Abstract
Coenzyme F420 is a deazaflavin hydride carrier with a lower reduction potential than most flavins. In Mycobacterium tuberculosis (Mtb), F420 plays an important role in activating PA-824, an antituberculosis drug currently used in clinical trials. Although F420 is important to Mtb redox metabolism, little is known about the enzymes that bind F420 and the reactions that they catalyze. We have identified a novel F420-binding protein, Rv1155, which is annotated in the Mtb genome sequence as a putative flavin mononucleotide (FMN)-binding protein. Using biophysical techniques, we have demonstrated that instead of binding FMN or other flavins, Rv1155 binds coenzyme F420. The crystal structure of the complex of Rv1155 and F420 reveals one F420 molecule bound to each monomer of the Rv1155 dimer. Structural, biophysical, and bioinformatic analyses of the Rv1155–F420 complex provide clues about its role in the bacterium.
Keywords: Mycobacterium tuberculosis, Rv1155, coenzyme F420, conserved hypothetical protein
Introduction
The causative bacterium of tuberculosis (TB), Mycobacterium tuberculosis (Mtb), is among the most widely distributed human pathogens, with an estimated one-third of the world population having evidence of infection by the bacterium.1 Drug-resistant strains of Mtb have emerged and pose a significant threat to the control of TB worldwide.2 New antibiotics are urgently needed to stem the spread of drug-resistant Mtb.3 TB drug design strategies can benefit from taking advantage of biochemical processes present in Mtb, but absent in human cells. One rich area is the biochemistry of coenzyme F420 or 7,8-didesmethyl-8-hydroxy 5-deazaflavin (Fig. 1), which is found in mycobacteria but not in humans. Coenzyme F420 is involved in the activation of PA-824,6 a prodrug that is currently used in human clinical trials for the treatment of tuberculosis.7
Figure 1.
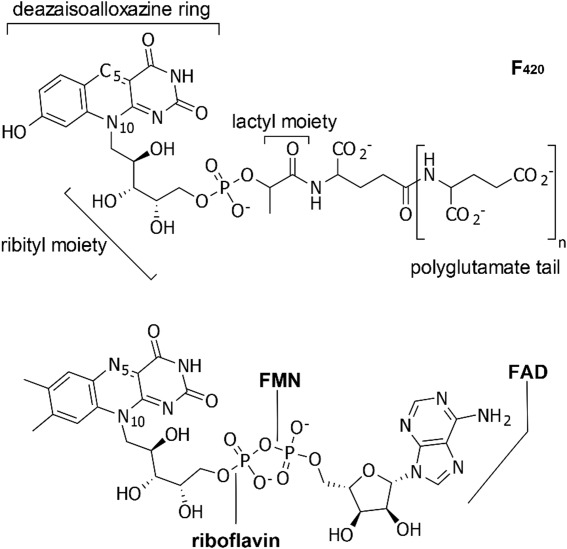
Coenzyme F420 compared to the structures of three other flavins, riboflavin, FMN (flavin mononucleotide), and FAD (flavin adenine dinucleotide). Oxidized F420 (upper) is shown. The predominant F420 isoforms observed in mycobacteria have polyglutamate tails consisting of glutamates (n = 5 or 6) that are linked by γ-glutamyl bonds.4,5 In the lower panel are shown the oxidized structures of riboflavin, FMN, and FAD.
Coenzyme F420 was first observed as a bright yellow and highly fluorescent pigment in mycobacteria in the early 1960s8,9 and was chemically identified after its purification from methanogenic Archaea.4 It is now known that F420 is present in organisms from all three domains of life, archaea, bacteria, and eukaryotes, but it is relatively rare in nature overall. F420 shares some structural similarities with more common flavins, such as riboflavin, flavin mononucleotide (FMN), and flavin adenine dinucleotide (FAD). However, the tricyclic ring system in F420 differs from that of FMN and FAD in having a central carbon atom, C5, at position 5 instead of a nitrogen atom as found in the other flavins (Fig. 1). For this reason, the F420 ring system is referred to as a deazaisoalloxazine ring, and its ground-state reactivity typically involves hydride transfer to or from this site rather than the usual single electron chemistry of flavins.10 Other differences from more familiar flavins occur beyond the phosphate moiety, where F420 has a lactyl group and polyglutamate tail using γ-glutamyl linkages (Fig. 1). Coenzyme F420 has a lower reduction potential (E° ≈ −360 mV)11 than most flavins and it has been suggested that this redox chemistry may help the bacterium survive in anaerobic conditions existing in human lesions.12
Little is known about the enzymes in Mtb that require coenzyme F420 for catalysis. A comparative genomics study of Mtb suggests that there are at least 28 unique F420-binding enzymes in Mtb that can be classified into three families: the deazaflavin-dependent nitroreductases (DDN), the luciferase-like monooxygenases (LLM), and the pyridoxine 5′-phosphate oxidases (PNPOx).13 All three classes have homologs in other organisms that are known to bind either FMN or FAD. Examples from two of the classes have been studied. In the DDN family, Rv3547, also known as Ddn, is an F420-dependent enzyme that catalyzes hydride transfer from the reduced coenzyme, F420H2, to the antituberculosis drug PA-824.14 In the LLM family, the F420-dependent glucose 6-phosphate dehydrogenase 1 (FGD1, Rv0407) catalyzes the oxidation of glucose 6-phosphate and produces F420H2.15,16 At present, it appears that the only studies on F420-dependent enzymes in Mtb are those on Ddn and FGD1, as no other F420-dependent enzymes have been identified and characterized experimentally.
The PNPOx family in Mtb includes the gene products Rv1155, Rv2991, Rv2607, Rv0121c, Rv2061c, Rv1875, Rv2074, and Rv3369. All members of this class are annotated in the Mtb genome as conserved hypothetical FMN-binding enzymes. We previously demonstrated that Rv2607 functions as a canonical PNPOx, which catalyzes the oxidation of pyridoxine 5′-phosphate into the bioactive pyridoxal 5′-phosphate (PLP).17 The cofactor requirements of other Mtb proteins in the PNPOx-like class have not been experimentally confirmed; although crystal structures have been determined of Rv1155 without bound FMN18 and with bound FMN,19 it has remained unclear if Rv1155 binds FMN outside of a crystal environment.
Using biophysical techniques, we report here that Rv1155 binds coenzyme F420 in solution, but does not bind FMN. In order to characterize the Rv1155–F420 interaction, we determined the crystal structure of Rv1155 bound to F420, which revealed how F420 is bound and the importance of its polyglutamate tail in coenzyme recognition.
Results
Rv1155 does not co-purify with bound flavins
Two of the Mtb PNPOx family members, Rv1155 and Rv2607, were individually expressed in Escherichia coli. Both Rv1155 and Rv2607 were identified as homodimers by size exclusion chromatography (SEC). We have previously shown by mass spectrometry that Rv2607 as isolated from E. coli already contains bound FMN.17 The Rv2607–FMN complex has a UV–visible absorbance profile that is typical of an FMN-binding protein, which is characterized by two peaks in the ∼350–500 nm region (Fig. 2). By contrast, Rv1155 lacks absorbance in this region, indicating that it does not co-purify with a flavin cofactor. As genes coding for enzymes involved in F420 synthesis are absent in the E. coli genome, neither Rv2607 nor Rv1155 would be expected to co-purify with F420.
Figure 2.
