Abstract
Alcohol abuse, either by acute intoxication or prolonged excessive consumption, leads to pathological changes in many organs and tissues including skeletal muscle. As muscle protein serves not only a contractile function but also as a metabolic reserve for amino acids, which are used to support the energy needs of other tissues, its content is tightly regulated and dynamic. This review focuses on the etiology by which alcohol perturbs skeletal muscle protein balance and thereby over time produces muscle wasting and weakness. The preponderance of data suggest that alcohol primarily impairs global protein synthesis, under basal conditions as well as in response to several anabolic stimuli including growth factors, nutrients, and muscle contraction. This inhibitory effect of alcohol is mediated, at least in part, by a reduction in mTOR kinase activity via a mechanism that remains poorly defined but likely involves altered protein-protein interactions within mTOR complex 1. Furthermore, alcohol can exacerbate the decrement in mTOR and/or muscle protein synthesis present in other catabolic states. In contrast, alcohol-induced changes in muscle protein degradation, either global or via specific modulation of the ubiquitin-proteasome or autophagy pathways, are relatively inconsistent and may be model dependent. Herein, changes produced by acute intoxication versus chronic ingestion are contrasted in relation to skeletal muscle metabolism, and limitations as well as opportunities for future research are discussed. As the proportion of more economically developed countries ages and chronic illness becomes more prevalent, a better understanding of the etiology of biomedical consequences of alcohol use disorders is warranted.
Keywords: ethanol, alcohol use disorder, skeletal muscle, protein synthesis, proteolysis
estimates from a national health survey show more than half of the adult population in the United States consume ethyl alcohol and ∼5% can be classified as “heavy” drinkers (e.g., more than 7 standard drinks per week for women and more than 14 drinks per week for men) (19). Moreover, demographic data also suggest an increase in episodic heavy (binge) drinking (12) which is associated with a higher odds ratio of mortality (37). Multi-criteria decision analysis of drug use in the United Kingdom reveals that alcohol (aka ethanol) is the drug that causes the greatest combined harm to the user and the wider society (101). While light to moderate alcohol consumption has a beneficial or protective effect on some organ systems (e.g., cardiovascular), both excessive chronic alcohol consumption and acute intoxication adversely affect multiple organ systems and ultimately increase mortality (49). While most studies and reviews have focused on the effect of alcohol on brain or liver, the potential toxic effects of alcohol on striated muscle are among the earliest recognized derangements and represent one of the most common forms of skeletal muscle myopathy (114).
This review focuses on the molecular etiology for the derangements in skeletal muscle protein metabolism and/or mass under conditions of acute alcohol intoxication and chronic alcohol abuse. The acute and chronic nature of the alcohol exposure, where possible, will be separated and defined, as the mechanisms for the observed changes often times differ. Moreover, although alcoholic cardiomyopathy is an important clinical manifestation of prolonged alcohol abuse (110), discussion has been restricted specifically to skeletal muscle to minimize any potential confusion related to the tissue-specific effects of alcohol. Moreover, other conditions (e.g., cancer, aging, disuse, sepsis, trauma, diabetes) are also associated with the erosion of lean body mass (LBM) and are not reviewed, as they may proceed via different mechanisms. The reader is referred to recent excellent reviews on these topics (6, 50, 71, 98). The final section does, however, provide analysis of pertinent studies where the interaction of alcohol with other catabolic states has been examined. Finally, we have highlighted limitations of various approaches that may complicate data interpretation and provide commentary on future research opportunities.
ALCOHOL DECREASES BASAL MUSCLE PROTEIN SYNTHESIS
Following early reports of patients presenting with skeletal muscle symptoms in greater frequency and independent of other alcohol-related pathologies (i.e., cirrhosis, cardiomyopathy, or neuropathy), attention turned to determining how alcohol causes such a substantial loss of muscle function and size (15, 17, 20, 33, 90). Early investigations in rats showed that chronic alcohol consumption reduced muscle protein content and protein synthesis in type II myofibers, and paved the way for subsequent studies elucidating how alcohol alters various components of the protein synthetic machinery and signal transduction pathways (92, 112). As protein degradation appears to be minimally impacted in this disease (see pathways for muscle protein degradation), the regulation of protein synthesis has been of primary importance. This first section highlights recent advances as well as past work that laid the foundation for these discoveries. Unless specifically noted, results were generated from work performed in animal models using either rats or mice and refer to skeletal muscle rich in type II myofibers like the gastrocnemius and/or plantaris.
Chronic alcoholic consumption.
One advantage of rodent models is the ability to tightly match nutritional intake so that all animals are provided an isocaloric, isonitrogenous diet differing only in the presence of alcohol (65, 85). Therefore, metabolic differences between control and alcohol-fed rodents can be attributed solely to alcohol intake. Rats ingesting the alcohol-containing diet typically derive up to 36% of their total daily caloric intake from ethanol. Extrapolation to humans would necessitate an intake of ∼700 kcal ethanol/day or the ingestion of approximately seven standard drinks. While the total caloric intake from alcohol is higher for rats than for humans, rodents also have a greater rate of alcohol metabolism. Hence, the blood alcohol level (BAL) achieved in chronic alcohol-fed rodents is quite comparable to that observed in humans (100–200 mg/dl) (18, 51).
Muscle mass and protein synthesis.
The protracted imbalance in protein homeostasis resulting from excessive chronic alcohol consumption manifests as a decrease in muscle mass and cross-sectional area (CSA) of type II fiber-rich muscle and the development of progressive proximal myopathy (23, 33, 90, 147). The prolonged imbalance between skeletal muscle protein accretion and breakdown represents the basic mechanism of alcohol-induced myopathy. However, the reduced rate of muscle protein synthesis per se appears to be the primary contributor to this catabolic condition. After being first reported in skeletal muscle from alcoholic patients (105), several independent investigators have modeled this pathology using a variety of alcohol feeding regimens in rats (62, 69, 72, 80, 113).
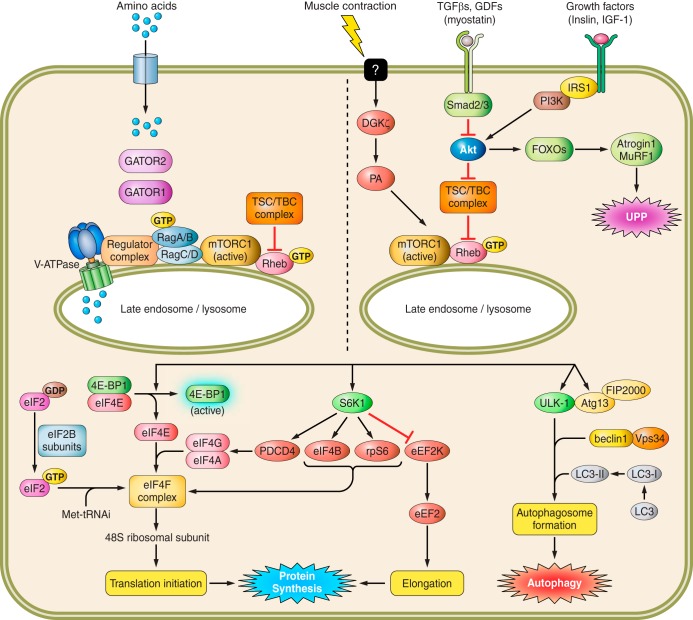
The synthesis of new proteins is highly regulated and controlled by multiple signals integrated by mTOR (mammalian/mechanistic target of rapamycin), which resides as part of two distinct protein complexes, mTORC1 and mTORC2 (83). While a primary role of mTORC1 is regulating protein synthesis, this protein complex also mediates autophagy, lysosome biogenesis, and lipid biosynthesis, thereby broadly integrating and regulating cellular energy metabolism (Fig. 1). The role of mTOR complex 2 (mTORC2) is less well understood but includes effects on cytoskeletal organization as well as growth, differentiation, and survival (31). However, the recent literature suggests that mTORC2 may have an important role in metabolic reprogramming (45, 94). The proteins forming mTORC1 (in addition to mTOR) include DEP domain-containing mTOR-interacting protein (Deptor), regulatory associated protein of mTOR (Raptor), mammalian lethal with SEC13 protein-8 (MLST8), and proline-rich Akt substrate of 40 kDa (PRAS40). In mTORC2 the rapamycin-insensitive companion of mTOR (Rictor) replaces Raptor, while PRAS40 is replaced by the mammalian stress-activated protein kinase interacting protein-1 (SIN1) and Protor. In general, chronic alcohol intake does not alter the total content of mTORC1 proteins in skeletal muscle (62). However, alcohol does disrupt protein-protein interactions within mTORC1, which may account for impaired kinase activity. Chronic alcohol consumption decreases the autophosphorylation of mTOR (Ser2481) (135), an indicator of mTOR catalytic activity, in conjunction with enhanced binding of Raptor to mTOR and Deptor (62). This latter association is posited to be of particular importance as Deptor is a recognized mTOR-inhibitory protein (109) and decreasing Deptor content in muscle partially prevents muscle wasting (55).
Fig. 1.
Pathways modulating skeletal muscle protein balance. See text for definitions. Signals from various anabolic stimuli enter the cell via membrane transporters (i.e., amino acids) or receptors (i.e., myostatin, hormones). Amino acids induce mTOR translocation and activation at the lysosomal surface via the Rag family of proteins and the GTP loading of Rheb. In contrast, TGFβs, GDFs, and growth factors converge at Akt to either activate mTOR at the lysosome via TSC1/TBC complex phosphorylation and subsequent Rheb GTP-loading or enhance proteasome activity [ubiquitin-proteasome pathway (UPP)] and muscle degradation. Upon lysosomal translocation and activation, mTOR phosphorylates several downstream targets, including 4E-BP1, S6K1, and ULK-1. Phosphorylation of 4E-BP1 and S6K1 enhances translation initiation and protein elongation, whereas ULK-1 regulates autophagosome formation and autophagy.
Protein synthesis is stimulated by events initiated by mTORC1, including phosphorylation of its two primary substrates, ribosomal protein S6 kinase (S6K)1 and eukaryotic initiation factor 4E binding protein-1 (4E-BP1). Phosphorylation of S6K1 enhances its kinase activity to subsequently phosphorylate multiple downstream proteins capable of stimulating mRNA translation (129). As chronic alcohol consumption suppresses ribosomal protein S6 (rpS6) phosphorylation in skeletal muscle (62), it is possible that the phosphorylation of other downstream targets (e.g., programed cell death protein-4 and eIF4B) is also impaired; however, this has not yet been assessed. Chronic alcohol also impairs 4E-BP1-dependent initiation of cap-dependent mRNA translation (69, 72, 80). The decreased phosphorylation of 4E-BP1 in response to chronic alcohol consumption enhances its association with eIF4E, which decreases binding of eIF4E with eIF4G and ultimately prevents the formation of the active eIF4F complex necessary for mRNA binding to the 43S preinitiation complex (2, 129). Conversely, these alcohol-induced effects on mTOR kinase activity are reversed in rats when alcohol is removed from the diet (155).
