Abstract
Microbiomes associated with multicellular organisms influence the disease susceptibility of hosts. The potential exists for such bacteria to protect wildlife from infectious diseases, particularly in the case of the globally distributed and highly virulent fungal pathogen Batrachochytrium dendrobatidis of the global panzootic lineage (B. dendrobatidis GPL), responsible for mass extinctions and population declines of amphibians. B. dendrobatidis GPL exhibits wide genotypic and virulence variation, and the ability of candidate probiotics to restrict growth across B. dendrobatidis isolates has not previously been considered. Here we show that only a small proportion of candidate probiotics exhibited broad-spectrum inhibition across B. dendrobatidis GPL isolates. Moreover, some bacterial genera showed significantly greater inhibition than others, but overall, genus and species were not particularly reliable predictors of inhibitory capabilities. These findings indicate that bacterial consortia are likely to offer a more stable and effective approach to probiotics, particularly if related bacteria are selected from genera with greater antimicrobial capabilities. Together these results highlight a complex interaction between pathogens and host-associated symbiotic bacteria that will require consideration in the development of bacterial probiotics for wildlife conservation. Future efforts to construct protective microbiomes should incorporate bacteria that exhibit broad-spectrum inhibition of B. dendrobatidis GPL isolates.
INTRODUCTION
The ability to withstand or mitigate pathogenic infection is partly determined by the host immune response. This has traditionally been examined in the context of immunity intrinsic to the host phenotype or genotype. However, all multicellular organisms coexist with a plethora of microbial organisms that are influential for host growth, development, and health (1). Although some of these microbes may be detrimental to the host, the importance of this microbiome in maintaining and improving host health is increasingly being recognized. The most obvious example of this is the gut community of humans: gut bacteria are essential for the digestion of food, but recent research has indicated that a healthy gut microbiome may also contribute to the prevention or moderation of liver, heart, and mental disease (reviewed in reference 2). The benefits to humans of a diverse microbiome are mirrored in other animal species, where the presence and composition of gut, buccal, and skin microbial communities have been linked to the occurrence and severity of both chronic and infectious disease (1).
Conservation practitioners are increasingly interested in manipulating host microbiomes as a management tool to combat infectious diseases that pose threats and welfare issues to wild animals. The use of host-associated bacteria to act as probiotics for disease mitigation is already common practice in agriculture and human health (e.g., see the reviews in references 3 and 4). The fundamental strategy is to identify bacterial genotypes that inhibit pathogens in vitro and apply these to susceptible hosts. Amphibians provide a particularly interesting example of this. This class of vertebrates is currently undergoing major population declines and extinctions in the wild, with 31% of species being listed as threatened by the International Union for Conservation of Nature (5, 6). This is in part due to pathogenic chytridiomycete fungi and the resulting chytridiomycosis disease (7, 8), which is arguably the most devastating infectious disease confronting wildlife today. Two chytridiomycete fungal species have been identified, Batrachochytrium dendrobatidis and Batrachochytrium salamandrivorans, and both of these species infect the skin of amphibian hosts and cause disease in an extraordinary range of species (8–11). Current methods to mitigate the disease (e.g., antifungals, heat treatment of hosts) cannot be practically used for wild populations, but one that holds some promise and has been the subject of significant scrutiny and research investment is the application of so-called probiotic bacteria (reviewed in reference 12). Several bacteria that reside on amphibian skin have been shown to inhibit the growth and survival of B. dendrobatidis in vitro. The presence of such bacteria on some host species or the application of such bacteria to some host species has proven to reduce the likelihood of infection and disease significantly (13–17). However, B. dendrobatidis is a rapidly evolving pathogen composed of multiple, deeply diverged lineages (18, 19). Studies of potential probiotics have not yet explored how reliable these bacteria are when confronted with the shifting targets that amphibian-associated chytridiomycete fungi present. The globally distributed and hypervirulent B. dendrobatidis of the global panzootic lineage (B. dendrobatidis GPL) is the genetic lineage of B. dendrobatidis that has been associated with mass mortalities and rapid population declines of amphibians (11, 19, 20). Isolates within this lineage exhibit enormous and unpredictable genetic variation (18) and significant variation in virulence, even within a single host species exposed under laboratory conditions (19). To date, single bacterial species have been used in the majority of amphibian probiotic studies, and although they have proven effective in inhibiting the growth of single B. dendrobatidis GPL isolates and can be effective at limiting disease when applied as supplements to augment amphibian microbiomes (e.g., see references 13, 14, and 16), it is not clear if this ability is universal across isolates of the B. dendrobatidis GPL. This would be essential because amphibian communities may be host to multiple B. dendrobatidis GPL genotypes, all of which may cause mortality in susceptible hosts (19).
