Abstract
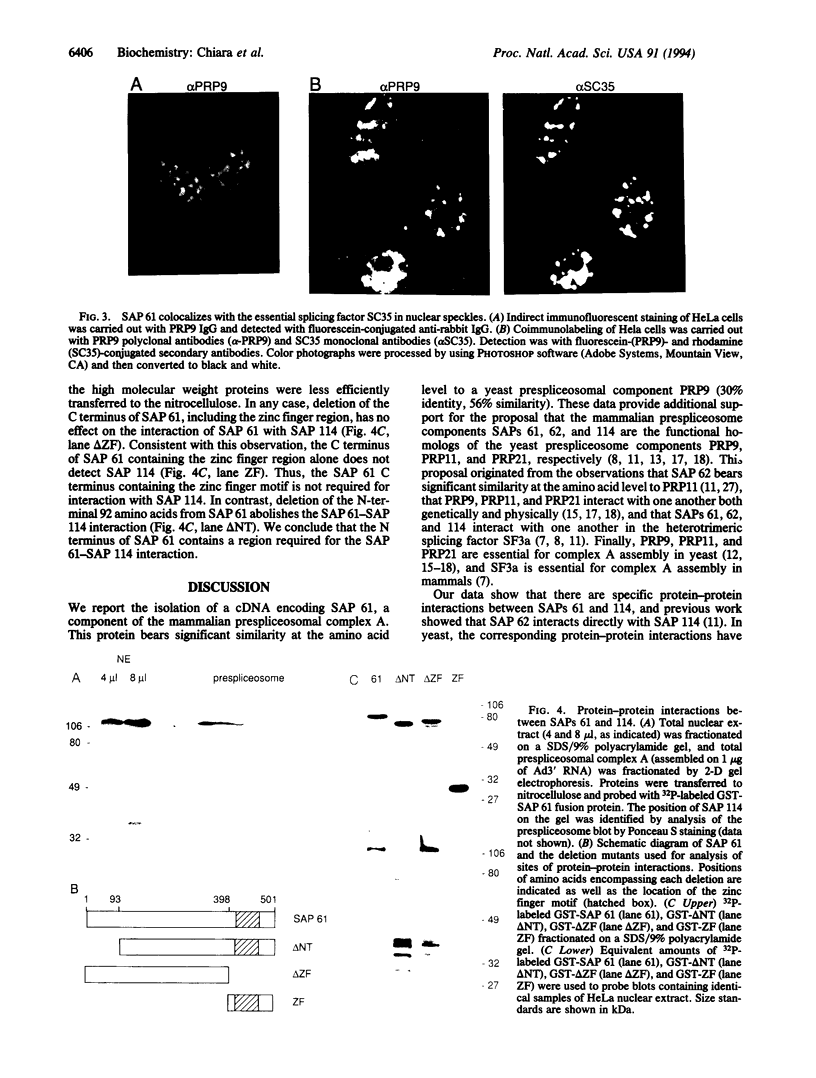
Spliceosome-associated proteins (SAPs) 61, 62, and 114 can be UV-crosslinked to pre-mRNA in purified spliceosomal complexes and are associated with U2 small nuclear ribonucleoproteins (snRNP). These proteins also compose the essential heterotrimeric splicing factor SF3a, and products of yeast pre-mRNA processing genes PRP9, PRP11, and PRP21 are their likely yeast counterparts. We report the isolation of a cDNA encoding SAP 61 and find that it is 30% identical in amino acid sequence to PRP9. A C-terminal Cys2His2 zinc-finger-like motif, which could be involved in the pre-mRNA binding, is the most highly conserved region of the protein. We also demonstrate specific protein-protein interactions between SAPs 61 and 114 and show that the N terminus of SAP 61 is required for this interaction. Significantly, the corresponding proteins are also known to interact in yeast: PRP9 interacts with PRP21, and the N-terminal portion of PRP9 is required. Previous work showed that direct interactions also occur between SAPs 62 and 114 and between the corresponding PRPs 11 and 21. These observations indicate that the specific protein-protein interactions that occur between the three prespliceosomal factors have been conserved between yeast and mammals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abovich N., Legrain P., Rosbash M. The yeast PRP6 gene encodes a U4/U6 small nuclear ribonucleoprotein particle (snRNP) protein, and the PRP9 gene encodes a protein required for U2 snRNP binding. Mol Cell Biol. 1990 Dec;10(12):6417–6425. doi: 10.1128/mcb.10.12.6417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Andres V., Nadal-Ginard B., Mahdavi V. Clox, a mammalian homeobox gene related to Drosophila cut, encodes DNA-binding regulatory proteins differentially expressed during development. Development. 1992 Oct;116(2):321–334. doi: 10.1242/dev.116.2.321. [DOI] [PubMed] [Google Scholar]
- Arenas J. E., Abelson J. N. The Saccharomyces cerevisiae PRP21 gene product is an integral component of the prespliceosome. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6771–6775. doi: 10.1073/pnas.90.14.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens S. E., Galisson F., Legrain P., Lührmann R. Evidence that the 60-kDa protein of 17S U2 small nuclear ribonucleoprotein is immunologically and functionally related to the yeast PRP9 splicing factor and is required for the efficient formation of prespliceosomes. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8229–8233. doi: 10.1073/pnas.90.17.8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens S. E., Tyc K., Kastner B., Reichelt J., Lührmann R. Small nuclear ribonucleoprotein (RNP) U2 contains numerous additional proteins and has a bipartite RNP structure under splicing conditions. Mol Cell Biol. 1993 Jan;13(1):307–319. doi: 10.1128/mcb.13.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M., Michaud S., Kingston J., Reed R. Protein components specifically associated with prespliceosome and spliceosome complexes. Genes Dev. 1992 Oct;6(10):1986–2000. doi: 10.1101/gad.6.10.1986. [DOI] [PubMed] [Google Scholar]
- Bennett M., Reed R. Correspondence between a mammalian spliceosome component and an essential yeast splicing factor. Science. 1993 Oct 1;262(5130):105–108. doi: 10.1126/science.8211113. [DOI] [PubMed] [Google Scholar]