Chronic alcohol intake also impairs translational efficiency (i.e., functioning of existing protein synthetic molecules); however, whether this occurs in addition to a reduction in ribosome number remains equivocal (80, 115, 120). While it was originally reported that alcohol consumption for 6 wk decreased total RNA content (assumed to be an indicator of ribosome number, as 80–90% of cellular RNA in muscle is ribosomal), subsequent work failed to confirm such an effect even when the duration of alcohol feeding was prolonged for several months (67, 68, 80, 81, 115, 153). Further support for the impact of impaired translational efficiency on alcoholic myopathy is garnered from reports of suppressed eIF2B activity in muscle of chronically fed rats (69, 80). Binding of met-tRNAiMet to the 40S ribosomal subunit, necessary for 43S preinitiation complex formation, is enhanced by eIF2B, as it catalyzes the exchange of eIF2-bound GDP for GTP and regenerates the active eIF2·GTP complex to which the met-tRNAiMet binds.
Peptide chain elongation and termination/release of the new protein complete translation and represent potential sites for alcohol action. Chronic alcohol feeding has divergent effects on various markers of peptide chain elongation. Alcohol decreases the distribution of 40S and 60S subunits in the type II psoas muscle, indicating that the movement of ribosomes along the mRNA (i.e., elongation) and subsequent release of the mRNA (i.e., termination) is unable to keep pace with binding of mRNA to the ribosome (i.e., initiation) (80). Similarly, a decrease in the protein content of eukaryotic elongation factor (eEF)1A occurred in the gastrocnemius of alcohol-fed rats, implicating a potential decrease in the transfer of aminoacyl-tRNAs to the A-site of the ribosome. However, it appears alcohol does not slow the movement of the tRNA to the P-site of the ribosome, as no change in eEF2 Thr56 phosphorylation was detected (154). The effect of chronic alcohol intake on termination and release of the polypeptide chain from the ribosome has not yet been assessed.
Regulation of mTORC1.
Progress has been made toward elucidating the mechanism by which chronic alcohol intake decreases muscle protein synthesis and how this relates to the developing myopathy. Nonetheless, gaps in our understanding remain, including exactly how alcohol induces the mTOR-associated decrease in translation initiation. The mTORC1 pathway is regulated by several upstream inputs, including (but not limited to) AMP-activated protein kinase (AMPK), regulated in development and DNA response (REDD1) protein, nutrients (e.g., amino acids), and growth factors (e.g., insulin). Activation of AMPK and REDD1 content in skeletal muscle is unchanged by chronic alcohol intake in adult male rats (62, 70) and therefore appear unlikely mediators of the suppression of basal mTORC1 activity. With regard to nutrients, chronic alcohol consumption does not alter plasma levels of the branched-chain amino acids (BCAAs) leucine, isoleucine, and valine, which are the most potent stimulators of protein synthesis (57, 62, 130). Similarly, chronic alcohol does not lower circulating levels of insulin (74). While these findings indicate that the prevailing concentrations of amino acids and insulin are not causally related to the alcohol-induced decrease in protein synthesis, they do not exclude the possibility that alcohol antagonizes the anabolic effects of these stimuli (see modulation of anabolic responsiveness in muscle).
In contrast, a significant decrease in the anabolic hormone insulin-like growth factor (IGF)-I is detected in both plasma and muscle and correlates with the decrease in muscle protein synthesis and development of alcohol myopathy (65, 69). Accordingly, administration of a binary complex consisting of IGF-I and IGF-binding protein-3 (IGFBP-3), which delays growth factor clearance and extends bioactivity, restored muscle protein synthesis to basal control values when given during the final week of chronic alcohol feeding (65, 69). However, unexpectedly, 4E-BP1 phosphorylation remained decreased in alcohol-fed rats treated with the binary complex, indicating that alcohol may have mTORC1-independent effects on protein synthesis or that the IGF-induced increase in synthesis was mediated by either mTORC1-induced phosphorylation of S6K1 or an mTOR-independent mechanism (69).
While these findings represent the generally accepted mechanism of how chronic alcohol consumption impairs muscle protein metabolism, differences in the sex and age of the experimental animals may complicate data interpretation. For example, in contrast to human studies, where women are more susceptible than men to developing alcoholic myopathy (148), female rats did not exhibit overt disease after 26 wk of alcohol consumption; no reduction in gastrocnemius weight, total protein, or rates of protein synthesis were observed (72). Furthermore, plasma IGF-I and the phosphorylation and/or binding of eIF4E, eIF4G, and 4E-BP1 was not altered in alcohol-fed female rats (72). Sex differences may be affected by muscle fiber type composition, as the RNA-to-protein ratio was decreased more in the plantaris than in the gastrocnemius or soleus of female compared with male rats (47). Strain differences may also influence the response of female rats to chronic alcohol, as adult Fischer F344 rats (62) show a reduction in protein synthesis and mTOR signaling whereas Sprague-Dawley (72) and Wistar (47) rats did not exhibit an overt myopathy. These findings are highlighted to underscore the importance of the recent NIH initiative toward the inclusion of both male and female animals in all experimental studies. This work emphasizes the need for models specifically mimicking the physiological impact of chronic heavy alcohol consumption in females, where variations in the hormonal milieu and smaller overall muscle mass with potentially different fiber type characteristics may influence disease development. Elucidating the cause for this sexual dimorphic response to alcohol could also potentially identify novel causative factors.
The age of the animal when alcohol feeding is initiated may also influence the development of myopathy. The use of young or immature rats (i.e., ∼6 wk of age, 100–150 g body wt) is common and allows for a shorter period of alcohol feeding prior to the development of myopathy (116). However, whether the myopathy present in young rodents results from a blunting of normal muscle growth and protein accretion or authentic muscle atrophy as seen in adult human subjects remains ill-defined. Accordingly, the muscle catabolic response to chronic alcohol intake was exaggerated in 18-mo-old compared with 4-mo-old mature rats, which was ascribed to greater inhibition of mTORC1 activity via activation of the stress-sensing proteins AMPK and REDD1 (62). Therefore, alcohol may have differential effects at both ends of the age continuum, and a consensus on the age of initiation and duration of feeding may prove effective for producing consistent results across studies utilizing this model.
Acute alcohol intoxication.
Acute ingestion of large doses of alcohol (i.e., binge drinking) antagonizes muscle protein synthesis in a dose- and time-dependent manner. The most commonly employed model of acute intoxication uses either intraperitoneal (ip) injection or oral gavage to deliver a single dose of alcohol in rats or mice. The standard dose administered to rats is 75 mmol/kg (∼3.5 g/kg), which produces a BAL of ∼300 mg/dl (∼65 mM) after 2.5 h (78). However, doses as low as 20 mmol/kg (78) and as high as 90 mmol/kg (79) have been reported. In mice, doses typically range from 3 to 5 g/kg body wt and produce a BAL of ∼275–350 mg/dl after 1 h (77, 140); a lower dose of 1.2 g/kg of alcohol is cleared from circulation within an hour (54). Although oral gavage is more physiological, both routes of administration yield comparable BALs (86) and have similar inhibitory effects on muscle protein synthesis (78).
Acute alcohol intoxication (75 mmol/kg) in rats decreases muscle protein synthesis, translational efficiency, and mTORC1 signaling (66, 72, 78, 79). Phosphorylation of rpS6, 4E-BP1, mTOR, and eIF4G is decreased, whereas the association of 4E-BP1 with eIF4E and mTOR with Raptor is increased by acute intoxication (63, 66, 72, 78, 79). Although some studies have also reported an alcohol-induced decrease in S6K1 phosphorylation (62, 78), this reduction is not consistently detected (63, 66). However, when observed, the alcohol-induced reduction in S6K1 phosphorylation was independent of the sex of the animal, route of alcohol administration (e.g., intraperitoneal vs. intragastric), and nutritional status (fed vs. fasted) (78). Last, despite decreases in translational efficiency, alcohol does not acutely alter the expression or activity of eIF2α, which mediates the formation of the 43S preinitiation complex, or eIF2B, which catalyzes the exchange of eIF2-bound GDP for GTP to regenerate the active eIF2·GTP complex necessary for translation initiation (68).
Similar changes following acute alcohol intoxication (5 g/kg, 1 h) are observed in mice as the rate of muscle protein synthesis, 4E-BP1 Thr37/46 phosphorylation, and the binding of 4E-BP1 to Raptor are decreased (77). Moreover, Raptor Ser792 phosphorylation was increased along with binding of mTOR to Raptor, likely contributing to the suppression of mTORC1 activity (77). Raptor is a scaffolding protein for mTORC1 and is a direct substrate for AMPK, implicating it as an essential component of AMPK-mediated suppression of mTORC1 during situations of cellular stress. ERK1/2 phosphorylation is also suppressed, indicating that alcohol antagonizes pathways that can alter protein synthesis independently of mTORC1 (141, 157). Finally, the inhibitory effect of acute alcohol on muscle protein synthesis is relatively long-lasting (>13 h) and persists for hours, even after clearance of alcohol from the circulation and normalization of mTORC1 signaling (140). Therefore, acute alcohol may damage the synthetic process in muscle to a greater extent than predicted by solely monitoring mTORC1 signaling at a single time point.
Supporting work has also been reported using the C2C12 myoblast model. Culturing C2C12 myoblasts with alcohol (80–100 mM) for 24 h reduces protein synthesis and mTORC1 signaling, including phosphorylation of its substrates 4E-BP1 and S6K1 among others (41, 44). In this system there appears to be a greater role for AMPK-mediated changes of the mTORC1 pathway or at least a greater ability to detect and/or manipulate activation of this protein. For example, alcohol-induced impairment of elongation assessed by eEF2 phosphorylation was mediated by AMPK (43). In vitro work has also permitted a greater mechanistic understanding of the direct effects of alcohol on muscle synthetic signaling and has revealed a potential role for mTORC2 signaling (42). Incubation of C2C12 myoblasts with alcohol (100 mM) increases mTORC2 activity assessed by increased phosphorylation of its substrate Akt (Ser473) as well as decreasing Rictor phosphorylation and its association with Deptor and the 14-3-3 protein (41, 42). These changes occur concomitantly with the alcohol-induced decrease in mTORC1 activity and protein synthesis, indicating a potential interaction between the two mTOR complexes. In this regard, Rictor knockdown using shRNA increases S6K1 Thr389 phosphorylation (i.e., mTORC1 activity) in control cells and prevents the alcohol-induced decrease in protein synthesis (41, 42). The importance of these alcohol-induced changes in mTORC2 signaling will need to be verified in vivo. For a more thorough description and analysis of the in vitro effects of alcohol on myocyte protein synthesis the reader is referred to the review by Hong-Brown et al. (45).
Limitations and opportunities.
One limitation to this field of study is the lack of mechanistic human data available from individuals with different types of alcohol use disorder (AUD) (34). Therefore, experimental evidence garnered from rat and mouse models is assumed to provide an accurate representation of the human disease. Moreover, while chronic alcohol-related myopathy has been consistently reported in various strains of rats, there is a paucity of data as to whether mice experience similar consequences. To our knowledge only one report exists showing significant loss of muscle mass and CSA in female C57BL/6 mice after 6 wk on an alcohol-containing liquid diet, but as that work focused on increased autophagy (e.g., protein degradation) it is unknown whether protein synthesis was reciprocally suppressed (144). Verification that mice develop chronic alcoholic myopathy similarly to humans is important to expand mechanistic research as more transgenic models are available (compared with rats), and genetic manipulations are easier to perform. Similarly, increased availability of muscle-specific and/or inducible knockout models should aid in the future identification or validation of the importance of specific components of the mTORC1 (and mTORC2) signaling pathway in alcoholic myopathy.
