Abstract
Foods and related processing environments are commonly contaminated with the pathogenic Listeria monocytogenes. To investigate potential environmental reservoirs of Listeria spp. and L. monocytogenes, surface water and point source pollution samples from an urban and a rural municipal water supply watershed in Nova Scotia, Canada, were examined over 18 months. Presumptive Listeria spp. were cultured from 72 and 35% of rural and urban water samples, respectively, with 24% of the positive samples containing two or three different Listeria spp. The L. innocua (56%) and L. welshimeri (43%) groups were predominant in the rural and urban watersheds, respectively. Analysis by the TaqMan assay showed a significantly (P < 0.05) higher prevalence of L. monocytogenes of 62% versus 17% by the culture-based method. Both methods revealed higher prevalences in the rural watershed and during the fall and winter seasons. Elevated Escherichia coli (≥100 CFU/100 ml) levels were not associated with the pathogen regardless of the detection method. Isolation of Listeria spp. were associated with 70 times higher odds of isolating L. monocytogenes (odds ratio = 70; P < 0.001). Serogroup IIa was predominant (67.7%) among the 285 L. monocytogenes isolates, followed by IVb (16.1%), IIb (15.8%), and IIc (0.4%). L. monocytogenes was detected in cow feces and raw sewage but not in septic tank samples. Pulsotyping of representative water (n = 54) and local human (n = 19) isolates suggested genetic similarities among some environmental and human L. monocytogenes isolates. In conclusion, temperate surface waters contain a diverse Listeria species population and could be a potential reservoir for L. monocytogenes, especially in rural agricultural watersheds.
INTRODUCTION
Listeria monocytogenes is a Gram-positive bacterium that is able to tolerate high salt concentrations, cold temperatures, and acid stress (1). Pathogenic Listeria species include L. monocytogenes and L. ivanovii (2), while 13 nonpathogenic species exist: L. innocua, L. welshimeri, L. grayi, L. seeligeri, L. rocourtiae, L. marthii, L. weihenstephanensis, L. fleischmannii, L. floridensis, L. aquatica, L. cornellensis, L. grandensis, and L. riparia (3, 4).
Listeria spp. are considered ubiquitous in the natural environment, and representatives of the genus, including L. monocytogenes, have been isolated from soil, surface waters, animal feeds, animal feces, sewage, food processing plants, and farm environments (5–11). In one New York study, L. seeligeri and L. welshimeri were dominant species in water and other environmental samples from pristine environments, while L. innocua and L. monocytogenes were associated with urban environments (8). Focusing on Listeria spp. diversity, Chapin et al. (12) reported finding Listeria spp. (excluding L. monocytogenes) in 22 and 51% of the surface water samples from pristine and produce growing areas, respectively, in New York. Linke et al. (11) detected Listeria spp. at similar levels (26%) in surface water samples from the pristine natural environment in different parts of Austria. As for the pathogenic L. monocytogenes in surface waters, recent culture-based studies have reported prevalence of 10% in Ontario, Canada (13), and 28 to 43% in various watersheds in New York and California (14–17).
Several methods of characterizing L. monocytogenes isolates in water- and food-borne outbreaks and ecological studies are available to elucidate the transmission of the pathogen through the environment and into our food and drinking water supply. A multiplex PCR method divides L. monocytogenes isolates into five serogroups (IIa [1/2a and 3a serovars], IIb [1/2b, 3b, and 7 serovars], IIc [1/2c and 3c serovars], IVa [4c serovar], and IVb [4b, 4d, and 4e serovars]) (18, 19). The serogroups represent evolutionary complexes and separate the four serovars (1/2a, 1/2c, 1/2b, and 4b) that cause >98% of human listeriosis cases (20, 21). For studies of strains at a more detailed level, genotyping methods such as ribotyping, pulsotyping using pulsed-field gel electrophoresis (PFGE), multiple-locus variable-number tandem-repeat analysis (MLVA), and multilocus-sequence based typing are useful (7, 22–24), and application of these methods has shown there to be a great biodiversity of L. monocytogenes in surface waters (11, 13, 14, 17).
Many communities rely on surface waters as a source of raw drinking water, as well as for recreational and agricultural uses. Protection of the source water is an important step in a proactive multibarrier approach to safeguard the safety of drinking and irrigation water (25). The quality of source water is commonly assessed by monitoring levels of fecal indicator bacteria (FIB) such as Escherichia coli. Although E. coli levels have been shown to predict the presence of zoonotic pathogens such as Salmonella spp. (26, 27), it is less certain that this approach can be used to predict the presence of an indigenous pathogen such as L. monocytogenes, which may or may not originate from fecal sources.
In this study, we set out to characterize the prevalence and diversity of Listeria spp., in particular L. monocytogenes, in an urban and a rural municipal source water system to understand the ecology and occurrence of this pathogen in the environment. Our specific objectives were to (i) determine the diversity of Listeria spp. in the urban and rural watersheds during an 18-month study period to cover all seasons, (ii) characterize the prevalence and diversity of L. monocytogenes in the surface waters, (iii) determine whether E. coli density, Listeria presence, or other water quality characteristics could predict L. monocytogenes presence in surface water, and (iv) compare PFGE patterns obtained for representative source water isolates to patterns from clinical L. monocytogenes strains representing all human clinical cases that occurred in Nova Scotia, Canada, during the study period.
MATERIALS AND METHODS
Collection of water and point source pollution samples.
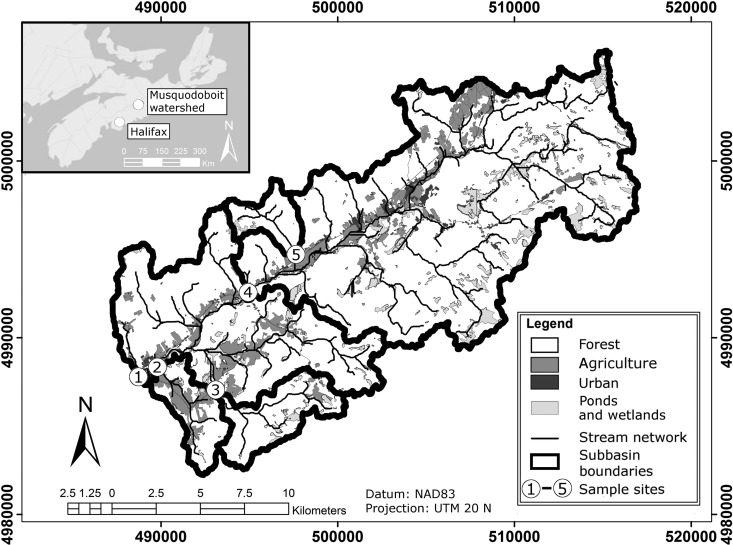
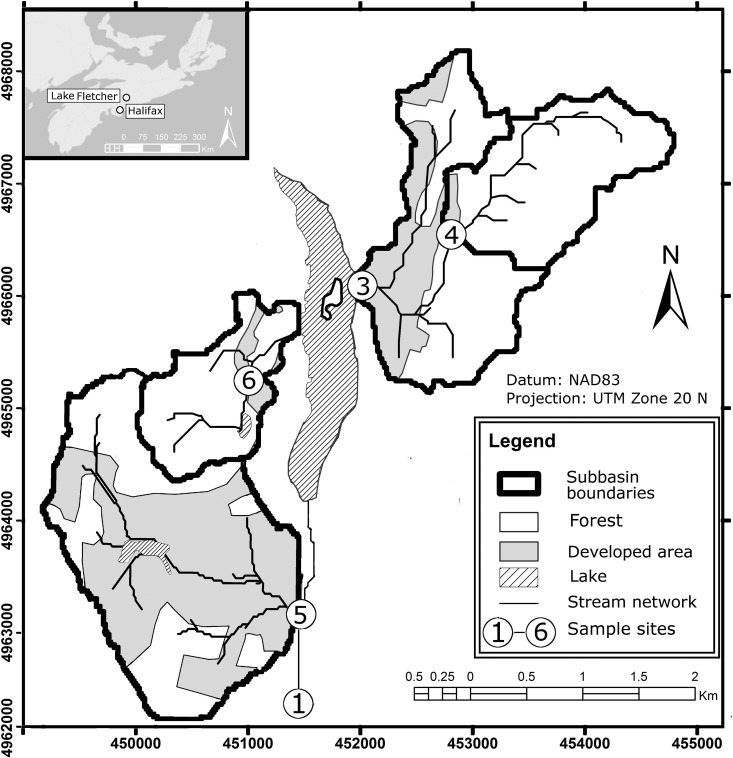
Surface water samples were collected in 33 separate sampling events from five sites in the rural and agricultural Musquodoboit River (MR) watershed (Fig. 1) and five sites in the urban/suburban Lake Fletcher (LF) watershed (Fig. 2) in Canada over the study period from January 2012 to August 2013 for a total of 329 water samples (one urban site was inaccessible in January 2012). The MR watershed is located upstream of the intake (site 1 in Fig. 1) to the community of Middle Musquodoboit's drinking water treatment plant. The watershed covers a drainage area of approximately 135 km2 and is home to several small dairy and beef cattle operations (approximately 500 animals), some of which are located along smaller tributaries flowing into the river (sites 2 and 3 in Fig. 1). The primary inflow to Lake Fletcher (site 1 in Fig. 2) drains a large (128 km2) urban catchment that originates in the city of Dartmouth, while additional smaller tributaries drain areas of suburban development (sites 3, 5, and 6 in Fig. 2).