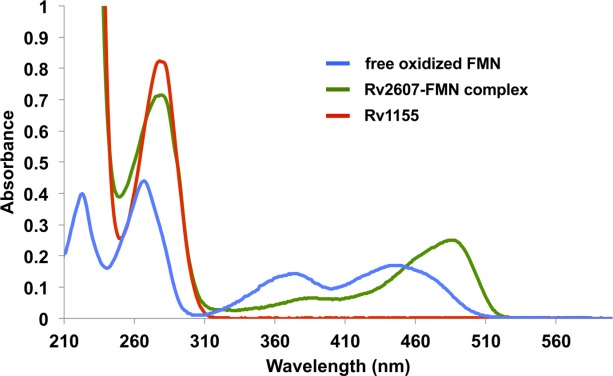
UV–visible absorbance spectra of Rv1155 and Rv2607, members of the Mtb PNPOx family, show that Rv1155 lacks the absorbance profile characteristic of an FMN-binding protein. The UV–visible spectrum of free, oxidized FMN (blue trace) has two peaks in ∼350–500 nm region. The UV–visible spectrum of recombinant Rv2607 (green trace) has two peaks in the same part of the spectrum, which is consistent with Rv2607 being a known FMN-binding protein. Rv1155 (red trace) lacks absorbance in this region of the spectrum, indicating that it does not co-purify with a flavin when expressed recombinantly in E. coli. Free oxidized FMN, Rv2607-FMN, and Rv1155 are present at 30 μM.
Rv1155 binds coenzyme F420
Several redox cofactors were tested for binding to Rv1155 using differential scanning fluorimetry (DSF), including F420, FMN, FAD, and nicotinamide adenine dinucleotide phosphate (NADPH) (Fig. 3). The intensity of the dye fluorescence versus temperature curve for Rv1155 has a stable baseline and sharp transition [Fig. 3(A)], making Rv1155 well suited for analysis using the DSF technique.20 In the absence of cofactor, the melting temperature (Tm) of Rv1155 is 51.9°C [Fig. 3(A), blue trace]. In the presence of F420 (50 µM), the Tm of Rv1155 increased by 5.3° [Fig. 3(A), red trace]. This higher melting temperature indicates that F420 binding to Rv1155 increases the free energy of protein unfolding and thus renders Rv1155 resistant to denaturation at higher temperatures. The Tm of Rv1155 did not increase significantly in the presence of FMN, FAD, or NADPH [Fig. 3(B)], indicating that these cofactors do not similarly stabilize this protein.
Figure 3.
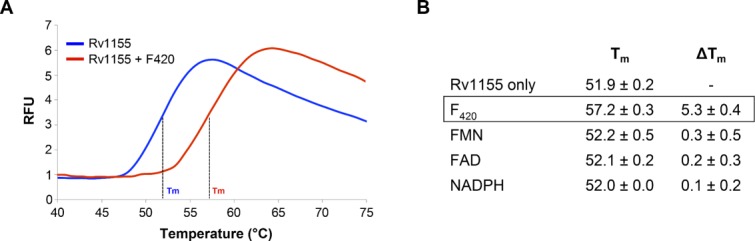
Rv1155 binds coenzyme F420 as assessed by differential scanning fluorimetry (DSF). (A) DSF melt curves in the presence of F420 (red trace) and in the absence of F420 (blue trace). Melt curves for FMN, FAD, and NADPH (not shown) were almost identical to the Rv1155 curve in the absence of F420. (B) Tm values for Rv1155 in the presence of 50 μM F420, FMN, FAD, and NADPH. The change in Tm (ΔTm) was calculated relative to the Rv1155 only sample. Each measurement was made in triplicate. RFU, relative fluorescence units.
The Rv1155–F420 binding interaction was further characterized by isothermal titration calorimetry (ITC) by titrating F420 into a 50 μM solution of Rv1155 (Fig. 4). Concentrations of Rv1155 >50 μM resulted in significant aggregation of the protein as determined by dynamic light scattering. Fitting the ITC data to a one binding site model yielded a KD of 170 μM for F420 binding to Rv1155. The measured binding affinity of F420 for Rv1155 is lower than that reported for the binding of F420 to other F420-dependent proteins in Mtb (Ddn: 1 μM,15 FGD1: 4.5 μM21).
Figure 4.
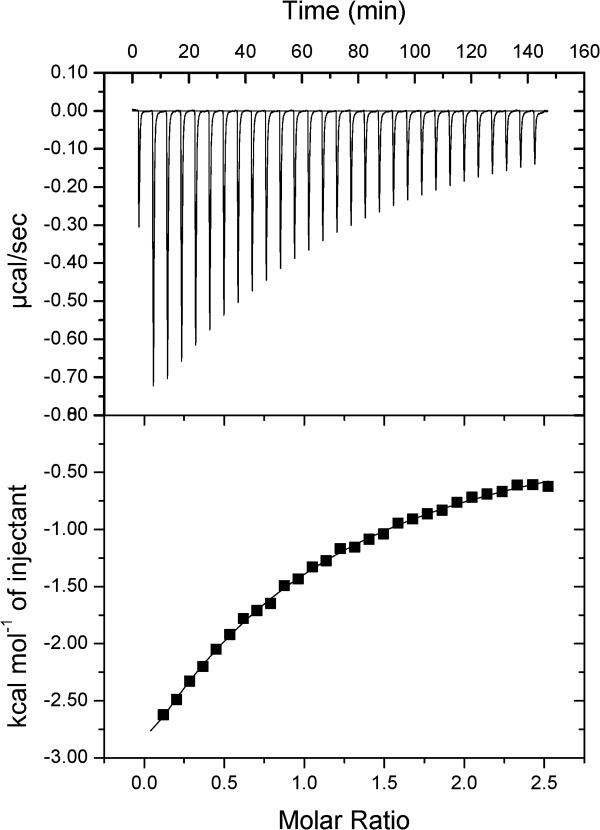
Isothermal titration calorimetry reveals the binding of F420 to Rv1155. The ligand, F420 (880 μM), was titrated into a solution of the protein, Rv1155 (50 μM as monomer). The top panel shows the ITC thermogram of the raw titration data that were measured for each injection of F420. The data points in the bottom panel form the binding isotherm, in which each integrated peak area in units of kcal/mol is plotted against the ratio of the number of moles F420 that were injected to the number of moles of Rv115 present in the experiment. The solid line represents the best fit of the isotherm data to a one-binding-site model, which yielded a KD value of 170 μM for the binding of F420 to Rv1155.
Trial enzymatic reactions with Rv1155 and F420
Enzymatic test reactions were performed with Rv1155 in the presence of either reduced or oxidized coenzyme F420. To detect a redox reaction mediated by Rv1155 and F420, the oxidation state of F420 was monitored by absorbance at 420 nm. Oxidized F420 has a strong absorbance at 420 nm, but reduced F420 (F420H2) exhibits weak absorbance in this region. No change in the absorbance of oxidized or reduced F420 was observed in the presence of Rv1155 and pyridoxine 5′-phosphate, pyridoxamine 5′-phosphate, or PLP, indicating that Rv1155–F420 does not bind pyridoxine 5′-phosphate or its analogs as substrates. This is unlike other members in the PNPOx class that catalyze the oxidation of pyridoxine 5′-phosphate, including Rv2607 from Mtb.17 In the presence of reduced F420, Rv1155 also does not metabolize the anti-TB drug PA-824, unlike the F420-dependent nitroreductase Ddn, which does activate PA-824.14 MSMEG_5170, an ortholog of Rv1155 in Mycobacterium smegmatis with 79% sequence identity to Rv1155, carries out the reduction of α,β-unsaturated esters in aflatoxins in the presence of F420H2.22 However, we did not detect a change in the absorbance of reduced F420 with Rv1155 in the presence of the aflatoxin analogs, coumarin, 7-hydroxycoumarin, 7-hydroxy-4-methylcoumarin, or imperatorin.