In the study described here, we used a quantitative in vitro assessment to determine whether potentially probiotic bacteria exhibit differences in inhibitory capabilities across isolates of B. dendrobatidis, focusing on isolates typed to the global panzootic lineage. All bacteria used in this study are amphibian associated and therefore have the potential to act as probiotics in the event that they inhibit B. dendrobatidis growth and reproduction. Our objectives were 2-fold: first, to determine if candidate bacterial isolates could inhibit any of the three B. dendrobatidis isolates that made up our panel of pathogens and, second, to ascertain if bacterial taxonomy, characterized using 16S rRNA typing, could be used to infer inhibitory capacity. This second objective is important for developing a strategy for mining amphibian microbiomes for target probiotics.
MATERIALS AND METHODS
Ethics statement.
Before it was started, this study was approved by the University of Manchester Research Ethics Committee. Bacteria were collected from wild populations of Agalychnis moreletii and Agalychnis callidryas frogs and exported with the permission of the Belize Forestry Department (research and export permit number CD/60/3/12) and imported into the United Kingdom by permission of the United Kingdom Department for Environment, Food & Rural Affairs (authorization number TARP/2012/224).
Bacterial sampling from Agalychnis frogs.
Eight A. moreletii frogs and eight A. callidryas frogs (four males and four females of each species) were collected from Elegans Pond at the Las Cuevas Research Station, Chiquibul Rainforest, Belize (16°43′N, 88°59′W), placed individually in sterile plastic bags, and returned to the research station (distance, ∼200 m). Sterile gloves were worn throughout handling and changed between frogs to minimize cross-contamination. Frogs were rinsed twice on their dorsal and ventral surfaces using sterile water to remove any transient bacteria from their skin and then swabbed all over their skin using sterile Eurotubo collection swabs (Deltalab, Spain), after which the swabs were placed into 1.5-ml sterile screw-top tubes containing 1 ml of 1 M NaCl2 solution. Care was taken to ensure that the frogs were not harmed during this process, and the frogs were released back at the pond the same evening they were collected. Tubes containing swabs were shaken vigorously for 30 s, and the contents were poured onto R2A agar plates [14], which were covered in Parafilm and inverted, and the bacteria were left to grow at ambient temperature (∼25°C) for 8 days. Morphologically distinct bacterial colonies were collected using individual sterile swabs and placed into screw-top tubes containing 1 ml R2A broth medium. The tubes were then shipped to the United Kingdom, where the contents were poured onto fresh R2A agar plates and incubated at 25°C until bacteria grew (∼3 days). These were then restreaked to ensure that a pure culture was obtained. In total, 56 strains of bacteria were isolated and sequenced as described previously (21).
In vitro B. dendrobatidis challenges.
We initially tested the anti-B. dendrobatidis capabilities of all 56 bacterial isolates using in vitro agar plate challenges against two B. dendrobatidis isolates (B. dendrobatidis GPL SFBC 014 and B. dendrobatidis GPL AUL 1.2) as described previously (21). Briefly, B. dendrobatidis cultures were grown in 1% tryptone gelatin hydrolysate lactose (TGhL) liquid medium at 18°C until the zoospore density and activity reached ∼10,000 zoospores/ml (at about 3 days post-passage). Three milliliters of active B. dendrobatidis zoospores was spread across the surface of 1% tryptone, 1% agar plates and left to dry in a sterile hood. Two bacterial pure cultures were then streaked onto opposing sides of each plate, which were inverted and incubated at 18°C for 10 days. Bacterial streaks were scored for the presence or absence of a zone of inhibition, characterized by dead B. dendrobatidis zoosporangia and zoospores and no evidence of continuing B. dendrobatidis growth and reproduction. If both bacterial streaks on one plate exhibited inhibition, the in vitro challenge was repeated for both bacterial isolates separately using a noninhibitory bacterial isolate as a control.
Based on the results of the initial screening, we selected four bacterial isolates that inhibited the growth of B. dendrobatidis GPL SFBC 014, four bacterial isolates that inhibited the growth of B. dendrobatidis GPL AUL 1.2, three bacterial isolates that inhibited the growth of both B. dendrobatidis isolates, and four bacterial isolates that had not shown any inhibition of B. dendrobatidis in vitro (n = 15 bacterial isolates). Three previously unassessed B. dendrobatidis isolates (B. dendrobatidis GPL CORN 3.2, isolated from a Mesotriton alpestris newt in the United Kingdom; B. dendrobatidis GPL JEL 423, isolated from a Agalychnis lemur frog in Panama; and B. dendrobatidis GPL VA05, isolated from a Alytes obstetricans toad in Spain) were cultured, and in vitro inhibition assays were conducted using the methods described above, with each bacterial isolate being replicated on three different plates and never being paired with the same bacterial isolate twice. These B. dendrobatidis isolates were chosen because their zoospores exhibited good growth on 1% tryptone, 1% agar plates, and one (JEL 423) originated from within the natural range of A. callidryas frogs from which some of the bacteria were isolated. Batrachochytrium dendrobatidis plate challenges were conducted as described above, again using 3 ml of B. dendrobatidis cultures containing ∼10,000 zoospores/ml. Care was taken to ensure that similarly sized colonies were picked for each streak for the three repeats of a given bacterial strain, as well as across bacterial strains, for all the inhibition assays.
