Abstract
Berardinelli-Seip congenital lipodystrophy 2-deficient (Bscl2−/−) mice recapitulate human BSCL2 disease with lipodystrophy. Bscl2-encoded seipin is detected in adipocytes and epithelium of mammary gland. Postnatal mammary gland growth spurt and vaginal opening signify pubertal onset in female mice. Bscl2−/− females have longer and dilated mammary gland ducts at 5-week old and delayed vaginal opening. Prepubertal exposure to 500 ppm genistein diet increases mammary gland area and accelerates vaginal opening in both control and Bscl2−/− females. However, genistein treatment increases ductal length in control but not Bscl2−/− females. Neither prepubertal genistein treatment nor Bscl2-deficiency affects phospho-estrogen receptor α or progesterone receptor expression patterns in 5-week old mammary gland. Interestingly, Bscl2-deficiency specifically reduces estrogen receptor β expression in mammary gland ductal epithelium. In summary, Bscl2−/− females have accelerated postnatal mammary ductal development but delayed vaginal opening; they display segregated responses in mammary gland development and vaginal opening to prepubertal genistein treatment.
Keywords: Bscl2/Seipin, lipodystrophy, mammary gland, vaginal opening, puberty, genistein
Introduction
Mammary gland development and vaginal opening are estrogen-dependent processes and markers for pubertal onset in mice [1–5]. Mammary gland development is also an indicator of pubertal onset in most girls [6]. Pubertal mammary gland growth is characterized by branching morphogenesis to form a ductal tree filling the fat pad [7–9]. Estrogen receptor alpha (ERα)-deficiency leads to failed mammary gland development beyond prepubertal stage [9]; ERβ−/− nulliparous mammary glands appear to have normal ductal growth but decreased side branching [10–12]. Estrogenic endocrine disruptors can affect both pubertal mammary gland development [1, 13] and vaginal opening, which is used as an external biomarker for pubertal onset in rodents [1, 4, 14–16].
Epidemiological studies have suggested that obesity may be causally related to earlier puberty in girls (reviewed in [17]). One study involving 135,223 girls (born between 1930 and 1969) indicates that heavier girls at age seven had earlier puberty; it also suggests that obesity epidemic is not solely responsible for the trend of younger age at puberty and that endocrine disruptors could contribute to this trend [18]. Indeed, we have demonstrated in mice that prepubertal exposure to endocrine disruptors accelerates pubertal onset [1, 4, 19, 20].
We hypothesized that both body fat and endocrine disruptors could affect pubertal onset and that body fat could influence the effect of endocrine disruptors on pubertal onset. This hypothesis was tested in Bscl2−/− mice [21] that recapitulate human Beinardinalli-Seipin Congenital Lipodystrophy type 2 (BSCL2) disease. BSCL2/Bscl2 gene encodes seipin, an integral endoplasmic reticulum membrane protein that plays an essential role in adipose tissue development [21–27]. Seipin is highly expressed in the adipose tissue, brain and testis of both human and mouse [22, 28–30]. Mutations or deletion of BSCL2/Bscl2 are associated with generalized lipodystrophy characterized by a near complete absence of adipose tissue and associated metabolic complications [21, 22, 25–27]. Although it was reported that among 45 patients (27 boys and 18 girls) with BSCL2 mutations, one girl had precocious puberty [31], it is unknown whether seipin deficiency and related lipodystrophy affect pubertal onset. It is also unknown whether lipodystrophy affects the responsiveness to prepubertal exposure of endocrine disruptors. These two aspects were investigated in this study. Genistein was used as a testing endocrine disruptor. A dose of 500 ppm in the diet was chosen because it was found in some soy products, such as soy bacon [32], and it was previously demonstrated to be an effective dose to influence pubertal onset in mice [1]. Pubertal mammary gland development and vaginal opening were two end points for determining pubertal onset in this study.
Materials and Methods
Animals
Bscl2-deficient mice (Bscl2−/−) on C57BL/6J background were derived from a colony at Georgia Regents University, which was originally derived from a colony at Baylor College of Medicine [21]. Mice with different genotypes were from Bscl2+/− (Het) females X Bscl2+/− (Het) males. They were genotyped as previously described [21]. All mice were maintained on PicoLab mouse diet 20 with soybean as a main protein source. They were housed in polypropylene cages with free access to food and water on a 12 h light/dark cycle (0700–1900) at 23±1°C with 30–50% relative humidity at the College of Veterinary Medicine animal facility at the University of Georgia. All methods used in this study were approved by the University of Georgia IACUC Committee and conform to National Institutes of Health guidelines and public law.
Treatment
Bscl2+/+ (WT) and Bscl2+/− (Het) mice had no difference in phenotypes and were both included in the genotype control for Bscl2−/− (Hom) mice. At weaning (3 weeks old), control females and their Bscl2−/− female littermates were randomly assigned into 0 ppm (vehicle control) or 500 ppm genistein diet groups, resulting in four groups: control mice, 0 ppm; control mice, 500 ppm; Bscl2−/− mice, 0 ppm; and Bscl2−/− mice, 500 ppm. The 0 ppm and 500 ppm genistein diets were prepared as described previously [1, 33]. Briefly, 0 g or 0.25 g genistein (LC Laboratories, Woburn, MA) was dissolved in 150 ml 70% ethanol, then mixed well with 500 g phytoestrogen-free AIN-93G diet (Bio-Serv, Frenchtown, NJ) in different glass bowls to obtain 0 ppm and 500 ppm genistein diets, respectively. Food pellets were hand squeezed, air dried at room temperature, and kept at 4°C. Fresh diets were prepared every two weeks. Body weight was measured at 3, 4, and 5 weeks old. At least 6 mice were included in each study group. Vaginal opening was evaluated daily from weaning until detection [1].
Tissue collection
All mice at 5 weeks old ± 1 day were dissected at estrus stage, which was determined by vaginal smear prior to dissection [1], except in Bscl2−/−, 0 ppm group, in which 6 out of the 7 mice had vaginal opening on PND34 or 35, and they were dissected on the day of vaginal opening detection. Both vaginal opening and estrus stage occur after an estrogen increase [16, 34–36]. The day at vaginal opening and the day at estrus stage were chosen as relatively comparable stages ~5 weeks old between control and Bscl2−/− females. The 4th inguinal mammary glands on both sides were collected, one was saved for whole mount analysis and the other was fixed in 10% formalin for histology and immunohistochemistry.