FIG 1.
Middle Musquodoboit watershed in Canada and sampling locations (MR1 to MR5). (Map created using ArcGIS v10.2.)
FIG 2.
Lake Fletcher/Collin's Park watershed system and sampling locations (LF1 to LF6). (Map created using ArcGIS v10.2.)
Water samples were collected monthly during the months of November to March and biweekly for the remainder of the year. The sample sites were selected for the purpose of monitoring the effects of key agricultural and residential land uses on source water quality. On each of the sampling events, four liters of surface water was collected aseptically into 1-liter sample bottles, which had been washed and rinsed in 70% (vol/vol) ethanol.
Cow fecal samples (n = 24 [9 in 2012 and 15 in 2013]) were obtained from one farm in the rural watershed. In addition, septic tank samples (n = 3) were collected from a home located in the urban watershed (in 2012). Incoming raw sewage samples (n = 3) were also collected on two occasions (in 2012 and 2013) from the wastewater treatment plant located within the urban watershed (site 5 area in Fig. 2). All samples were transported back to the laboratory in coolers (maximum temperature, 5°C), and the analyses were begun immediately upon arrival (i.e., a maximum of 5 h after being sampled).
Enrichment and isolation of Listeria spp. from water and point source pollution samples.
Aliquots of 500 ml of sample water were filtered through 0.45-μm-pore-size membrane filters (Millipore, Etobicoke, Ontario, Canada). These filters were then placed in Listeria enrichment broth (LEB; Oxoid, Nepean, Ontario, Canada) and incubated for 24 h at 37°C. Next, 1 ml from the LEB culture was transferred to 9 ml of Fraser broth (Oxoid), followed by incubation for another 24 h at 37°C.
Fecal samples (250 mg) were also enriched in LEB, followed by Fraser broth, according to the protocol described above. Septic tank and raw sewage samples (500 ml) were mixed well prior to transferring 10 ml into a 15-ml Falcon tube for centrifugation at 3,200 × g for 10 min. The pellets (ca. 250 mg) were then spiked into LEB and enriched for Listeria spp. as described above.
To isolate Listeria spp., an aliquot from the Fraser broth culture was streaked onto Palcam agar (Oxoid) and incubated for 48 h at 35°C. Up to eight black, presumptive Listeria colonies were picked from each positive plate, grown in brain heart infusion broth (Oxoid) for 48 h at 37°C, followed by the addition of glycerol (15% [vol/vol]; Sigma, Oakville, Ontario, Canada) and storage at −80°C until further analysis.
Diversity of Listeria species.
Frozen cultures of the presumptive Listeria isolates were streaked directly on RAPID'L. Mono chromogenic agar (Bio-Rad Laboratories, Hercules, CA) which separates Listeria species based on their phosphoinositide phospholipase C activity and xylose metabolism. The inoculated plates were incubated aerobically at 37°C for 30 h. This method allowed us to divide the presumptive Listeria isolates into four groups: L. monocytogenes, L. ivanovii, the L. innocua group (L. seeligeri, L. marthii, and L. grayi), and the L. welshimeri group, which includes L. rocourtiae, L. fleischmannii, L. weihenstephanensis, L. floridensis, L. aquatic, L. cornellensis, L. grandensis, and L. riparia (see Table S1 in the supplemental material).
Serogrouping of L. monocytogenes isolates.
A multiplex PCR method was performed to confirm the identity (prfA positive) and determine the serogroup of each L. monocytogenes isolate (Table 1). The method was based on the work by Doumith et al. (18) and Kérouanton et al. (19) and was performed in a Biometra T-Gradient thermocycler (Biometra, Gottingen, Germany) using the following PCR program: initial denaturation for 3 min at 94°C, followed by 40 cycles of denaturation at 94°C for 40 s, annealing at 53°C for 45 s, and extension at 72°C for 1 min 15 s, and then a final extension for 7 min at 72°C. Each colony PCR (25 μl) consisted of ∼0.5 μl of colony mass, 1 U of Taq DNA polymerase (IDTaq Taq polymerase kit; IDTaq, London, Ontario, Canada), 1× MgCl2-free buffer, 3 mM MgCl2, 1 mM concentrations of deoxynucleoside triphosphates, 0.4 μM concentrations of each of the lmo737F/R, lmo1118F/R, orf2819F/R, and orf2110F/R primer sets, and a 0.2 μM concentration of the LIP1/LIP2a (prfA-F/R) primer set and diethyl pyrocarbonate-water.
TABLE 1.
Primers and probes used for the TaqMan assay and multiplex PCR experiments
| Assay and primer | Sequence (5′–3′)a | Annealing temp (°C) | Amplicon size (bp) | Reference |
|---|---|---|---|---|
| TaqMan assay for detection of L. monocytogenes | ||||
| HlyQF | CATGGCACCACCAGCATCT | |||
| HlyQR | ATCCGCGTGTTTCTTTTCGA | 56 | 64 | 28 |
| HlyQP | FAM-CGCCTGCAAGTCCTAAGACGCCA-TAMRA | |||
| Serogrouping of L. monocytogenes (by PCR) | ||||
| Lmo737F | AGGGCTTCAAGGACTTACCC | 53 | 691 | 18 |
| Lmo737R | ACGATTTCTGCTTGCCATTC | |||
| Lmo1118F | AGGGGTCTTAAATCCTGGAA | 53 | 906 | |
| Lmo1118R | CGGCTTGTTCGGCATACTTA | |||
| Orf2819F | AGCAAAATGCCAAAACTCGT | 53 | 471 | |
| Orf2819R | CATCACTAAAGCCTCCCATTG | |||
| Orf2110F | AGTGGACAATTGATTGGTGAA | 53 | 597 | |
| Orf2110R | CATCCATCCCTTACTTTGGAC | |||
| LIP1 (prfAF) | GATACAGAAACATCGGTTGGC | 53 | 274 | 19 |
| LIP2a (prfAR) | GTGTAATCTTGATGCCATCAGG |
TaqMan probe labels: FAM, fluorescein; TAMRA, 5(6)-carboxytetramethylrhodamine.
The amplified PCR products were transferred to the wells of a 2% (wt/vol) agarose (Fisher Scientific, Oakville, Ontario, Canada) gel, containing 0.016% (vol/vol) GelRed (Biotium, Hayward, CA) for visualization, in 1× Tris-borate-EDTA buffer (Sigma) and resolved by electrophoresis (80 min, 85 V) in a Hoefer submarine HE33 (GE Health Care Life Sciences, Baie d'Urfe, Quebec, Canada). The resulting gel band patterns were then used to confirm the identity and assign the L. monocytogenes isolates to their respective serogroups.
L. monocytogenes presence or absence testing using a TaqMan assay.
For TaqMan real-time PCR (TaqMan assay) detection of L. monocytogenes in the water samples, 2 ml of the Fraser enrichment broth was centrifuged at 3,200 × g for 10 min. The supernatant was removed, and 250 μl of resuspended pellet was subjected to DNA extraction using the PowerSoil DNA extraction kit (MoBio Laboratories, Carlsbad, CA) according to the manufacturer's instructions.