Emerging evidence supports the role of another signaling pathway upstream of mTORC1 in the control of muscle atrophy; the Smad family of proteins are activated in response to transforming growth factor (TGF)β, activin, or select growth and differentiation factors like myostatin and are essential mediators of muscle atrophy (30). For instance, Smad signaling is required for the myostatin-mediated inhibition of Akt/mTORC1 signaling and myotube atrophy, as well as for TGFβ-induced fiber atrophy (30, 128, 146). Nevertheless, mTORC1-independent effects of myostatin signaling may also occur (159). Smad signaling in muscle has not been assessed following alcohol intoxication; however, both TGFβ and myostatin mRNA are increased in response to alcohol feeding (69, 102). Collectively, the increase in myostatin and TGFβ suggests the Smad pathway may be activated and contributing to the impairment of mTORC1. This increased TGFβ also suggests that alcohol may increase fibrosis and remodeling in the skeletal muscle over time, which could also potentially impair muscle function (38, 119).
Appropriate translocation of mTOR to the lysosomal surface is necessary for its activation and is a rapidly evolving area in the study. Positioning of mTOR at or near the lysosomal membrane is essential to its interaction with and subsequent activation by GTP-loaded Rheb. GTP/GDP loading of Rheb is regulated by the TSC1/TSC2/TBC complex (aka the Rhebulator), which, when phosphorylated by Akt, dissociates from the lysosome and allows Rheb to remain GTP loaded and active (14, 162). Currently, the effects of alcohol on the lysosome in general, or translocation of mTOR to the lysosomal membrane specifically, remain unknown. Modulation of the Rhebulator complex and Rheb GDP/GTP loading state by alcohol also remain unstudied. As the lysosomal positioning of mTOR is at the crux of amino acid-dependent activation of protein synthesis (125), the importance of these observations cannot be underestimated. Should alcohol prevent the appropriate interactions of mTOR at the lysosomal surface, it could be inferred that alcohol may also prevent the normal stimulation by amino acids. Such a defect may contribute to the development of myopathy in chronic alcoholics in the absence of overt malnutrition. As discussed in pathways for muscle protein degradation, alcohol may adversely impact other important lysosome functions, including autophagy (118).
Finally, a major shortcoming in the literature associated with the prevalent use of animal models to mimic chronic alcohol myopathy is the lack of accompanying functional end points. Much of the seminal work describing alcoholic myopathy was based upon functional deficits in humans, which included muscle pain and weakness (114); however, muscle function per se is rarely measured in animals likely due to methodological difficulties or limitations. Being able to quantify the deleterious effects of alcohol on contractile function in the same muscle for which corresponding synthetic signaling data are available would be invaluable.
PATHWAYS FOR MUSCLE PROTEIN DEGRADATION
Tissue protein content is also governed by protein degradation, which is primarily distributed between the ubiquitin-proteasome pathway (UPP) and the autophagic-lysosomal system (6, 126). The role of intracellular protein degradation in the development of alcoholic myopathy as well as the relative contributions of these two pathways are ill-defined and an issue of some debate. The paucity of data in this area relates, at least in part, to the lack of a convenient method for assessing in vivo rates of proteolysis, causing data to be limited by their inferential nature. In this regard, studies have used urinary 3-methylhistidine (3-MH) excretion as a marker of whole body protein breakdown, with the understanding that release of this amino acid can originate from myofibrillar degradation in both skeletal and smooth (i.e., intestine) muscle (35). Although not extensive, there are reports that 3-MH excretion is either decreased (91) or unchanged (122) in humans chronically consuming alcohol and increased in alcohol-consuming (6 wk) mice (100). Hence, data on 3-MH excretion provide little definitive insight into the effect of alcohol on muscle proteolysis, thereby necessitating other approaches.
UPP and proteases.
In mature rats, acute alcohol intoxication decreased activity of the cytoplasmic proteases alanyl aminopeptidase, arginyl amino peptidase and leucyl aminopeptidase at 2.5 h post-alcohol administration (59). Furthermore, while dipeptidyl aminopeptidase II was decreased by acute alcohol, there was no alcohol-induced change in dipeptidyl aminopeptidase I (59). As these relatively modest alcohol-induced changes were transient in nature and their activity did not differ in muscle from chronic alcohol-fed and pair-fed control rats (59, 121), the contribution of these enzymes to the development of alcoholic myopathy appears limited. In addition, the cysteine proteases referred to as calpains, which are calcium but not ATP dependent, could potentially contribute to the degradation of cytoplasmic proteins (138). However, the activity of calpain 1 and 2 (μ- and m-calpains, respectively), and their inhibitor calpastatin, did not differ in alcohol-fed and control rats (59). Chronic alcohol consumption also did not alter muscle caspase-3 activity, which contributes to apoptotic cell death by both the extrinsic (death ligand) and intrinsic (mitochondrial) pathways (133). In this regard, a surrogate marker for caspase-3-mediated cleavage of actomyosin in skeletal muscle, a 14-KDa degradation product of actin, did not differ between control and alcohol-fed rats (152). Likewise, neither acute nor chronic alcohol abuse altered other end points related to apoptosis [i.e., DNA fragmentation or TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) assays] in rat skeletal muscle (106).
The role of the UPP as a major regulator of overall muscle proteolysis and the degradation of myofibrillar proteins have been areas of recent focus, and the specifics of this ATP-dependent proteolytic system have been reviewed elsewhere (126). A diverse array of atrophic stimuli increases two muscle-specific ubiquitin E3 ligases, muscle atrophy F-box (MAFbx or atrogin-1) and muscle RING finger-1 (MuRF1), and the mRNA content of these proteins is routinely used as a biomarker of UPP activity (7, 29). These “atrogenes” have been reported to be increased in fast-twitch muscles in chronic alcohol-fed rats (62, 104). Moreover, acute alcohol intoxication increased atrogene mRNA expression in a dose- and time-dependent manner in skeletal muscle (152). However, independent lines of investigation suggest that these changes do not increase global proteolysis in the presence of alcohol. First, in vitro-determined proteasome activity was either unchanged (62, 152) or decreased (59) in muscle from alcohol-treated compared with control rats. Second, alcohol did not alter the rate of protein degradation of in vitro-incubated epitrochlearis muscle (143, 152). Third, tyrosine and 3-MH release from the isolated perfused hindlimb was the same in control and alcohol-treated rats (152). Finally, protein breakdown in vitro did not differ between control and alcohol (100 mM) -treated C2C12 myotubes (44). Hence, the alcohol-induced increase in atrogene mRNA is not associated with a corresponding increase in muscle proteolysis, a discordant observation that has been reported in other catabolic conditions (3, 82). One caveat to this general conclusion might involve conditions where a secondary stress is combined with alcohol, leading to enhanced proteasome activity (84).
Autophagy.
Cellular organelles as well as specific intracellular proteins can undergo autophagy-mediated degradation in the lysosome. Early studies detected no change in the lysosomal cathepsins B, D, H, and L in muscle from control and alcohol-fed mature rats or following acute alcohol intoxication (59). Thapaliya et al. (143) have reported increased autophagy in skeletal muscle of alcohol-fed mice as indicated by increased LC3B-II, autophagy-related gene (Atg)7 mRNA, and BECN1/Beclin1 protein expression; however, other work shows no change in LC3-II, p62, or ULK-1 phosphorylation between mice fed alcohol and time-matched control values (142). Increased LC3B lipidation was also detected in muscle from alcoholic patients with cirrhosis in association with a loss of muscle mass. Furthermore, complementary in vitro studies indicated short-term (e.g., 6 h) incubation of C2C12 myotubes with alcohol increased expression of autophagy-related genes, enhanced global proteolysis, and decreased myocyte size. These latter two alcohol-induced changes were largely prevented in myocytes by knockdown of Atg7 or by chemical inhibition of autophagy. Such an alcohol-induced increase in autophagy would be consistent with the above-mentioned inhibition of mTOR by alcohol, as mTOR activation is a potent inhibitor of autophagy (32). While these data demonstrate the ability of alcohol to stimulate autophagy in skeletal muscle, it is difficult to reconcile these data with the numerous in vivo studies where alcohol intoxication or long-term consumption has not been shown to increase overall muscle proteolysis (62, 152). Furthermore, the alcohol-induced activation of the autophagy pathway observed in mice contrasts with the lack of change in autophagic markers in alcohol-consuming rhesus macaques (131).
Limitations and opportunities.
Many of the data in this area are inferential, with the majority suggesting that acute alcohol intoxication and chronic alcohol consumption do not accelerate global protein breakdown. The inability to easily quantitate in vivo muscle proteolysis remains a limiting factor in assessing its contribution to the associated wasting syndrome. Alcohol-induced changes in the mRNA content of atrogenes and other UPP components are often reported; however, few studies provide corroborating evidence related to protein levels for these factors. As a discordance between alcohol-induced changes in mRNA and protein might be anticipated under circumstances where mRNA translation is impaired (74), extrapolating mRNA changes in atrogenes or components of the UPP to global protein degradation should be avoided. Utilizing mice with a tissue-specific and inducible promoter to knock down individual elements of the proteolytic signaling network (e.g., MuRF1 or atrogin-1) could provide more definitive mechanistic information regarding the relative importance of proteolytic processes as mediators of alcohol-induced muscle wasting.
Cell culture systems are commonly employed to overcome the challenges associated with in vivo research; however, relatively high concentrations (e.g., 100 mM) of ethanol over a long period of time (e.g., 24 h) are often required to induce changes in protein balance, thereby raising concerns regarding the physiological significance of this model. Moreover, the use of myoblasts versus differentiated myotubes further complicates the clinical relevance of this system. The concept of cross-talk between the various proteolytic pathways (e.g., activation of one pathway leading to a compensatory inhibition of another) has been proposed but largely unexplored in relation to alcohol (107). It is also paramount to determine exactly which cellular proteins undergo altered rates of proteolysis in response to alcohol. Finally, it remains to be assessed whether therapeutic inhibition of autophagy and/or the UPP in an attempt to minimize alcoholic muscle wasting may actually prove problematic, as this could lead to the accumulation of damaged proteins and enhanced cellular stress (127), and even greater muscle wasting (93) and increased mortality (73).
MITOCHONDRIAL ENERGY PRODUCTION AND ROS PRODUCTION
Muscle protein balance and function rely heavy on normal mitochondrial function; hence, deficits in ATP production may be causally related to the development of alcoholic myopathy (36). Early light and electron microscopic studies demonstrated diffuse morphological changes in mitochondria in both acute and chronic alcoholic myopathy, although these were not quantified and their functional significance not assessed (58, 149). Other studies reported that mitochondrial size (136, 150) and/or number (56) may be decreased in humans chronically abusing alcohol. However, functional studies in both humans and rats detected either few or no alcohol-induced change in the oxidation rates of various substrates, activity of individual respiratory chain complexes, or cytochrome content in skeletal muscle (8, 9). These data are consistent with the observation that ATP content and energy charge in the gastrocnemius did not differ between control and alcohol-fed rats (80).
