver disease with an hypothesis for the mechanism of hepatic coma. J Clin Invest. 1955 Apr;34(4):622–628. doi: 10.1172/JCI103111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIHLER I., CRANE R. K. Studies on the mechanism of intestinal absorption of sugars. V. The influence of several cations and anions on the active transport of sugars, in vitro, by various preparations of hamster small intestine. Biochim Biophys Acta. 1962 May 7;59:78–93. doi: 10.1016/0006-3002(62)90699-6. [DOI] [PubMed] [Google Scholar]
- BROMBERG P. A., ROBIN E. D., FORKNER C. E., Jr The existence of ammonia in blood in vivo with observations on the significance of the NH4 plus minus NH3 system. J Clin Invest. 1960 Feb;39:332–341. doi: 10.1172/JCI104044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPERSTEIN I. L., HOGBEN C. A. Ionic transfer across the isolated frog large intestine. J Gen Physiol. 1959 Jan 20;42(3):461–473. doi: 10.1085/jgp.42.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F. Na, Cl, and water transport by rat ileum in vitro. J Gen Physiol. 1960 Jul;43:1137–1148. doi: 10.1085/jgp.43.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., SOLOMON A. K. Ion and water fluxes in the ileum of rats. J Gen Physiol. 1957 Sep 20;41(1):143–168. doi: 10.1085/jgp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON A. M., SHERLOCK S., SUMMERSKILL W. H. The treatment and prognosis of hepatic coma. Lancet. 1956 Oct 6;271(6945):689–694. doi: 10.1016/s0140-6736(56)92383-2. [DOI] [PubMed] [Google Scholar]
- EWE K., SUMMERSKILL W. H. TRANSFER OF AMMONIA IN THE HUMAN JEJUNUM. J Lab Clin Med. 1965 May;65:839–847. [PubMed] [Google Scholar]
- LAWRENCE W., Jr, JACQUEZ J. A., DIENST S. G., POPPELL J. W., RANDALL H. T., ROBERTS K. E. The effect of changes in blood pH on the plasma total ammonia level. Surgery. 1957 Jul;42(1):50-9; discussion, 60. [PubMed] [Google Scholar]
- MANNING R. T. CHEMISTRY OF AMMONIA INTOXICATION. Biochem Clin. 1964;3:225–244. [PubMed] [Google Scholar]
- MILNE M. D., SCRIBNER B. H., CRAWFORD M. A. Non-ionic diffusion and the excretion of weak acids and bases. Am J Med. 1958 May;24(5):709–729. doi: 10.1016/0002-9343(58)90376-0. [DOI] [PubMed] [Google Scholar]
- PHILLIPS G. B., SCHWARTZ R., GABUZDA G. J., Jr, DAVIDSON C. S. The syndrome of impending hepatic coma in patients with cirrhosis of the liver given certain nitrogenous substances. N Engl J Med. 1952 Aug 14;247(7):239–246. doi: 10.1056/NEJM195208142470703. [DOI] [PubMed] [Google Scholar]
- SELIGSON D., HIRAHARA K. The measurement of ammonia in whole blood, erythrocytes, and plasma. J Lab Clin Med. 1957 Jun;49(6):962–974. [PubMed] [Google Scholar]
- SHORE P. A., BRODIE B. B., HOGBEN C. A. The gastric secretion of drugs: a pH partition hypothesis. J Pharmacol Exp Ther. 1957 Mar;119(3):361–369. [PubMed] [Google Scholar]
- SILEN W., HARPER H. A., MAWDSLEY D. L., WEIRICH W. L. Effect of antibacterial agents on ammonia production within the intestine. Proc Soc Exp Biol Med. 1955 Jan;88(1):138–140. doi: 10.3181/00379727-88-21516. [DOI] [PubMed] [Google Scholar]
- SUMMERSKILL W. H., WOLFE S. J., DAVIDSON C. S. The management of hepatic coma in relation to protein withdrawal and certain specific measures. Am J Med. 1957 Jul;23(1):59–76. doi: 10.1016/0002-9343(57)90358-3. [DOI] [PubMed] [Google Scholar]
- SUMMERSKILL W. H., WOLFE S. J., DAVIDSON C. S. The metabolism of ammonia and alpha-keto-acids in liver disease and hepatic coma. J Clin Invest. 1957 Mar;36(3):361–372. doi: 10.1172/JCI103432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartorius O. W., Roemmelt J. C., Pitts R. F., Calhoon D., Miner P. THE RENAL REGULATION OF ACID-BASE BALANCE IN MAN. IV. THE NATURE OF THE RENAL COMPENSATIONS IN AMMONIUM CHLORIDE ACIDOSIS. J Clin Invest. 1949 May;28(3):423–439. doi: 10.1172/JCI102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN K. S., NATHAN D. G. The passage of ammonia across the blood-brain-barrier and its relation to blood pH. J Clin Invest. 1958 Dec;37(12):1724–1728. doi: 10.1172/JCI103764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBSTER L. T., Jr, DAVIDSON C. S., GABUZDA G. J. Effect on portal blood ammonium of administering nitrogenous substances to patients with chronic hepatic disease. J Lab Clin Med. 1958 Oct;52(4):501–514. [PubMed] [Google Scholar]
- WHITE L. P., PHEAR E. A., SUMMERSKILL W. H., SHERLOCK S. Ammonium tolerance in liver disease: observations based on catheterization of the hepatic veins. J Clin Invest. 1955 Feb;34(2):158–168. doi: 10.1172/JCI103070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON T. H., KAZYAK L. Acid-base changes across the wall of hamster and rat intestine. Biochim Biophys Acta. 1957 Apr;24(1):124–132. doi: 10.1016/0006-3002(57)90154-3. [DOI] [PubMed] [Google Scholar]
- WILSON T. H., WISEMAN G. The use of sacs of everted small intestine for the study of the transference of substances from the mucosal to the serosal surface. J Physiol. 1954 Jan;123(1):116–125. doi: 10.1113/jphysiol.1954.sp005036. [DOI] [PMC free article] [PubMed] [Google Scholar]