Structure of Rv1155 with bound F420
X-ray datasets were obtained from yellow crystals of the Rv1155–F420 complex, which diffracted X-rays to resolutions of 1.9–2.8 Å. An initial electron density map was obtained by molecular replacement. The Rv1155–F420 structure was refined at 2.3 Å resolution resulting in R-factors of 0.25 (Rfree) and 0.19 (Rwork) (Table1). One Rv1155 homodimer was present in the asymmetric unit of the Rv1155–F420 crystals. There was continuous electron density in monomer A from Gln4A to Pro144A and in monomer B from Gln4B to Arg147B. At the interface between the monomers and near the surface of the dimer, there was difference electron density at two sites that enabled the unambiguous placement of two molecules of F420 [Fig. 5(A)]. For the molecule of F420 in binding site 1, we observed electron density for the deazaisoalloxazine ring system, the ribityl moiety, the phosphate, the lactyl moiety, and one residue of the polyglutamate tail [Fig. 5(B)]. For the F420 molecule in binding site 2, we did not see electron density for the deazaisoalloxazine ring system, but as in binding site 1, we observed difference electron density for the ribityl moiety, the phosphate, the lactyl moiety, and two glutamate residues out of the five or six residues in the polyglutamate tail of F420 [Fig. 5(C)]. Electron density was not observed in either site for the third glutamate and additional glutamates of the polyglutamate tail as they were likely disordered in the crystal.
Table 1.
Rv1155 X-Ray Data and Refinement Statistics
| X-ray data | |
| X-ray source | APS 22-BM |
| Resolution limit (Å) | 49–2.30 (2.36–2.30) |
| Space group | P212121 |
| Unit cell dimensions (Å) | 53.6, 65.2, 77.1 |
| Completeness (%) | 99.8 (100) |
| Multiplicity | 7.0 |
| Wilson B-factor (Å2) | 45 |
| Rmerge (%) | 3.7 (25.0) |
| <I/σ(I)> | 33.6 (8.5) |
| Refinement | |
| Resolution limits (Å) | 33–2.30 (2.38–2.30) |
| No. of reflections in working set | 12,470 |
| No. of reflections in test set | 634 |
| Rwork (%) | 18.6 (22.1) |
| Rfree (%) | 25.2 (34.6) |
| Number of atoms | |
| Protein; H2O; F420 and other | 2206; 59; 138 |
| Geometry r.m.s. deviations | |
| Bond lengths (Å) | 0.008 |
| Bond angles (°) | 1.13 |
| B-factors (Å2) | |
| Monomer A | 37.3 |
| Monomer B | 35.6 |
| H2O | 37.8 |
| F420 and other atoms | 49.7 |
Figure 5.
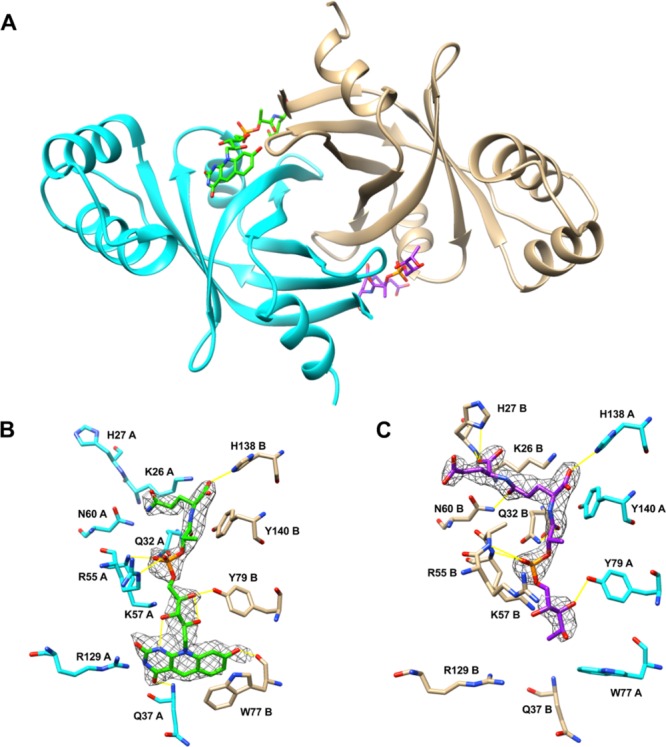
Crystal structure of Rv1155 bound to F420 at 2.3 Å resolution. (A) Structure of the Rv1155 homodimer with two F420 molecules (green, purple) bound at the interface between chain A (cyan) and chain B (tan). (B) Detailed view of binding site 1. (C) Detailed view of binding site 2. Electron density for the ring system of F420 is not visible in binding site 2 (see text). The 2Fo–Fc electron density for F420 is contoured at 1.0σ. Hydrogen bonds are represented as yellow lines.
Each F420 binding site is formed by residues from both Rv1155 monomers. The negative charge of the phosphate group and the first two residues of the polyglutamate tail of F420 are accommodated by a tunnel of positively charged and polar residues at the dimer interface. The portion of the polyglutamate tail visible in the structure makes hydrogen-bonding interactions with His27, Asn60, and His138 (Fig. 5). Several additional basic residues that could interact with additional glutamates in the polyglutamate tail are Arg30, Arg59, Arg63, and Arg66. The lactyl moiety of F420 forms a hydrogen bond with Gln32 and the phosphate is held in place via hydrogen bonds to Arg55 and Lys57. The ribityl moiety interacts with Tyr79, hydrogen bonds with Lys57, and is stabilized by intra-F420 hydrogen bonds. The deazaisoalloxazine ring system forms hydrogen bonds with main chain atoms of Gln37 and Trp77. A stacking interaction occurs between the side chain of Trp77 and the benzene ring portion of the deazaflavin.
Even though the two coenzyme binding sites in Rv1155 are identical, there was little electron density for the deazaisoalloxazine ring of F420 in binding site 2. The positions of the Trp77 and Arg55 side chains in binding site 2 suggest that the F420 ring system in binding site 2 may be missing or disordered [compare Fig. 5(B) and 5(C)]. Rv1155–F420 crystals that were transferred to a cryoprotectant solution that was not supplemented with F420 yielded structures with a bound F420 in binding site 2, but without a F420 in binding site 1. This observation indicates that the incomplete F420 in binding site 2 may have co-crystallized with the protein, while the F420 in binding site 1 may have soaked into its binding site.
The electron density for F420 in binding site 1 clearly demonstrates that the ring system assumes a conformation in which the two outer rings are bent relative to the central ring containing C5 [Fig. 6(B)]. This “butterfly” conformation is commonly seen in protein-bound flavins.24 The butterfly angle of the coenzyme F420 in the structure was determined to be 16.5° by measuring the angle between the mean planes of the central and benzene rings.25
Figure 6.
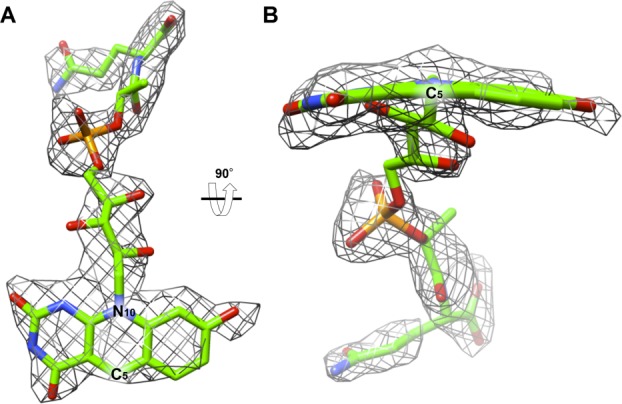
The 2Fo–Fc omit electron density of the F420 in the crystal structure of Rv1155 contoured at 1.0σ. (A) F420 modeled into the density found in the binding site 1 viewed from the interface of chains A and B. (B) F420 as viewed after 90° rotation around the horizontal axis relative to panel A. The density indicates that the ring assumes a butterfly conformation, meaning the ring system is not fully planar, but that the pyrimidine and benzene rings of the deazaisoalloxazine ring system are bent below the central ring, which contains C5 and N10. The butterfly angle is 16.5°, defined as the angle between the plane of the pyrimidine ring and the plane of the benzene ring. The angle was measured using Chimera.23
A substrate molecule that can be oxidized or reduced by F420 would likely bind near the deazaisoalloxazine ring system where hydride transfer is known to occur in other proteins. Adjacent to the ring system in the structure there is a pocket lined with polar and hydrophobic residues where a substrate may bind (Fig. 7). The volume of the pocket is 130 Å3 as calculated using Computed Atlas of Surface Topography of proteins server (CASTp).26 This pocket is formed by the small domain of the PNPOx-like fold, which is the known binding site of substrates PNP and PMP in the E. coli27 and human28 PNPOx enzymes.