Inhibition scores.
Each plate was photographed, and the areas of the zone of B. dendrobatidis inhibition around each bacterial streak along with the areas of the bacterial streaks were measured with ImageJ software (http://imagej.nih.gov/ij/). The inhibitory capabilities of each bacterium were quantified by dividing the area of the zone of inhibition by the area of the bacterial streak, and the result was rounded to the nearest integer to give an inhibition score. The inclusion of the size of the bacterial streak in this data conversion step ensured that bacterial density was controlled for in the analyses. An alternative method of quantifying B. dendrobatidis inhibition using 96-well plates may be more accurate and quantifiable than plate challenges, but it does not allow consideration of the direct competition (e.g., for space and resources) that may occur between B. dendrobatidis and bacteria and that may also occur on the skin of amphibians (22).
Statistical analyses.
The effects of B. dendrobatidis isolate, bacterial isolate, and their interaction on inhibition scores were analyzed using a generalized linear model with a Poisson error structure and log link. To control for the phylogenetic structure in the data, models initially contained bacterial isolate nested within genus as random effects, but this model structure was too complex, given the data, and the models would not converge, and so generalized linear models were used. In addition, individual generalized linear models with a Poisson error structure and log link were run for each bacterial strain separately to determine differences in inhibition between B. dendrobatidis isolates.
Multiple bacterial isolates of four genera (Acinetobacter, Chryseobacterium, Enterobacter, and Serratia) were tested, and so differences in the overall propensity of a given genus to inhibit B. dendrobatidis GPL isolates were analyzed using a generalized linear mixed model with a Poisson error structure and log link. Genus, B. dendrobatidis isolate, and their interaction were fitted as fixed effects, and bacterial isolate nested within genus was fitted as a random effect to control for the phylogenetic structure in the data. Statistical significance was determined by stepwise removal of terms from the maximal model (B. dendrobatidis × genus) and performing likelihood ratio tests between nested models. Where appropriate, post hoc tests were performed on the models by collapsing factor levels within an explanatory variable (e.g., by combining multiple B. dendrobatidis isolates into one factor level) and testing the simplified model against the original model with a likelihood ratio test. A nonsignificant result suggests that the combined factor levels all have a similar influence on the response variable and that the simpler model explains the data equally well.
Poisson models make distributional assumptions about the data, including the assumption that the variance is equal to the fitted mean. To test the robustness of the distributional assumptions of the models, analyses were rerun using ordinal models and the package MCMCglmm (23). Five competing models were fitted, and all had the same random effects structure described above (genus/bacterium). The most complex model contained B. dendrobatidis GPL, bacterial genus, and their interaction as fixed effects. All four nested models were also fitted: B. dendrobatidis GPL and bacterial isolate as main effects without their interaction, the B. dendrobatidis GPL isolate only, bacterial genus only, and an intercept-only model. All five models were compared using the deviance information criterion (DIC). All models were run for 100,000 iterations following a burn-in of 20,000 iterations, with a thinning interval of 100 being used. Uninformative priors were used for the random effects (G) structure, specifying shape parameters V and nu to be equal to 1 and 0.002, respectively. As the residual variance is not identifiable for ordinal models, it was fixed at 1.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the 56 strains of bacteria collected from the frogs are KC853168 to KC853184, KC853186 to KC853194, KC853196 to KC853224, and KF444793.