Mammary gland whole mount and quantification of mammary gland development
Mammary gland whole mount and quantification were done as previously described [1, 37]. ImageJ (National Institutes of Health, Bethesda, MD, USA) was used to quantify the size of each lymph node, the length of the longest duct from the nipple, the diameter of the widest mammary gland duct at the position near each lymph node, the occupied area of each mammary gland (approximated by a polygon area that covered all the ducts), the width of the ductal tree passing the lymph node, and the number of terminal end buds (TEB) with diameters larger than 70 mm.
Histology
Mammary gland histology was done as previously described [1].
Immunohistochemistry
Paraffin sections (5 μm) of the 4th inguinal mammary glands were used in immunohistochemistry as previously described [1, 38]. Seipin expression was detected in 5 weeks old Bscl2+/+ and Bscl2−/− mammary glands as well as 3 months old Bscl2+/+ and Bscl2−/− testes using our customized rabbit polyclonal anti-seipin antibody (1:1,000, 2.21 μg/ml, Thermo Scientific), which was raised against the C-terminal 17 amino acids of mouse seipin. Bscl2+/+ mammary gland sections and testis sections without primary antibody were also included. To determine the effects of Bscl2-deficiency and genistein treatment on the expression of phospho-estrogen receptor α (P-ESR1/P-ERα), estrogen receptor β (ESR2/ERβ), and progesterone receptor (PR) in the mammary gland, mammary gland sections from three mice in each of the four groups (control mice, 0 ppm; control mice, 500 ppm; Bscl2−/− mice, 0 ppm; and Bscl2−/− mice, 500 ppm) were evaluated using immunohistochemistry for P-ERα (rabbit anti-phospho-ERα antibody, 1:100, 10 μg/ml, Abcam), ERβ (anti-ERβ antibody, 1:50, 20 μg/ml, Abcam), and PR (rabbit anti-PR antibody, 1:200, 6 μg/ml, Daco, Denmark) as previously described [1, 38, 39]. Sections were counterstained with Harris Hematoxylin.
Statistical analysis
Nonparametric Two-Sample Kolmogorov-Smirnov test was used to compare the ages at vaginal opening, the sizes of lymph nodes, the ductal lengths and the areas of mammary glands, the diameters of mammary gland ducts, the widths of ductal trees passing the lymph nodes, and the numbers of TEB. Two-tail unequal variance student’s t-test was used to compare the weaning body weight. ANOVA with repeated measure was used to compare the body weight from 3 to 5 weeks old in different genotypes and treatments. Error bars represented standard deviation. The significance level was set at p<0.05.
Results and Discussion
Body weight
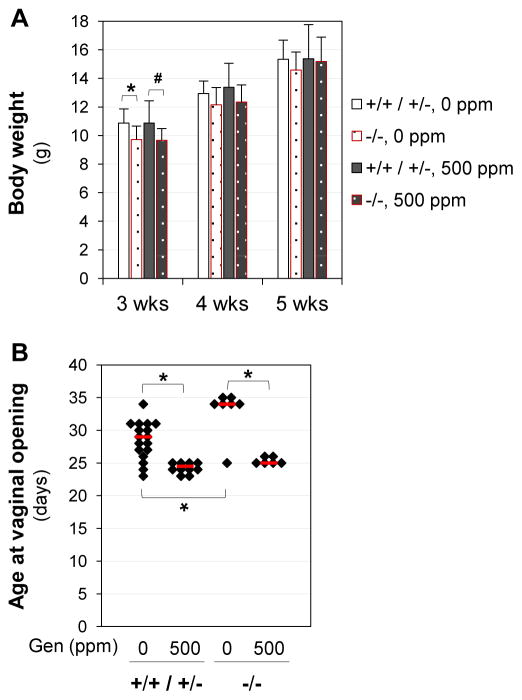
Bscl2−/− females had significantly lower body weight than the control mice only at weaning (3 weeks old) but not at 4 or 5 weeks old (Fig. 1A), in agreement with our previous report [21]. The average weaning weights were 10.87±1.18 g (N=23) for control females and 9.69±0.85 g (N=13, P<0.01) for Bscl2−/− females.
Figure 1.
Body weight and age at vaginal opening. A. Body weight from weaning (3 weeks old, 3wks) to dissection (5 wks). Body weight at weaning: two-tail unequal variance t-test; * P=0.022; # P=0.086. Body weight from 3 to 5 weeks old: ANOVO repeated measures; no treatment related difference (P=0.645). Error bars, standard deviations. N=6–15. B. Age at vaginal opening. * p<0.05; nonparametric Two-Sample Kolmogorov-Smirnov test. Black diamonds indicate data from individual mice; red lines indicate median in each group. N=6–17. +/+ / +/−, Bscl2+/+ (WT) and Bscl2+/− (Het) mice as the genotype control for Bscl2−/− (Hom) mice; −/−, Bscl2−/− mice. Genistein (Gen) diets: 0 ppm or 500 ppm.
Interestingly, we noticed a dramatic difference in the weaning body weight of the control C57BL/6J females (10.87±1.18 g, N=23) in this study compared to that of the control C57BL/6J females in our previous study, which was 7.22±1.05 g (N=111, P<0.001, two-tailed unequal variance t-test) [1]. The main difference in these two studies was the diet. In the previous study, our C57BL/6J colony was maintained on phytoestrogen-free AIN-93G diet (3.8 kcal/g), and the ancestors of the females used in the previous study were already on this diet for at least 2 generations. In this current study, all the female pups were from parents maintained on PicoLab mouse diet 20 (4.6 kcal/g).
Vaginal opening
Although it has been debated for its accuracy as a biomarker for puberty [40], vaginal opening has been used as a standard endpoint for assessing pubertal development by U.S. Environmental Protection Agency (EPA) (http://www.epa.gov/endo/pubs/pubertal_protocol_2007_v7.2c.pdf) and it has been used in many rodent studies to indicate pubertal onset [15, 41–44]. Analyses of the original data from our previous study [1] revealed a significant correlation between age at vaginal opening and age at first copulation following vaginal opening. In addition, other biomarkers for puberty, such as vaginal estrus, vaginal plug, and ovulation, also showed a consistent sequential pattern following vaginal opening [15, 40]. Therefore, vaginal opening can be used as an easily obtainable noninvasive biomarker for pubertal onset in rodents [15].