Figure 7.
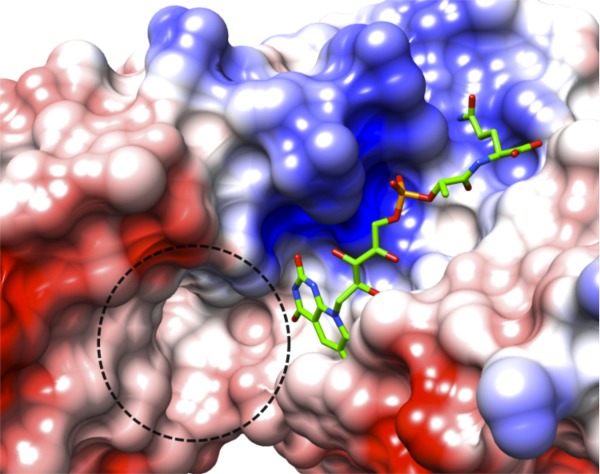
Putative active site in Rv1155–F420. Surface representation of the Rv1155 colored by electrostatic potential (red, negative; blue, positive; white, neutral, which corresponds to areas occupied by polar or hydrophobic residues). The dashed line encircles a putative substrate-binding pocket near the F420 deazaisoalloxazine ring system, which has a volume of 130 Å3.
Comparison of Rv1155–F420 complex with other structures
Rv1155–F420 was compared with all other structures deposited in the PDB using the distance alignment matrix method (Dali).29 The PDB entries with the greatest global-fold similarity to Rv1155 are predominantly other PNPOx-like proteins of unknown function. The highest scoring hits were the two PNPOx-like proteins, Rv2991 and Rv2074 from Mtb, and one protein, MSMEG_3380, which is the M. smegmatis ortholog of Rv2074. The root-mean-square (r.m.s.) deviations on α-carbons of Rv2991 (PDBID: 1RFE), Rv2074 (PDBID: 2ASF30), and MSMEG_3380 (PDBID: 3F7E22) relative to Rv1155–F420 are 2.7, 2.5, and 2.6 Å, respectively. The available X-ray crystal structures of Rv2991, Rv2074, and MSMEG_3380 have no bound cofactors or substrates. We superimposed the crystal structure of Rv1155–F420 with those of Rv2991, Rv2074, and MSMEG_3380 to compare the F420 binding site in Rv1155 to the analogous sites in the three other mycobacterial PNPOx-like proteins. This analysis revealed that most of the residues in Rv1155 that interact with F420 [shown in Fig. 5(A,B)] are conserved in Rv2991, Rv2074, and MSMEG_3380. For example, Rv1155 residues Q32A, K57A, N60A, Y79B, R129A, and H138B are mostly conserved in Rv2991, Rv2074, and MSMEG_3380 (Table2). Based on the structural similarities that Rv2991 and Rv2074 share with Rv1155, we propose that Rv2991 and Rv2074 also bind coenzyme F420. MSMEG_3380 is known to bind F420H2 and to use it to reduce alfatoxins.22 From its comparison with the Rv1155 structure, MSMEG_3380 likely binds F420 at an analogous site. Crystal structures are not available for Rv1875 and Rv0121c, but sequence alignments indicate that they also bind F420 (Table2).
Table 2.
Other PNPOx-Like Proteins in Mycobacteria Have Analogous Amino Acid Residues Positioned Similarly to Those of Rv1155 That Interact with the Coenzyme and May Also Bind F420
| F420 moieties | ||||||
|---|---|---|---|---|---|---|
| Protein | Lactyl | Ribityl, P | PolyGlu | Ribityl, P | Ring system | Lactyl, polyGlu |
| Rv1155 | Q32A | K57A | N60A | Y79B | R129A | H138B |
| Rv2991a | H38A | K61A | N64A | G86B | R132A | R141B |
| Rv2074a | H36A | K61A | N60A | W81B | R117A | R126B |
| MSMEG_3380a | Q30A | K53A | N56A | Y76B | A125B | R80B |
| Rv1875b | Q30 | K58 | N61 | W78 | R133 | R142 |
| Rv0121cb | H28 | R66 | N69 | W92 | G129 | R138 |
Analogous residues identified by structural superposition with Rv1155.
Analogous residues identified by sequence alignment with Rv1155.
Metabolic pathway analysis for Rv1155
Proteomic investigation has shown that Rv1155 is found in the membrane fraction of Mtb cell lysates.31 There is no signal sequence in Rv1155 to target it to the membrane, nor does it have any predicted transmembrane regions, amphipathic membrane anchors, or lipidation sites. In order to identify possible interaction partners that can localize Rv1155 to the membrane, a metabolic pathway analysis was performed on 1133 genomes using the Search Tool for Retrieval of Interacting Genes/Proteins (STRING) database32 (http://string-db.org/). One way to predict functional pathways is to compare gene co-occurrence across many genomes, that is, to identify genes that tend to be either present or absent together because they form a functional unit in the cell.33 Of the 10 genes identified that co-occur with Rv1155, seven are also found in the membrane fraction of Mtb cells,31 including Ddn, a Ddn homolog Rv1558, and PNPOx-like proteins Rv2074 and Rv2061c (Supporting Information Table1). Like Rv1155, none of the co-occurring gene products contain predicted signal peptides or transmembrane regions.
Discussion
Rv1155 is a member of a class of proteins that are related to one another by a common domain type classified as “PNPOx-like” in the Pfam database34 (Pfam ID: Pyridox_oxidase, PF01243). Selengut and Haft used computational techniques to identify potential F420-dependent protein families in Mtb and to distinguish proteins that bind F420 from those that recognize FMN.13 This approach pointed to Rv1155 as an F420-binding protein along with other PNPOx-like proteins in Mtb (Rv2991, Rv0121c, Rv2061c, Rv1875, Rv2074, and Rv3369). Our data provide experimental evidence for the use of F420 by Rv1155. The remaining PNPOx-like proteins in Mtb may constitute a novel class of F420-binding proteins.
The differential scanning fluorimetry assay demonstrated that the Tm of Rv1155 remains unchanged in the presence and absence of FMN, showing that FMN does not bind Rv1155. This result is consistent with the absorbance profile of pure recombinant Rv1155, which indicates that it does not co-purify with FMN when expressed in E. coli. These findings corroborate previous observations that Rv1155 does not behave as a canonical FMN-dependent enzyme,18 but they are conflicting with the reported structures of Rv1155–FMN (PDBID: 1Y3019) and Rv1155–PLP (PDBID: 2AQ619). We suggest that the crystal structure of Rv1155 bound to FMN may be the result of weak FMN binding that is stabilized by the crystal lattice. Comparison of the Rv1155–FMN and Rv1155–F420 structures shows that the phosphate moieties in FMN and F420 bind at nearly the same site where they form hydrogen bonds with Arg55 and Lys57 (Supporting Information Fig. 1A). The ribityl moieties, which are identical in FMN and F420, make the same hydrogen bond with Tyr79 in Rv1155. A structural superimposition of Rv1155–FMN and Rv1155–PLP showed that simultaneous binding of FMN and PLP is improbable because the molecules would occupy the same space in the binding site (Supporting Information Fig. 1B). In addition, FMN and PLP are found in only one of the two binding sites per Rv1155 homodimer.19
The present crystallographic data reveal that F420 binds to Rv1155 at the homodimer interface on the surface of the protein. F420 is accommodated by a cleft in the protein lined with basic residues that can form hydrogen bonds with the polyglutamate tail and the phosphate of F420. The binding mode of F420 in Rv1155 is similar to that of F420 bound to Ddn (Supporting Information Fig. 2). Rv1155 and Ddn also share some global-fold similarities, including a beta-barrel core and a small, alpha helical domain, although the two proteins have only 12% sequence identity.