RESULTS
Fifty-six bacterial strains isolated from wild Agalychnis callidryas and Agalychnis moreletii frogs were initially screened for their antifungal capabilities against two B. dendrobatidis GPL isolates. Of these, six inhibited isolate AUL 1.2, six inhibited isolate SFBC 014, and three inhibited both isolates (see Table S1 in the supplemental material). Because these challenges were not replicated, no statistical analyses were performed. Four bacterial isolates that inhibited the growth of SFBC 014, four bacterial isolates that inhibited AUL 1.2, three bacterial isolates that inhibited both B. dendrobatidis isolates, and four bacterial isolates that had not previously shown any inhibition of B. dendrobatidis in vitro (n = 15 bacterial isolates) were then used for a quantitative assessment of anti-B. dendrobatidis capabilities using three previously unassessed B. dendrobatidis GPL isolates (CORN 3.2, VA05, and JEL 423). Inhibition scores were significantly predicted by bacterial strain (χ2 = 53.442, degrees of freedom [df] = 14, P < 0.001), B. dendrobatidis isolate (χ2 = 20.270, df = 2, P > 0.001), and the interaction between bacterial strain and B. dendrobatidis isolate (χ2 = 68.173, df = 28, P > 0.001). The host species from which the bacteria were isolated had no significant effect on the overall inhibition capabilities of the bacteria (χ2 = 0.001, df = 1, P = 0.981; see Table S1 in the supplemental material). Individual models for each bacterial strain indicated that 10 of the 15 bacteria exhibited inconsistent inhibition across the B. dendrobatidis isolates (Table 1; Fig. 1). Only three bacteria consistently inhibited all three B. dendrobatidis isolates in the quantitative inhibition assessment, and only one of these also inhibited both B. dendrobatidis GPL isolates used for the initial screening (Chryseobacterium sp. strain 2; see Table S1 in the supplemental material). Two bacteria exhibited no or negligible inhibition of any of the three B. dendrobatidis GPL isolates in the quantitative assessment (Fig. 1), although, interestingly, of these, the Agrobacterium sp. inhibited both B. dendrobatidis GPL isolates in the initial screening, whereas Enterobacter sp. strain 2 inhibited neither isolate (see Table S1 in the supplemental material). Even though Serratia sp. strains 1, 2, and 3 all typed as identical bacterial species at the 16S rRNA locus and all were isolated from the same host species (A. moreletii), only Serratia sp. 3 showed a comprehensive ability to inhibit all three isolates of B. dendrobatidis (Fig. 1). The growth of two of the B. dendrobatidis isolates (B. dendrobatidis GPL CORN 3.2 and JEL 423) was consistently inhibited by the candidate bacteria, while the growth of the third isolate (B. dendrobatidis GPL VA05) was rarely impaired (Fig. 1).
TABLE 1.
Statistical significance values for generalized linear models with Poisson error structure and log link to analyze the effect of each bacterial isolate on inhibition scores against the three B. dendrobatidis isolates
| Bacterial isolate | χ2 value | P valuea |
|---|---|---|
| Acinetobacter sp. strain 1 | 9.843 | 0.007* |
| Acinetobacter sp. strain 2 | 1.567 | 0.457 |
| Agrobacterium sp. | 0.000 | 1.000 |
| Arthrobacter sp. | 14.756 | <0.001* |
| Chryseobacterium sp. strain 1 | 14.120 | <0.001* |
| Chryseobacterium sp. strain 2 | 23.789 | <0.001* |
| Chryseobacterium sp. strain 3 | 3.170 | 0.205 |
| Enterobacter sp. strain 1 | 9.442 | 0.009* |
| Enterobacter sp. strain 2 | 3.915 | 0.141 |
| Lysobacter sp. | 10.109 | 0.006* |
| Serratia sp. strain 1 | 11.046 | 0.004* |
| Serratia sp. strain 2 | 9.825 | 0.007* |
| Serratia sp. strain 3 | 1.273 | 0.529 |
| Serratia sp. strain 4 | 17.723 | <0.001* |
| Stenotrophomonas sp. | 25.994 | <0.001* |
*, a statistically significant result (P < 0.05), meaning statistically significantly different inhibition scores against the three B. dendrobatidis isolates for a given bacterial isolate. For all models, the degrees of freedom are equal to 2.
FIG 1.
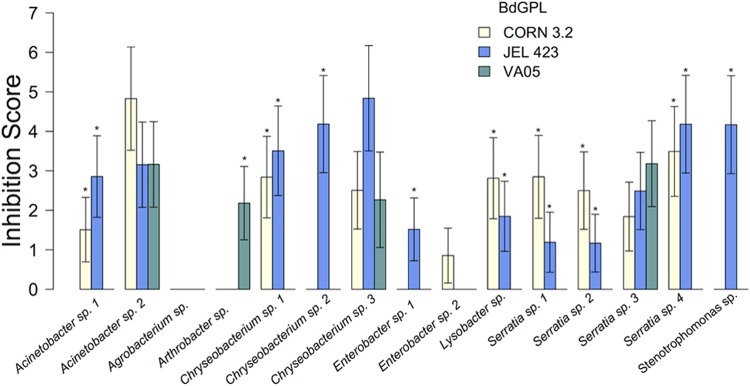
Average (±1 SEM) inhibition scores for 15 bacteria from quantitative in vitro challenges against three B. dendrobatidis GPL (BdGPL) isolates. *, within each bacterium, B. dendrobatidis isolates with inhibition scores significantly different from those for B. dendrobatidis isolates without an asterisk.
Genus-level models.