DNA was directly extracted from the bovine feces samples (250 mg; n = 24) by using a PowerSoil DNA extraction kit (MoBio Laboratories). Septic tank and raw sewage samples (500 ml) were mixed well prior to transferring 10 ml into 15-ml Falcon tubes for centrifugation (3,200 × g, 10 min). The supernatant was decanted, and 250 mg of resuspended pellet was used for DNA extraction using a PowerSoil DNA extraction kit (MoBio). Along with the samples, DNA was also extracted from a negative extraction control (250 μl of sterile water) and other negative controls (enrichment media). All DNA samples were stored at −20°C until further analysis.
Each TaqMan assay reaction mixture (25 μl) consisted of the following: DNase-free water (7.7 μl; Fisher Scientific, Ottawa, Ontario, Canada), master mix (12.5 μl; Applied Biosystems Fast Advanced 2×; Applied Biosystems, Burlington, Ontario, Canada), 0.3 μl each of 10 μM concentrations of forward and reverse primers, 0.2 μl of 10 μM concentrations of TaqMan probes, and 4 μl of sample DNA. Primers and probes (Sigma, Oakville, Ontario, Canada) for L. monocytogenes targeted the hly gene as outlined by Rodríguez-Lázaro et al. (28) (Table 1). Amplification and detection were done using a StepOne Plus system (Applied Biosystems) and the following thermocycler program: initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 20 s, annealing at 56°C for 30 s, and extension at 72°C for 1 min.
The test results were scored as L. monocytogenes being present or absent in 500 ml of water, 10 ml of wastewater, or 250 mg of bovine feces. DNA extracted from L. monocytogenes 568 (serogroup IIa) was used as the positive control in each TaqMan assay along with negative or no-template controls. The TaqMan assay PCR efficiencies ranged from 88 to 105% (within the standard operating procedures for pathogen detection), as determined using a 10-fold dilution series of the positive-control sample. The limit of detection (LOD) for the (enriched) water samples was 1 CFU in 500 ml (enriched to minimum levels of 50 CFU/ml in the Fraser enrichment broth), while LODs for wastewater and bovine feces samples were 10 and 100 CFU/g, respectively. The absence of PCR inhibitors in each sample type was confirmed in spiking assays with the positive-control strain (data not shown).
PFGE on a subset of L. monocytogenes isolates.
Results from the serogrouping multiplex PCR showed that all six to eight L. monocytogenes isolates from a culture-positive sample belonged to the same serogroup. Therefore, one L. monocytogenes isolate was selected from each culture-positive water sample, for a total of 54 L. monocytogenes strains, to be subjected to the PFGE analysis (see Table 3; 2 of the 56 positive samples were inadvertently omitted from the analyses).
TABLE 3.
Prevalence of L. monocytogenes as determined using TaqMan assay and culture methods in surface water samples from a rural and urban watershed in Nova Scotia, Canada, during an 18-month monitoring period from 2012 to 2013
| Watershed | Type | Detection (% positive)a |
L. monocytogenes serotype diversity (%)b |
||||
|---|---|---|---|---|---|---|---|
| TaqMan assay | Culture based | IIa | IIb | IIc | IVb | ||
| Musquodoboit River | Rural | 67.3 (111/165) | 21.8 (36/165) | 76.8 | 12.5 | 0.6 | 10.1 |
| MR1 | WTP intake/river | 66.7 (22/33) | 15.2 (5/33) | 34.8 | 65.2 | 0 | 0 |
| MR2 | Tributary A | 75.8 (25/33) | 18.2 (6/33) | 66.7 | 33.3 | 0 | 0 |
| MR3 | Tributary B | 57.6 (22/33) | 21.2 (7/33) | 96.7 | 0 | 3.3 | 0 |
| MR4 | River (upstream) | 72.7 (24/33) | 33.3 (11/33) | 71.7 | 0 | 0 | 28.8 |
| MR5 | Tributary C | 72.7 (24/33) | 21.2 (7/33) | 100 | 0 | 0 | 0 |
| Lake Fletcher | Urban | 56.1 (92/164) | 12.2 (20/164) | 54.7 | 20.5 | 0 | 24.8 |
| LF1 | Lake Fletcher run/inlet | 51.5 (17/33) | 0 | 0 | 0 | 0 | 0 |
| LF3 | Tributary A (outlet to lake) | 48.5 (16/33) | 6.1 (2/33) | 0 | 100 | 0 | 0 |
| LF4 | Tributary A (headwater) | 60.6 (20/33) | 21.2 (7/33) | 76.6 | 0 | 0 | 23.4 |
| LF5 | Tributary B | 60.6 (20/33) | 18.2 (6/33) | 71.0 | 25.8 | 0 | 54.8 |
| LF6 | Tributary C | 59.4 (19/32) | 15.6 (5/32) | 54.7 | 20.5 | 0 | 24.8 |
| Total | 61.7 (203/329) | 17.0 (56/329) | 67.7 | 15.8 | 0.4 | 16.1 | |
Values are expressed as the percentage of L. monocytogenes-positive samples (number of positive results/total number of samples).
Serogroup diversity among L. monocytogenes isolates (n = 285).
PFGE was performed following the standard CDC PulseNet Protocol (29) on a Bio-Rad Chef Mapper (Bio-Rad, Hercules, CA). Isolates were grown overnight at 37°C in tryptic soy broth (Oxoid). Plugs were created using 1% (wt/vol) Megabase agarose (Bio-Rad) and digested separately with the restriction enzymes AscI (New England BioLabs [NEB], Whitby, Ontario, Canada) and ApaI (NEB) for 4.5 h at 37°C (AscI) and 25°C (ApaI). Salmonella enterica serotype Braenderup (H9812) was digested for 4.5 h with the XbaI enzyme (NEB) and used as a reference standard (30). Run conditions on the Chef Mapper were as follows: 18 h at 6 V/cm with switch times of 4 s to 40.01 s. PFGE images were captured with a Bio-Rad Versa Doc. Patterns from PFGE images were analyzed by using BioNumerics software version 7 (Applied Maths, Saint-Martine, Belgium). Included in the analyses were the PFGE patterns from the 19 human clinical listeriosis cases that occurred in Nova Scotia, Canada, from 2011 to 2013, which were generously provided by the Pulsenet Canada Team at the National Microbiology Laboratory (NML), Public Health Agency of Canada (Winnipeg, Canada) and R. Davidson (Departments of Microbiology and Immunology/Medicine, Dalhousie University). The human L. monocytogenes strains were originally isolated from blood, cerebrospinal, or peritoneal fluids from affected individuals at the Anchor Laboratories of the Provincial Public Health Laboratory Network, Nova Scotia and subsequently pulsotyped at NML using the CDC PulseNet Protocol.
Water quality testing and enumeration of E. coli and total coliforms.
The turbidity in all water samples was determined using a 2100AN IS laboratory turbidimeter (Hach, Mississauga, Ontario, Canada). Using a handheld 600R Sonde device (YSI, Yellow Springs, OH), water temperature (°C) was measured during each sampling event.
E. coli and total coliforms were enumerated using the m-ColiBlue medium (Millipore) according to the manufacturer's instructions. Briefly, sample (undiluted or diluted 10-fold in phosphate-buffered saline) volumes of 100 ml were filtered through a 0.45-μm-pore-size membrane (Millipore). Filters were transferred aseptically to petri dishes (37 mm in diameter; Millipore) containing absorbent pads with 2 ml of m-Coliblue broth. The plates were incubated at 37°C for 24 h and enumerated.
Statistical analysis.
The normality of the data was evaluated by using Shapiro-Wilk normality tests in SYSTAT software (Chicago, IL). Binary logistic regression analyses were carried out in SYSTAT to determine the odds ratios (ORs) for the ability of the L. monocytogenes TaqMan assay results to predict the presence of culturable Listeria spp. and L. monocytogenes in water samples (ORs with 95% confidence intervals [CI]). Other OR calculations included evaluating whether the detection of Listeria spp. predicted the presence of culturable L. monocytogenes and explored possible associations between elevated E. coli (≥100 CFU/100 ml) and turbidity (nephelometric turbidity units [NTU] ≥ 1.0) levels and presence of Listeria spp. and L. monocytogenes (as detected by the molecular and culture-based methods). Multidimensional scaling (MDS) and cluster analysis using the Dice coefficient and unweighted pair group matching algorithm (UPGMA) with a tolerance of 1.5% of PFGE pattern data were completed in BioNumerics 7 (Applied Maths). Simpson's index of discrimination (D) for Listeria spp. and L. monocytogenes pulsotype diversity in the watersheds was calculated as outlined by Hunter and Gaston (31).
RESULTS
Prevalence and diversity of presumptive Listeria spp. in water and point source pollution samples.