- Brosi R., Gröning K., Behrens S. E., Lührmann R., Krämer A. Interaction of mammalian splicing factor SF3a with U2 snRNP and relation of its 60-kD subunit to yeast PRP9. Science. 1993 Oct 1;262(5130):102–105. doi: 10.1126/science.8211112. [DOI] [PubMed] [Google Scholar]
- Brosi R., Hauri H. P., Krämer A. Separation of splicing factor SF3 into two components and purification of SF3a activity. J Biol Chem. 1993 Aug 15;268(23):17640–17646. [PubMed] [Google Scholar]
- Carmo-Fonseca M., Pepperkok R., Carvalho M. T., Lamond A. I. Transcription-dependent colocalization of the U1, U2, U4/U6, and U5 snRNPs in coiled bodies. J Cell Biol. 1992 Apr;117(1):1–14. doi: 10.1083/jcb.117.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. H., Clark M. W., Lustig A. J., Cusick M. E., Abelson J. RNA11 protein is associated with the yeast spliceosome and is localized in the periphery of the cell nucleus. Mol Cell Biol. 1988 Jun;8(6):2379–2393. doi: 10.1128/mcb.8.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. D., Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature. 1990 Feb 1;343(6257):437–441. doi: 10.1038/343437a0. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lamm G. M., Lamond A. I. Non-snRNP protein splicing factors. Biochim Biophys Acta. 1993 Jun 25;1173(3):247–265. doi: 10.1016/0167-4781(93)90122-t. [DOI] [PubMed] [Google Scholar]
- Legrain P., Chapon C., Galisson F. Interactions between PRP9 and SPP91 splicing factors identify a protein complex required in prespliceosome assembly. Genes Dev. 1993 Jul;7(7B):1390–1399. doi: 10.1101/gad.7.7b.1390. [DOI] [PubMed] [Google Scholar]
- Legrain P., Chapon C. Interaction between PRP11 and SPP91 yeast splicing factors and characterization of a PRP9-PRP11-SPP91 complex. Science. 1993 Oct 1;262(5130):108–110. doi: 10.1126/science.8211114. [DOI] [PubMed] [Google Scholar]
- Legrain P., Choulika A. The molecular characterization of PRP6 and PRP9 yeast genes reveals a new cysteine/histidine motif common to several splicing factors. EMBO J. 1990 Sep;9(9):2775–2781. doi: 10.1002/j.1460-2075.1990.tb07465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud S., Reed R. An ATP-independent complex commits pre-mRNA to the mammalian spliceosome assembly pathway. Genes Dev. 1991 Dec;5(12B):2534–2546. doi: 10.1101/gad.5.12b.2534. [DOI] [PubMed] [Google Scholar]
- Ruby S. W., Chang T. H., Abelson J. Four yeast spliceosomal proteins (PRP5, PRP9, PRP11, and PRP21) interact to promote U2 snRNP binding to pre-mRNA. Genes Dev. 1993 Oct;7(10):1909–1925. doi: 10.1101/gad.7.10.1909. [DOI] [PubMed] [Google Scholar]
- Sillekens P. T., Beijer R. P., Habets W. J., van Venrooij W. J. Human U1 snRNP-specific C protein: complete cDNA and protein sequence and identification of a multigene family in mammals. Nucleic Acids Res. 1988 Sep 12;16(17):8307–8321. doi: 10.1093/nar/16.17.8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L. Macromolecular domains within the cell nucleus. Annu Rev Cell Biol. 1993;9:265–315. doi: 10.1146/annurev.cb.09.110193.001405. [DOI] [PubMed] [Google Scholar]
- Staknis D., Reed R. Direct interactions between pre-mRNA and six U2 small nuclear ribonucleoproteins during spliceosome assembly. Mol Cell Biol. 1994 May;14(5):2994–3005. doi: 10.1128/mcb.14.5.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore P. D., Green M. R. Identification, purification, and biochemical characterization of U2 small nuclear ribonucleoprotein auxiliary factor. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9243–9247. doi: 10.1073/pnas.86.23.9243. [DOI] [PMC free article] [PubMed] [Google Scholar]