There was no evidence for a significant interaction between bacterial genus and B. dendrobatidis isolate (χ2 = 5.2, df = 6, P = 0.51). However, both bacterial genus (χ2 = 9.32, df = 3, P = 0.025) and B. dendrobatidis isolate (χ2 = 14.8, df = 2, P < 0.001) were significant predictors of inhibition of B. dendrobatidis growth. Post hoc comparisons showed that there were no significant differences in the inhibitory capabilities of the genera Acinetobacter, Chryseobacterium, and Serratia (χ2 = 0.54, df = 1, P = 0.76) but that Enterobacter species had significantly lower inhibitory capabilities than the other three genera (Acinetobacter, Chryseobacterium, and Serratia; χ2 = 8.77, df = 1, P = 0.003; Fig. 2). Similarly, there was no significant difference in the degree of inhibition of CORN 3.2 and JEL 423 by the four bacterial genera (χ2 = 0.46, df = 1, P = 0.47), but all four bacterial genera were significantly less likely to inhibit the growth of B. dendrobatidis GPL VA05 (χ2 = 14.2, df = 1, P < 0.001) than the growth of any of the other B. dendrobatidis isolates (Fig. 2).
FIG 2.
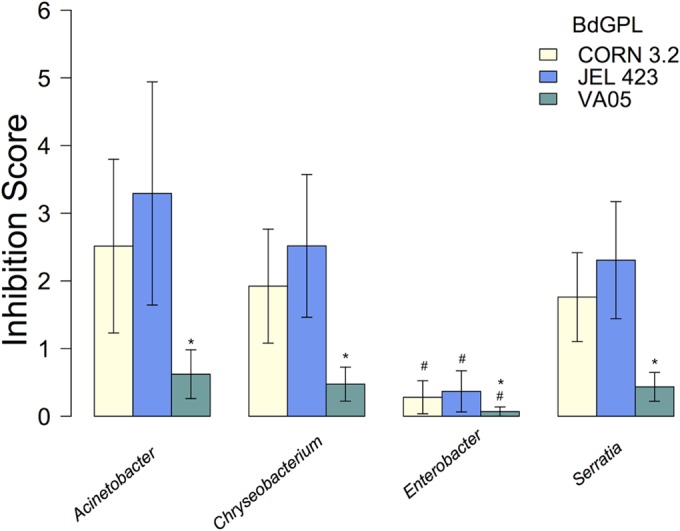
Average (±1 SEM) inhibition scores for multiple bacteria from four genera used to challenge three B. dendrobatidis GPL isolates. Inhibition scores against VA05 isolates were significantly lower than those against the other B. dendrobatidis GPL isolates (*), and Enterobacter spp. showed a significantly lower inhibition of the range of B. dendrobatidis GPL isolates than the other bacteria (#).
The results from the ordinal analyses mirrored the results from the Poisson mixed models; the model with the lowest DIC (the best-supported model) contained B. dendrobatidis GPL isolate and bacterial genus as main effects, without an interaction. The genus Enterobacter was associated with significantly lower inhibition scores (mean difference = −2.03; 95% credible interval = −3.53 to −0.57). In addition, B. dendrobatidis GPL VA05 was also associated with significantly lower inhibition scores (mean difference = −1.42, 95% credible interval = −2.46 to −0.43). Parameter estimates for the best-supported model, as well as a model selection table containing DIC values for all five models, are provided in Tables S2 and S3 in the supplemental material.
DISCUSSION
Here we show that symbiotic bacteria from the skin of amphibians exhibit differences in inhibitory capabilities across B. dendrobatidis GPL isolates, with only a small proportion of candidate probiotics showing broad-spectrum inhibition against the global panzootic B. dendrobatidis lineage. This is strong evidence that candidate bacteria tested in vitro for use in probiotic B. dendrobatidis mitigation in situ are unlikely to be consistently successful when confronting a variety of fungal genotypes. Because of the enormous genetic variability of B. dendrobatidis GPL (10, 18, 19, 24, 25), the propensity for B. dendrobatidis to rapidly evolve in situ (10, 18, 26), and the panglobal, ongoing dissemination of B. dendrobatidis through numerous vectors (11, 27), amphibians and their microbiomes can be expected to confront an ever changing and diverse distribution of B. dendrobatidis genotypes. Thus, the pathogen represents a “moving target” for potential interventions (28), and the mitigation of chytridiomycosis in the wild also needs to account for complex interactions between the host, the pathogen, and the environment, as well as multiple pathogen genotypes, in order to be successful (28–30).
We did not test our wild study animals for the presence of B. dendrobatidis; however, between 2006 and 2008 Kaiser and Pollinger (31) sampled amphibians at the same study site in Belize and found only a 5% B. dendrobatidis prevalence on A. moreletii frogs and a 0% prevalence on A. callidryas frogs. Museum specimens date the arrival of B. dendrobatidis in the general region (Mexico and Guatemala) to the late 1960s or early 1970s (32), suggesting that both host species are persisting in spite of the long-term presence of B. dendrobatidis in the area. The finding that a reasonable proportion of the bacteria isolated from these two host species in this study inhibited at least one of the B. dendrobatidis isolates suggests that these populations may possess a microbiome capable of at least partially mitigating B. dendrobatidis infection.