In vivo muscle energy metabolism studied using 31P magnetic resonance spectroscopy (MRS) indicated the intracellular muscle pH and phosphocreatine (PCr) content did not differ between controls and humans challenged acutely with alcohol under both resting basal conditions and after exercise (160). Similarly, chronic alcohol consumption without accompanying hepatic disease did not alter basal pH or PCr in muscle. However, alcohol ingestion was associated with a greater reduction in both end points during exercise and a slower recovery of pH (but not PCr) postexercise. These data suggest that chronic alcohol abuse may alter muscle blood flow and/or produce a yet to be defined defect in mitochondrial function. While similar MRS results were reported for chronic alcoholic patients with mild cirrhosis (Child-Pugh class A), alcoholics with more extensive liver derangements (class B and C) showed a lower PCr/Pi ratio and decreased maximal rate of mitochondrial ATP synthesis (48). These data imply that cumulative alcohol consumption may have a dose-dependent effect (either direct or indirect) on muscle mitochondrial function. Overall, the lack of an alcohol-induced change in basal ATP content or synthesis in response to chronic intake is consistent with the absence of an increase in the phosphorylation of the muscle energy sensor AMPK (62).
The possibility that chronic alcohol consumption induces mitochondrial changes independently of ATP production cannot be excluded. For example, mitochondrial fusion and connectivity are inhibited in muscle from alcohol-fed rats, and this may result, in part, from a reduction in the outer mitochondrial membrane fusion protein mitofusin-1 (Mfn1) (16). Such changes may be causally related to the observed calcium dysregulation and thereby impair both bioenergetics and excitation-contraction coupling. Mitochondria also play a pivotal role in regulating apoptosis, but, as noted above, there is little definitive evidence of alcohol-induced apoptosis in skeletal muscle.
In addition to their energy producing function, mitochondria are a major source of reactive oxygen species (ROS). While the current review is not intended to provide a comprehensive description of alcohol-induced oxidative damage, some studies have examined the potential role of oxidants in the developing myopathy which are pertinent. In general, tissue damage after alcohol could be due to an increased ROS production and/or decreased antioxidant mechanisms. In this regard, the decrease in CSA in chronic alcohol-fed rats was associated with a decrease in total and free glutathione (GSH) content as well as reduced activity of glutathione reductase and glutathione peroxidase and the mRNA for the mitochondrial form of the superoxide scavenging enzyme superoxide dismutase (SOD)2 (Mn-SOD2) (102, 104). Moreover, some studies have reported that alcoholic patients have a reduction in the circulating concentration of antioxidants such as α-tocopherol (158). Consistent with these changes, redox damage in muscle from alcohol-fed rats was evidenced by an increase in protein carbonyl (61, 102) and cholesterol hydroperoxide content (1), and malondialdehyde (MDA), which primarily reflects lipid oxidation (156). In contrast, other studies have reported no alcohol-induced change in MDA in muscle from rats (61) and no change in α-tocopherol, ascorbic acid, or retinol (22) or muscle antioxidant changes in alcohol consuming patients with myopathy (21). Finally, while exogenous treatment with α-tocopherol failed to blunt the decrease in muscle protein synthesis seen with either alcohol or chronic alcohol treatment (60), administration of the anti-oxidant GSH precursor procysteine to chronic alcohol-fed rats ameliorated some of the oxidant stress response and prevented at least part of the loss of muscle CSA (103, 104). Hence, evaluation of the current data does not unequivocally support a causative role for oxidative stress in the etiology of alcoholic myopathy. However, the advent of mitochondria-targeted antioxidants may yet prove useful in limiting alcohol-induced mitochondrial dysfunction, increased production of ROS, and the resultant myopathy (123).
Limitations and opportunities.
To date, the extent and physiological importance of alcohol-induced changes in mitochondrial bioenergetics in muscle remain enigmatic. There is increasing evidence indicating discordant results obtained using isolated (in vitro) mitochondria compared with studies using in situ- or in vivo-based approaches (64). As alterations in mitochondrial structure and function are common during aging, it remains to be determined whether defects in this organelle might be responsible for the increased sensitivity of muscle to the catabolic effects of alcohol in aged rats (62). Systematic studies on mitochondrial fission and the mitochondrial stress response are needed, as the ability of alcohol to increase the latter via inhibition of mTOR signaling might be predicted (111). Finally, the relative importance and the connection between alcohol-induced mitochondrial dysfunction and tissue injury produced by ROS production specifically in skeletal muscle requires clarification, as this represents a nexus for potential therapeutic intervention.
MODULATION OF ANABOLIC RESPONSIVENESS IN MUSCLE
A strategy to prevent or attenuate the development of muscle atrophy involves administration of an anabolic agent designed to reverse and/or protect muscle from the catabolic insult. While in theory this approach appears poised for success, independent reports show that alcohol antagonizes the desired anabolic response to several exogenously administered stimuli. As presented below, alcohol-induced anabolic resistance would be expected to limit the therapeutic effectiveness of exogenously administered agents as well as the accretion of muscle protein by these same mediators when they are synthesized endogenously.
Hormone resistance.
IGF-I is a well-characterized, growth-promoting hormone that activates the canonical AKT/mTORC1 signaling pathway to increase protein synthesis (25). Chronic alcohol consumption has been consistently shown to decrease plasma and muscle IGF-I (65, 75, 99, 137). Short-term (4-day) treatment of alcohol-fed rats with an IGF-I/IGFBP3 binary complex reversed the decrease in plasma IGF-I and restored muscle protein synthesis, translational efficiency, eIF4E·eIF4G binding, eIF4G Ser1108 phosphorylation, and myostatin back to levels seen in untreated control rats (69). However, chronic alcohol feeding prevented the maximal response IGF-I/IGFBP3 stimulation, as these end points remained below those observed in the control rats treated with the binary complex (69). Furthermore, the binary complex did not reverse the alcohol-induced decrease in 4E-BP1 phosphorylation or the increase in 4E-BP1·eIF4E binding (69). Endogenously produced IGF-I can also be acutely increased with recombinant human growth hormone (GH) in chronic alcohol-fed rats; however, it is unknown whether this GH-induced increase of IGF-I is physiologically meaningful, as neither protein synthesis nor mTORC1 activity was assessed (75).
A more systematic evaluation of IGF-I action in muscle has been pursued using acute alcohol intoxication. While these alcohol models do not acutely lower blood-borne IGF-I, alcohol intoxication dose-dependently impairs IGF-I-stimulated muscle protein synthesis and mTORC1 signaling (63, 78). Resistance to IGF-I, as assessed by phosphorylation of S6K1 and rpS6, was present 2.5 h after administration of 20, 50, or 75 mmol/kg alcohol; however, partial recovery was noted at the lowest dose (63, 78). Furthermore, at the highest alcohol dose, partial IGF-I resistance was still detected 8 h after alcohol administration, and the normal anabolic response fully recovered only at 24 h. This alcohol-induced IGF-I resistance was independent of animals' sex, nutritional status (fasted/fed), or the route of alcohol administration (oral gavage/IP) (78). Acute alcohol also blunted the IGF-I-stimulated increase in 4E-BP1 phosphorylation in mice (76) but not rats (63), and the reason for this difference is not known. In vitro data do not clarify this issue, as neither S6K1 nor 4E-BP1 was measured in a previous report, showing that 3 days of alcohol exposure suppressed IGF-I stimulation of protein synthesis in myoblasts (44). Similarly, acute alcohol intoxication also decreased insulin-stimulated phosphorylation of S6K1 and S6 (but again not 4E-BP1), which was independent of a change in insulin receptor phosphorylation (63).
The effect of alcohol, whether acute or chronic, on muscle could be direct or regulated indirectly by secondary mediators released into the systemic circulation. This issue was addressed using an isolated perfused muscle preparation, and the data revealed that the inhibition was attributable to the local actions of alcohol on muscle rather than by increased concentrations of glucocorticoid or acetaldehyde (63, 78). Moreover, as little alcohol metabolism occurs in this muscle preparation, these data strongly support the concept that alcohol, and not one of its metabolites (e.g., acetaldehyde) is responsible for inhibiting protein synthesis. When assessing the mechanism of alcohol action, we emphasize that caution should be exercised when extrapolating data from other tissues. For example, in the liver, alcohol is actively metabolized, producing high levels of acetaldehyde that are present only in low concentrations in skeletal muscle because of the nominal capacity to oxidatively metabolize ethanol. Overall, alcohol produces a growth factor resistance related to protein synthesis in skeletal muscle that is in part mediated by a reduction in mTOR kinase activity and may contribute to the development of myopathy either alone or in conjunction with nutrient stimulation (see next section).
Feeding/nutrients/amino acids.
Feeding or nutrient intake stimulates muscle protein synthesis, as mTORC1 is activated both directly by select amino acids and indirectly by the coordinated rise in circulating growth factors. Moreover, these stimuli are translational in nature, as food and alcohol consumption often occur in close temporal proximity. Alcohol blunted the stimulation of muscle protein synthesis and mTORC1 activity when a nutritionally complete supplement was administered intragastrically in 24-h-fasted rats (134). In contrast, alcohol did not antagonize the stimulation of protein synthesis when nutrition was infused intravenously. While these data may suggest that alcohol inhibits nutrient absorption from the gut, subsequent studies showed that alcohol does not slow leucine distribution from the intestine or the secondary increase in plasma insulin (66).
Stimulation of mTORC1 signaling by feeding is dependent not only on hormonal activation of the canonical Akt pathway (26) but also amino acid-specific stimulation of a separate pathway composed of the Rag GTPases v-ATPase, Ragulator, GATOR1, and GATOR2 protein complexes (4). It has been shown that amino acids induce GATOR (GAP activity toward Rags)-2-mediated inhibition of GATOR1 while the Ragulator and v-ATPase mediate GTP binding of RagA and GDP binding of RagC (5). As a result, mTORC1 is recruited to the lysosomal surface, where it colocalizes with and is activated by Rheb-GTP. While addition of leucine to C2C12 myoblasts after a 24-h exposure to alcohol returned protein synthesis, binding of mTOR to Rheb and the phosphorylation of mTOR and its substrates 4E-BP1, S6K1, and rpS6 back to untreated control levels (41), these changes were all attenuated compared with the response of control (no alcohol) cells challenged with leucine. Acute alcohol intoxication in rats also blunted the leucine-induced increase in muscle protein synthesis and mTORC1 kinase activity (66). Just as exogenous leucine does not overcome the inhibitory actions of alcohol, increasing circulating levels of leucine (and the other BCAAs) via disruption of the gene encoding the mitochondrial branched-chain aminotransferase isozyme (BCATm) also does not prevent the alcohol-induced decrease in protein synthesis and mTORC1 substrate phosphorylation (77). It is also noteworthy that intracerbroventricular injection of alcohol decreased basal muscle protein synthesis and recapitulated the leucine-resistant state observed when alcohol was administered systemically, suggesting that some of the muscle metabolic effects of alcohol may be mediated via the central nervous system (117).
The effect of alcohol on Rag protein-mediated mTOR activation is not well characterized in muscle despite the recognized importance of this pathway. The simultaneous constitutive activation of RagA and RagC increased the phosphorylation of S6K1 and 4E-BP1 back to control levels in myocytes cultured with alcohol (41). However, alcohol did not impair the leucine-induced increase in RagA/C binding to mTOR (41). Supporting data have not been obtained in animals following alcohol, although chronic alcohol intake does decrease the association of RagA with Raptor (presumably decreasing mTORC1 activation) in the rat gastrocnemius (62). Collectively, current evidence indicates acute alcohol intoxication prevents the maximal anabolic response to feeding and the amino acid leucine and may contribute to the development of alcoholic myopathy.
Muscle contraction.