Presumptive Listeria spp. were isolated from 177 (53.8%) of the 329 water samples, which were collected over the course of the study, with the detection rates being higher in the rural watershed (72.1%) than in the urban watershed (35.4%) (Table 2). Consequently, more Listeria isolates were obtained from rural watersheds (n = 860) than the urban watersheds (n = 424) for a total of 1,284 isolates.
TABLE 2.
Prevalence and diversity of Listeria spp. in water samples from the rural (MR) and urban (LF) source watershedsa
| Site | Listeria spp. (%)b | No. (%) of water samples |
||||||
|---|---|---|---|---|---|---|---|---|
| Single speciesc |
Mixed speciesd |
|||||||
| L. innocua | L. welshimeri | L. ivanovii | L. monocytogenes | Two spp. | Three spp. | Mixed | ||
| Rural | 72.1 | 45 (37.8) | 23 (19.3) | 2 (1.7) | 18 (15.1) | 24 (20.2) | 7 (5.9) | 18 (15.1) |
| MR1 WTPe intake/river | 84.8 | 12 | 8 | 0 | 1 | 7 | 0 | 4 |
| MR2 tributary A | 75.8 | 13 | 2 | 1 | 2 | 4 | 3 | 4 |
| MR3 tributary B | 78.8 | 14 | 4 | 0 | 4 | 3 | 1 | 3 |
| MR4 river (upstream) | 69.7 | 4 | 3 | 1 | 7 | 6 | 2 | 4 |
| MR5 tributary C | 51.5 | 2 | 6 | 0 | 4 | 4 | 1 | 3 |
| Urban | 35.4 | 13 (22.4) | 18 (31.0) | 3 (5.2) | 13 (22.4) | 11 (19.0) | 0 (0) | 7 (12.1) |
| LF1 Lake Fletcher run/inlet | 18.2 | 3 | 0 | 1 | 0 | 2 | 0 | 0 |
| LF3 tributary A (outlet to lake) | 27.3 | 5 | 2 | 0 | 1 | 1 | 0 | 1 |
| LF4 tributary A (headwater) | 48.5 | 1 | 4 | 2 | 5 | 4 | 0 | 2 |
| LF5 tributary B | 60.9 | 4 | 10 | 0 | 3 | 3 | 0 | 3 |
| LF6 tributary C | 21.9 | 0 | 2 | 0 | 4 | 1 | 0 | 1 |
| Total | 53.8 | 58 (32.8) | 41 (23.2) | 5 (2.8) | 31 (17.5) | 35 (19.8) | 7 (4.0) | 25 (14.1) |
Listeria spp. were detected after enrichment and plating on Palcam agar, followed by determination of the species for up to eight Listeria isolates per water sample on RAPID'L. Mono agar to yield Listeria special diversity.
Percent positive samples (33 samples per site [32 for LF6] for a total of 165 and 164 for the rural and urban watersheds, respectively).
Number of water samples (% of all Listeria spp.-containing samples) where all Listeria spp. isolates belonged to the same species. The L. innocua group includes L. seeligeri, L. marthii, and L. grayi; the L. welshimeri group includes L. rocourtiae, L. fleischmannii, L. weihenstephanensis, L. floridensis, L. aquatica, L. cornellensis, L. grandensis, and L. riparia.
Number of water samples that contained two or three different Listeria spp. “Mixed” refers to water samples in which L. monocytogenes cooccurred with one (n = 20) or two (n = 5) other Listeria spp.
WTP was the sampling site located immediately adjacent to the intake of raw source water from the river to the local drinking water treatment plant.
Species determination on RAPID'L. Mono agar of up to eight isolates obtained from each Listeria-positive sample showed that two or three Listeria spp. were present in 42 (23.8%) of the samples (Table 2). Of the 56 samples that tested positive for L. monocytogenes by the culture method, the pathogen was found to co-occur with other Listeria species in 25 (44.6%) samples. The diversity of Listeria spp. in the rural watershed was dominated by the L. innocua group, which was found in a total of 56.3% of single and mixed species Listeria genus-positive samples, followed by the L. welshimeri group (37%), L. monocytogenes (30.3%), and L. ivanovii (8.4%). Members of the L. welshimeri group (43.1%) were more common in water samples from the urban watershed, followed by the L. innocua group and L. monocytogenes (both 34.5%) and L. ivanovii (6.9%). Only 5 of the 177 Listeria-positive samples from the watersheds contained L. ivanovii alone with another 9 samples being positive for L. ivanovii, together with other Listeria spp., including L. monocytogenes, indicating that L. ivanovii occurred in 7.9% of the Listeria-positive samples and in 4.3% of all samples. The Listeria diversity was slightly higher for the urban watershed listerial population (D = 0.687) compared to the rural watershed population (D = 0.622).
The culture-based method yielded no L. monocytogenes isolates from the point source samples (cow feces [n = 15; only samples from 2013 were tested], sewage [n = 3], and septic tanks [n = 3]). Other Listeria species were isolated, including the L. innocua group and L. ivanovii from two cow fecal samples and L. ivanovii from one of the raw municipal sewage samples.
L. monocytogenes detection in samples from the rural and urban watersheds.
Analyses by the TaqMan assay revealed detection rates of L. monocytogenes ranging from 57.6 to 75.8% (average, 67.3%) and 48.5 to 60.6% (average, 56.1%) at the rural and urban watershed sampling sites, respectively (Table 3). Of the sampling sites, MR2 (MR tributary A in close vicinity of cattle farms), LF4 (undeveloped forested area, headwaters of LF tributary A), and LF5 (LF tributary B, close to urban development) showed the highest levels of L. monocytogenes detection of 75.8 and 60.6% in their respective watersheds. In contrast, the culture-based detection gave fewer positives for L. monocytogenes, with detection rates ranging from 15.2 to 33.3% (average, 21.8%) and 0 to 21.2% (average, 12.2%) for the sampling sites in the rural and urban watersheds, respectively (Table 3). It should be noted that the TaqMan assay was performed on DNA extracted from the enrichment cultures, making it less likely that the positive amplification results were due to amplification of DNA from dead cells.
Both detection methods pointed to L. monocytogenes being more prevalent in the rural watershed than in the urban watershed. Detection of L. monocytogenes by the TaqMan assay was associated with a 4.7 times higher risk of obtaining L. monocytogenes isolates from the same sample (OR = 4.7, CI = 2.1 to 10.3, P < 0.001, rho2 = 0.064). A positive TaqMan assay result predicted a 2.3-fold higher risk of Listeria spp. being cultured from the same water samples (OR = 2.3, CI = 1.4 to 3.6, P < 0.001, rho2 = 0.030). Importantly, detection of Listeria spp. was associated with a 70-fold higher risk of obtaining a culture-based positive L. monocytogenes result (OR = 69.9, CI = 9.5 to 512.2, P < 0.001, rho2 = 0.232).
In 2012, the majority of dairy cows (randomly selected across all age groups) tested positive for L. monocytogenes (88.8%, or 8/9) by the TaqMan assay, while the opposite occurred in 2013, where only 13% (2/15) of the bovine feces contained L. monocytogenes. All raw municipal sewage samples tested positive for L. monocytogenes (3/3) in the TaqMan assay, while the septic tank samples tested negative (0/3).
PCR-based serogrouping of the 285 L. monocytogenes isolates showed that serogroup IIa strains dominated at most watershed sampling sites, with the exception of MR1 (main river, adjacent to the intake to the water treatment plant) and LF3 (bottom of tributary A, outlet to lake) where serogroup IIb strains prevailed, and LF6 (tributary C in developed urban area) where serogroup IVb strains formed the majority (Table 3). Serogroup IIc accounted for only 0.4% of the L. monocytogenes isolates, followed by IIb (15.8%), IVb (16.1%), and IIa (67.7%).
E. coli and turbidity as predictors of the presence of Listeria spp. and L. monocytogenes in surface waters.
E. coli levels in the water samples ranged from below the detection limit of 1 to 2,080 CFU/100 ml, with the concentrations of E. coli being significantly (P < 0.05) lower in the urban watershed (median, 2 CFU/100 ml) than in the rural watershed (median, 22 CFU/100 ml). E. coli concentrations of ≥100 CFU/100 ml occurred in 12 and 35 water samples from the urban and rural watersheds, respectively, and were associated with a 5.2-fold increased likelihood of the simultaneous presence of Listeria spp. (OR = 5.2, CI 2.3 to 11.4, P < 0.001, rho2 = 0.046) but not L. monocytogenes (P > 0.05) regardless of the detection method.
