Abstract
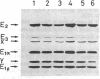
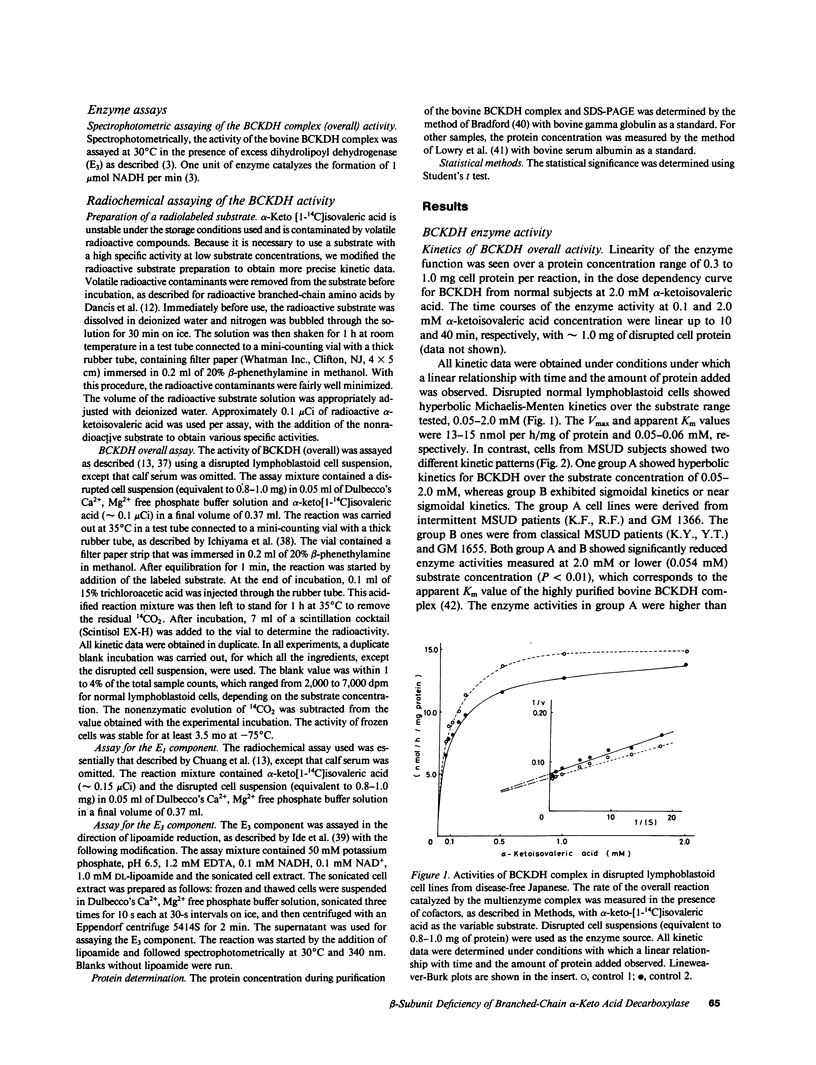
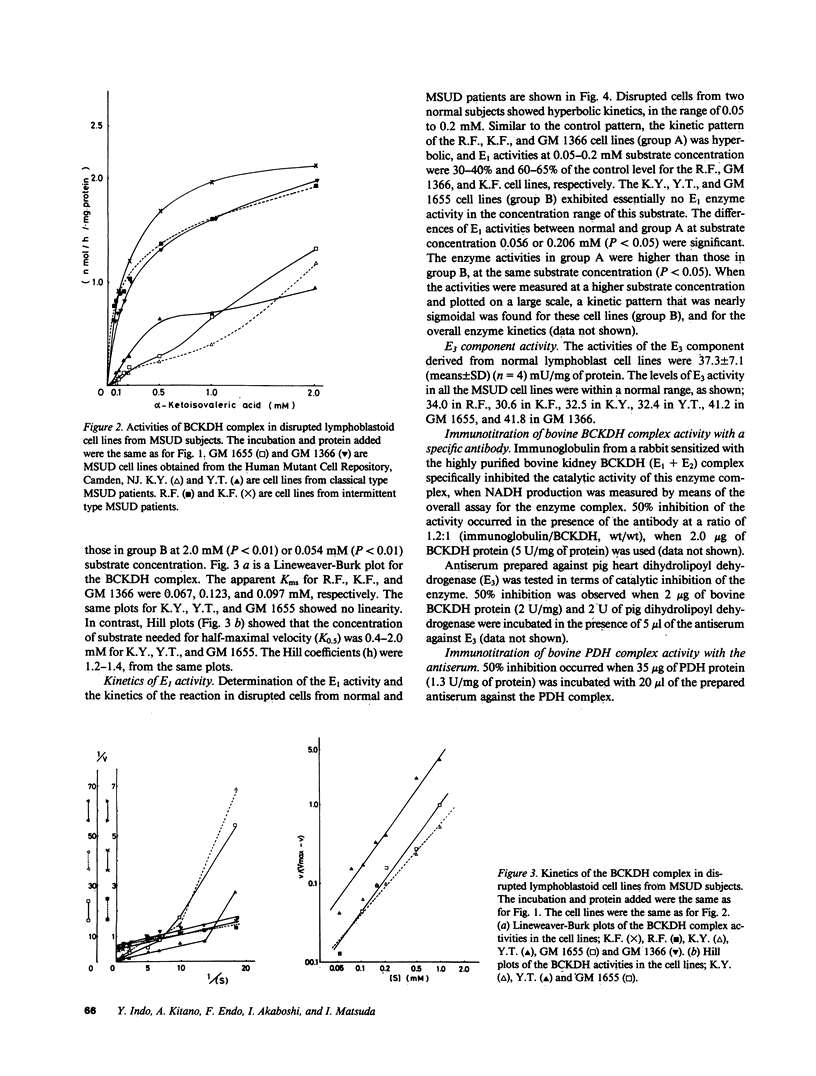
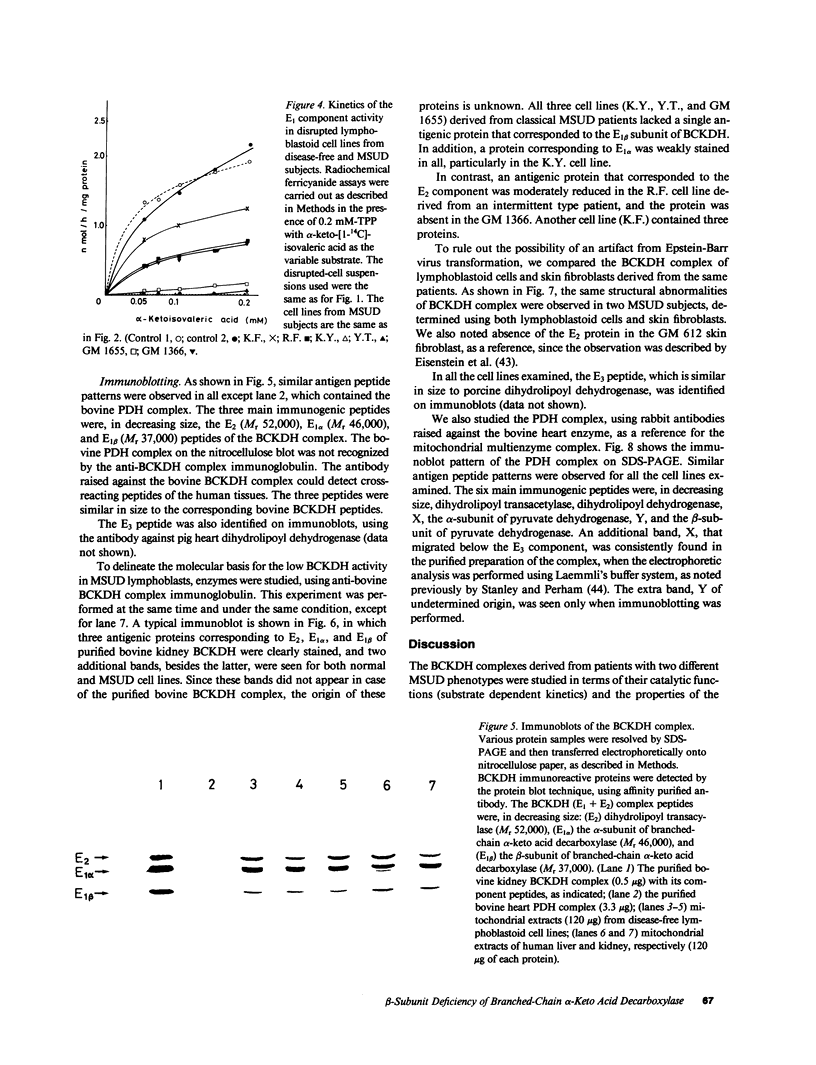
Branched-chain alpha-keto acid dehydrogenase (BCKDH) complexes of lymphoblastoid cell lines derived from patients with classical maple syrup urine disease (MSUD) phenotypes were studied in terms of their catalytic functions and analyzed by immunoblotting, using affinity purified anti-bovine BCKDH antibody. Kinetic studies on three cell lines derived from patients with the classical phenotype showed sigmoidal or near sigmoidal kinetics for overall BCKDH activity and a deficiency of the E1 component activity. An immunoblot study revealed a markedly decreased amount of the E1 beta subunit accompanied by weak staining of the E1 alpha subunit. The E2 and E3 component exhibited a cross-reactive peptide. Thus, in at least some patients with MSUD, mutations of the E1 beta subunit might provide an explanation for the altered kinetic properties of the BCKDH complex.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chuang D. T., Niu W. L., Cox R. P. Activities of branched-chain 2-oxo acid dehydrogenase and its components in skin fibroblasts from normal and classical-maple-syrup-urine-disease subjects. Biochem J. 1981 Oct 15;200(1):59–67. doi: 10.1042/bj2000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANCIS J., LEVITZ M., MILLER S., WESTALL R. G. Maple syrup urine disease. Br Med J. 1959 Jan 10;1(5114):91–93. doi: 10.1136/bmj.1.5114.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancis J., Hutzler J., Rokkones T. Intermittent branched-chain ketonuria. Variant of maple-syrup-urine disease. N Engl J Med. 1967 Jan 12;276(2):84–89. doi: 10.1056/NEJM196701122760204. [DOI] [PubMed] [Google Scholar]