An anabolic response in skeletal muscle is also generated by fiber contraction (i.e., exercise). Until recently, little attention had been paid to the potential ability of alcohol to impair this stimulus or the therapeutic potential of muscle contraction in the treatment of muscle disease in individuals with AUD. Induction of muscle contractions using electrical stimulation is currently being investigated as a clinical therapy to reduce illness- and immobilization-induced muscle atrophy (88). In mice, acute alcohol intoxication (3 g/kg) immediately prior to a bout of maximal muscle contractions prevented the increase in protein synthesis and S6K1/4E-BP1 phosphorylation for at least 4 h (140). While the suppressive effect of alcohol waned over time, contraction-stimulated increases in protein synthesis and mTORC1 were still partially suppressed up to 12 h after alcohol. Such data suggest that inducing muscle contraction in individuals with elevated BALs may be ineffective as a treatment for alcoholic myopathy and demonstrate that alcohol-induced anabolic resistance is a generalizable muscle response.
Alcohol administered prior to an anabolic agent clearly impairs stimulation of protein synthesis/mTORC1 signaling. However, whether alcohol also reduced rates of synthesis which had already been increased by an anabolic agent remained unexplored despite its direct societal relevance (e.g., alcohol is commonly consumed in the evening following a meal or exercise). This concept has now been addressed in both humans and mice, although unfortunately protocols and reported results differ considerably. For example, alcohol administered in mice 2 h after muscle contraction completely reversed the contraction-induced increase in protein synthesis (141). It is noteworthy that this decrease in protein synthesis was not due to suppressed mTORC1 signaling but was associated with inhibition of translation elongation by alcohol intoxication. In humans, alcohol was ingested after a high-intensity exercise session that included both endurance and resistance exercise (108). Unfortunately, the lack of appropriate control groups (i.e., exercise alone and alcohol alone) precluded direct comparison with the aforementioned mouse study, but the data did show that alcohol suppressed the combined stimulatory effects of exercise and protein supplementation on muscle S6K1 Thr389 phosphorylation. In contradistinction, alcohol did not suppress myofibrillar fractional synthetic rates compared with the unexercised control group.
Finally, chronic alcohol feeding did not prevent the normal mTOR-dependent increase in protein synthesis or hypertrophic response of muscle whereby overload was produced by synergistic ablation (142). Additional work is needed to reconcile the apparently divergent responses observed between mice and humans and to assess the importance of the prevailing BAL in modulating contraction and hypertrophic signaling.
Limitations and opportunities.
While multiple studies have characterized the existence of alcohol-induced anabolic resistance, the physiological importance of growth factor-, nutrient-, and contraction-induced resistance is not known nor is the relative contribution of each to the erosion of LBM in AUD; the underlying mechanism(s) for each remains to be fully elucidated. Anabolic resistance caused by alcohol intoxication represents a major barrier to the successful treatment of alcoholic myopathy, as it prevents the full repertoire of responses necessary for the normal accretion of muscle protein. As both growth factor and amino acid signaling is required for maximal mTORC1 stimulation, mechanistic studies investigating the potential effects of alcohol on lysosomal regulation as well as on the activity of the proteins (i.e., Rags, v-ATPase, Ragulator, Rheb-GTP), which are integral in each signaling process would be of value. Finally, the majority of work in this area has been performed using a model of acute intoxication, but it is unclear whether chronic alcohol consumption produces a similar level of anabolic resistance.
INTERACTION OF ALCOHOL AND CATABOLIC STATES
This final section reviews studies on the interaction of alcohol and various catabolic stressors on skeletal muscle protein balance, primarily protein synthesis. The most thoroughly investigated pairing is that of alcohol and acquired immunodeficiency syndrome (AIDS) (95), due in part to the disproportionately high percentage of human immunodeficiency virus (HIV)-infected individuals with AUD (53, 124). Several studies have combined simian immunodeficiency virus (SIV) infection, a widely accepted model of HIV, and chronic alcohol ingestion in rhesus monkeys. During the early asymptomatic period of SIV infection, alcohol feeding increases the proinflammatory environment of muscle [e.g., increased tumor necrosis factor (TNF)α and interleukin (IL)-6 mRNA] but does not alter muscle protein synthesis or degradation (97). However, as the SIV disease progressed in the presence of alcohol, a greater decrease in limb muscle mass was detected (96). These changes appeared independent of alterations in IL-6, IGF-I, or myostatin mRNA in the affected muscle, although the upregulation of TNFα mRNA expression was exaggerated. In vitro-determined 20S proteasome activity of muscle from alcohol-fed animals with late-stage SIV infection was increased compared with SIV infection alone (84). A similar exacerbation of muscle wasting was reported in HIV-1 transgenic rats fed an alcohol-containing diet (10). Finally, while antiretroviral therapy has decreased morbidity and mortality from AIDS, several of the HIV-1 protease inhibitors have been reported to impair basal protein synthesis and accentuate alcohol-induced changes in skeletal muscle (39, 40, 46). For example, the coincubation of myocytes with both alcohol and the HIV protease inhibitor indinavir led to a greater reduction in mTOR activity and cap-dependent initiation than produced by either condition alone (39). Collectively, these studies demonstrate an adverse interaction between alcohol, HIV, and/or selective drugs used to treat the disease, but definitive mechanistic studies are largely lacking.
Despite the extensive literature on sepsis-induced changes in muscle protein balance per se (24, 132) and the recognized detrimental interaction of alcohol plus sepsis on other organ systems (e.g., lung, liver, and intestine) (13, 52, 161), information is lacking on the effect of alcohol on muscle protein metabolism produced by bacterial infection. Related to this topic is a report where E. coli endotoxin injected 24-h post-acute alcohol intoxication exaggerated the increase in muscle IL-6 mRNA and protein as well as the late-phase cytokine high-mobility group protein-1 (28) which, because of their proinflammatory properties, might be expected to impair protein synthesis and/or increase proteolysis (27, 145). That report is limited by the lack of accompanying data on muscle protein balance. As chronic alcohol consumption decreases survival following bacterial infection (161), this would appear to be a fruitful area for future research.
Epidemiological studies report that excessive alcohol consumption can increase or decrease the incidence of certain types of cancer in humans. Such studies will not be reviewed here, but we will instead focus on the potential interaction of alcohol and cancer cachexia. Chronic alcohol consumption exacerbates the loss of body weight in melanoma-bearing mice compared with tumor-bearing control-fed mice. This response was associated with a reduction in carcass and gonadal fat mass but also with a decrease in urinary 3-MH excretion (e.g., decreased muscle proteolysis) (100). These results could not be explained by changes in food consumption or energy production, but they suggest that impaired protein synthetic processes were the principal cause for the loss of body mass. As alcohol consumption is a recognized risk factor for a variety of cancers (87), research in this area has as high translational relevance.
Muscle disuse can occur in isolation (e.g., extended bed rest and immobilization) or concomitant with general catabolic illness; hence, alcohol may modulate either the disuse-induced loss of muscle or the accretion of muscle during the reloading phase. In the sole study in this category, oral gavage of alcohol for 3 days exaggerated the mTOR-independent decrease in muscle protein synthesis produced by unilateral hindlimb immobilization (151). Additionally, alcohol ingestion during the atrophic immobilization period exaggerated the disuse-induced increases in atrogin-1 and MuRF1 (implying increased proteolysis), and accordingly, the proteasome inhibitor Velcade prevented the exaggerated loss of muscle mass in alcohol-fed rats. Furthermore, when administered during the reloading phase, alcohol suppressed mTORC1 signaling and increased atrogene expression in association with AMPK activation (151). Collectively, these data suggest that alcohol has the potential to negatively interact with other catabolic stressors to induce derangements in protein metabolism not observed in response to the individual conditions.
An erosion of LBM commonly occurs as part of the aging process (i.e., sarcopenia), resulting in age-related muscle dysfunction (11). As the relative age of the population in the US and other developed countries continues to increase, the prevalence of AUD among the elderly represents an increasing medical concern (139). Similar to heart disease, low- to moderate-level alcohol consumption does not appear to contribute to age-induced loss of muscle, but sarcopenia is exacerbated when alcohol consumption is sustained and excessive (89, 148). When adult (4 mo) and aged (18 mo) rats were placed on an alcohol-containing diet for 20 wk, the latter demonstrated greater decreases in LBM and gastrocnemius weight (62). The ability of alcohol to accentuate the age-induced decrease in muscle mass resulted primarily from a reduction in protein synthesis (both sarcoplasmic and myofibrillar) but not from activation of the UPP. Alcohol-fed aged rats had enhanced dephosphorylation of 4E-BP1, suggesting that the underlying mechanism might be mTOR mediated. Additionally, alcohol-fed aged rats showed enhanced binding of Deptor (a negative regulator) with mTOR, which was associated with activation of AMPK signaling (e.g., increased LKB1 and AMPK phosphorylation) and total REDD1 protein (62). Finally, the accentuation of the age-induced impairment in protein synthesis and mTORC1 signaling was not associated with an exaggerated cytokine (TNFα, IL-6) response in muscle but did correlate with the reduction in muscle IGF-I.
Limitations and opportunities.
With the exception of the work on alcohol and SIV infection, research on the interaction of alcohol either prior to or during the recovery phase of different catabolic stresses represents singular studies for which the underlying etiology has not been defined and the fidelity of the preclinical models employed not critically evaluated. Moreover, many of the studies have been conducted in young growing animals in which there is a net accretion of muscle protein over time. Regardless, the translational importance of such studies is evident as the population ages and there are more individuals living with one or more chronic catabolic illnesses (aging, chronic obstructive pulmonary disease, heart failure) or convalescing from traumatic injury (surgery, falls, disuse).
SUMMARY
The effects of alcohol, like all drugs, can be beneficial or detrimental based on the dose and duration of exposure. Low to moderate doses of alcohol have little to no effect, either directly or indirectly, on muscle protein balance, whereas acute alcohol intoxication and chronic alcohol abuse decrease basal muscle protein synthesis via what appears to be a largely mTOR-dependent mechanism. Of potentially greater importance is the ability of alcohol to induce a resistance to a variety of anabolic stimuli all of which converge on mTORC1 to influence muscle protein homeostasis. It is unclear whether this diminished responsiveness to growth factors, nutrients, and muscle contraction is mediated via a common mechanism or, more likely, via defects in multiple pathways that intersect and result in mTORC1 inhibition. In addition, alcohol has the potential to interact with and exacerbate the derangements in muscle protein balance produced by other catabolic conditions. As protein synthesis and degradation are coordinately controlled, what appears to be the relative selective alcohol-induced decrease of protein synthesis in muscle is somewhat unexpected. With methodological advances, future studies should be able to illuminate more subtle or nuanced effects of alcohol on the synthesis and degradation of individual proteins. Such studies will yield insight into the mechanism of action for alcohol and how such changes negatively impact muscle structure and function, potentially contributing to the increased morbidity associated with alcohol use disorders.
GRANTS
This work was supported in part by NIAAA Grant R37 AA-011290 (C. H. Lang) and F32 AA23422 (J. L. Steiner).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L.S. and C.H.L. interpreted results of experiments; J.L.S. and C.H.L. prepared figure; J.L.S. and C.H.L. drafted manuscript; J.L.S. and C.H.L. edited and revised manuscript; J.L.S. and C.H.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our many collaborators who helped to make our work possible, especially Drs. L. Jefferson, S. Kimball, C. Lynch, and the late T Vary. We apologize to those investigators whose work was not cited due to space limitations or our oversight.