The butterfly bend angle of the flavin tricyclic ring system is related to its redox properties. The protein environment mediates the extent of ring puckering, which allows for fine-tuning of the reduction potential required to carry out its biological function.25 The butterfly bend angle of F420 in the structure is 16.5°. This value falls within the range of bend angles measured in other F420-bound structures deposited in the Protein Databank: 13.9° (PDBID: 1JAY35), 15.0° (PDBID: 3IQE36), 20.4° (PDBID: 3B4Y15), 31.7° (PDBID: 1RHC37), and 52.7° (PDBID: 1Z6938). Flavins with larger bend angles tend to have lower reduction potentials.25 Larger flavin butterfly bend angles are also associated with a less stable semiquinone (one electron) intermediate.25 The semiquinone state is not observed in deazaflavins like F420 because the radical anion at C5 is too unstable.11 F420 is able to carry out low reduction potential reactions inaccessible by other flavins such as FMN and FAD.11
The butterfly bend angle of the deazaisoalloxazine ring in the current structure provides some insight into the enzymatic properties of this enzyme–coenzyme system. Oxidized flavins tend to be more planar than the one- and two-electron reduced forms. The butterfly bend angle observed for F420 bound to Rv1155 is similar to that (20.4°) of F420-dependent glucose 6′-phosphate dehydrogenase from Mtb (PDBID: 3B4Y15), which catalyzes the reduction of F420, and to that (15.0°) of F420-dependent methylene-tetrahydromethanopterin dehydrogenase (PDBID: 3IQE36) from Methanopyrus kandleri, which is capable of reversibly transferring a hydride using F420 as the carrier. Rv1155 may exhibit similar redox properties to these F420-dependent systems in which F420 can be used as an oxidizing agent.
The precise enzymatic reaction catalyzed by Rv1155 remains unknown. Trial enzymatic reactions conducted with Rv1155 and reduced or oxidized F420 have ruled out the possibility that Rv1155 can function as a PNPOx, a Ddn, or an enzyme capable of aflatoxin degradation. Adjacent to the F420-binding pocket in Rv1155 is a hydrophobic cavity measuring 130 Å3 (Fig. 7). The pocket may be the site of substrate binding as it is located near the F420 deazaisoalloxazine ring system responsible for redox chemistry. This putative substrate-binding space is similar in size to that of the F420-dependent secondary alcohol dehydrogenase (128 Å3), which binds isopropanol (PDBID: 1RHC37). Alternatively, this location may be where a partner protein may bind that would use the redox properties of the bound F420.
Rv1155 is found in the membrane fraction of Mtb cell lysates,31 but analysis of its primary sequence reveals that it lacks features that target it to the membrane. In order to explore potential functional roles for Rv1155, we used the STRING database to predict other gene products in Mtb that may participate in the same pathway as Rv1155. Some of the predicted pathway members may physically interact with Rv1155, particularly if they are located in the same subcellular region. The gene co-occurrence analysis on Rv1155 identified seven gene products that are experimentally confirmed membrane-associated proteins (Supporting Information Table1). It is possible that Rv1155 forms a complex with one or more membrane-associated proteins, which would tether it to the membrane and allow it to carry out its function in the bacterium.
The gene product Rv1155 was annotated in the Mtb genome as an FMN-binding protein belonging to the PNPOx family based on sequence comparison and previous structural analysis. We have provided experimental evidence showing that Rv1155 does not bind FMN nor does it exhibit canonical PNPOx activity, that is, oxidation of pyridoxine 5′-phosphate or pyridoxamine 5′-phosphate to produce PLP. Using biophysical and crystallographic methods, we have shown that Rv1155 instead binds to a redox deazaflavin coenzyme found in mycobacteria called F420, and thus Rv1155 takes its place beside Ddn and FGD1 as one of a small number of experimentally validated F420-binding proteins in Mtb.
Materials and Methods
Production and purification of F420
The protocol for F420 purification was developed by modifying existing procedures.39,40 M. smegmatis cells were transformed with genes fbiA, fbiB, and fbiC, which were obtained in a pYUBDuet vector from Prof. Edward N. Baker (University of Auckland, New Zealand). The genes fbiA, fbiB, and fbiC encode enzymes involved in the biosynthesis of F420.41 The vector was electroporated into M. smegmatis strain mc2155 as previously described.42 M. smegmatis cells overexpressing the F420 biosynthetic genes were grown in kilogram quantities in fermenters in Luria broth (LB) broth. When the culture reached an OD600nm of 0.6, IPTG was added to a final concentration of 0.25 mM and further incubated for 2 days. For one round of purification, typically around 800 g of M. smegmatis transformed with fbiABC was thawed at 4°C and resuspended in a minimal volume of 25 mM potassium phosphate buffer pH 7.5, avoiding large clumps of cells (∼1.6 L). The suspension was autoclaved (121°C) for 20 min, cooled at 4°C, and centrifuged at 40,000g for 50 min. The bright yellow supernatant was filtered with a 0.45-μm pore membrane filter (Millipore). The cell pellet was again resuspended in a minimal volume of 25 mM potassium phosphate buffer, pH 7.5 (∼1 L) and the mixture was again autoclaved, centrifuged, and filtered as before.
The supernatants from both autoclave cycles were pooled and loaded onto a quaternary aminoethyl anion exchange column equilibrated with 25 mM potassium phosphate buffer pH 7.5 at a flow rate of 0.5 mL/min at 4°C. The column was then washed at a constant flow rate of 1 mL/min with 25 mM potassium phosphate buffer pH 7.5 containing increasing concentrations of NaCl. The column was first washed with 100 mM NaCl in potassium phosphate buffer (2 L). A precursor to F420, F0, was eluted with 250 mM NaCl in potassium phosphate buffer (2 L). The column was further washed with 400 mM NaCl (2 L) in potassium phosphate buffer. F420 eluted from the column with 600 mM NaCl (2 L) potassium phosphate buffer. The eluate was monitored spectrophotometrically at λ = 420 nm and 300 or 500 mL fractions were collected. Each fraction was analyzed by LC-MS, which detected F420 species with five glutamates, F420−5 (m/z: 1161), and with six glutamates, F420−6 (m/z: 1290). Under these conditions, M. smegmatis cells transformed with the vector containing fbiABC have about five times higher F420 production levels than do wild-type M. smegmatis cells.
The fractions containing F420 were removed from the high salt buffer using solid-phase extraction (SPE) C18 cartridges according to the manufacturer's protocol (Waters SEP-Pak Long). Conductivity measurements indicated that the SPE cartridges removed 97% of salts in the sample. The desalted F420 fractions were lyophilized and then dissolved in a minimal volume of water. The F420 was further purified by reverse-phase HPLC on a Varian Prep Star instrument using a Phenomenex Luna C18 (250 × 21.20 mm2, 10 µm) column. Solution A was 0.1% formic acid in water and solution B was acetonitrile. The column was equilibrated with 95% solution A and 5% solution B for 2 min. The following linear elution gradients were used: 95–80% solution A and 5–20% solution B from 2 to 32 min, then 80–5% solution A and 20–95% solution B from 32 to 35 min, and 5% solution A and 95% solution B for 5 min. The fractions containing F420 were pooled, lyophilized, and dissolved in a minimal volume of water. The concentration of F420 was determined with the extinction coefficient43 at 420 nm, which is 25.9 mM−1 cm−1. The purity of F420 was assessed by LC-MS. Yield was determined to be 104 mg of F420 per 800 g M. smegmatis cells overexpressing the F420 biosynthetic genes.