Genistein treatment significantly advanced vaginal opening in both control and Bscl2−/− mice (Fig. 1B). Interestingly, without genistein treatment, Bscl2−/− mice had significantly delayed vaginal opening compared to the control mice (Fig. 1B). The lower body weight in the newly-weaned Bscl2−/− mice (Fig. 1A) might contribute to the delayed vaginal opening. This difference seemed to be erased by 500 ppm genistein treatment because no significant difference in the ages at vaginal opening was observed between genistein-treated control and Bscl2−/− mice (Fig. 1B). These results indicated that although Bscl2 deficiency delayed vaginal opening, it did not seem to affect the responsiveness to genistein treatment.
In our previous study [1], the age at vaginal opening in the vehicle control group was 32.2±2.8 days old (N=35). It was significantly younger in the vehicle control mice (28.5±2.9 days old, N=17, P<0.001) in this study. There could be two related potential explanations: body weight and diet. The body weight in the previous study [1] was significantly lower than that in the current study (Fig. 1A). Normally, higher body weight is correlated with earlier pubertal development (reviewed in [45]). Females in the previous study were not exposed to phytoestrogen-containing diet directly or indirectly from gestation to weaning because they were from a colony maintained on phytoestrogen-free AIN-93G diet [1]. Females in the current study were indirectly exposed to PicoLab mouse diet 20 from gestation to weaning. Soybean was a main protein source in this diet, and it contained phytoestrogens genistein and daidzein [46–48]. It has been reported that in utero and lactational exposure to phytoestrogens could promote pubertal onset [49, 50] and the phytoestrogen content in the diet could affect the age at vaginal opening [51].
Mammary gland
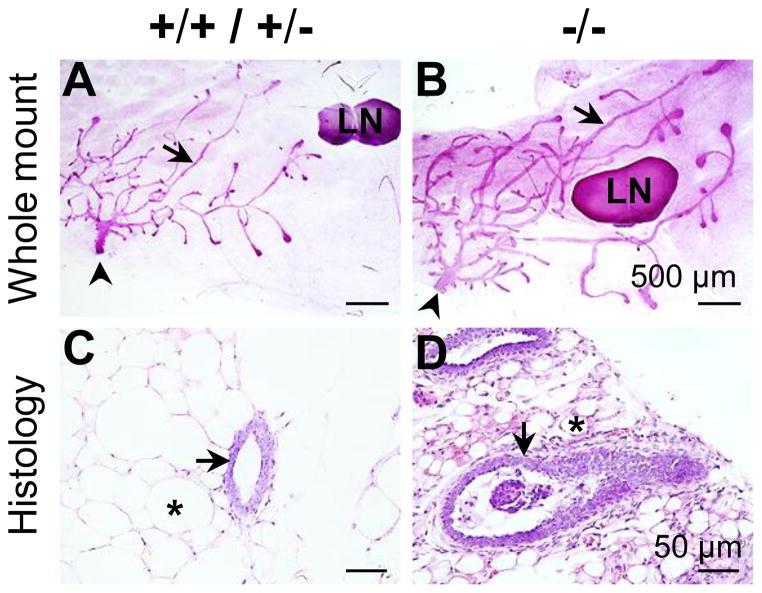
Since seipin-deficiency is associated with generalized lipodystrophy characterized by a near complete absence of adipose tissue [21, 22, 25–27], it was expected that adipocyte-rich mammary gland would have abnormalities in the Bscl2−/−female mice. Indeed, several obvious differences were observed. Whole mount mammary glands showed enlarged lymph nodes (1.23±0.24 mm2 (N=7) vs. 0.54±0.22 mm2 (N=9) in the control, P<0.001), and longer and wider mammary gland ducts in the 5 weeks old Bscl2−/− female mice (Figs. 2A, 2B). Histology of mammary gland confirmed enlarged ductal lumen and smaller adipocytes in these mice (Figs. 2C, 2D). The average diameters of the widest mammary gland ducts near the lymph nodes were 0.027±0.006 mm (N=9) for the control females and 0.063±0.007 mm (N=7, P<0.001) for the Bscl2−/− females. Interestingly, cells and patches of cells were often seen in the Bscl2−/− mammary gland ductal lumen (Fig. 2D). It was possible that they were sloughed ductal epithelial cells.
Figure 2.
Representative images of whole mount and histology of mammary gland of females at 5 weeks old on vehicle control diet. A. Whole mount of Bscl2+/+ / Bscl2+/−control mammary gland. B. Whole mount of Bscl2−/− mammary gland. Scale bars in A & B, 500 μm. C. Histology of Bscl2+/+ / Bscl2+/− control mammary gland. D. Histology of Bscl2−/− mammary gland. H & E stain. Scale bars in C & D, 50 μm. A~D: arrows, mammary gland ducts; arrowheads, nipples; *, mammary gland adipocyte; LN, lymph node.
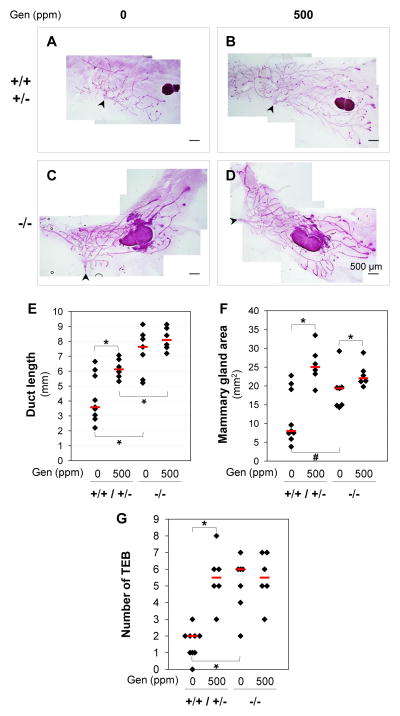
Upon postweaning 500 ppm genistein treatment, the control mice had significantly increased ductal length and area of mammary gland at 5 weeks old (Figs. 3A, 3B, 3E, 3F). This effect was consistent with our previous study in C57BL/6J females derived from a colony maintained on phytoestrogen-free AIN-93G diet [1]. In Bscl2−/−mammary glands, segregated responses upon 500 ppm genistein treatment were observed. Significantly increased mammary gland area but not mammary gland ductal length was observed in the 500 ppm genistein-treated Bscl2−/− females (Figs. 3C–3F). This could be attributed to the accelerated ductal growth in the Bscl2−/− females, which had significantly longer duct than the control females on either 0 or 500 ppm genistein diet (Figs. 3A–3E). It was possible that the ductal lengths in the Bscl2−/− females had reached the maximum and 500 ppm genistein treatment could not further extend the ductal lengths. In support of this speculation, we found that the number of TEB (highly proliferative structures located at the tips of the invading ducts) in the Bscl2−/− females on 0 ppm genistein diet was comparable to those in the control females and the Bscl2−/−females on 500 ppm genistein diet (Fig. 3G).