Turbidity (NTU) is regularly measured by water utilities as a source water quality indicator. NTU levels of ≥1.0 came with a 17.3-fold higher risk (OR = 17.3, CI 4.1 to 73.1, P < 0.001, rho2 = 0.130) of detecting E. coli levels at or above 100 CFU/100 ml and a 2.1-fold (OR = 2.1, CI 1.3 to 3.4, P < 0.01, rho2 = 0.023) higher likelihood of detecting Listeria spp. in the samples. However, there was no significant (P > 0.05) relationship between NTU levels of ≥1.0 and the detection of L. monocytogenes.
Influences of season and storm events on prevalence of L. monocytogenes.
L. monocytogenes was detected year round in both watersheds, with TaqMan assay-positive detection in 76.9, 59.1, 55.4, and 72.0% of the water samples obtained during the winter (December to February, n = 39, average water temperature of 1.2°C), spring (March to May, n = 110, average water temperature of 8.1°C), summer (June to August, n = 130, average water temperature of 17.8°C), and fall (September to November, n = 50, average water temperature of 13.0°C) periods, respectively.
Six and three storm events (>20-mm rainfall) were captured in the rural and urban watershed, respectively, during the study. Storm event-associated detection rates of L. monocytogenes in the TaqMan assay tended to be higher than base-flow detection rates in the rural watershed with 73.3% of the 30 storm samples in the rural watershed testing positive versus 65.8% of the 135 base-flow samples. The opposite was observed in the urban watershed, where the L. monocytogenes TaqMan assay yielded lower detection rates of 40% (6 of the 15 samples) during storm events compared to the 57.7% testing positive found in 149 base-flow water samples.
Pulsotype comparison of representative L. monocytogenes surface water and NS human isolates.
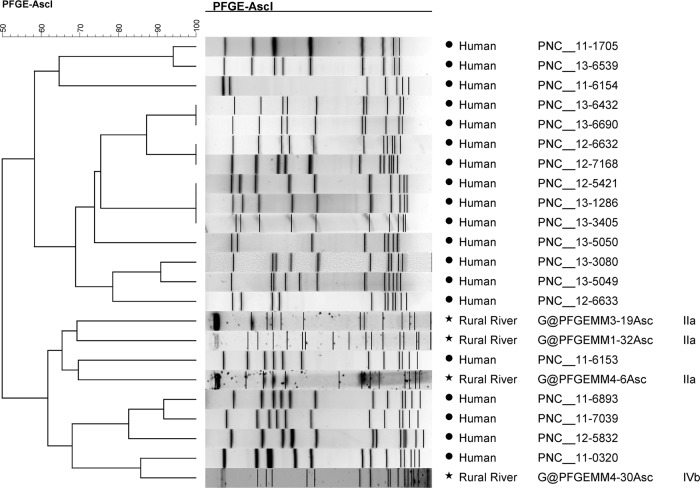
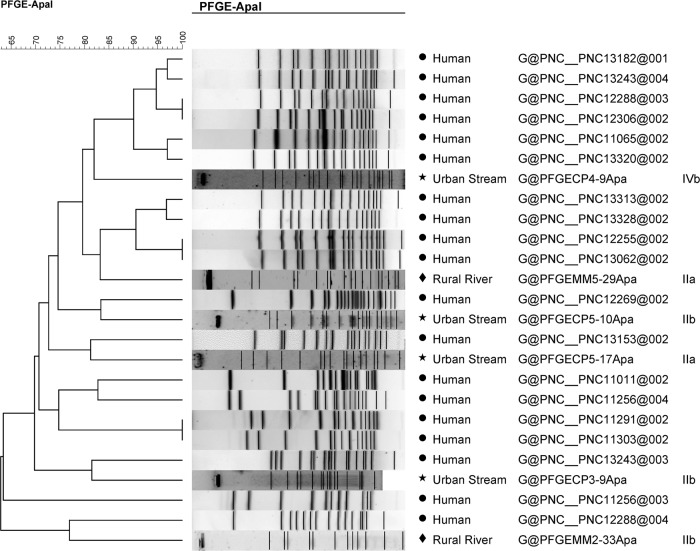
A large diversity of pulsotypes (n = 52) was found among isolates collected from water samples (n = 54) (see Fig. S1 and S2 in the supplemental material), leading to a Simpson's index of discrimination (D) of 0.99 for both watersheds. One strain (LF6-5) did not produce a pattern. Only two isolates collected during the same sampling run (MR2-4 and MR3-4, collected ∼8 km apart in the rural watershed) showed matching AscI/ApaI combination patterns (>80% similarity). Fourteen PFGE types were found among the human clinical isolates and several (9/19) showed identical AscI/ApaI patterns with >95% similarity (Fig. 3 and 4).
FIG 3.
UPGMA clustering analysis of human L. monocytogenes isolate AscI PFGE patterns and selected L. monocytogenes watershed isolates using the Dice coefficient with a tolerance of 1.5%. L. monocytogenes NS human food-borne outbreak isolates are labeled “human,” MR L. monocytogenes isolates are labeled “rural river,” and LF isolates are labeled “urban stream.”
FIG 4.
UPGMA clustering analysis of human L. monocytogenes isolate ApaI PFGE patterns and selected L. monocytogenes watershed isolates using the Dice coefficient with a tolerance of 1.5%. L. monocytogenes NS human food-borne outbreak isolates are labeled “human,” MR L. monocytogenes isolates are labeled “rural river,” and LF isolates are labeled “urban stream.”
When AscI and ApaI patterns were analyzed separately (Fig. 3 and 4), the AscI pattern of human isolate 11-0320 showed 85% similarity with the rural river water isolate MR4-30. Similarly, the human isolate 12-5832 shared 83% similarity with the urban water strain LF5-10 using ApaI patterns, while human isolates 13-3080 and 13-5049 both shared 80% similarity with urban watershed strains LF5-17 and LF3-9.
The pulsotypes did not cluster by serogroup (see Fig. S1 and S2 in the supplemental material). Isolates from water samples that were highly similar to human isolates using AscI patterns were not similar to the same human isolates using ApaI patterns. Furthermore, water isolates that were highly similar in regard to their AscI patterns were not grouped together using ApaI patterns and vice versa, with the exception of MR2-4 and MR3-4 and human isolates noted above.
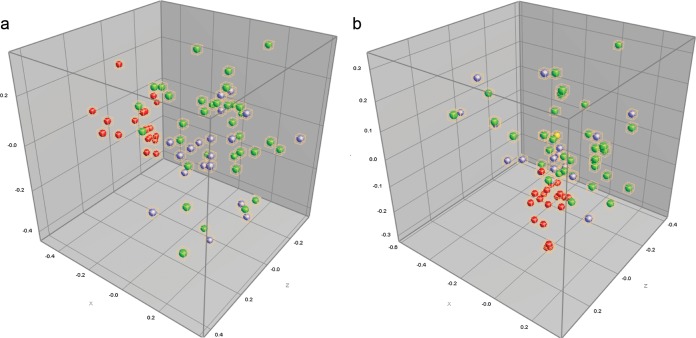
Results from cluster analysis and multidimensional scaling (MDS) of AscI and ApaI patterns from the pulsotyped L. monocytogenes isolates (n = 53 of the original 285 isolates) and 2011-3 Nova Scotia human isolates (n = 19; total n = 72) indicated a higher level of similarity among the rural and urban watershed isolates. The MDS analysis (Fig. 5) did not separate strains based on their origin, indicating overlaps in genetic traits among clinical and environmental isolates from the rural and urban watershed. However, some but not all human isolates formed a distinct cluster MDS plot based on the ApaI patterns (Fig. 5).
FIG 5.
AscI multidimensional scaling in three dimensions in the coordinate space (a), followed by ApaI multidimensional scaling in three dimensions (b). Samples from the rural watershed (MR) are green/yellow, while samples from the urban watershed (LF) are represented by purple symbols. NS human food-borne outbreak isolates are indicated in red. The proportion of the data variance (r2) accounted for by MDS was 0.72.
DISCUSSION
Prevalence and diversity of Listeria spp. in surface water and point source pollution samples.