Expression and purification of Rv1155
The Rv1155 bacterial expression plasmid was obtained as an amino terminal 6x-histidine (His6x)-maltose-binding protein (MBP) fusion construct with TEV protease cleavage site from Dr. Yves Bourne (Architecture et Fonction des Macromolécules Biologiques, CNRS, Marseille, France).18 The Rv1155 construct included an ampicillin resistance gene and was transformed into E. coli BL21(DE3) cells. An overnight culture of a single colony was diluted 1:100 into 2 L LB that was supplemented with 100 µg/mL ampicillin. The culture was incubated with shaking at 37°C and when an OD600nm of 0.4 – 0.6 was reached, protein expression was induced with the addition of IPTG to a final concentration of 1 mM. The culture was further incubated at 25°C for 16 h with agitation. The cells were then harvested by centrifugation (15 min, 14,000g), resuspended in equilibration buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM imidazole), and subjected to sonication on ice. The lysate was cleared by centrifugation (50 min, 10,000g) and the supernatant was loaded onto HiTrap HP nickel affinity column 5 mL (GE Healthcare) pre-equilibrated with equilibration buffer. The column was then washed with equilibration buffer (20× column volume). The His6x-MBP-Rv1155 fusion protein was released from the column with elution buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 300 mM imidazole). The eluted protein was exchanged into a buffer optimized for the TEV protease cleavage reaction (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.5 mM EDTA, 1 mM DTT) using 10 kDa cutoff centrifugation filters (Millipore). The tag was efficiently cleaved by incubation with TEV protease (1:100 molar ratio) at 25°C for 2 h. The His6x-TEV and any uncleaved His6x-MBP-Rv1155 were removed from the reaction mixture by loading it onto a second HiTrap HP nickel affinity column with a 50 mL superloop (GE Healthcare). The cleaved Rv1155 without a His6x tag also bound the nickel column, but was eluted with high salt buffer (50 mM Tris-HCl, pH 7.5, 600 mM NaCl, 10 mM imidazole), leaving the His6x-tagged proteins on the nickel affinity resin. The Rv1155 that was eluted from the second HiTrap column was pooled and exchanged into equilibration buffer.
For protein to be used for crystallization experiments, F420 was added to Rv1155 (4:1 molar ratio of coenzyme to enzyme) and incubated at 4°C overnight. The complex was then purified by SEC using a Superdex-75 10/300 GL column (GE Healthcare) pre-equilibrated with 10 mM Tris-HCl, pH 7.5, 50 mM NaCl, and 50 μM F420. The eluate was monitored at two wavelengths: λ1 = 280 nm (protein) and λ2 = 420 nm (F420). The column fractions that absorbed at both 280 nm and 420 nm were analyzed by SDS–PAGE. Fractions containing pure Rv1155–F420 were pooled and concentrated to 10 mg/mL with 10 kDa cutoff centrifugation filters (Millipore). For protein to be used for ITC experiments, the pooled cleaved Rv1155 eluted from the second HiTrap column was purified by SEC with a 16/60 Superdex-75 column (GE Healthcare) pre-equilibrated with 50 mM potassium phosphate buffer, pH 7.5, 50 mM NaCl. Fractions containing pure Rv1155 were pooled and concentrated to 10 mg/mL with 10 kDa cutoff centrifugation filters (Millipore). The yield was 10 mg of Rv1155 per 1 L of cells. Rv1155 purified by SEC was a >95% pure homodimer as assessed by SDS–PAGE of column fractions. The mass of one untagged Rv1155 monomer was determined to be 16227.0 Da by mass spectrometry, which is the calculated molecular weight of Rv1155 without the initiating methionine and with an extra glycine residue remaining after TEV cleavage.
Differential scanning fluorimetry
Samples (100 µL) contained 5 µM Rv1155, 2.5× SYPRO® Orange (Invitrogen), 100 mM potassium phosphate buffer pH 7.8, 100 mM NaCl, and either 50 μM cofactor (FMN, FAD, NADPH, or F420) or an equivalent volume of water as a negative control.20 Samples were placed in a MicroAmp® optical 96-well reaction plate and sealed with optical adhesive film (Applied Biosystems). All samples were tested in triplicate over a temperature range of 40–75°C with a ramp rate of 0.01°C/s, collecting a scan every 1 s. Fluorescence data were collected on a 7500 Real Time PCR instrument using the ROX™ filter (λex: 587 nm, λem: 607 nm). Data were analyzed using Protein Thermal Shift v1.0 software (Applied Biosystems). The Tm was determined by calculating the negative derivative of the melt curve and identifying the local minimum.
Isothermal titration calorimetry
Untagged Rv1155 (without added F420) underwent dialysis overnight at 4°C with stirring in a 10 kDa MW cutoff Slide-A-Lyzer dialysis cassette (ThermoScientific, US) against 600 mL of 100 mM potassium phosphate buffer pH 7.4 and 20 mM NaCl. F420 was dialyzed into the same buffer using a 500 Da cutoff Float-A-Lyzer dialysis cassette (Spectrum Laboratories, CA). The dialysate was retained to rinse the instrument before use. For the titration of F420 into Rv1155, 879 μM F420 and 50 μM Rv1155 were used.
All titrations were performed on a MicroCal VP-ITC calorimeter (GE Healthcare). In general, experimental parameters were as follows: thirty 10 μL injections (5 μL for first injection) with 20 s duration (10 s for the first injection), 5–7 μcal/s reference power, 120 s initial delay, and 300 s spacing. The cell temperature was held at 30°C. The titration of F420 into Rv1155 (50 μM monomer) was conducted under low c value conditions, where c = [M]t/KD and [M]t is the total protein concentration.44 To achieve a higher c value in the ITC experiment, protein concentrations >50 μM were attempted, but led to protein aggregation. Data were analyzed and figures were generated using Origin software (OriginLab Corp).
Trial enzymatic reactions
All activity assays for Rv1155 were conducted using a Varian Cary 300 Bio UV-Visible spectrophotometer in 1 cm path length quartz cuvettes (Varian). Where present, the reagents were at the following concentrations: 1 µM Rv1155, 100 µM test substrate, 20 µM F420 or F420H2. Reaction mixtures (150 µL total) contained: (1) Rv1155, substrate, and F420 or F420H2, (2) Rv1155 and substrate, (3) substrate and F420 or F420H2, (4) Rv1155 and F420 or F420H2. Test substrates were pyridoxine 5′-phosphate, pyridoxamine 5′-phosphate, PLP, PA-824, coumarin, 7-hydroxycoumarin, 7-hydroxy-4-methylcoumarin, and imperatorin. All reactions were carried out in 25 mM potassium phosphate, pH 7.5. The reactions were initiated with the addition of enzyme and a wavelength scan (200–500 nm) was collected every 2 min for 1 h.
Rv1155–F420 crystals, X-ray data, and structure determination
Crystallization trials were carried out using a Crystal Phoenix crystallization robot (Art Robbins Instruments LLC) to set up the binary complex Rv1155–F420 with the conditions from the Classics, JCSG Core I–IV, pH Clear, and PEGs Suite crystallization kits (Qiagen). A number of buffer conditions produced crystals, including 0.1M MES, 25% PEG-3350 or PEG-4000 or PEG-6000 or PEG-8000 and 0.2M sodium or potassium fluoride, 20% PEG-3350, and 0.2M sodium or potassium formate, 20% PEG-3350. Crystals grown in 0.2M sodium fluoride, 20% PEG-3350 or in 0.2M sodium formate, 20% PEG-3350 exhibited the highest resolution diffraction.
Crystallization trials with Rv1155–F420 yielded initial crystals of the Rv1155 dimer in which only one monomer contained a bound F420 molecule. Optimization studies succeeded in filling the second binding site by soaking briefly in a cryoprotectant solution with additional F420 (1 mM). All Rv1155–F420 crystals, whether there were one or two molecules of F420 bound, exhibited the same space group (P212121) with unit cell dimensions of about 54, 65, and 77 Å. Rv1155 apoenzyme crystallized in the space group P21 and had unit cell dimensions of about 47, 55, 55 Å, β = 108°, similar to other apoenzyme crystals.18,19 In the absence of F420, the crystals grew as thin plates, but in the presence of F420 the crystals appeared as tetragonal bipyramids. For X-ray data collection, Rv1155 crystals were briefly soaked in a cryoprotectant buffer consisting of 0.2M sodium formate, 20% PEG-3350, 25% glycerol, and 1 mM F420, and then were rapidly frozen in liquid nitrogen. X-ray data were collected at −180°C at beamline 22 at the Advanced Photon Source (Argonne National Laboratory, Lemont, IL). Initial phases were obtained by molecular replacement with an Rv1155 model (PDBID: 1Y3019) after removing ligands. Refinement of the structure with Crystallography and NMR System (CNS),45 Phenix,46 and model rebuilding with the O software47 resulted in an Rfree of 0.25 and an Rwork of 0.19 at 2.3 Å resolution. Structural images were produced with Chimera.23 The Rv1155–F420 coordinates and structure factors were deposited into the Protein Data Bank (http://www.rcsb.org) as PDBID 4QVB.