If manipulation of amphibian skin microbiota is to be of value for mitigating B. dendrobatidis infection in the wild, amphibian microbiomes will need to be managed for a functional redundancy that provides a broad-spectrum capacity against the evolving threat represented by B. dendrobatidis. Studies have repeatedly illustrated the importance in a complex microbiome for resilience of the community in response to a pathogenic infection (33–35). A bacterial consortium approach that treats microbiomes as a suite of functional traits rather than a substrate for the insertion of candidate bacteria is likely to offer a more comprehensive protection of hosts from B. dendrobatidis and other threatening amphibian pathogens (12, 28, 36). How the different members of such consortia will be determined is currently unknown, but our results highlight the limitations of a taxonomic approach for understanding what bacterial communities may afford resistance to B. dendrobatidis: both species and genus showed a limited potential to identify potentially inhibitory bacteria in our study. That said, devising probiotic strategies that incorporate bacterial genus as a criterion might yield better results than bacterial species-specific approaches, and a recently developed open access database for antifungal bacterial isolates from amphibian skin will allow researchers to optimize approaches to identifying candidate probiotics (37). Ultimately, understanding functional redundancy in amphibian skin microbiomes will require a deeper understanding of how bacteria inhibit B. dendrobatidis growth and of their ability to infect hosts. Mining of the B. dendrobatidis genome for virulence factors will be fraught with difficulty, as aneuploidy and polyploidy are common across B. dendrobatidis isolates and changes in ploidy levels do not map to infectivity and virulence in any predictable fashion (18). However, our identification of some bacteria exhibiting broad-spectrum B. dendrobatidis inhibition capabilities and a significant effect of the genus on B. dendrobatidis growth and reproduction suggests some bacterial phylogenetic conservation of the ability to inhibit B. dendrobatidis. This bodes well for the presence of bacterial genetic factors that are responsible for impairment of the ability of B. dendrobatidis to infect and cause disease in amphibian hosts. Current criteria for selecting candidate probiotic bacteria include successful inhibition of B. dendrobatidis, residency in the normal microbiota of the host, and an ability to persist on the skin of inoculated individuals (12). We propose that candidate probiotics should also exhibit inhibitory activity against a range of B. dendrobatidis isolates, particularly the hypervirulent B. dendrobatidis GPL.
Supplementary Material
ACKNOWLEDGMENTS
This project was funded by a BBSRC studentship and a North-West University postdoctoral research fellowship to R.E.A.
We thank Olivia Daniel and Lola Brookes for providing culturing assistance and Mat Fisher for providing Batrachochytrium dendrobatidis isolates. We are particularly grateful to the Belize Forestry Department and Rasheda Sampson for providing sampling and export permits.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00010-15.
REFERENCES
- 1.McFall-Ngai M, Hadfield MG, Bosch TC, Carey HV, Domazet-Lovso T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci U S A 110:3229–3236. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarchum I, Pamer EG. 2011. Regulation of innate and adaptive immunity by the commensal microbiota. Curr Opin Immunol 23:353–360. doi: 10.1016/j.coi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newaj-Fyzul A, Al-Harbi H, Austin B. 2014. Review: developments in the use of probiotics for disease control in aquaculture. Aquaculture 431:1–11. doi: 10.1016/j.aquaculture.2013.08.026. [DOI] [Google Scholar]
- 4.Smith JM. 2014. A review of avian probiotics. J Avian Med Surg 28:87–94. doi: 10.1647/2012-031. [DOI] [PubMed] [Google Scholar]
- 5.Kilpatrick AM, Briggs CJ, Daszak P. 2010. The ecology and impact of chytridiomycosis: an emerging disease of amphibians. Trends Ecol Evol 25:109–118. doi: 10.1016/j.tree.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 6.International Union for Conservation of Nature. 2014. The IUCN Red List of Threatened Species: summary statistics. International Union for Conservation of Nature, Cambridge, United Kingdom: http://www.iucnredlist.org/about/summary-statistics Accessed July 2014. [Google Scholar]