Figure 3.
Effects of Bscl2/seipin and genistein on mammary gland development. A~D. Representative images of whole mount mammary glands in Bscl2+/+ / Bscl2+/−, 0 ppm genistein group (A), Bscl2+/+ / Bscl2+/−, 500 ppm genistein group (B), Bscl2−/−, 0 ppm genistein group (C), and Bscl2−/−, 500 ppm genistein group (D) at 5 weeks old. E. Lengths of the longest mammary gland ducts. * P<0.05. F. Areas occupied by mammary gland ducts. * P<0.05; # P=0.092. G. Numbers of terminal end buds (TEB). * P<0.05. E–G: nonparametric Two-Sample Kolmogorov-Smirnov test. Black diamonds indicate data from individual mice; red lines indicate median in each group. N=6–9. +/+ / +/−, Bscl2+/+ and Bscl2+/− control mice; −/−, Bscl2−/− mice; Genistein (Gen) diets, 0 ppm or 500 ppm. A–D: scale bars, 500 μm; arrowheads, nipples.
However, there was still limited room to expand the mammary gland area in the Bscl2−/− females. In the control females, the average increase of mammary gland area was ~200% upon genistein treatment; while in the Bscl2−/− females, it was ~15% in response to 500 ppm genistein treatment (Figs. 3A~3D, 3F). Although the mammary gland ductal tree was supported by the surrounding fat tissue, which was not well developed in the Bscl2−/− females (Fig. 2D), it did not seem to limit the ductal growth. However, the width of ductal tree passing the lymph node was narrower in the 500 ppm genistein-treated Bscl2−/− females (2.91±0.46 mm (N=6), Fig. 3D) compared to 500 ppm genistein-treated control mice (3.99±0.37 mm (N=6), P=0.026, Fig. 3B). This observation could be supported by the longer ductal length in the Bscl2−/− mammary gland (Fig. 3E) but comparable area of mammary gland between control and Bscl2−/−mammary glands upon 500 ppm genistein treatment (Fig. 3F).
Expression of seipin in 5 weeks old mammary gland
Seipin has been demonstrated to be highly expressed in adipose tissue, brain and testis [22, 28, 29, 52]. Its expression in the mammary gland had not been previously reported. Immunohistochemistry showed seipin expression in the adipocytes and the ductal epithelial cells of 5 weeks old Bscl2+/+ mammary gland (Fig. 4A). However, there were still lower levels of staining in the Bscl2−/− mammary gland (Fig. 4B) but no staining in the mammary gland without the primary antibody (Fig. 4C). Seipin was highly detected in the Bscl2+/+ spermatids but not in the Bscl2−/− spermatids (Figs. 4D, 4E) as previously reported [29, 52]. However, compared to the testis section without the primary antibody (Fig. 4F), the Bscl2−/− testis had background immunostaining throughout all the cell types (Fig. 4E). The expression of seipin in the mammary gland supported its potential role in mammary gland development and/or function.
Figure 4.
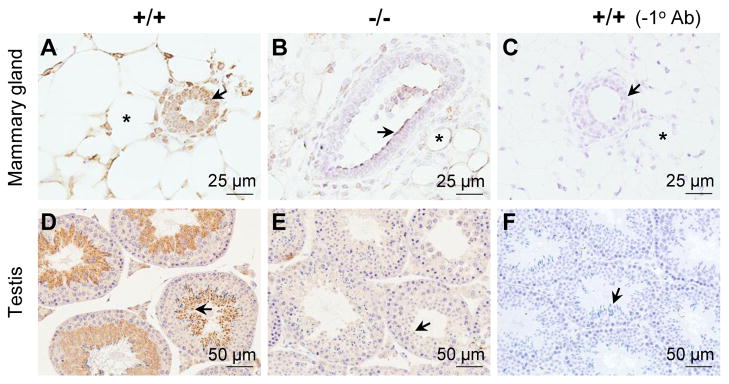
Immunohistochemistry detection of seipin expression in 5 weeks old mammary glands and 3 month old testes. A. Wild type (+/+) mammary gland with anti-seipin antibody. B. Bscl2−/− mammary gland with anti-seipin antibody. C. Wild type (+/+) mammary gland without primary antibody (−1° Ab). A~C: arrows, mammary gland ductal luminal epithelial cells; *, mammary gland adipocytes; scale bars, 25 μm. D. Wild type (+/+) testis with anti-seipin antibody. E. Bscl2−/− testis with anti-seipin antibody. F. Wild type (+/+) testis without primary antibody (−1° Ab). D~F: arrows, spermatids; scale bars, 50 μm. Brown signal, positive staining.
ER and PR expression
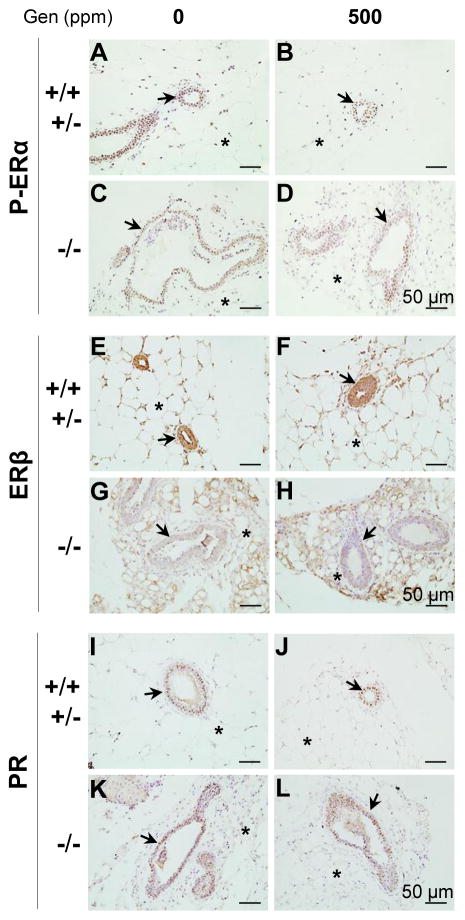
ERα is critical for pubertal mammary gland development [9]. ERβ seems to be important for side branching [10], presumably through its involvement in progesterone production from corpora lutea [10–12] because PR-mediated progesterone signaling is critical for side branching [53, 54]. It was previously demonstrated that postweaning genistein treatment did not influence P-ERα, ERβ, and PR expression in C57BL/6 mammary gland at 5 weeks old [1]. Here we further examined P-ERα, ERβ, and PR expression in 5 weeks old mammary gland to determine if any of these receptors had altered expression in the Bscl2−/− mammary glands treated with 0 or 500 ppm genistein diets.