REFERENCES
- 1.Adachi J, Kudo R, Asano M, Ueno Y, Hunter R, Rajendram R, Martin C, Preedy VR. Skeletal muscle and liver oxysterols during fasting and alcohol exposure. Metabolism 55: 119–127, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Aitken CE, Lorsch JR. A mechanistic overview of translation initiation in eukaryotes. Nat Struct Mol Biol 19: 568–576, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Baehr LM, Tunzi M, Bodine SC. Muscle hypertrophy is associated with increases in proteasome activity that is independent of MuRF1 and MAFbx expression. Front Physiol 5: 69, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science 340: 1100–1106, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol 24: 400–406, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodine SC. Disuse-induced muscle wasting. Int J Biochem Cell Biol 45: 2200–2208, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Cardellach F, Galofre J, Grau JM, Casademont J, Hoek JB, Rubin E, Urbano-Marquez A. Oxidative metabolism in muscle mitochondria from patients with chronic alcoholism. Ann Neurol 31: 515–518, 1992. [DOI] [PubMed] [Google Scholar]
- 9.Cardellach F, Taraschi TF, Ellingson JS, Stubbs CD, Rubin E, Hoek JB. Maintenance of structural and functional characteristics of skeletal-muscle mitochondria and sarcoplasmic-reticular membranes after chronic ethanol treatment. Biochem J 274: 565–573, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clary CR, Guidot DM, Bratina MA, Otis JS. Chronic alcohol ingestion exacerbates skeletal muscle myopathy in HIV-1 transgenic rats. AIDS Res Ther 8: 30, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa-de-Araujo R, Hadley E. Skeletal muscle function deficit: a new terminology to embrace the evolving concepts of sarcopenia and age-related muscle dysfunction. J Gerontol A Biol Sci Med Sci 69: 591–594, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courtney KE, Polich J. Binge drinking in young adults: Data, definitions, and determinants. Psychol Bull 135: 142–156, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deaciuc IV, McDonough KH, Bagby GJ, Spitzer JJ. Alcohol consumption in rats potentiates the deleterious effect of gram-negative sepsis on hepatic hyaluronan uptake. Alcohol Clin Exp Res 17: 1002–1008, 1993. [DOI] [PubMed] [Google Scholar]
- 14.Dibble CC, Elis W, Menon S, Qin W, Klekota J, Asara JM, Finan PM, Kwiatkowski DJ, Murphy LO, Manning BD. TBC1D7 is a third subunit of the TSC1-TSC2 complex upstream of mTORC1. Mol Cell 47: 535–546, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duane P, Peters TJ. Nutritional status in alcoholics with and without chronic skeletal muscle myopathy. Alcohol Alcohol 23: 271–277, 1988. [PubMed] [Google Scholar]
- 16.Eisner V, Lenaers G, Hajnoczky G. Mitochondrial fusion is frequent in skeletal muscle and supports excitation-contraction coupling. J Cell Biol 205: 179–195, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekbom K, Hed R, Kirstein L, Astrom KE. Muscular affections in chronic alcoholism. Arch Neurol 10: 449–458, 1964. [DOI] [PubMed] [Google Scholar]
- 18.Erickson CK. Ethanol clearance in nine inbred rat strains. Alcohol Clin Exp Res 8: 491–494, 1984. [DOI] [PubMed] [Google Scholar]
- 19.Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS. Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Prev Chronic Dis 11: E206, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Estruch R, Nicolás JM, Villegas E, Junqué A, Urbano-Márquez A. Relationship between ethanol-related diseases and nutritional status in chronically alcoholic men. Alcohol Alcohol 28: 543–550, 1993. [PubMed] [Google Scholar]
- 21.Fernandez-Sola J, Garcia G, Elena M, Tobias E, Sacanella E, Estruch R, Nicolas JM. Muscle antioxidant status in chronic alcoholism. Alcohol Clin Exp Res 26: 1858–1862, 2002. [PubMed] [Google Scholar]
- 22.Fernandez-Sola J, Villegas E, Nicolas JM, Deulofeu R, Antunez E, Sacanella E, Estruch R, Urbano-Marquez A. Serum and muscle levels of alpha-tocopherol, ascorbic acid, and retinol are normal in chronic alcoholic myopathy. Alcohol Clin Exp Res 22: 422–427, 1998. [PubMed] [Google Scholar]
- 23.Freilich R, Kirsner R, Whelan G, Chmiel R, Byrne E. Quantitative measure of muscle strength and size in chronic alcoholism: an early indication of tissue damage. Drug Alcohol Rev 15: 277–287, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Frost RA, Lang CH. mTOR signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiology (Bethesda) 26: 83–96, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frost RA, Lang CH. Multifaceted role of insulin-like growth factors and mammalian target of rapamycin in skeletal muscle. Endocrinol Metab Clin North Am 41: 297–322, vi, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frost RA, Lang CH. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J Appl Physiol (1985) 103: 378–387, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Frost RA, Lang CH. Regulation of muscle growth by pathogen-associated molecules. J Anim Sci 86: E84–E93, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Frost RA, Nystrom G, Burrows PV, Lang CH. Temporal differences in the ability of ethanol to modulate endotoxin-induced increases in inflammatory cytokines in muscle under in vivo conditions. Alcohol Clin Exp Res 29: 1247–1256, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA 98: 14440–14445, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman CA, Hornberger TA. New roles for Smad signaling and phosphatidic acid in the regulation of skeletal muscle mass. F1000Prime Rep 6: 20, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haissaguerre M, Saucisse N, Cota D. Influence of mTOR in energy and metabolic homeostasis. Mol Cell Endocrinol 397: 67–77, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Hands SL, Proud CG, Wyttenbach A. mTOR's role in ageing: protein synthesis or autophagy? Aging (Albany NY) 1: 586–597, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanid A, Slavin G, Mair W, Sowter C, Ward P, Webb J, Levi J. Fibre type changes in striated muscle of alcoholics. J Clin Pathol 34: 991–995, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 64: 830–842, 2007. [DOI] [PubMed] [Google Scholar]
- 35.Haverberg LN, Omstedt PT, Munro HN, Young VR. Ntau-methylhistidine content of mixed proteins in various rat tissues. Biochim Biophys Acta 405: 67–71, 1975. [DOI] [PubMed] [Google Scholar]
- 36.Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology 122: 2049–2063, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holahan CJ, Schutte KK, Brennan PL, Holahan CK, Moos RH. Episodic heavy drinking and 20-year total mortality among late-life moderate drinkers. Alcohol Clin Exp Res 38: 1432–1438, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hong-Brown LQ, Brown C, Navaratnarajah M, Lang CH. Adamts1 mediates ethanol-induced alterations in collagen and elastin via a FoxO1-sestrin3-AMPK signaling cascade in myocytes. J Cell Biochem 116: 91–101, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Alcohol and indinavir adversely affect protein synthesis and phosphorylation of MAPK and mTOR signaling pathways in C2C12 myocytes. Alcohol Clin Exp Res 30: 1297–1307, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Hong-Brown LQ, Brown CR, Huber DS, Lang CH. Lopinavir impairs protein synthesis and induces eEF2 phosphorylation via the activation of AMP-activated protein kinase. J Cell Biochem 105: 814–823, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong-Brown LQ, Brown CR, Kazi AA, Navaratnarajah M, Lang CH. Rag GTPases and AMPK/TSC2/Rheb mediate the differential regulation of mTORC1 signaling in response to alcohol and leucine. Am J Physiol Cell Physiol 302: C1557–C1565, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong-Brown LQ, Brown CR, Navaratnarajah M, Huber DS, Lang CH. Alcohol-induced modulation of rictor and mTORC2 activity in C2C12 myoblasts. Alcohol Clin Exp Res 35: 1445–1453, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong-Brown LQ, Brown CR, Navaratnarajah M, Lang CH. Activation of AMPK/TSC2/PLD by alcohol regulates mTORC1 and mTORC2 assembly in C2C12 myocytes. Alcohol Clin Exp Res 37: 1849–1861, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong-Brown LQ, Frost RA, Lang CH. Alcohol impairs protein synthesis and degradation in cultured skeletal muscle cells. Alcohol Clin Exp Res 25: 1373–1382, 2001. [PubMed] [Google Scholar]
- 45.Hong-Brown LQ, Kazi AA, Lang CH. Mechanisms mediating the effects of alcohol and HIV anti-retroviral agents on mTORC1, mTORC2 and protein synthesis in myocytes. World J Biol Chem 3: 110–120, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong-Brown LQ, Pruznak AM, Frost RA, Vary TC, Lang CH. Indinavir alters regulators of protein anabolism and catabolism in skeletal muscle. Am J Physiol Endocrinol Metab 289: E382–E390, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Hunter RJ, Neagoe C, Järveläinen HA, Martin CR, Lindros KO, Linke WA, Preedy VR. Alcohol affects the skeletal muscle proteins, titin and nebulin in male and female rats. J Nutr 133: 1154–1157, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Jacobsen EB, Hamberg O, Quistorff B, Ott P. Reduced mitochondrial adenosine triphosphate synthesis in skeletal muscle in patients with Child-Pugh class B and C cirrhosis. Hepatology 34: 7–12, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Jayasekara H, English DR, Room R, MacInnis RJ. Alcohol consumption over time and risk of death: a systematic review and meta-analysis. Am J Epidemiol 179: 1049–1059, 2014. [DOI] [PubMed] [Google Scholar]
- 50.Johns N, Stephens NA, Fearon KC. Muscle wasting in cancer. Int J Biochem Cell Biol 45: 2215–2229, 2013. [DOI] [PubMed] [Google Scholar]
- 51.Jones MK, Jones BM. Ethanol metabolism in women taking oral contraceptives. Alcohol Clin Exp Res 8: 24–28, 1984. [DOI] [PubMed] [Google Scholar]
- 52.Joshi PC, Guidot DM. The alcoholic lung: epidemiology, pathophysiology, and potential therapies. Am J Physiol Lung Cell Mol Physiol 292: L813–L823, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Kalichman SC, Amaral CM, White D, Swetsze C, Pope H, Kalichman MO, Cherry C, Eaton L. Prevalence and clinical implications of interactive toxicity beliefs regarding mixing alcohol and antiretroviral therapies among people living with HIV/AIDS. AIDS Patient Care STDS 23: 449–454, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karavitis J, Murdoch EL, Gomez CR, Ramirez L, Kovacs EJ. Acute ethanol exposure attenuates pattern recognition receptor activated macrophage functions. J Interferon Cytokine Res 28: 413–422, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kazi AA, Hong-Brown L, Lang SM, Lang CH. Deptor knockdown enhances mTOR Activity and protein synthesis in myocytes and ameliorates disuse muscle atrophy. Mol Med 17: 925–936, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiessling KH, Pilstrom L, Bylund AC, Piehl K, Saltin B. Effects of chronic ethanol abuse on structure and enzyme activities of skeletal muscle in man. Scand J Clin Lab Invest 35: 601–607, 1975. [DOI] [PubMed] [Google Scholar]