- Dancis J., Hutzler J., Snyderman S. E., Cox R. P. Enzyme activity in classical and variant forms of maple syrup urine disease. J Pediatr. 1972 Aug;81(2):312–320. doi: 10.1016/s0022-3476(72)80301-9. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Armstrong N., Heffelfinger S. C., Sewell E. T., Priest J. H., Elsas L. J. Absence of branched chain acyl-transferase as a cause of maple syrup urine disease. J Clin Invest. 1985 Mar;75(3):858–860. doi: 10.1172/JCI111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danner D. J., Elsas L. J., 2nd Subcellular distribution and cofactor function of human branched chain alpha-ketoacid dehydrogenase in normal and mutant cultured skin fibroblasts. Biochem Med. 1975 May;13(1):7–22. doi: 10.1016/0006-2944(75)90135-0. [DOI] [PubMed] [Google Scholar]
- Danner D. J., Lemmon S. K., Besharse J. C., Elsas L. J., 2nd Purification and characterization of branched chain alpha-ketoacid dehydrogenase from bovine liver mitochondria. J Biol Chem. 1979 Jun 25;254(12):5522–5526. [PubMed] [Google Scholar]
- Goudie R. B., Horne C. H., Wilkinson P. C. A simple method for producing antibody specific to a single selected diffusible antigen. Lancet. 1966 Dec 3;2(7475):1224–1226. doi: 10.1016/s0140-6736(66)92305-1. [DOI] [PubMed] [Google Scholar]
- Heffelfinger S. C., Sewell E. T., Danner D. J. Antibodies to bovine liver branched-chain 2-oxo acid dehydrogenase cross-react with this enzyme complex from other tissues and species. Biochem J. 1983 Aug 1;213(2):339–344. doi: 10.1042/bj2130339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffelfinger S. C., Sewell E. T., Danner D. J. Identification of specific subunits of highly purified bovine liver branched-chain ketoacid dehydrogenase. Biochemistry. 1983 Nov 22;22(24):5519–5522. doi: 10.1021/bi00293a011. [DOI] [PubMed] [Google Scholar]
- Ho L., Hu C. W., Packman S., Patel M. S. Deficiency of the pyruvate dehydrogenase component in pyruvate dehydrogenase complex-deficient human fibroblasts. Immunological identification. J Clin Invest. 1986 Sep;78(3):844–847. doi: 10.1172/JCI112651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama A., Nakamura S., Nishizuka Y., Hayaishi O. Enzymic studies on the biosynthesis of serotonin in mammalian brain. J Biol Chem. 1970 Apr 10;245(7):1699–1709. [PubMed] [Google Scholar]