Metabolic pathway analysis for Rv1155
We used the Search Tool for Retrieval of Interacting Genes/Proteins (STRING) v9.148 with Rv1155 as the seed protein to identify possible functional partners using the following prediction methods: gene fusion, co-occurrence, co-expression, experiments, databases, and text mining. A high confidence combined score of 0.7 was used as a cutoff. From the top 20 hits, those co-occurring with Rv1155 were selected for further analysis including signal peptide prediction with SignalP 4.149 (http://www.cbs.dtu.dk/services/SignalP/), transmembrane prediction using the TMHMM server v2.050 (http://www.cbs.dtu.dk/services/TMHMM/), amphipathic membrane anchors using AmphipaSeeK51 (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_amphipaseek.html), and cell localization using the TubercuList database52 (http://tuberculist.epfl.ch/index.html).
Acknowledgments
The authors thank Dr. Tathagata Mukherjee (Gennova Biopharmaceuticals) for his assistance in developing the protocol to purify coenzyme F420. They also thank Dr. Marko Hyvönen (Department of Biochemistry, University of Cambridge) for useful discussions. EHM acknowledges the support of the NIH Oxford-Cambridge Scholars Program. X-ray data were collected at the Southeast Regional Collaborative Access Team (SER-CAT) 22-ID and 22-BM beam lines at the Advanced Photon Source (APS), which is supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences under contract W-31–109-Eng-38.
Glossary
- CASTp
computed atlas of surface topography of proteins server
- Dali
distance alignment matrix method
- DDN
deazaflavin-dependent nitroreductases
- DSF
differential scanning fluorimetry
- F420H2
reduced F420
- FGD1
F420-dependent glucose 6-phosphate dehydrogenase 1
- His6x
6x-histidine
- IPTG
isopropyl β-d−1-thiogalacto-pyranoside
- ITC
isothermal titration calorimetry
- LB
Luria broth
- LLM
luciferase-like monooxygenases
- MBP
maltose-binding protein
- Mtb
Mycobacterium tuberculosis
- PLP
pyridoxal 5′-phosphate
- PNPOx
pyridoxine 5′-phosphate oxidases
- r.m.s.
root-mean-square
- SEC
size exclusion chromatography
- SPE
solid-phase extraction
- STRING
search tool for retrieval of interacting genes/proteins
- TB
tuberculosis
- Tm
melting temperature
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Information
References
- Koul A, Arnoult E, Lounis N, Guillemont J, Andries K. The challenge of new drug discovery for tuberculosis. Nature. 2011;469:483–490. doi: 10.1038/nature09657. [DOI] [PubMed] [Google Scholar]
- Raviglione M, Marais B, Floyd K, Lonnroth K, Getahun H, Migliori GB, Harries AD, Nunn P, Lienhardt C, Graham S, Chakaya J, Weyer K, Cole S, Kaufmann SH, Zumla A. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet. 2012;379:1902–1913. doi: 10.1016/S0140-6736(12)60727-2. [DOI] [PubMed] [Google Scholar]
- Barry CE, III, Boshoff HI, Dowd CS. Prospects for clinical introduction of nitroimidazole antibiotics for the treatment of tuberculosis. Curr Pharm Des. 2004;10:3239–3262. doi: 10.2174/1381612043383214. [DOI] [PubMed] [Google Scholar]
- Cheeseman P, Toms-Wood A, Wolfe RS. Isolation and properties of a fluorescent compound, factor 420, from Methanobacterium strain M.o.H. J Bacteriol. 1972;112:527–531. doi: 10.1128/jb.112.1.527-531.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair TB, Isabelle DW, Daniels L. Structures of coenzyme F420 in Mycobacterium species. Arch Microbiol. 2001;176:37–43. doi: 10.1007/s002030100290. [DOI] [PubMed] [Google Scholar]
- Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, Langhorne MH, Anderson SW, Towell JA, Yuan Y, McMurray DN, Kreiswirth BN, Barry CE, III, Baker WR. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405:962–966. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- Ginsberg AM, Laurenzi MW, Rouse DJ, Whitney KD, Spigelman MK. Safety, tolerability, and pharmacokinetics of PA-824 in healthy subjects. Antimicrob Agents Chemother. 2009;53:3720–3725. doi: 10.1128/AAC.00106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins FB. The prosthetic group of a chromoprotein from mycobacteria. Biochim Biophys Acta. 1960;40:532–534. doi: 10.1016/0006-3002(60)91396-2. [DOI] [PubMed] [Google Scholar]
- Sutton WB. Properties of a new TPN-like electron transport component from Mycobacterium phlei. Biochem Biophys Res Commun. 1964;15:414–419. doi: 10.1016/0006-291x(64)90477-2. [DOI] [PubMed] [Google Scholar]
- Walsh C. Naturally occurring 5-deazaflavin coenzymes: biological redox roles. Acc Chem Res. 1986;19:216–221. [Google Scholar]
- Bugg TDH. Introduction to enzyme and coenzyme chemistry. Oxford: Blackwell Publishing Ltd; 2004. [Google Scholar]
- Boshoff HI, Barry CE., III Tuberculosis—metabolism and respiration in the absence of growth. Nat Rev Microbiol. 2005;3:70–80. doi: 10.1038/nrmicro1065. [DOI] [PubMed] [Google Scholar]
- Selengut JD, Haft DH. Unexpected abundance of coenzyme F420-dependent enzymes in Mycobacterium tuberculosis and other actinobacteria. J Bacteriol. 2010;192:5788–5798. doi: 10.1128/JB.00425-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjunatha UH, Boshoff H, Dowd CS, Zhang L, Albert TJ, Norton JE, Daniels L, Dick T, Pang SS, Barry CE. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2006;103:431–436. doi: 10.1073/pnas.0508392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashiri G, Squire CJ, Moreland NJ, Baker EN. Crystal structures of F420-dependent glucose-6-phosphate dehydrogenase FGD1 involved in the activation of the anti-tuberculosis drug candidate PA-824 reveal the basis of coenzyme and substrate binding. J Biol Chem. 2008;283:17531–17541. doi: 10.1074/jbc.M801854200. [DOI] [PubMed] [Google Scholar]
- Purwantini E, Daniels L. Purification of a novel coenzyme F420-dependent glucose-6-phosphate dehydrogenase from Mycobacterium smegmatis. J Bacteriol. 1996;178:2861–2866. doi: 10.1128/jb.178.10.2861-2866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashalidis EH, Mukherjee T, Sledz P, Matak-Vinkovic D, Boshoff H, Abell C, Barry CE., III Rv2607 from Mycobacterium tuberculosis is a pyridoxine 5'-phosphate oxidase with unusual substrate specificity. PLoS One. 2011;6:e27643. doi: 10.1371/journal.pone.0027643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaan S, Sulzenbacher G, Roig-Zamboni V, Scappuccini-Calvo L, Frassinetti F, Maurin D, Cambillau C, Bourne Y. Crystal structure of the conserved hypothetical protein Rv1155 from Mycobacterium tuberculosis. FEBS Lett. 2005;579:215–221. doi: 10.1016/j.febslet.2004.11.069. [DOI] [PubMed] [Google Scholar]
- Biswal BK, Cherney MM, Wang M, Garen C, James MN. Structures of Mycobacterium tuberculosis pyridoxine 5'-phosphate oxidase and its complexes with flavin mononucleotide and pyridoxal 5'-phosphate. Acta Crystallogr D Biol Crystallogr. 2005;61:1492–1499. doi: 10.1107/S0907444905026673. [DOI] [PubMed] [Google Scholar]
- Mashalidis EH, Sledz P, Lang S, Abell C. A three-stage biophysical screening cascade for fragment-based drug discovery. Nat Protoc. 2013;8:2309–2324. doi: 10.1038/nprot.2013.130. [DOI] [PubMed] [Google Scholar]