- 57.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr 136: 227S–231S, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Klinkerfuss G, Bleisch V, Dioso MM, Perkoff GT. A spectrum of myopathy associated with alcoholism. II. Light and electron microscopic observations. Ann Intern Med 67: 493–510, 1967. [DOI] [PubMed] [Google Scholar]
- 59.Koll M, Ahmed S, Mantle D, Donohue TM, Palmer TN, Simanowski UA, Seltz HK, Peters TJ, Preedy VR. Effect of acute and chronic alcohol treatment and their superimposition on lysosomal, cytoplasmic, and proteosomal protease activities in rat skeletal muscle in vivo. Metabolism 51: 97–104, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Koll M, Beeso JA, Kelly FJ, Simanowski UA, Seitz HK, Peters TJ, Preedy VR. Chronic alpha-tocopherol supplementation in rats does not ameliorate either chronic or acute alcohol-induced changes in muscle protein metabolism. Clin Sci (Lond) 104: 287–294, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Koo-Ng R, Falkous G, Reilly M, Peters TJ, Mantle D, Preedy VR. Carbonyl levels in type I and II fiber-rich muscles and their response to chronic ethanol feeding in vivo and hydroxyl and superoxide radicals in vitro. Alcohol Clin Exp Res 24: 1862–1868, 2000. [PubMed] [Google Scholar]
- 62.Korzick DH, Sharda DR, Pruznak AM, Lang CH. Aging accentuates alcohol-induced decrease in protein synthesis in gastrocnemius. Am J Physiol Regul Integr Comp Physiol 304: R887–R898, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar V, Frost RA, Lang CH. Alcohol impairs insulin and IGF-I stimulation of S6K1 but not 4E-BP1 in skeletal muscle. Am J Physiol Endocrinol Metab 283: E917–E928, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Kuznetsov AV, Javadov S, Guzun R, Grimm M, Saks V. Cytoskeleton and regulation of mitochondrial function: the role of beta-tubulin II. Front Physiol 4: 82, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lang CH, Fan J, Lipton BP, Potter BJ, McDonough KH. Modulation of the insulin-like growth factor system by chronic alcohol feeding. Alcohol Clin Exp Res 22: 823–829, 1998. [PubMed] [Google Scholar]
- 66.Lang CH, Frost RA, Deshpande N, Kumar V, Vary TC, Jefferson LS, Kimball SR. Alcohol impairs leucine-mediated phosphorylation of 4E-BP1, S6K1, eIF4G, and mTOR in skeletal muscle. Am J Physiol Endocrinol Metab 285: E1205–E1215, 2003. [DOI] [PubMed] [Google Scholar]
- 67.Lang CH, Frost RA, Kumar V, Vary TC. Impaired myocardial protein synthesis induced by acute alcohol intoxication is associated with changes in eIF4F. Am J Physiol Endocrinol Metab 279: E1029–E1038, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Lang CH, Frost RA, Kumar V, Wu D, Vary TC. Impaired protein synthesis induced by acute alcohol intoxication is associated with changes in eIF4E in muscle and eIF2B in liver. Alcohol Clin Exp Res 24: 322–331, 2000. [PubMed] [Google Scholar]
- 69.Lang CH, Frost RA, Svanberg E, Vary TC. IGF-I/IGFBP-3 ameliorates alterations in protein synthesis, eIF4E availability, and myostatin in alcohol-fed rats. Am J Physiol Endocrinol Metab 286: E916–E926, 2004. [DOI] [PubMed] [Google Scholar]
- 70.Lang CH, Frost RA, Vary TC. Acute alcohol intoxication increases REDD1 in skeletal muscle. Alcohol Clin Exp Res 32: 796–805, 2008. [DOI] [PubMed] [Google Scholar]
- 71.Lang CH, Frost RA, Vary TC. Regulation of muscle protein synthesis during sepsis and inflammation. Am J Physiol Endocrinol Metab 293: E453–E459, 2007. [DOI] [PubMed] [Google Scholar]
- 72.Lang CH, Frost RA, Vary TC. Skeletal muscle protein synthesis and degradation exhibit sexual dimorphism after chronic alcohol consumption but not acute intoxication. Am J Physiol Endocrinol Metab 292: E1497–E1506, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Lang CH, Huber D, Frost RA. Burn-induced increase in atrogin-1 and MuRF-1 in skeletal muscle is glucocorticoid independent but downregulated by IGF-I. Am J Physiol Regul Integr Comp Physiol 292: R328–R336, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Lang CH, Kimball SR, Frost RA, Vary TC. Alcohol myopathy: impairment of protein synthesis and translation initiation. Int J Biochem Cell Biol 33: 457–473, 2001. [DOI] [PubMed] [Google Scholar]
- 75.Lang CH, Liu X, Nystrom G, Wu D, Cooney RN, Frost RA. Acute effects of growth hormone in alcohol-fed rats. Alcohol Alcohol 35: 148–158, 2000. [DOI] [PubMed] [Google Scholar]
- 76.Lang CH, Lynch CJ, Vary TC. Alcohol-induced IGF-I resistance is ameliorated in mice deficient for mitochondrial branched-chain aminotransferase. J Nutr 140: 932–938, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lang CH, Lynch CJ, Vary TC. BCATm deficiency ameliorates endotoxin-induced decrease in muscle protein synthesis and improves survival in septic mice. Am J Physiol Regul Integr Comp Physiol 299: R935–R944, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lang CH, Pruznak AM, Deshpande N, Palopoli MM, Frost RA, Vary TC. Alcohol intoxication impairs phosphorylation of S6K1 and S6 in skeletal muscle independently of ethanol metabolism. Alcohol Clin Exp Res 28: 1758–1767, 2004. [DOI] [PubMed] [Google Scholar]
- 79.Lang CH, Pruznak AM, Nystrom GJ, Vary TC. Alcohol-induced decrease in muscle protein synthesis associated with increased binding of mTOR and raptor: Comparable effects in young and mature rats. Nutr Metab (Lond) 6: 4, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lang CH, Wu D, Frost RA, Jefferson LS, Kimball SR, Vary TC. Inhibition of muscle protein synthesis by alcohol is associated with modulation of eIF2B and eIF4E. Am J Physiol Endocrinol Metab 277: E268–E276, 1999. [DOI] [PubMed] [Google Scholar]
- 81.Lang CH, Wu D, Frost RA, Jefferson LS, Vary TC, Kimball SR. Chronic alcohol feeding impairs hepatic translation initiation by modulating eIF2 and eIF4E. Am J Physiol Endocrinol Metab 277: E805–E814, 1999. [DOI] [PubMed] [Google Scholar]
- 82.Lang SM, Kazi AA, Hong-Brown L, Lang CH. Delayed recovery of skeletal muscle mass following hindlimb immobilization in mTOR heterozygous mice. PLoS One 7: e38910, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci 126: 1713–1719, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.LeCapitaine NJ, Wang ZQ, Dufour JP, Potter BJ, Bagby GJ, Nelson S, Cefalu WT, Molina PE. Disrupted anabolic and catabolic processes may contribute to alcohol-accentuated SAIDS-associated wasting. J Infect Dis 204: 1246–1255, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lieber CS, DeCarli LM. Liquid diet technique of ethanol administration: 1989 update. Alcohol Alcohol 24: 197–211, 1989. [PubMed] [Google Scholar]
- 86.Livy DJ, Parnell SE, West JR. Blood ethanol concentration profiles: a comparison between rats and mice. Alcohol 29: 165–171, 2003. [DOI] [PubMed] [Google Scholar]
- 87.Longnecker MP. Alcohol consumption and risk of cancer in humans: an overview. Alcohol 12: 87–96, 1995. [DOI] [PubMed] [Google Scholar]
- 88.Maddocks M, Gao W, Higginson IJ, Wilcock A. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst Rev 1: CD009419, 2013. [DOI] [PubMed] [Google Scholar]
- 89.Maraldi C, Harris TB, Newman AB, Kritchevsky SB, Pahor M, Koster A, Satterfield S, Ayonayon HN, Fellin R, Volpato S, and . Health ABCs. Moderate alcohol intake and risk of functional decline: the Health, Aging, and Body Composition Study. J Am Geriatr Soc 57: 1767–1775, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martin F, Ward K, Slavin G, Levi J, Peters TJ. Alcoholic skeletal myopathy, a clinical and pathological study. Q J Med 55: 233–251, 1985. [PubMed] [Google Scholar]
- 91.Martin FC, Peters TJ. Assessment in vitro and in vivo of muscle degradation in chronic skeletal muscle myopathy of alcoholism. Clin Sci (Lond) 68: 693–700, 1985. [DOI] [PubMed] [Google Scholar]
- 92.Marway JS, Preedy VR, Peters TJ. Experimental alcoholic skeletal muscle myopathy is characterised by a rapid and sustained decrease in muscle RNA content. Alcohol Alcohol 25: 401–406, 1990. [PubMed] [Google Scholar]
- 93.Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M. Autophagy is required to maintain muscle mass. Cell Metab 10: 507–515, 2009. [DOI] [PubMed] [Google Scholar]
- 94.Masui K, Cavenee WK, Mischel PS. mTORC2 in the center of cancer metabolic reprogramming. Trends Endocrinol Metab 25: 364–373, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Molina PE, Bagby GJ, Nelson S. Biomedical consequences of alcohol use disorders in the HIV-infected host. Curr HIV Res 12: 265–275, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Molina PE, Lang CH, McNurlan M, Bagby GJ, Nelson S. Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res 32: 138–147, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Molina PE, McNurlan M, Rathmacher J, Lang CH, Zambell KL, Purcell J, Bohm RP, Zhang P, Bagby GJ, Nelson S. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol Clin Exp Res 30: 2065–2078, 2006. [DOI] [PubMed] [Google Scholar]
- 98.Muscaritoli M, Lucia S, Molfino A, Cederholm T, Rossi Fanelli F. Muscle atrophy in aging and chronic diseases: is it sarcopenia or cachexia? Intern Emerg Med 8: 553–560, 2013. [DOI] [PubMed] [Google Scholar]
- 99.Nguyen VA, Le T, Tong M, Silbermann E, Gundogan F, de la Monte SM. Impaired insulin/IGF signaling in experimental alcohol-related myopathy. Nutrients 4: 1058–1075, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nunez NP, Carter PA, Meadows GG. Alcohol consumption promotes body weight loss in melanoma-bearing mice. Alcohol Clin Exp Res 26: 617–626, 2002. [DOI] [PubMed] [Google Scholar]
- 101.Nutt DJ, King LA, Phillips LD, and Independent Scientific Committee on D. Drug harms in the UK: a multicriteria decision analysis. Lancet 376: 1558–1565, 2010. [DOI] [PubMed] [Google Scholar]
- 102.Otis JS, Brown LA, Guidot DM. Oxidant-induced atrogin-1 and transforming growth factor-beta1 precede alcohol-related myopathy in rats. Muscle Nerve 36: 842–848, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Otis JS, Guidot DM. Procysteine increases alcohol-depleted glutathione stores in rat plantaris following a period of abstinence. Alcohol Alcohol 45: 495–500, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Otis JS, Guidot DM. Procysteine stimulates expression of key anabolic factors and reduces plantaris atrophy in alcohol-fed rats. Alcohol Clin Exp Res 33: 1450–1459, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pacy PJ, Preedy VR, Peters TJ, Read M, Halliday D. The effect of chronic alcohol ingestion on whole body and muscle protein synthesis—a stable isotope study. Alcohol Alcohol 26: 505–513, 1991. [DOI] [PubMed] [Google Scholar]
- 106.Paice AG, Hesketh JE, Towner P, Hirako M, Peters TJ, Preedy VR. No change in apoptosis in skeletal muscle exposed acutely or chronically to alcohol. Addict Biol 8: 97–105, 2003. [DOI] [PubMed] [Google Scholar]