- Ide S., Hayakawa T., Okabe K., Koike M. Lipoamide dehydrogenase from human liver. J Biol Chem. 1967 Jan 10;242(1):54–60. [PubMed] [Google Scholar]
- Jinno Y., Akaboshi I., Katsuki T., Matsuda I. Study on established lymphoid cells in maple syrup urine disease. Correlation with clinical heterogeneity. Hum Genet. 1984;65(4):358–361. doi: 10.1007/BF00291560. [DOI] [PubMed] [Google Scholar]
- Jinno Y., Akaboshi I., Matsuda I. Complementation analysis in lymphoid cells from five patients with different forms of maple syrup urine disease. Hum Genet. 1984;68(1):54–56. doi: 10.1007/BF00293872. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson R., Cook K. G., Yeaman S. J. Rapid purification of bovine kidney branched-chain 2-oxoacid dehydrogenase complex containing endogenous kinase activity. FEBS Lett. 1983 Jun 27;157(1):54–58. doi: 10.1016/0014-5793(83)81115-6. [DOI] [PubMed] [Google Scholar]
- Loewenstein J., Scholte H. R., Wit-Peeters E. M. A rapid and simple procedure to deplete rat-liver mitochondria of lysosomal activity. Biochim Biophys Acta. 1970 Dec 8;223(2):432–436. doi: 10.1016/0005-2728(70)90201-x. [DOI] [PubMed] [Google Scholar]
- Lyons L. B., Cox R. P., Dancis J. Complementation analysis of maple syrup urine disease in heterokaryons derived from cultured human fibroblasts. Nature. 1973 Jun 29;243(5409):533–535. doi: 10.1038/243533a0. [DOI] [PubMed] [Google Scholar]
- MENKES J. H., HURST P. L., CRAIG J. M. A new syndrome: progressive familial infantile cerebral dysfunction associated with an unusual urinary substance. Pediatrics. 1954 Nov;14(5):462–467. [PubMed] [Google Scholar]
- MORRIS M. D., LEWIS B. D., DOOLAN P. D., HARPER H. A. Clinical and biochemical observations on an apparently nonfatal variant of branched-chain ketoaciduria (maple syrup urine disease). Pediatrics. 1961 Dec;28:918–923. [PubMed] [Google Scholar]
- Mackall J., Meredith M., Lane M. D. A mild procedure for the rapid release of cytoplasmic enzymes from cultured animal cells. Anal Biochem. 1979 May;95(1):270–274. doi: 10.1016/0003-2697(79)90216-1. [DOI] [PubMed] [Google Scholar]