P-ERα was mainly detected in the nuclei of epithelial cells and adipocytes; there was no obvious difference in the P-ERα expression patterns among all four groups (Figs. 5A–5D). ERβ was comparably highly expressed in the epithelial cells and adipocytes of the control mammary glands (both 0 and 500 ppm genistein-treated) (Figs. 5E, 5F). However, its expression was lower in the epithelial cells compared to that in the surrounding adipocytes of the Bscl2−/− mammary glands (both 0 and 500 ppm genistein-treated) (Figs. 5G, 5H). The expression levels of ERβ in the adipocytes of the control mammary gland and of the Bscl2−/− mammary gland seemed to be comparable (Figs. 5E–5H). PR was mainly detected in the nuclei of mammary gland epithelial cells, and no obvious difference in the epithelial PR expression pattern was observed among all four groups (Figs. 5I–5L).
Figure 5.
Expression of phospho-estrogen receptor alpha (P-ERα/P-ESR1), estrogen receptor beta (ERβ/ESR2), and progesterone receptor (PR) in 5 weeks old mammary gland. Sections from 3 mice in each group were examined and representative images were shown. A. P-ERα, Bscl2+/+ / Bscl2+/−, 0 ppm genistein group. B. P-ERα, Bscl2+/+ / Bscl2+/−, 500 ppm genistein group. C. P-ERα, Bscl2−/−, 0 ppm genistein group. D. P-ERα, Bscl2−/−, 500 ppm genistein group. E. ERβ, Bscl2+/+ / Bscl2+/−, 0 ppm genistein group. F. ERβ, Bscl2+/+ / Bscl2+/−, 500 ppm genistein group. G. ERβ, Bscl2−/−, 0 ppm genistein group. H. ERβ, Bscl2−/−, 500 ppm genistein group. I. PR, Bscl2+/+ / Bscl2+/−, 0 ppm genistein group. J. PR, Bscl2+/+ / Bscl2+/−, 500 ppm genistein group. K. PR, Bscl2−/−, 0 ppm genistein group. L. PR, Bscl2−/−, 500 ppm genistein group. Dark brown, immunostaining; purple-blue, counter staining with Harris Hematoxylin; no specific immunostaining in the minus primary antibody negative control (Fig. 4C). Arrows, mammary gland ducts; *, mammary gland adipocytes.
The mechanism of ERβ downregulation in the Bscl2−/− ductal epithelium and the consequence of such cell-type specific downregulation are unclear. It was demonstrated that glucocorticoid receptor (GR) deficiency (GR−/−) could lead to accelerated pubertal mammary ductal growth and distention [55], similar to what was seen in the Bscl2−/−mammary gland (Figs. 2, 3). Based on a mammary gland transplant study, the driving force for the GR−/− mammary gland duct phenotypes was from the transplanted GR−/−duct itself and not the host wild type fat pad [55]. ERβ has an antiproliferative function in the uterus [8, 56]. It may also have an antiproliferative function in the mammary gland. With this assumption, reduced ERβ expression specifically in the Bscl2−/− ductal epithelium could lead to increased proliferation of the ductal epithelium, leading to longer and dilated Bscl2−/− mammary gland duct (Figs. 2, 3).
Leptin and genistein on puberty
There is a leptin surge during the second postnatal week, preceding the developmental estrogen increase in the female mice [57]. Leptin might promote puberty by indirectly stimulating gonadotropin-releasing hormone (GnRH) production [58]. Leptin is synthesized in the adipocytes and leptin levels are positvely correlated with fat mass [59]. Therefore, lipodystrophy is often accompanied with reduced leptin levels [60]. Indeed, our Bscl2−/− mice were previously demonstrated to have greatly reduced leptin levels [21], which might cause the delayed pubertal onset indicated by delayed vaginal opening (Fig. 1B). However, prepubertal exposure to 500 ppm genistein significantly accelerated vaginal opening in the Bscl2−/− females to a level comparable to that in the genistein-treated control females (Fig. 1B).
Genistein can induce GnRH pulsatile production in the prepubertal mice [61]. It advances pubertal onset possibly via suppressing inhibitory and activating stimulatory components of the GnRH network [62]. Leptin administration could restore pubertal onset in the leptin-deficient mice [63]. However, in vitro studies showed that genistein could decrease leptin production in adipocytes [64, 65], while an in vivo study on adult ovariectomized C57BL/6 mice treated with 1500 ppm genistein diet for 21 days did not show any effect of genistein on blood leptin levels [66]. These observations suggested that genistein advanced pubertal onset in the Bscl2−/− females but it might not act through increasing leptin levels. It could possibly enhance leptin sensitivity in the brain in a way similar to what estrogen does [67]. It is also possible that genistein could circumvent the defect(s) leading to delayed pubertal onset in Bscl2−/− females via an unknown pathway.
Fat and pubertal mammary gland development
Pubertal mammary gland growth is characterized by ductal morphogenesis with extensive epithelial cell proliferation, a process that is believed to be regulated by paracrine signaling [7–9, 68]. Adipocytes play a key role in this paracrine regulation of pubertal mammary gland development [69]. Ablation of adipocytes during puberty could inhibit pubertal mammary gland growth, and restoration of adipocytes could rescue it to a considerable extent [70]. A-ZIP/F1 transgenic mice without white adipose tissue have short and dilated mammary gland ducts and lack normal pubertal mammary gland growth, resulting from the lack of adipose tissue but not any defects in the ductal epithelial cells [71]. Although the Bscl2−/− females also lack well-developed fat tissue [21], they have long and dilated mammary gland ducts at 5 weeks old (Figs. 3A–3E). It is possible that the longer mammary gland ducts in the Bscl2−/− females (Fig. 3E) are caused by the local loss of seipin in the ductal epithelium (Fig. 4A), potentially involving downregulation of ERβ in the ductal epithelium (Fig. 5G, 5H).