The present study discovered a diverse Listeria population in 35.4 and 72.1% of the urban and rural watershed samples, respectively, indicating differences among the watersheds. Other studies also report widespread but variable occurrence of Listeria spp. in the environment. Sauders et al. (8) reported an overall Listeria prevalence of 16 and 33% in surface waters from urban and natural environments, respectively, in New York State (NYS). A survey of five produce farms and five natural sites in NYS over a 2-year period yielded 51% culture-positive water samples (n = 264), which was higher than the prevalence in soil (17%), drag swab (21%), and fecal samples (16%) (12). Interestingly, we found a similar culture-based prevalence of just 14% in our fecal point source samples (cow feces, raw sewage, and septic tank). In Austria, Linke et al. (11) isolated Listeria from 26.5% of the water samples, while Frances et al. (32) in a small culture-based study of surface waters (n = 21) in the United Kingdom reported finding Listeria in 27% of samples.
Isolation of up to eight strains from each Listeria-positive sample (average of 7.25 strains/sample) showed that 23.8% of the samples contained two or three Listeria spp., which is higher than the 6 to 8% previously reported in studies where three or four isolates per sample were characterized (7, 11). The diversity in the rural watersheds was slightly lower (D = 0.622) and dominated by the L. innocua group (including L. seeligeri) compared to urban samples (D = 0.687), which were dominated by the L. welshimeri group. The diversity of Listeria spp. in surface water samples appear to vary among regions as studies in NYS reported high levels of L. seeligeri and L. welshimeri in pristine environments and L. innocua in produce farming and urban environments (7, 12). The Austrian study similarly showed significant geographical variations in the dominant Listeria populations (11). Taken together, this indicates considerable geographical variation in listerial biodiversity, which likely is linked to farming practices and other anthropogenic impacts.
Our culture-based analysis of the cow feces and raw municipal sewage samples yielded only L. innocua group and L. ivanovii isolates. A molecular-detection-based survey of urban wastewater treatment effluent in France indicated the presence of Listeria spp. in 84% of samples with L. monocytogenes and L. innocua in 75 and 63% of the samples, respectively, indicating co-occurrence (5).
L. monocytogenes in surface water and point source pollution samples.
Higher detection rates of L. monocytogenes occurred when the water and point source pollution samples were analyzed by the TaqMan assay (61.7%) compared to the culture-based method (17%). This discrepancy may be due to L. innocua, and possibly other Listeria spp., outcompeting L. monocytogenes during the selective enrichment process (33–36). Oravcová et al. (37) reported that culture-based detection of L. monocytogenes in the presence of L. innocua was consistently lower than when the same samples were analyzed by PCR, indicating that the TaqMan assay results in our study may more accurately reflect the prevalence of the pathogen. Also, the use of Palcam and RAPID'L. Mono agar may result in lower recoveries of less virulent strains (38), which might have been detected in our TaqMan assay. Interestingly, a recent European validation study found >90% agreement between plating methods using Palcam and quantitative PCR (qPCR) detection (39). Lastly, our culture-based approach characterized eight isolates per sample, meaning that L. monocytogenes populations constituting <12.5% of the Listeria population in a given sample might have been missed and possibly contributing to the discrepancy between culture-based and molecular methods.
Detection of L. monocytogenes was a common occurrence in both natural and anthropogenically impacted sampling sites (Table 3), pointing to watershed as an important reservoir. The reported culture-based prevalence of L. monocytogenes in different geographical regions ranges from 50% in South African rivers (40), 43% in five Californian watersheds (14), 30% in water samples from a produce-producing region in NYS (15), 30% in river, lake and stream samples (n = 206) from the Central California Coast (17), 12.8% of spring and river samples (n = 148) from Switzerland (41), 10% of water samples (n = 314) from a mixed-use watershed in Ontario, Canada (13), 1.7 and 10.9% of natural and urban water samples (n = 486), respectively, in NYS (8), and 3.9% in surface water samples (n = 128) in Northern Greece (42) to none in water samples (n = 30) obtained in Cheshire and Northern Wales (32) and from the natural environment (n = 68) in Austria (11).
Although we detected more L. monocytogenes-positive samples in the rural agricultural watershed, Sauders et al. (8) reported a significant association between culturable L. monocytogenes (and L. innocua) and urban watersheds. This is likely due to differences in local farming practices, urban development, drainage, wastewater management, etc.
Most of the cattle tested positive for L. monocytogenes (by the TaqMan assay) in 2012; however, only 13% tested positive in 2013, indicating variability in carriage among farm animals. L. monocytogenes has previously been cultured from animal feeds and feces (43–46) and L. monocytogenes was among the most abundant pathogens found in feedlot cattle manure in Australia (47).
In our study, serogroup IIa (1/2a and 3a serovars, 68%) were predominant among the L. monocytogenes isolates from the watersheds. These results are similar those reported in another Canadian study, where serogroups IIa and IVb constituted 50 and 32% of the isolates, respectively, in a mixed-use watershed (13) but different from the findings of predominantly serogroup IVb (>80%) isolates in water ways on the Central Coast of California (14, 17), suggesting that L. monocytogenes populations are diverse in surface waters and the natural environment.
E. coli, turbidity, and Listeria spp. as predictors of L. monocytogenes in surface water.
Elevated E. coli levels (≥100 CFU/100 ml) were associated with 5.2-fold-higher likelihood of detecting Listeria spp. but were not related to the presence of L. monocytogenes as determined using a culture-based or molecular detection method. Other studies have shown absence of relationships between E. coli/FIB and specific pathogens (48, 49), which may be due to differences in physiological factors between the pathogen and indicator organisms (50). However, in the case of L. monocytogenes it is most likely due to it being naturalized in the environment, as also indicated by our detection of the pathogen at the pristine headwater of tributary A in the urban watershed (LF4, Table 3). Turbidity levels of ≥1 NTU were associated with higher risks of detecting elevated E. coli levels and Listeria spp. but not L. monocytogenes, indicating the limitations of this widely used source water quality indicator. Culture-based detection of Listeria spp. came with a 70-fold-higher risk of also isolating L. monocytogenes from the sample, supporting the suggestion of Chapin et al. (12) that Listeria spp. can be used as index organisms for the presence of L. monocytogenes.
Seasonality and storm-related detection of L. monocytogenes.
In contrast to the findings of detection of L. monocytogenes being associated with storms and flooding in Austria (11), the prevalence of L. monocytogenes was not related to storm events in the present study. Although L. monocytogenes was detected year round in water samples, detection rates tended to be higher in the temperate to colder months (September to February), possibly due to less competition from the autochthonous microflora and adaptation to colder temperatures (1, 51). Seasonal differences in L. monocytogenes prevalence were also found in Ontario (Canada), California, and NYS, where abundance tended to be higher during cooler periods (14, 16, 52).
Comparison of the pulsotypes of representative L. monocytogenes surface water and NS human isolates.
The finding of 52 pulsotypes among 53 representative L. monocytogenes isolates revealed large biodiversity (D = 0.99) in both watersheds. The presence of host organisms such as wild animals, cattle in the rural watershed, and humans in both watersheds may have contributed to this diversity. Twenty-one pulsotypes were discerned among 32 L. monocytogenes-positive samples from a watershed in Ontario, leading to D = 0.885 (13). Similarly, a large diversity of MLVA types were found in a study of watersheds on the Central California Coast (17) and in sigB allelic types in NYS surface water samples (16). Taken together, these studies point toward a large biodiversity of L. monocytogenes in surface waters.
The two catchments in the present study may potentially serve as a reservoir for pathogenic L. monocytogenes, as shown by genetic similarity among some of the human and water L. monocytogenes isolates and the lack of distinct origin-based clusters in the MDS plots (Fig. 5). Although certain genotypes have been associated with specific sources such as humans and farms, some L. monocytogenes subtypes are widely distributed, including in the natural environment (53). Other studies have indicated that L. monocytogenes subtype populations found in farming environments overlap with human listeriosis strains (13, 44). In the former study, the majority of the pulsotypes of fecal L. monocytogenes isolates from livestock, wildlife, and humans in Ontario, Canada, matched clinical isolate patterns in the Pulsenet Canada National Listeria Database (13). Such virulent L. monocytogenes can possibly be transferred to soil and water ways via nonsanitized sludge being spread onto agricultural fields (54) or from failing on-site septic systems. The risk of transmission of L. monocytogenes from different point sources into surface waters warrants further study, including the possible connectivity to L. monocytogenes subtypes in food plant environments and potential to infect humans and animals.
Supplementary Material
ACKNOWLEDGMENTS
We thank NSERC, Canadian Water Network, and Ross Davidson, Lorelee Tschetter, Halifax Water for funding this project.