- Matsuda I., Yamamoto J., Nagata N., Ninomiya N., Akaboshi I. Lysosomal enzyme activities in cultured lymphoid cell lines. Clin Chim Acta. 1977 Nov 1;80(3):483–486. doi: 10.1016/0009-8981(77)90141-3. [DOI] [PubMed] [Google Scholar]
- Matuda S., Shirahama T., Saheki T., Miura S., Mori M. Purification and immunochemical studies of pyruvate dehydrogenase complex from rat heart, and cell-free synthesis of lipoamide dehydrogenase, a component of the complex. Biochim Biophys Acta. 1983 Oct 13;741(1):86–93. doi: 10.1016/0167-4781(83)90013-1. [DOI] [PubMed] [Google Scholar]
- Pettit F. H., Yeaman S. J., Reed L. J. Purification and characterization of branched chain alpha-keto acid dehydrogenase complex of bovine kidney. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4881–4885. doi: 10.1073/pnas.75.10.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L. J., Damuni Z., Merryfield M. L. Regulation of mammalian pyruvate and branched-chain alpha-keto acid dehydrogenase complexes by phosphorylation-dephosphorylation. Curr Top Cell Regul. 1985;27:41–49. doi: 10.1016/b978-0-12-152827-0.50011-6. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Taylor J., Sherwood W. G. Deficiency of dihydrolipoyl dehydrogenase (a component of the pyruvate and alpha-ketoglutarate dehydrogenase complexes): a cause of congenital chronic lactic acidosis in infancy. Pediatr Res. 1977 Dec;11(12):1198–1202. doi: 10.1203/00006450-197712000-00006. [DOI] [PubMed] [Google Scholar]
- Schulman J. D., Lustberg T. J., Kennedy J. L., Museles M., Seegmiller J. E. A new variant of maple syrup urine disease (branched chain ketoaciduria). Clinical and biochemical evaluation. Am J Med. 1970 Jul;49(1):118–124. doi: 10.1016/s0002-9343(70)80121-8. [DOI] [PubMed] [Google Scholar]
- Scriver C. R., Mackenzie S., Clow C. L., Delvin E. Thiamine-responsive maple-syrup-urine disease. Lancet. 1971 Feb 13;1(7694):310–312. doi: 10.1016/s0140-6736(71)91041-5. [DOI] [PubMed] [Google Scholar]