- 7.Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, Slocombe R, Ragan MA, Hyatt AD, McDonald KR, Hines HB, Lips KR, Marantelli G, Parkes H. 1998. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci U S A 95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martel A, Spitzen-van der Sluijs A, Blooi M, Bert W, Ducatelle R, Fisher MC, Woeltjes A, Bosman W, Chiers K, Bossuyt F, Pasmans F. 2013. Batrachochytrium salamandrivorans sp. nov. causes lethal chytridiomycosis in amphibians. Proc Natl Acad Sci U S A 110:15325–15329. doi: 10.1073/pnas.1307356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longcore JE, Pessier AP, Nichols DK. 1999. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia 91:219–227. doi: 10.2307/3761366. [DOI] [Google Scholar]
- 10.Fisher MC, Garner TWJ, Walker SF. 2009. Global emergence of Batrachochytrium dendrobatidis and amphibian chytridiomycosis in space, time, and host. Annu Rev Microbiol 63:291–310. doi: 10.1146/annurev.micro.091208.073435. [DOI] [PubMed] [Google Scholar]
- 11.Olson DH, Aanensen DM, Ronnenberg KL, Powell CI, Walker SF, Bielby J, Garner TWJ, Weaver G, Fisher MC. 2013. Mapping the global emergence of Batrachochytrium dendrobatidis, the amphibian chytrid fungus. PLoS One 8:e56802. doi: 10.1371/journal.pone.0056802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bletz MC, Loudon AH, Becker MH, Bell SC, Woodhams DC, Minbiole KPC, Harris RN. 2013. Mitigating amphibian chytridiomycosis with bioaugmentation: characteristics of effective probiotics and strategies for their selection and use. Ecol Lett 16:807–820. doi: 10.1111/ele.12099. [DOI] [PubMed] [Google Scholar]
- 13.Harris RN, Lauer A, Simon MA, Banning JL, Alford RA. 2009. Addition of antifungal skin bacteria to salamanders ameliorates the effects of chytridiomycosis. Dis Aquat Organ 83:11–16. doi: 10.3354/dao02004. [DOI] [PubMed] [Google Scholar]
- 14.Harris RN, Brucker RM, Walke JB, Becker MH, Schwantes CR, Flaherty DC, Lam BA, Woodhams DC, Briggs CJ, Vredenburg VT, Minbiole KP. 2009. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J 3:818–824. doi: 10.1038/ismej.2009.27. [DOI] [PubMed] [Google Scholar]
- 15.Lam BA, Walke JB, Vredenburg VT, Harris RN. 2010. Proportion of individuals with anti-Batrachochytrium dendrobatidis skin bacteria is associated with population persistence in the frog Rana muscosa. Biol Conserv 143:529–531. doi: 10.1016/j.biocon.2009.11.015. [DOI] [Google Scholar]
- 16.Muletz CR, Myers JM, Domangue RJ, Herrick JB, Harris RN. 2012. Soil bioaugmentation with amphibian cutaneous bacteria protects amphibian hosts from infection by Batrachochytrium dendrobatidis. Biol Conserv 152:119–126. doi: 10.1016/j.biocon.2012.03.022. [DOI] [Google Scholar]
- 17.Woodhams DC, Vredenburg VT, Simon MA, Billheimer D, Shakhtour B, Shyr Y, Briggs CJ, Rollins-Smith LA, Harris RN. 2007. Symbiotic bacteria contribute to innate immune defenses of the threatened mountain yellow-legged frog, Rana muscosa. Biol Conserv 138:390–398. doi: 10.1016/j.biocon.2007.05.004. [DOI] [Google Scholar]
- 18.Farrer RA, Henk DA, Garner TWJ, Balloux F, Woodhams DC, Fisher MC. 2013. Chromosomal copy number variation, selection and uneven rates of recombination reveal cryptic genome diversity linked to pathogenicity. PLoS Genet 9:e1003703. doi: 10.1371/journal.pgen.1003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farrer RA, Weinert LA, Bielby J, Garner TWJ, Balloux F, Clare F, Bosch J, Cunningham AA, Weldon C, du Preez LH, Anderson L, Pond SL, Shahar-Golan R, Henk DA, Fisher MC. 2011. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc Natl Acad Sci U S A 108:18732–18736. doi: 10.1073/pnas.1111915108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher MC, Bosch J, Yin Z, Stead DA, Walker J, Selway L, Brown AJP, Walker LA, Gow NAR, Stajich JE, Garner TWJ. 2009. Proteomic and phenotypic profiling of the amphibian pathogen Batrachochytrium dendrobatidis shows that genotype is linked to virulence. Mol Ecol 18:415–429. doi: 10.1111/j.1365-294X.2008.04041.x. [DOI] [PubMed] [Google Scholar]