Summary
Both mammary gland development and vaginal opening are markers for pubertal onset in mice. These two processes are segregated in the Bscl2−/− female mice with lipodystrophy, indicated by accelerated mammary gland ductal growth but delayed vaginal opening. Bscl2−/− females are responsive to genistein treatment, indicated by accelerated vaginal opening and increased mammary gland area. Mammary gland development is not as responsive as vaginal opening upon genistein treatment in the Bscl2−/− females. This can be explained by accelerated pubertal mammary gland ductal growth but limited mammary fat pad in the Bscl2−/− females. Reduced expression of ERβ may contribute to the phenotypes in the Bscl2−/− mammary gland ducts.
Highlights.
Accelerated pubertal mammary gland ductal development in Bscl2−/− mice.
Delayed vaginal opening in Bscl2−/− mice.
Accelerated pubertal onset by postweaning dietary genistein exposure.
Segregated responses of mammary gland development and vaginal opening to prepubertal genistein exposure in Bscl2−/− mice.
Decreased ERβ expression in Bscl2−/− mammary gland ductal epithelium.
Acknowledgments
The authors thank the Department of Pathology in the College of Veterinary Medicine, University of Georgia for access to the imaging system; the Office of the Vice President for Research, Interdisciplinary Toxicology Program, and Department of Physiology and Pharmacology at the University of Georgia, and the National Institutes of Health (NIH R15HD066301 and NIH R01HD065939 (co-funded by ORWH and NICHD) to X.Y.) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rong Li, Email: lirong9@uga.edu.
Ahmed E. El Zowalaty, Email: ahmdezat@uga.edu.
Weiqin Chen, Email: wechen@gru.edu.
Elizabeth A. Dudley, Email: ldudley9@uga.edu.
Xiaoqin Ye, Email: ye@uga.edu.
References
- 1.Li R, Zhao F, Diao H, Xiao S, Ye X. Postweaning dietary genistein exposure advances puberty without significantly affecting early pregnancy in C57BL/6J female mice. Reprod Toxicol. 2014;44:85–92. doi: 10.1016/j.reprotox.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlin J, McBryan J, Martin F. Pubertal mammary gland development: insights from mouse models. J Mammary Gland Biol Neoplasia. 2006;11:283–97. doi: 10.1007/s10911-006-9024-2. [DOI] [PubMed] [Google Scholar]
- 3.McNally S, Martin F. Molecular regulators of pubertal mammary gland development. Ann Med. 2011;43:212–34. doi: 10.3109/07853890.2011.554425. [DOI] [PubMed] [Google Scholar]
- 4.Zhao F, Li R, Xiao S, Diao H, Viveiros MM, Song X, et al. Postweaning exposure to dietary zearalenone, a mycotoxin, promotes premature onset of puberty and disrupts early pregnancy events in female mice. Toxicol Sci. 2013;132:431–42. doi: 10.1093/toxsci/kfs343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walters LM, Rourke AW, Eroschenko VP. Purified methoxychlor stimulates the reproductive tract in immature female mice. Reprod Toxicol. 1993;7:599–606. doi: 10.1016/0890-6238(93)90036-7. [DOI] [PubMed] [Google Scholar]
- 6.Wheeler MD. Physical changes of puberty. Endocrinol Metab Clin North Am. 1991;20:1–14. [PubMed] [Google Scholar]
- 7.Macias H, Hinck L. Mammary gland development. Wiley interdisciplinary reviews. Developmental biology. 2012;1:533–57. doi: 10.1002/wdev.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, et al. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149:2970–9. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korach KS, Couse JF, Curtis SW, Washburn TF, Lindzey J, Kimbro KS, et al. Estrogen receptor gene disruption: molecular characterization and experimental and clinical phenotypes. Recent Prog Horm Res. 1996;51:159–86. discussion 86–8. [PubMed] [Google Scholar]
- 10.Palmieri C, Cheng GJ, Saji S, Zelada-Hedman M, Warri A, Weihua Z, et al. Estrogen receptor beta in breast cancer. Endocr Relat Cancer. 2002;9:1–13. doi: 10.1677/erc.0.0090001. [DOI] [PubMed] [Google Scholar]
- 11.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, et al. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci U S A. 1998;95:15677–82. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forster C, Makela S, Warri A, Kietz S, Becker D, Hultenby K, et al. Involvement of estrogen receptor beta in terminal differentiation of mammary gland epithelium. Proc Natl Acad Sci U S A. 2002;99:15578–83. doi: 10.1073/pnas.192561299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padilla-Banks E, Jefferson WN, Newbold RR. Neonatal exposure to the phytoestrogen genistein alters mammary gland growth and developmental programming of hormone receptor levels. Endocrinology. 2006;147:4871–82. doi: 10.1210/en.2006-0389. [DOI] [PubMed] [Google Scholar]
- 14.Thigpen JE, Setchell KD, Padilla-Banks E, Haseman JK, Saunders HE, Caviness GF, et al. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague-Dawley rats. Environ Health Perspect. 2007;115:1717–26. doi: 10.1289/ehp.10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen E, Doisy EA. The induction of a sexually mature condition in immature females by injection of the ovarian follicular hormone. Am J Physiol. 1924;69:577–88. [Google Scholar]
- 16.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.nsa04is48. Appendix 4:Appendix 4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplowitz PB. Link between body fat and the timing of puberty. Pediatrics. 2008;121 (Suppl 3):S208–17. doi: 10.1542/peds.2007-1813F. [DOI] [PubMed] [Google Scholar]