We thank Barry Geddes, Anna McCarron, Tristan Goulden, Greg Piorkowski, Ross Davidson, Lorelee Tschetter, and Amy Jackson for their involvement and help with the project.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00416-15.
REFERENCES
- 1.Ghandi M, Chikindas ML. 2007. Listeria: a foodborne pathogen that knows how to survive. Int J Food Microbiol 118:1–15. doi: 10.1016/j.ijfoodmicro.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Guillet C, Join-Lambert O, Le Monnier A, Leclercq A, Mechaï F, Mamzer-Bruneel MF, Lecuit M. 2010. Human listeriosis caused by Listeria ivanovii. Emerg Infect Dis 16:136–138. doi: 10.3201/eid1601.091155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orsi RH, den Bakker HC, Wiedmann M. 2011. Listeria monocytogenes lineages: genomics, evolution, ecology, and phenotypic characteristics. Int J Med Microbiol 301:79–96. doi: 10.1016/j.ijmm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 4.den Bakker HC, Warchocki S, Wright EM, Allred AF, Ahlstrom C, Manuel CS, Stasiewicz MJ, Burrell A, Roof S, Strawn LK, Fortes E, Nightingale KK, Kephart D, Wiedmann M. 2014. Listeria floridensis sp. nov., Listeria aquatica sp. nov., Listeria cornellensis sp. nov., Listeria riparia sp. nov. and Listeria grandensis sp. nov., from agricultural and natural environments. Int J Syst Evol Microbiol 64:1882–1889. doi: 10.1099/ijs.0.052720-0. [DOI] [PubMed] [Google Scholar]
- 5.Paillard D, Dubois V, Thiebaut R, Nathier F, Hoogland E, Caumette P, Quentin C. 2005. Occurrence of Listeria spp. in effluents of French urban wastewater treatment plants. Appl Environ Microbiol 71:7562–7566. doi: 10.1128/AEM.71.11.7562-7566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyautey E, Hartmann A, Pagotto F, Tyler K, Lapen DR, Wilkes G, Piveteau P, Rieu A, Robertson WJ, Medeiros DT, Edge TA, Gannon V, Topp E. 2007. Characteristics and frequency of detection of fecal Listeria monocytogenes shed by livestock, wildlife, and humans. Can J Microbiol 53:1158–1167. doi: 10.1139/W07-084. [DOI] [PubMed] [Google Scholar]
- 7.Sauders BD, Wiedmann M. 2007. Ecology of Listeria species and L. monocytogenes in the natural environment, p 21–44. In Ryser ET, Marth EH (ed), Listeria, listeriosis, and food safety, 3rd ed Taylor and Francis Group, Boca Raton, FL. [Google Scholar]
- 8.Sauders BD, Overdevest J, Fortes E, Schukken Y, Lembo A, Windham K, Wiedmann M. 2012. Diversity of Listeria species in urban and natural environments. Appl Environ Microbiol 78:4420–4433. doi: 10.1128/AEM.00282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vivant A-L, Garmyn D, Piveteau P. 2013. Listeria monocytogenes, a down-to-earth pathogen. Front Cell Infect Microbiol 3:87. doi: 10.3389/fcimb.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira V, Wiedmann M, Teixeira P, Stasiewicz M. 2014. Listeria monocytogenes persistence in food-associated environments: epidemiology, strain characteristics, and implications for public health. J Food Prot 77:150–170. doi: 10.4315/0362-028X.JFP-13-150. [DOI] [PubMed] [Google Scholar]
- 11.Linke K, Ruckerl I, Bruger K, Karpiskova JW, Muri-Klinger S, Tichy A, Wagner M, Stessl B. 2014. Reservoirs of Listeria species in three environmental ecosystems. Appl Environ Microbiol 80:5583–5592. doi: 10.1128/AEM.01018-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapin TK, Nightingale KK, Worobo RW, Wiedmann M, Strawn LK. 2014. Geographical and meteorological factors associated with isolation of Listeria species in New York state produce production and natural environments. J Food Prot 77:1919–1928. doi: 10.4315/0362-028X.JFP-14-132. [DOI] [PubMed] [Google Scholar]
- 13.Lyautey E, Lapen DR, Wilkes G, McCleary K, Pagotto F, Tyler K, Topp E. 2007. Distribution and characteristics of Listeria monocytogenes isolates from surface waters of the South Nation River watershed, Ontario, Canada. Appl Environ Microbiol 73:5401–5410. doi: 10.1128/AEM.00354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooley MB, Quinones B, Oryang D, Mandrell RE, Gorski L. 2014. Prevalence of Shiga toxin producing Escherichia coli, Salmonella enterica and Listeria monocytogenes at public access watershed sites in a California central coast agricultural region. Front Cell Infect Microbiol 4:30. doi: 10.3389/fcimb.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strawn LK, Fortes ED, Bihn EA, Nightingale KK, Gröhn YT, Worobo RW, Wiedmann M, Bergholz PW. 2013. Landscape and meteorological factors affecting prevalence of three food-borne pathogens in fruit and vegetable farms. Appl Environ Microbiol 79:588–600. doi: 10.1128/AEM.02491-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strawn LK, Gröhn YT, Warchocki S, Worobo RW, Bihn EA, Wiedmann M. 2013. Risk factors associated with Salmonella and Listeria monocytogenes contamination of produce fields. Appl Environ Microbiol 79:7618–7627. doi: 10.1128/AEM.02831-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorski L, Walker S, Liang AS, Nguyen KM, Govoni J, Carychao D, Cooley MB, Mandrell RE. 2014. Comparison of subtypes of Listeria monocytogenes isolates from naturally contaminated watershed samples with or without a selective secondary enrichment. PLoS One 9:e92467. doi: 10.1371/journal.pone.0092467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doumith M, Buchrieser C, Glaser P, Jacquet C, Martin P. 2004. Differentiation of the major Listeria monocytogenes serovars by multiplex PCR. J Clin Microbiol 42:3819–3822. doi: 10.1128/JCM.42.8.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kérouanton A, Marault M, Petit L, Grout J, Dao TT, Brisabois A. 2010. Evaluation of a multiplex PCR assay as an alternative method for Listeria monocytogenes serotyping. J Microbiol Methods 80:134–137. doi: 10.1016/j.mimet.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Jacquet C, Gouin E, Jeannel D, Cossart P, Rocourt J. 2002. Expression of ActA, Ami, InlB, and listeriolysin O in Listeria monocytogenes of human and food origin. Appl Environ Microbiol 68:616–622. doi: 10.1128/AEM.68.2.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward TJ, Usgaard T, Evans P. 2010. A targeted multilocus genotyping assay for lineage, serogroup, and epidemic clone typing of Listeria monocytogenes. Appl Environ Microbiol 76:6680–6684. doi: 10.1128/AEM.01008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy M, Corcoran D, Buckley JF, O'Mahony M, Whyte P, Fanning S. 2007. Development and application of multiple-locus variable number of tandem repeat analysis (MLVA) to subtype a collection of Listeria monocytogenes. Int J Food Microbiol 115:187–194. doi: 10.1016/j.ijfoodmicro.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Li J, Saleh-Lakha S, Allen V, Odumeru J. 2011. Multiple-locus variable number of tandem repeat analysis (MLVA) of Listeria monocytogenes directly in food samples. Int J Food Microbiol 148:8–14. doi: 10.1016/j.ijfoodmicro.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Jadhav S, Bhave M, Palombo EA. 2012. Methods used for the detection and subtyping of Listeria monocytogenes. J Microbiol Methods 88:327–341. doi: 10.1016/j.mimet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Bartram J, Corrales L, Davison A, Deere D, Drury D, Gordon B, Howard G, Rinehold A, Stevens M. 2009. Water safety plan manual: step-by-step risk management for drinking water suppliers. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2009/9789241562638_eng.pdf. [Google Scholar]