- 21.Antwis RE, Garcia G, Fidgett AL, Preziosi RF. 2014. Tagging frogs with passive integrated transponders causes disruption of the cutaneous bacterial community and proliferation of opportunistic fungi. Appl Environ Microbiol 80:4779–4784. doi: 10.1128/AEM.01175-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell SC, Alford RA, Garland S, Padilla G, Thomas AD. 2013. Screening bacterial metabolites for inhibitory effects against Batrachochytrium dendrobatidis using a spectrophotometric assay. Dis Aquat Organ 103:77–85. doi: 10.3354/dao02560. [DOI] [PubMed] [Google Scholar]
- 23.Hadfield JD. 2010. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stats Softw 33:1–22. [Google Scholar]
- 24.Retallick RW, Miera V. 2007. Strain differences in the amphibian chytrid Batrachochytrium dendrobatidis and non-permanent, sub-lethal effects of infection. Dis Aquat Organ 75:201–207. doi: 10.3354/dao075201. [DOI] [PubMed] [Google Scholar]
- 25.Doddington BJ, Bosch J, Oliver JA, Grassly NC, Garcia G, Schmidt BR, Garner TW, Fisher MC. 2013. Context-dependent amphibian host population response to an invading pathogen. Ecology 94:1795–1804. doi: 10.1890/12-1270.1. [DOI] [PubMed] [Google Scholar]
- 26.Phillips BL, Puschendorf R. 2013. Do pathogens become more virulent as they spread? Evidence from the amphibian declines in Central America. Proc Biol Sci 280:20131290. doi: 10.1098/rspb.2013.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMahon TA, Sears BF, Venesky MD, Bessler SM, Brown JM, Deutsch K, Halstead NT, Lentz G, Tenouri N, Young S, Civitello DJ, Ortega N, Fites JS, Reinert LK, Rollins-Smith LA, Raffel TR, Rohr JR. 2014. Amphibians acquire resistance to live and dead fungus overcoming fungal immunosuppression. Nature 511:224–227. doi: 10.1038/nature13491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venesky MD, Raffel TR, McMahon TA, Rohr JR. 2014. Confronting inconsistencies in the amphibian-chytridiomycosis system: implications for disease management. Biol Rev Camb Philos Soc 89:477–483. doi: 10.1111/brv.12064. [DOI] [PubMed] [Google Scholar]
- 29.Woodhams DC, Bosch J, Briggs CJ, Cashins S, Davis LR, Lauer A, Muths E, Puschendorf R, Schmidt BR, Sheafor B. 2011. Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Front Zool 8:8. doi: 10.1186/1742-9994-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheele BC, Guarino F, Osborne W, Hunter DA, Skerratt LF, Driscoll DA. 2014. Decline and re-expansion of an amphibian with high prevalence of chytrid fungus. Biol Conserv 170:86–91. doi: 10.1016/j.biocon.2013.12.034. [DOI] [Google Scholar]
- 31.Kaiser K, Pollinger J. 2012. Batrachochytrium dendrobatidis shows high genetic diversity and ecological niche specificity among haplotypes in the Maya Mountains of Belize. PLoS One 7:e32113. doi: 10.1371/journal.pone.0032113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng TL, Rovito SM, Wake DB, Vredenburg VT. 2011. Coincident mass extirpation of neotropical amphibians with the emergence of the infectious fungal pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci U S A 108:9502–9507. doi: 10.1073/pnas.1105538108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dillon RJ, Vennard CT, Buckling A, Charnley AK. 2005. Diversity of locust gut bacteria protects against pathogen invasion. Ecol Lett 8:1291–1298. doi: 10.1111/j.1461-0248.2005.00828.x. [DOI] [Google Scholar]
- 34.Eisenhauer N, Schulz W, Scheu S, Jousset A, Pfrender M. 2013. Niche dimensionality links biodiversity and invasibility of microbial communities. Funct Ecol 27:282–288. doi: 10.1111/j.1365-2435.2012.02060.x. [DOI] [Google Scholar]
- 35.Van Elsas JD, Chiurazzi M, Mallon CA, Elhottova D, Kristufek V, Salles JF. 2012. Microbial diversity determines the invasion of soil by a bacterial pathogen. Proc Natl Acad Sci U S A 109:1159–1164. doi: 10.1073/pnas.1109326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loudon AH, Holland JA, Umile TP, Burzynski EA, Minbiole KPC, Harris RN. 2014. Interactions between amphibians' symbiotic bacteria cause the production of emergent anti-fungal metabolites. Front Microbiol 5:441. doi: 10.3389/fmicb.2014.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Woodhams DC, Alford R, Antwis RE, Archer H, Becker MH, Belden LK, Bell SC, Bletz M, Daskin JH, Davis LA, Flechas SV, Lauer A, Peña AG, Harris RN, Holden WM, Hughey MC, Ibañez R, Knight R, Kueneman J, Rabemananjara F, Reinert LK, Rollins-Smith LA, Roman-Rodriguez F, Shaw SD, Walke JB, McKenzie V. 2015. Antifungal isolates database of amphibian skin-associated bacteria and function against emerging fungal pathogens. Ecology 96:595. doi: 10.1890/14-1837.1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