- 18.Aksglaede L, Juul A, Olsen LW, Sorensen TI. Age at puberty and the emerging obesity epidemic. PLoS One. 2009;4:e8450. doi: 10.1371/journal.pone.0008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao F, Li R, Xiao S, Diao H, El Zowalaty AE, Ye X. Multigenerational exposure to dietary zearalenone (ZEA), an estrogenic mycotoxin, affects puberty and reproduction in female mice. Reprod Toxicol. 2014 doi: 10.1016/j.reprotox.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao F, Zhou J, El Zowalaty AE, Li R, Dudley EA, Ye X. Timing and recovery of postweaning exposure to diethylstilbestrol on early pregnancy in CD-1 mice. Reprod Toxicol. 2014;49C:48–54. doi: 10.1016/j.reprotox.2014.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W, Chang B, Saha P, Hartig SM, Li L, Reddy VT, et al. Berardinelli-seip congenital lipodystrophy 2/seipin is a cell-autonomous regulator of lipolysis essential for adipocyte differentiation. Mol Cell Biol. 2012;32:1099–111. doi: 10.1128/MCB.06465-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Magre J, Delepine M, Khallouf E, Gedde-Dahl T, Jr, Van Maldergem L, Sobel E, et al. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet. 2001;28:365–70. doi: 10.1038/ng585. [DOI] [PubMed] [Google Scholar]
- 23.Lundin C, Nordstrom R, Wagner K, Windpassinger C, Andersson H, von Heijne G, et al. Membrane topology of the human seipin protein. FEBS Lett. 2006;580:2281–4. doi: 10.1016/j.febslet.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 24.Sim MF, Talukder MU, Dennis RJ, Edwardson JM, Rochford JJ. Analyzing the functions and structure of the human lipodystrophy protein seipin. Methods Enzymol. 2014;537:161–75. doi: 10.1016/B978-0-12-411619-1.00009-4. [DOI] [PubMed] [Google Scholar]
- 25.Cui X, Wang Y, Tang Y, Liu Y, Zhao L, Deng J, et al. Seipin ablation in mice results in severe generalized lipodystrophy. Hum Mol Genet. 2011;20:3022–30. doi: 10.1093/hmg/ddr205. [DOI] [PubMed] [Google Scholar]
- 26.Prieur X, Dollet L, Takahashi M, Nemani M, Pillot B, Le May C, et al. Thiazolidinediones partially reverse the metabolic disturbances observed in Bscl2/seipin-deficient mice. Diabetologia. 2013;56:1813–25. doi: 10.1007/s00125-013-2926-9. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Yechoor VK, Chang BH, Li MV, March KL, Chan L. The human lipodystrophy gene product Berardinelli-Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology. 2009;150:4552–61. doi: 10.1210/en.2009-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal AK, Garg A. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol Metab. 2003;14:214–21. doi: 10.1016/s1043-2760(03)00078-x. [DOI] [PubMed] [Google Scholar]
- 29.Jiang M, Gao M, Wu C, He H, Guo X, Zhou Z, et al. Lack of testicular seipin causes teratozoospermia syndrome in men. Proc Natl Acad Sci U S A. 2014;111:7054–9. doi: 10.1073/pnas.1324025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cartwright BR, Goodman JM. Seipin: from human disease to molecular mechanism. J Lipid Res. 2012;53:1042–55. doi: 10.1194/jlr.R023754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Maldergem L, Magre J, Khallouf TE, Gedde-Dahl T, Jr, Delepine M, Trygstad O, et al. Genotype-phenotype relationships in Berardinelli-Seip congenital lipodystrophy. J Med Genet. 2002;39:722–33. doi: 10.1136/jmg.39.10.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.USDA. USDA database for the isoflavone content of selected foods. 2008 http://wwwarsusdagov/nutrientdata/isoflav.
- 33.Zhao F, Li R, Xiao S, Diao H, Viveiros MM, Song X, et al. Postweaning exposure to dietary zearalenone, a mycotoxin, promotes premature onset of puberty and disrupts early pregnancy events in female mice. Toxicol Sci. 2013;132:431–42. doi: 10.1093/toxsci/kfs343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murr SM, Geschwind II, Bradford GE. Plasma LH and FSH during different oestrous cycle conditions in mice. J Reprod Fertil. 1973;32:221–30. doi: 10.1530/jrf.0.0320221. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7:185–99. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 36.Walmer DK, Wrona MA, Hughes CL, Nelson KG. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology. 1992;131:1458–66. doi: 10.1210/endo.131.3.1505477. [DOI] [PubMed] [Google Scholar]
- 37.Fenton SE, Hamm JT, Birnbaum LS, Youngblood GL. Persistent abnormalities in the rat mammary gland following gestational and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Toxicol Sci. 2002;67:63–74. doi: 10.1093/toxsci/67.1.63. [DOI] [PubMed] [Google Scholar]
- 38.Xiao S, Diao H, Smith MA, Song X, Ye X. Preimplantation exposure to bisphenol A (BPA) affects embryo transport, preimplantation embryo development, and uterine receptivity in mice. Reprod Toxicol. 2011;32:434–41. doi: 10.1016/j.reprotox.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diao H, Paria BC, Xiao S, Ye X. Temporal expression pattern of progesterone receptor in the uterine luminal epithelium suggests its requirement during early events of implantation. Fertil Steril. 2011;95:2087–93. doi: 10.1016/j.fertnstert.2011.01.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safranski TJ, Lamberson WR, Keisler DH. Correlations among three measures of puberty in mice and relationships with estradiol concentration and ovulation. Biol Reprod. 1993;48:669–73. doi: 10.1095/biolreprod48.3.669. [DOI] [PubMed] [Google Scholar]
- 41.Delclos KB, Weis CC, Bucci TJ, Olson G, Mellick P, Sadovova N, et al. Overlapping but distinct effects of genistein and ethinyl estradiol (EE(2)) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reprod Toxicol. 2009;27:117–32. doi: 10.1016/j.reprotox.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Risma KA, Hirshfield AN, Nilson JH. Elevated luteinizing hormone in prepubertal transgenic mice causes hyperandrogenemia, precocious puberty, and substantial ovarian pathology. Endocrinology. 1997;138:3540–7. doi: 10.1210/endo.138.8.5313. [DOI] [PubMed] [Google Scholar]
- 43.Jefferson WN, Padilla-Banks E, Newbold RR. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol Reprod. 2005;73:798–806. doi: 10.1095/biolreprod.105.041277. [DOI] [PubMed] [Google Scholar]
- 44.Lomniczi A, Loche A, Castellano JM, Ronnekleiv OK, Bosch M, Kaidar G, et al. Epigenetic control of female puberty. Nat Neurosci. 2013;16:281–9. doi: 10.1038/nn.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagner IV, Sabin MA, Pfaffle RW, Hiemisch A, Sergeyev E, Korner A, et al. Effects of obesity on human sexual development. Nat Rev Endocrinol. 2012;8:246–54. doi: 10.1038/nrendo.2011.241. [DOI] [PubMed] [Google Scholar]