- 26.Walters SP, Thebo AL, Boehm AB. 2011. Impact of urbanization and agriculture on the occurrence of bacterial pathogens and stx genes in coastal waterbodies of central California. Water Res 45:1752–1762. doi: 10.1016/j.watres.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 27.McEgan R, Mootian G, Goodridge LD, Schaffner DW, Danyluk MD. 2013. Predicting Salmonella populations from biological, chemical, and physical indicators in Florida surface waters. Appl Environ Microbiol 79:4094–4105. doi: 10.1128/AEM.00777-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez-Lázaro D, Hernández M, Scortti M, Esteve T, Vázquez-Boland JA, Pla M, Herna M. 2004. Quantitative detection of Listeria monocytogenes and Listeria innocua by real-time PCR: assessment of hly, iap, and lin02483 targets and amplifluor technology. Appl Environ Microbiol 70:1366–1377. doi: 10.1128/AEM.70.3.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graves LM, Swaminathan B. 2001. PulseNet standardized protocol for subtyping Listeria monocytogenes by macrorestriction and pulsed-field gel electrophoresis. Int J Food Microbiol 65:55–62. doi: 10.1016/S0168-1605(00)00501-8. [DOI] [PubMed] [Google Scholar]
- 30.Hunter SB, Vauterin P, Lambert-Fair MA, Van Duyne MS, Kubota K, Graves L, Wrigley D, Barrett T, Ribot E. 2005. Establishment of a universal size standard strain for use with the PulseNet standardized pulsed-field gel electrophoresis protocols: converting the national databases to the new size standard. J Clin Microbiol 43:1045–1050. doi: 10.1128/JCM.43.3.1045-1050.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunter PR, Gaston MA. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J Clin Microbiol 26:2465–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frances N, Hornby H, Hunter PR. 1991. The isolation of Listeria species from fresh-water sites in Cheshire and North Wales. Epidemiol Infect 107:235–238. doi: 10.1017/S0950268800048858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald F, Sutherland AD. 1994. Important differences between the generation times of Listeria monocytogenes and Listeria innocua in two Listeria enrichment broths. J Dairy Res 61:433–436. [DOI] [PubMed] [Google Scholar]
- 34.Bruhn JB, Vogel BF, Gram L. 2005. Bias in the Listeria monocytogenes enrichment procedure: lineage 2 strains outcompete lineage I strains in University of Vermont selective enrichments. Appl Environ Microbiol 71:961–967. doi: 10.1128/AEM.71.2.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zitz U, Zunabovic M, Domig KJ, Wilrich P-T, Kneifel W. 2011. Reduced detectability of Listeria monocytogenes in the presence of Listeria innocua. J Food Prot 74:1282–1287. doi: 10.4315/0362-028X.JFP-11-045. [DOI] [PubMed] [Google Scholar]
- 36.Fgaier H, Kalmokoff M, Ells T, Eberl HJ. 2014. An allelopathy based model for the Listeria overgrowth phenomenon. Math Biosci 247:13–26. doi: 10.1016/j.mbs.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Oravcová K, Trncíková T, Kuchta T, Kaclíková E. 2008. Limitation in the detection of Listeria monocytogenes in food in the presence of competing Listeria innocua. J Appl Microbiol 104:429–437. doi: 10.1111/j.1365-2672.2007.03554.x. [DOI] [PubMed] [Google Scholar]
- 38.Gracieux P, Roche SM, Pardon P, Velge P. 2003. Hypovirulent Listeria monocytogenes strains are less frequently recovered than virulent strains on Palcam and Rapid'L.Mono. Int J Food Microbiol 83:133–145. doi: 10.1016/S0168-1605(02)00321-5. [DOI] [PubMed] [Google Scholar]
- 39.Dalmasso M, Bolocan AS, Hernandez M, Kapetanakou AE, Kuchta T, Manios SG, Melero B, Minaroviová J, Muhterem M, Nicolau AI, Rovira J, Skandamis PN, Stessl B, Wagner M, Jordan K, Rodríguez-Lázaro D. 2014. Comparison of polymerase chain reaction methods and plating for analysis of enriched cultures of Listeria monocytogenes when using the ISO11290-1 method. J Microbiol Methods 98:8–14. doi: 10.1016/j.mimet.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 40.Ijabadeniyi OA, Debusho LK, Vanderlinde M, Buys EM. 2011. Irrigation water as a potential preharvest source of bacterial contamination of vegetables. J Food Safety 31:452–461. doi: 10.1111/j.1745-4565.2011.00321.x. [DOI] [Google Scholar]
- 41.Schaffter N, Parriaux A. 2002. Pathogenic-bacterial water contamination in mountainous catchments. Water Res 36:131–139. doi: 10.1016/S0043-1354(01)00242-1. [DOI] [PubMed] [Google Scholar]
- 42.Arvanitidou M, Papa A, Constantinidis TC, Danielides V, Katsouyannopoulos V. 1997. The occurrence of Listeria spp. and Salmonella spp. in surface waters. Microbiol Res 152:395–397. [DOI] [PubMed] [Google Scholar]
- 43.Skovgaard N, Morgen C-Å. 1988. Detection of Listeria spp. in faeces from animals, in feeds, and in raw food of animal origin. Int J Food Microbiol 6:229–242. [DOI] [PubMed] [Google Scholar]
- 44.Nightingale KK, Shuckken YH, Nightingale CR, Fortes ED, Ho AJ, Her Z, Gröhn YT, McDonough PL, Wiedmann M. 2004. Ecology and transmission of Listeria monocytogenes infecting ruminants and in the farm environment. Appl Environ Microbiol 70:4458–4467. doi: 10.1128/AEM.70.8.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atil E, Ertas HB, Ozbey G. 2011. Isolation and molecular characterization of Listeria spp. from animals, food, and environmental samples. Vet Med 56:386–394. [Google Scholar]
- 46.Hasegawa M, Iwabuchi E, Yamamoto S, Muramatsu M, Takashima I, Hirai K. 2014. Prevalence and characteristics of Listeria monocytogenes in feces of black beef cattle reared in three geographically distant areas in Japan. Foodborne Pathog Dis 11:96–103. doi: 10.1089/fpd.2013.1616. [DOI] [PubMed] [Google Scholar]
- 47.Klein M, Brown L, Tucker RW, Ashbolt NJ, Stuetz RM, Roser DJ. 2010. Diversity and abundance of zoonotic pathogens and indicators in manures of feedlot cattle in Australia. Appl Environ Microbiol 76:6947–6950. doi: 10.1128/AEM.01095-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed W, Sawant S, Huygens F, Goonetilleke A, Gardner T. 2009. Prevalence and occurrence of zoonotic bacterial pathogens in surface waters determined by quantitative PCR. Water Res 43:4918–4928. doi: 10.1016/j.watres.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 49.Drozd M, Merrick NN, Sanad YM, Dick LK, Dick WA, Rajashekara G. 2012. Evaluating the occurrence of host-specific, general fecal indicators, and bacterial pathogens in a mixed-use watershed. J Environ Qual 42:713–725. doi: 10.2134/jeq2012.0359. [DOI] [PubMed] [Google Scholar]
- 50.Harwood VJ, Stanley C, Badgley BD, Borges K, Korajkic A. 2013. Microbial source tracking markers for detection of fecal contamination in environmental waters: relationships between pathogens and human health outcomes. FEMS Microbiol Rev 38:1–40. doi: 10.1111/1574-6976.12031. [DOI] [PubMed] [Google Scholar]
- 51.McLaughlin HP, Casey PG, Cotter J, Gahan CGM, Hill C. 2011. Factors affecting survival of Listeria monocytogenes and Listeria innocua in soil samples. Arch Microbiol 193:775–785. doi: 10.1007/s00203-011-0716-7. [DOI] [PubMed] [Google Scholar]
- 52.Wilkes G, Edge TA, Gannon VPJ, Jokinen C, Lyautey E, Neumann NF, Ruecker N, Scott A, Sunohara M, Topp E, Lapen DR. 2011. Associations among pathogenic bacteria, parasites, and environmental and land use factors in multiple mixed-use watersheds. Water Res 45:5807–5825. doi: 10.1016/j.watres.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 53.Fugett EB, Schoonmaker-Bopp D, Dumas NB, Corby J, Wiedmann M. 2007. Pulsed-field gel electrophoresis (PFGE) analysis of temporally matched Listeria monocytogenes isolates from human clinical cases, foods, ruminant farms, and urban and natural environments reveals source-associated as well as widely distributed PFGE types. J Clin Microbiol 45:865–873. doi: 10.1128/JCM.01285-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kérouanton A, Roche SM, Marault M, Velge P, Pourcher AM, Brisabois A, Federighi Garrec MN. 2010. Characterization of isolates of Listeria monocytogenes from sludge using pulsed-field gel electrophoresis and virulence assays. J Appl Microbiol 108:1380–1388. doi: 10.1111/j.1365-2672.2009.04531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.