- Cellitti SE, Shaffer J, Jones DH, Mukherjee T, Gurumurthy M, Bursulaya B, Boshoff HI, Choi I, Nayyar A, Lee YS, Cherian J, Niyomrattanakit P, Dick T, Manjunatha UH, Barry CE, III, Spraggon G, Geierstanger BH. Structure of Ddn, the deazaflavin-dependent nitroreductase from Mycobacterium tuberculosis involved in bioreductive activation of PA-824. Structure. 2012;20:101–112. doi: 10.1016/j.str.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MC, Jackson CJ, Tattersall DB, French N, Peat TS, Newman J, Briggs LJ, Lapalikar GV, Campbell PM, Scott C, Russell RJ, Oakeshott JG. Identification and characterization of two families of F420H2-dependent reductases from Mycobacteria that catalyse aflatoxin degradation. Mol Microbiol. 2010;78:561–575. doi: 10.1111/j.1365-2958.2010.07356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Moonen CT, Vervoort J, Muller F. Carbon-13 nuclear magnetic resonance study on the dynamics of the conformation of reduced flavin. Biochemistry. 1984;23:4868–4872. doi: 10.1021/bi00316a008. [DOI] [PubMed] [Google Scholar]
- Walsh JD, Miller A-F. Flavin reduction potential tuning by substitution and bending. J Mol Struct: Theochem. 2003;623:185–195. [Google Scholar]
- Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, Liang J. CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Res. 2006;34:W116–W118. doi: 10.1093/nar/gkl282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo MK, Musayev FN, di Salvo ML, Schirch V. X-ray structure of Escherichia coli pyridoxine 5'-phosphate oxidase complexed with pyridoxal 5'-phosphate at 2.0 Å resolution. J Mol Biol. 2001;310:817–826. doi: 10.1006/jmbi.2001.4734. [DOI] [PubMed] [Google Scholar]
- Musayev FN, Di Salvo ML, Ko TP, Schirch V, Safo MK. Structure and properties of recombinant human pyridoxine 5'-phosphate oxidase. Protein Sci. 2003;12:1455–1463. doi: 10.1110/ps.0356203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, Rosenstrom P. Dali server: conservation mapping in 3D. Nucleic Acids Res. 2010;38:W545–W549. doi: 10.1093/nar/gkq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BK, Au K, Cherney MM, Garen C, James MN. The molecular structure of Rv2074, a probable pyridoxine 5'-phosphate oxidase from Mycobacterium tuberculosis, at 1.6 Å resolution. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62:735–742. doi: 10.1107/S1744309106025012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malen H, Pathak S, Softeland T, de Souza GA, Wiker HG. Definition of novel cell envelope associated proteins in Triton X-114 extracts of Mycobacterium tuberculosis H37Rv. BMC Microbiol. 2010;10:132–142. doi: 10.1186/1471-2180-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Mering C, Huynen M, Jaeggi D, Schmidt S, Bork P, Snel B. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. 2003;31:258–261. doi: 10.1093/nar/gkg034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Marcotte EM, Thompson MJ, Eisenberg D, Yeates TO. Assigning protein functions by comparative genome analysis: protein phylogenetic profiles. Proc Natl Acad Sci USA. 1999;96:4285–4288. doi: 10.1073/pnas.96.8.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A, Coin L, Durbin R, Finn RD, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer EL, Studholme DJ, Yeats C, Eddy SR. The Pfam protein families database. Nucleic Acids Res. 2004;32:D138–D141. doi: 10.1093/nar/gkh121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warkentin E, Mamat B, Sordel-Klippert M, Wicke M, Thauer RK, Iwata M, Iwata S, Ermler U, Shima S. Structures of F420H2:NADP+ oxidoreductase with and without its substrates bound. EMBO J. 2001;20:6561–6569. doi: 10.1093/emboj/20.23.6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceh K, Demmer U, Warkentin E, Moll J, Thauer RK, Shima S, Ermler U. Structural basis of the hydride transfer mechanism in F420-dependent methylenetetrahydromethanopterin dehydrogenase. Biochemistry. 2009;48:10098–10105. doi: 10.1021/bi901104d. [DOI] [PubMed] [Google Scholar]
- Aufhammer SW, Warkentin E, Berk H, Shima S, Thauer RK, Ermler U. Coenzyme binding in F420-dependent secondary alcohol dehydrogenase, a member of the bacterial luciferase family. Structure. 2004;12:361–370. doi: 10.1016/j.str.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Aufhammer SW, Warkentin E, Ermler U, Hagemeier CH, Thauer RK, Shima S. Crystal structure of methylenetetrahydromethanopterin reductase (Mer) in complex with coenzyme F420: architecture of the F420/FMN binding site of enzymes within the nonprolyl cis-peptide containing bacterial luciferase family. Protein Sci. 2005;14:1840–1849. doi: 10.1110/ps.041289805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashiri G, Rehan AM, Greenwood DR, Dickson JM, Baker EN. Metabolic engineering of cofactor F420 production in Mycobacterium smegmatis. PLoS One. 2010;5:e15803. doi: 10.1371/journal.pone.0015803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isabelle D, Simpson DR, Daniels L. Large-scale production of coenzyme F420−5,6 by using Mycobacterium smegmatis. Appl Environ Microbiol. 2002;68:5750–5755. doi: 10.1128/AEM.68.11.5750-5755.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KP, Kendrick N, Daniels L. Demonstration that fbiC is required by Mycobacterium bovis BCG for coenzyme F420 and FO biosynthesis. J Bacteriol. 2002;184:2420–2428. doi: 10.1128/JB.184.9.2420-2428.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashiri G, Squire CJ, Baker EN, Moreland NJ. Expression, purification and crystallization of native and selenomethionine labeled Mycobacterium tuberculosis FGD1 (Rv0407) using a Mycobacterium smegmatis expression system. Protein Expr Purif. 2007;54:38–44. doi: 10.1016/j.pep.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Ashby KD, Casey TA, Rasmussen MA, Petrich JW. Steady-state and time-resolved spectroscopy of F420 extracted from methanogen cells and its utility as a marker for fecal contamination. J Agric Food Chem. 2001;49:1123–1127. doi: 10.1021/jf000689r. [DOI] [PubMed] [Google Scholar]
- Turnbull WB, Precious BL, Homans SW. Dissecting the cholera toxin-ganglioside GM1 interaction by isothermal titration calorimetry. J Am Chem Soc. 2004;126:1047–1054. doi: 10.1021/ja0378207. [DOI] [PubMed] [Google Scholar]
- Brunger AT. Version 1.2 of the crystallography and NMR system. Nat Protoc. 2007;2:2728–2733. doi: 10.1038/nprot.2007.406. [DOI] [PubMed] [Google Scholar]
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA. Interactive electron-density map interpretation: from INTER to O. Acta Crystallogr D Biol Crystallogr. 2004;60:2115–2125. doi: 10.1107/S0907444904023509. [DOI] [PubMed] [Google Scholar]
- Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C, Jensen LJ. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–D815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Sapay N, Guermeur Y, Deleage G. Prediction of amphipathic in-plane membrane anchors in monotopic proteins using a SVM classifier. BMC Bioinformatics. 2006;7:255. doi: 10.1186/1471-2105-7-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew JM, Kapopoulou A, Jones LM, Cole ST. TubercuList—10 years after. Tuberculosis (Edinb) 2011;91:1–7. doi: 10.1016/j.tube.2010.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