- 46.Thigpen JE, Setchell KDR, Ahlmark KB, Locklear J, Spahr T, Caviness GF, et al. Phytoestrogen content of purified, open- and closed-formula laboratory animal diets. Lab Anim Sci. 1999;49:530–6. [PubMed] [Google Scholar]
- 47.Thigpen JE, Setchell KD, Kissling GE, Locklear J, Caviness GF, Whiteside T, et al. The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol A studies. Journal of the American Association for Laboratory Animal Science : JAALAS. 2013;52:130–41. [PMC free article] [PubMed] [Google Scholar]
- 48.Degen GH, Janning P, Diel P, Bolt HM. Estrogenic isoflavones in rodent diets. Toxicol Lett. 2002;128:145–57. doi: 10.1016/s0378-4274(02)00009-7. [DOI] [PubMed] [Google Scholar]
- 49.Takashima-Sasaki K, Komiyama M, Adachi T, Sakurai K, Kato H, Iguchi T, et al. Effect of exposure to high isoflavone-containing diets on prenatal and postnatal offspring mice. Biosci Biotechnol Biochem. 2006;70:2874–82. doi: 10.1271/bbb.60278. [DOI] [PubMed] [Google Scholar]
- 50.Casanova M, You L, Gaido KW, Archibeque-Engle S, Janszen DB, Heck HD. Developmental effects of dietary phytoestrogens in Sprague-Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro (vol 51, pg 236, 1999) Toxicol Sci. 1999;52:Cp2–Cp. doi: 10.1093/toxsci/51.2.236. [DOI] [PubMed] [Google Scholar]
- 51.Thigpen JE, Setchell KD, Saunders HE, Haseman JK, Grant MG, Forsythe DB. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. ILAR journal / National Research Council, Institute of Laboratory Animal Resources. 2004;45:401–16. doi: 10.1093/ilar.45.4.401. [DOI] [PubMed] [Google Scholar]
- 52.Ito D, Fujisawa T, Iida H, Suzuki N. Characterization of seipin/BSCL2, a protein associated with spastic paraplegia 17. Neurobiol Dis. 2008;31:266–77. doi: 10.1016/j.nbd.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 53.Brisken C, Park S, Vass T, Lydon JP, O’Malley BW, Weinberg RA. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc Natl Acad Sci U S A. 1998;95:5076–81. doi: 10.1073/pnas.95.9.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shyamala G, Yang X, Silberstein G, Barcellos-Hoff MH, Dale E. Transgenic mice carrying an imbalance in the native ratio of A to B forms of progesterone receptor exhibit developmental abnormalities in mammary glands. Proc Natl Acad Sci U S A. 1998;95:696–701. doi: 10.1073/pnas.95.2.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kingsley-Kallesen M, Mukhopadhyay SS, Wyszomierski SL, Schanler S, Schutz G, Rosen JM. The mineralocorticoid receptor may compensate for the loss of the glucocorticoid receptor at specific stages of mammary gland development. Mol Endocrinol. 2002;16:2008–18. doi: 10.1210/me.2002-0103. [DOI] [PubMed] [Google Scholar]
- 56.Weihua Z, Saji S, Makinen S, Cheng G, Jensen EV, Warner M, et al. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci U S A. 2000;97:5936–41. doi: 10.1073/pnas.97.11.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahima RS, Prabakaran D, Flier JS. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest. 1998;101:1020–7. doi: 10.1172/JCI1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elias CF. Leptin action in pubertal development: recent advances and unanswered questions. Trends Endocrinol Metab. 2012;23:9–15. doi: 10.1016/j.tem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Friedman JM. Leptin at 14 y of age: an ongoing story. Am J Clin Nutr. 2009;89:973s–9s. doi: 10.3945/ajcn.2008.26788B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Savage DB. Mouse models of inherited lipodystrophy. Dis Model Mech. 2009;2:554–62. doi: 10.1242/dmm.002907. [DOI] [PubMed] [Google Scholar]
- 61.Bhattarai JP, Abraham IM, Han SK. Genistein excitation of gonadotrophin-releasing hormone neurones in juvenile female mice. J Neuroendocrinol. 2013;25:497–505. doi: 10.1111/jne.12020. [DOI] [PubMed] [Google Scholar]
- 62.Mueller JK, Heger S. Endocrine disrupting chemicals affect the gonadotropin releasing hormone neuronal network. Reprod Toxicol. 2014;44:73–84. doi: 10.1016/j.reprotox.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 63.Cunningham MJ, Clifton DK, Steiner RA. Leptin’s actions on the reproductive axis: perspectives and mechanisms. Biol Reprod. 1999;60:216–22. doi: 10.1095/biolreprod60.2.216. [DOI] [PubMed] [Google Scholar]
- 64.Relic B, Zeddou M, Desoroux A, Beguin Y, de Seny D, Malaise MG. Genistein induces adipogenesis but inhibits leptin induction in human synovial fibroblasts. Lab Invest. 2009;89:811–22. doi: 10.1038/labinvest.2009.41. [DOI] [PubMed] [Google Scholar]
- 65.Szkudelski T, Nogowski L, Pruszynska-Oszmalek E, Kaczmarek P, Szkudelska K. Genistein restricts leptin secretion from rat adipocytes. J Steroid Biochem Mol Biol. 2005;96:301–7. doi: 10.1016/j.jsbmb.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 66.Kim HK, Nelson-Dooley C, Della-Fera MA, Yang JY, Zhang W, Duan J, et al. Genistein decreases food intake, body weight, and fat pad weight and causes adipose tissue apoptosis in ovariectomized female mice. J Nutr. 2006;136:409–14. doi: 10.1093/jn/136.2.409. [DOI] [PubMed] [Google Scholar]
- 67.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–87. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 68.McBryan J, Howlin J, Napoletano S, Martin F. Amphiregulin: role in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:159–69. doi: 10.1007/s10911-008-9075-7. [DOI] [PubMed] [Google Scholar]
- 69.Hovey RC, Aimo L. Diverse and active roles for adipocytes during mammary gland growth and function. J Mammary Gland Biol Neoplasia. 2010;15:279–90. doi: 10.1007/s10911-010-9187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Landskroner-Eiger S, Park J, Israel D, Pollard JW, Scherer PE. Morphogenesis of the developing mammary gland: stage-dependent impact of adipocytes. Dev Biol. 2010;344:968–78. doi: 10.1016/j.ydbio.2010.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Couldrey C, Moitra J, Vinson C, Anver M, Nagashima K, Green J. Adipose tissue: a vital in vivo role in mammary gland development but not differentiation. Dev Dyn. 2002;223:459–68. doi: 10.1002/dvdy.10065. [DOI] [PubMed] [Google Scholar]