- Seegmiller J. E. Collection and uses of lymphocyte cultures. Monogr Hum Genet. 1978;9:244–247. doi: 10.1159/000401645. [DOI] [PubMed] [Google Scholar]
- Shigematsu Y., Kikuchi K., Momoi T., Sudo M., Kikawa Y., Nosaka K., Kuriyama M., Haruki S., Sanada K., Hamano N. Organic acids and branched-chain amino acids in body fluids before and after multiple exchange transfusions in maple syrup urine disease. J Inherit Metab Dis. 1983;6(4):183–189. doi: 10.1007/BF02310879. [DOI] [PubMed] [Google Scholar]
- Singh S., Willers I., Goedde H. W. Heterogeneity in maple syrup urine disease: aspects of cofactor requirement and complementation in cultured fibroblasts. Clin Genet. 1977 Apr;11(4):277–284. doi: 10.1111/j.1399-0004.1977.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Skaper S. D., Molden D. P., Seegmiller J. E. Maple syrup urine disease: branched-chain amino acid concentrations and metabolism in cultured human lymphoblasts. Biochem Genet. 1976 Aug;14(7-8):527–539. doi: 10.1007/BF00485832. [DOI] [PubMed] [Google Scholar]
- Stanley C. J., Perham R. N. Purification of 2-oxo acid dehydrogenase multienzyme complexes from ox heart by a new method. Biochem J. 1980 Oct 1;191(1):147–154. doi: 10.1042/bj1910147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp L. R., Reed L. J. Active-site modification of mammalian pyruvate dehydrogenase by pyridoxal 5'-phosphate. Biochemistry. 1985 Dec 3;24(25):7187–7191. doi: 10.1021/bi00346a026. [DOI] [PubMed] [Google Scholar]
- Taylor J., Robinson B. H., Sherwood W. G. A defect in branched-chain amino acid metabolism in a patient with congenital lactic acidosis due to dihydrolipoyl dehydrogenase deficiency. Pediatr Res. 1978 Jan;12(1):60–62. doi: 10.1203/00006450-197801000-00018. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Baugher B. W., Mattes P. M., Daddona P. E., Kelley W. N. Human hypoxanthine-guanine phosphoribosyltransferase. Demonstration of structural variants in lymphoblastoid cells derived from patients with a deficiency of the enzyme. J Clin Invest. 1982 Mar;69(3):706–715. doi: 10.1172/JCI110499. [DOI] [PMC free article] [PubMed] [Google Scholar]