- 107.Park C, Cuervo AM. Selective autophagy: talking with the UPS. Cell Biochem Biophys 67: 3–13, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parr EB, Camera DM, Areta JL, Burke LM, Phillips SM, Hawley JA, Coffey VG. Alcohol ingestion impairs maximal post-exercise rates of myofibrillar protein synthesis following a single bout of concurrent training. PLoS One 9: e88384, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell 137: 873–886, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piano MR. Alcoholic cardiomyopathy: clinical characteristics, pathophysiology, incidence. Chest 121: 1638–1650, 2002. [DOI] [PubMed] [Google Scholar]
- 111.Picard M, Shirihai OS, Gentil BJ, Burelle Y. Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am J Physiol Regul Integr Comp Physiol 304: R393–R406, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Preedy VR, Bateman CJ, Salisbury JR, Price AB, Peters TJ. Ethanol-induced skeletal muscle myopathy: biochemical and histochemical measurements on type I and type II fibre-rich muscles in the young rat. Alcohol Alcohol 24: 533–539, 1989. [DOI] [PubMed] [Google Scholar]
- 113.Preedy VR, Keating JW, Peters TJ. The acute effects of ethanol and acetaldehyde on rates of protein synthesis in type I and type II fibre-rich skeletal muscles of the rat. Alcohol Alcohol 27: 241–251, 1992. [PubMed] [Google Scholar]
- 114.Preedy VR, Peters TJ. Alcohol and skeletal muscle disease. Alcohol Alcohol 25: 177–187, 1990. [DOI] [PubMed] [Google Scholar]
- 115.Preedy VR, Peters TJ. Changes in protein, RNA and DNA and rates of protein synthesis in muscle-containing tissues of the mature rat in response to ethanol feeding: a comparative study of heart, small intestine and gastrocnemius muscle. Alcohol Alcohol 25: 489–498, 1990. [PubMed] [Google Scholar]
- 116.Preedy VR, Peters TJ. The effect of chronic ethanol ingestion on protein metabolism in type-I- and type-II-fibre-rich skeletal muscles of the rat. Biochem J 254: 631–639, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pruznak AM, Nystrom J, Lang CH. Direct central nervous system effect of alcohol alters synthesis and degradation of skeletal muscle protein. Alcohol Alcohol 48: 138–145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Puertollano R. mTOR and lysosome regulation. F1000Prime Rep 6: 52, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Purohit V, Brenner DA. Mechanisms of alcohol-induced hepatic fibrosis: a summary of the Ron Thurman Symposium. Hepatology 43: 872–878, 2006. [DOI] [PubMed] [Google Scholar]
- 120.Reilly ME, Erylmaz EI, Amir A, Peters TJ, Preedy VR. Skeletal muscle ribonuclease activities in chronically ethanol-treated rats. Alcohol Clin Exp Res 22: 876–883, 1998. [PubMed] [Google Scholar]
- 121.Reilly ME, Mantle D, Salisbury J, Peters TJ, Preedy VR. Comparative effects of acute ethanol dosage on liver and muscle protein metabolism. Biochem Pharmacol 60: 1773–1785, 2000. [DOI] [PubMed] [Google Scholar]
- 122.Reinus JF, Heymsfield SB, Wiskind R, Casper K, Galambos JT. Ethanol: relative fuel value and metabolic effects in vivo. Metabolism 38: 125–135, 1989. [DOI] [PubMed] [Google Scholar]
- 123.Romano AD, Greco E, Vendemiale G, Serviddio G. Bioenergetics and mitochondrial dysfunction in aging: recent insights for a therapeutical approach. Curr Pharm Des 20: 2978–2992, 2014. [DOI] [PubMed] [Google Scholar]
- 124.Samet JH, Cheng DM, Libman H, Nunes DP, Alperen JK, Saitz R. Alcohol consumption and HIV disease progression. J Acquir Immune Defic Syndr 46: 194–199, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol 45: 2121–2129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sandri M, Coletto L, Grumati P, Bonaldo P. Misregulation of autophagy and protein degradation systems in myopathies and muscular dystrophies. J Cell Sci 126: 5325–5333, 2013. [DOI] [PubMed] [Google Scholar]
- 128.Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, Sandri M. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol 296: C1248–C1257, 2009. [DOI] [PubMed] [Google Scholar]
- 129.Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab 279: E715–E729, 2000. [DOI] [PubMed] [Google Scholar]
- 130.She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab 6: 181–194, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Simon L, LeCapitaine N, Berner P, Vande Stouwe C, Mussell JC, Allerton T, Primeaux SD, Dufour J, Nelson S, Bagby GJ, Cefalu W, Molina PE. Chronic binge alcohol consumption alters myogenic gene expression and reduces in vitro myogenic differentiation potential of myoblasts from rhesus macaques. Am J Physiol Regul Integr Comp Physiol 306: R837–R844, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Smith IJ, Lecker SH, Hasselgren PO. Calpain activity and muscle wasting in sepsis. Am J Physiol Endocrinol Metab 295: E762–E771, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smith MA, Schnellmann RG. Calpains, mitochondria, apoptosis. Cardiovasc Res 96: 32–37, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sneddon AA, Koll M, Wallace MC, Jones J, Miell JP, Garlick PJ, Preedy VR. Acute alcohol administration inhibits the refeeding response after starvation in rat skeletal muscle. Am J Physiol Endocrinol Metab 284: E874–E882, 2003. [DOI] [PubMed] [Google Scholar]
- 135.Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, Fingar DC. mTOR Ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem 285: 7866–7879, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Song SK, Rubin E. Ethanol produces muscle damage in human volunteers. Science 175: 327–328, 1972. [DOI] [PubMed] [Google Scholar]
- 137.Sonntag WE, Boyd RL. Diminished insulin-like growth factor-1 levels after chronic ethanol: relationship to pulsatile growth hormone release. Alcohol Clin Exp Res 13: 3–7, 1989. [DOI] [PubMed] [Google Scholar]
- 138.Sorimachi H, Ono Y. Regulation and physiological roles of the calpain system in muscular disorders. Cardiovasc Res 96: 11–22, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sorocco KH, Ferrell SW. Alcohol use among older adults. J Gen Psychol 133: 453–467, 2006. [DOI] [PubMed] [Google Scholar]
- 140.Steiner JL, Lang CH. Alcohol impairs skeletal muscle protein synthesis and mTOR signaling in a time-dependent manner following electrically stimulated muscle contraction. J Appl Physiol (1985) 117: 1170–1179, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Steiner JL, Lang CH. Alcohol intoxication following muscle contraction in mice decreases muscle protein synthesis but not mTOR signal transduction. Alcohol Clin Exp Res 39: 1–10, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Steiner JL, Lang CH. Moderate alcohol consumption does not impair overload-induced muscle hypertrophy and protein synthesis. Physiol Rep. 2015March3(3). pii: e12333. doi: 10.14814/phy2.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Thapaliya S, Runkana A, McMullen MR, Nagy LE, McDonald C, Naga Prasad SV, Dasarathy S. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy 10: 677–690, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thapaliya S, Runkana A, McMullen MR, Nagy LE, McDonald C, Prasad SV, Dasarathy S. Alcohol-induced autophagy contributes to loss in skeletal muscle mass. Autophagy 10: 677–690, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tisdale MJ. Loss of skeletal muscle in cancer: biochemical mechanisms. Front Biosci 6: D164–D174, 2001. [DOI] [PubMed] [Google Scholar]
- 146.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296: C1258–C1270, 2009. [DOI] [PubMed] [Google Scholar]
- 147.Trounce I, Byrne E, Dennett X, Santamaria J, Doery J, Peppard R. Chronic alcoholic proximal wasting: physiological, morphological and biochemical studies in skeletal muscle. Aust NZ J Med 17: 413–419, 1987. [DOI] [PubMed] [Google Scholar]
- 148.Urbano-Marquez A, Estruch R, Fernandez-Sola J, Nicolas JM, Pare JC, Rubin E. The greater risk of alcoholic cardiomyopathy and myopathy in women compared with men. JAMA 274: 149–154, 1995. [DOI] [PubMed] [Google Scholar]
- 149.Urbano-Marquez A, Estruch R, Navarro-Lopez F, Grau JM, Mont L, Rubin E. The effects of alcoholism on skeletal and cardiac muscle. N Engl J Med 320: 409–415, 1989. [DOI] [PubMed] [Google Scholar]
- 150.Urbano-Marquez A, Fernandez-Sola J. Effects of alcohol on skeletal and cardiac muscle. Muscle Nerve 30: 689–707, 2004. [DOI] [PubMed] [Google Scholar]
- 151.Vargas R, Lang CH. Alcohol accelerates loss of muscle and impairs recovery of muscle mass resulting from disuse atrophy. Alcohol Clin Exp Res 32: 128–137, 2008. [DOI] [PubMed] [Google Scholar]
- 152.Vary TC, Frost RA, Lang CH. Acute alcohol intoxication increases atrogin-1 and MuRF1 mRNA without increasing proteolysis in skeletal muscle. Am J Physiol Regul Integr Comp Physiol 294: R1777–R1789, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vary TC, Lynch CJ, Lang CH. Effects of chronic alcohol consumption on regulation of myocardial protein synthesis. Am J Physiol Heart Circ Physiol 281: H1242–H1251, 2001. [DOI] [PubMed] [Google Scholar]
- 154.Vary TC, Nairn AC, Deiter G, Lang CH. Differential effects of alcohol consumption on eukaryotic elongation factors in heart, skeletal muscle, and liver. Alcohol Clin Exp Res 26: 1794–1802, 2002. [PubMed] [Google Scholar]
- 155.Vary TC, Nairn AC, Lang CH. Restoration of protein synthesis in heart and skeletal muscle after withdrawal of alcohol. Alcohol Clin Exp Res 28: 517–525, 2004. [DOI] [PubMed] [Google Scholar]
- 156.Wang J, Chu H, Zhao H, Cheng X, Liu Y, Jin W, Zhao J, Liu B, Ding Y, Ma H. Nitricoxide synthase-induced oxidative stress in prolonged alcoholic myopathies of rats. Mol Cell Biochem 304: 135–142, 2007. [DOI] [PubMed] [Google Scholar]
- 157.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 21: 362–369, 2006. [DOI] [PubMed] [Google Scholar]
- 158.Ward RJ, Peters TJ. The antioxidant status of patients with either alcohol-induced liver damage or myopathy. Alcohol Alcohol 27: 359–365, 1992. [PubMed] [Google Scholar]
- 159.Welle S, Burgess K, Mehta S. Stimulation of skeletal muscle myofibrillar protein synthesis, p70 S6 kinase phosphorylation, and ribosomal protein S6 phosphorylation by inhibition of myostatin in mature mice. Am J Physiol Endocrinol Metab 296: E567–E572, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yazaki K, Haida M, Kurita D, Shinohara Y. Effect of chronic alcohol intake on energy metabolism in human muscle. Alcohol Clin Exp Res 20: 360A–362A, 1996. [PubMed] [Google Scholar]
- 161.Yoseph BP, Breed E, Overgaard CE, Ward CJ, Liang Z, Wagener ME, Lexcen DR, Lusczek ER, Beilman GJ, Burd EM, Farris AB, Guidot DM, Koval M, Ford ML, Coopersmith CM. Chronic alcohol ingestion increases mortality and organ injury in a murine model of septic peritonitis. PLoS One 8: e62792, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zheng X, Liang Y, He Q, Yao R, Bao W, Bao L, Wang Y, Wang Z. Current models of mammalian target of rapamycin complex 1 (mTORC1) activation by growth factors and amino acids. Int J Mol Sci 15: 20753–20769, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]